Abstract
Subtraction hybridization identified genes displaying differential expression as meta-static human melanoma cells terminally differentiated and lost tumorigenic properties by treatment with recombinant fibroblast interferon and mezerein. This approach permitted cloning of multiple genes displaying enhanced expression when melanoma cells terminally differentiated, called melanoma differentiation associated (mda) genes. One mda gene, mda-7, has risen to the top of the list based on its relevance to cancer and now inflammation and other pathological states, which based on presence of a secretory sequence, chromosomal location, and an IL-10 signature motif has been named interleukin-24 (MDA-7/IL-24). Discovered in the early 1990s, MDA-7/IL-24 has proven to be a potent, near ubiquitous cancer suppressor gene capable of inducing cancer cell death through apoptosis and toxic autophagy in cancer cells in vitro and in preclinical animal models in vivo. In addition, MDA-7/IL-24 embodied profound anti-cancer activity in a Phase I/II clinical trial following direct injection with an adenovirus (Ad.mda-7; INGN-241) in tumors in patients with advanced cancers. In multiple independent studies, MDA-7/IL-24 has been implicated in many pathological states involving inflammation and may play a role in inflammatory bowel disease, psoriasis, cardiovascular disease, rheumatoid arthritis, tuberculosis, and viral infection. This review provides an up-to-date review on the multifunctional gene mda-7/IL-24, which may hold potential for the therapy of not only cancer, but also other pathological states.
1. INTRODUCTION
Melanoma differentiation associated gene-7 (MDA-7), also known as interleukin-24 (IL-24), is a secreted cytokine and a member of the IL-10 gene family. Although MDA-7/IL-24 was discovered several decades ago, new discoveries of the role that MDA-7/IL-24 plays in normal physiology as well as in multiple human pathologies are still unfolding. So far, researchers have confirmed that MDA-7/IL-24 is not only involved in normal immune function and wound healing, but it also has several additional beneficial effects in a variety of human diseases. As examples, MDA-7/IL-24 functions as an anticancer gene in multiple diverse cancers including melanoma (Lebedeva et al., 2002; Sarkar et al., 2008), prostate cancer (Greco et al., 2010; Lebedeva, Sarkar, et al., 2003; Lebedeva, Su, Sarkar, & Fisher, 2003), breast cancer (Bhutia et al., 2013; Menezes et al., 2015; Pradhan et al., 2017; Sarkar et al., 2005), osteosarcoma (Zhuo et al., 2017), neuroblastoma (Bhoopathi et al., 2016), pancreatic cancer (Sarkar, Quinn, Shen, Dent, et al., 2015), renal carcinoma (Park et al., 2009), leukemia (Rahmani et al., 2010), lung cancer (Lv, Su, Liang, Hu, & Yuan, 2016; Shapiro et al., 2016), esophageal squamous cell carcinoma (Ma, Jin, et al., 2016), and hepatocellular carcinoma (Wang, Ye, Zhong, Xiang, & Yang, 2007). MDA-7/IL-24 provides protection against autoimmune diseases and bacterial infections (Leng, Pan, Tao, & Ye, 2011; Ma et al., 2009). MDA-7/IL-24 is also relevant in inflammation (Pasparakis, Haase, & Nestle, 2014), rheumatoid arthritis (RA) (Kragstrup et al., 2008), and cardiovascular diseases (Vargas-Alarcon et al., 2014). In this review, we discuss in detail the roles of MDA-7/IL-24 in both normal physiology as well as the various disease states mentioned earlier. We begin with a discussion of the characteristic features of MDA-7/IL-24 that allows this molecule to play a key role in normal cellular function as well as contributing to a variety of disease states.
2. CHARACTERISTIC FEATURES OF MDA-7/IL-24
We begin with an overview of the initial cloning of the MDA-7/IL-24 gene, followed by its structure, isoforms, and modifications that have helped enhance MDA-7/IL-24 potency. We then discuss the receptors that MDA-7/IL-24 utilizes for cellular signaling.
2.1. Identification of MDA-7/IL-24
As the name suggests, MDA-7 was initially identified and cloned from terminally differentiating human melanoma cells in the Fisher laboratory by Jiang in 1993 and reported in detail in 1995 (Jiang & Fisher, 1993; Jiang, Lin, Su, Goldstein, & Fisher, 1995). HO-1 human metastatic melanoma cells were treated with a combination of recombinant human fibroblast interferon (IFN-beta) and mezerein (MEZ) to induce terminal differentiation and suppression of growth and tumorigenic abilities. Next subtraction hybridization of cDNA libraries was performed to assess genes that were differentially expressed in melanoma cells before and after terminal differentiation (Jiang & Fisher, 1993). MDA-7 was identified as one of the transcripts that was induced in terminally differentiating melanoma cells (Jiang et al., 1995; Jiang & Fisher, 1993). In subsequent years, MDA-7 was found to have tumor suppressive abilities against several different cancer indications, while leaving normal counterparts unharmed (Jiang et al., 1996; Su et al., 1998). In 2001, Huang and colleagues in the Fisher laboratory identified the genomic structure and chromosomal localization of MDA-7 (Huang et al., 2001). They determined that MDA-7 was located in a region of the chromosome that contained a cluster of genes associated with the IL-10 cytokine family (Huang et al., 2001). MDA-7 also had an IL-10 signature sequence and was specifically expressed in tissues associated with the immune system including the spleen, thymus, and peripheral blood leukocytes (Huang et al., 2001). Given the conserved chromosomal location, presence of a putative secretory motif, an IL-10 signature sequence and the expression profile of MDA-7, the Human Gene Organization (HUGO) designated this gene as IL-24 (Sarkar et al., 2002a). Additionally in 2002, Caudell and colleagues provided evidence that MDA-7/IL-24 had functional immunostimulatory attributes justifying its designation as an interleukin (Caudell et al., 2002).
2.2. Structure of MDA-7/IL-24
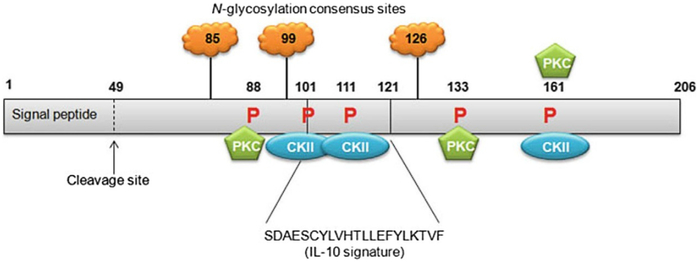
Located on chromosome 1q32–33 in humans, MDA-7/IL-24 is a secreted cytokine that belongs to the IL-10 gene family (Caudell et al., 2002; Huang et al., 2001). MDA-7/IL-24 contains seven exons and six introns. The cDNA of MDA-7/IL-24 is 1718 base pairs and the protein encodes 206-amino acids (Huang et al., 2001). Being a secreted cytokine, MDA-7/IL-24 has a 49-amino acid N-terminal hydrophobic signal peptide that allows for protein secretion (Fig. 1). Sauane and colleagues in the Fisher laboratory utilized the Prosite database to analyze the peptide sequence of MDA-7/IL-24 and identified three putative N-glycosylation sites at amino acids 85, 99, and 126 (Sauane, Gopalkrishnan, Sarkar, et al., 2003). In addition, an IL-10 signature motif was identified from amino acids 101–121; three protein kinase C consensus phosphorylation sites were identified at amino acids 88, 133, and 161; and three casein kinase II consensus phosphorylation sites were identified at amino acids 101, 111, and 161 using this database (Sauane, Gopalkrishnan, Sarkar, et al., 2003). The predicted tertiary structure of MDA-7/IL-24 is that of a compact globular molecule comprised of four strongly helical regions interspersed by loops of unpredicted structure (Sauane, Gopalkrishnan, Sarkar, et al., 2003). MDA-7/IL-24 can also form N-linked glycosylated dimers through intermolecular disulfide bonds and these dimers are functionally active (Mumm, Ekmekcioglu, Poindexter, Chada, & Grimm, 2006).
Fig. 1.
Schematic representation of MDA-7/IL-24 protein with predicted and established domains and protein modification sites indicated. Cleavage of the 49-amino acid signal peptide allows for secretion of the MDA-7/IL-24 protein. The IL-10 signature sequence is located between amino acids 101 and 121. N-glycosylation can occur at amino acids 85, 99, and 126. Protein kinase C consensus phosphorylation sites are present at amino acids 88, 133, and 161. Casein kinase II (CKII) consensus phosphorylation sites are present at amino acids 101, 111, and 161. Numbers indicate amino acids. Not drawn to scale. Figure reproduced from Menezes, M. E., Bhatia, S., Bhoopathi, P., Das, S. K., Emdad, L., Dasgupta, S., et al. (2014). MDA-7/IL-24: Multifunctional cancer killing cytokine. Advances in Experimental Medicine and Biology, 818, 127–153.
2.3. Splice Variants/Isoforms of MDA-7/IL-24
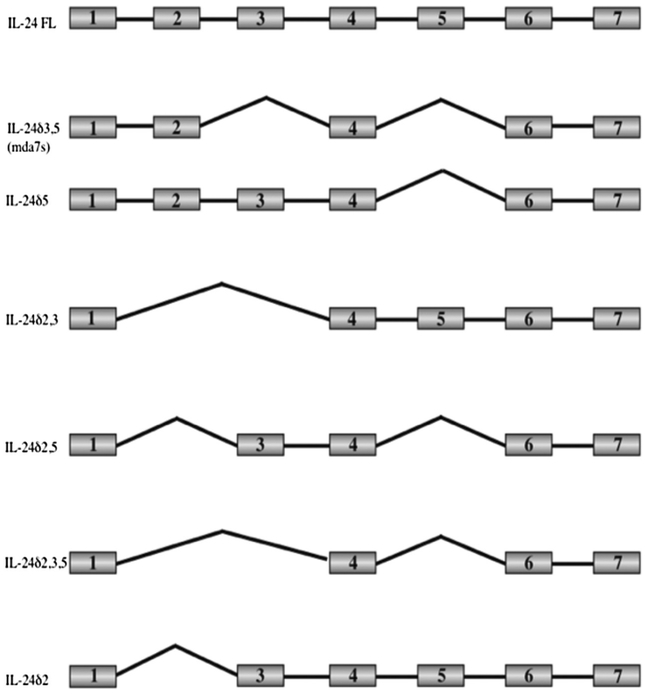
Allen and colleagues identified a splice variant of MDA-7/IL-24, mda-7s, which lacked exons 3 and 5 (Allen et al., 2004). They observed that MDA-7S could heterodimerize with full-length MDA-7/IL-24 but noted that this interaction did not affect the apoptotic abilities of MDA-7/IL-24 in melanoma cells. Since the expression of mda-7s was reduced or absent in melanoma as compared to normal melanocytes, the authors also suggested an association between loss of mda-7s and metastatic melanoma (Allen et al., 2004). This same group also identified and published a short study on the presence of two splice variants that lacked exons 3 and 5, respectively, that were expressed in normal human melanocytes but not in metastatic melanoma (Allen et al., 2005). Filippov and colleagues identified splice isoforms of MDA-7/IL-24 while studying the effects of a ubiquitous splicing factor SRp55, that is upregulated by DNA damage in the absence of p53 and whose inactivation enhanced DNA damage resistance in a p53-dependent manner (Filippov, Schmidt, Filippova, & Duerksen-Hughes, 2008). U2OS human osteosarcoma cells treated with siRNA to SRp55 were assessed using a splice-specific microarray analysis to identify the relevance of SRp55 on the splicing patterns of genes involved in apoptosis. At least four isoforms of MDA-7 were identified, out of which one isoform (that lacks exons 2 and 3) was sensitive to splicing by SRp55 and silencing SRp55 splicing activity caused an increase in this isoform. In a follow-up study, Whitaker and colleagues identified and characterized five alternatively spliced isoforms of MDA-7/IL-24 (Whitaker, Filippov, Filippova, Guerrero-Juarez, & Duerksen-Hughes, 2011). Overall, they observed six differentially spliced transcripts of MDA-7/IL-24 in addition to the full-length transcript (Fig. 2). The splice variants identified were: mda-7/IL-24δ3,5—lacking exons 3 and 5 (described and characterized previously by Allen et al., 2004); mda-7/IL-24δ5—lacking exon 5; mda-7/IL-24δ2,3—lacking exons 2 and 3; mda-7/IL-24δ2,5—lacking exons 2 and 5; mda-7/IL-24δ2,3,5—lacking exons 2, 3, and 5; and mda-7/IL-24δ2—lacking exon 2. All seven exons were present in the full-length transcript. An important point to note is that the expression and distribution of mda-7/IL-24 isoforms might vary based on different cell types. Full-length MDA-7/IL-24 as well as spliced isoforms δ5, δ2,3,5, δ2,5, and δ2 were capable of reducing U2OS cell viability with no effect on the viability of noncancerous immortalized NOK cells (Whitaker et al., 2011). Interestingly, apoptosis was higher in U2OS cells expressing mda-7/IL-24δ2,3,5 than cells expressing full-length MDA-7/IL-24.
Fig. 2.
Schematic representation of the splice isoforms of MDA-7/IL-24. Figure reproduced from Whitaker, E. L., Filippov, V., Filippova, M., Guerrero-Juarez, C. F., Duerksen-Hughes, P. J. (2011). Splice variants of mda-7/IL-24 differentially affect survival and induce apoptosis in U2OS cells. Cytokine, 56, 272–281.
2.4. Deletions, Modifications, and Enhancing Stability of MDA-7/IL-24
In an effort to identify the molecular basis of tumor cell selectivity of MDA-7/IL-24, Gupta and colleagues in the Fisher laboratory constructed several amino terminal deletion mutants of MDA-7/IL-24 and labeled them M1–M6 (Gupta, Walter, et al., 2006). The signal peptide was deleted in M1; α-helical domain A was disrupted in M2; α-helical domain B was disrupted in M3; α-helical domains C, D, E, and F were present in M4; α-helical domains D, E, and F were present in M5; and α-helical domains E and F were present in M6. As would be expected, deletion of the signal peptide (M1) did not disrupt the tumor inhibitory effects of MDA-7/IL-24. Interestingly, however, all the other deletions, except M4, caused a loss of tumor inhibitory effects. M4 showed tumor suppressive effects in HeLa and DU-145 cells but did not affect normal prostate epithelial P69 cells and was capable of inducing cancer cell-specific apoptosis (Gupta, Walter, et al., 2006).
MDA-7/IL-24 protein gets ubiquitinated and degraded via the 26S proteasome. In order to determine the exact site of ubiquitination, Tian and colleagues mutated each of the 10 lysine sites within the MDA-7/IL-24 protein and converted them to arginine (Tian et al., 2012). They suggested lysine 123 as the critical internal lysine involved in MDA-7/IL-24 ubiquitination. They report that further conversion of lysine 123 to arginine enhanced MDA-7/IL-24 protein stability as well as tumor suppressive abilities (Tian et al., 2012). Based on the original sequence of the MDA-7/IL-24 protein, as reported in the database, the lysine site at 122 is the key site, which when we changed to arginine resulted in enhanced stability of the MDA-7/IL-24 protein (M. Menezes et al., unpublished data).
2.5. Receptors of MDA-7/IL-24
IL-10 cytokine family members signal through receptor dimers that consist of an R1 type receptor (with a long cytoplasmic domain) and an R2 type receptor (with a short cytoplasmic domain). The IL-10 cytokine family of receptors has three R1 and two R2 subunits. The R1 subunits are IL-10R1, IL-20R1, and IL-22R1 and the R2 subunits are IL-20R2 and IL-10R2. In order to identify the receptors of MDA-7/IL-24, Wang and colleagues utilized a biochemical approach using IL-24 affinity tagged to the secreted human placental alkaline phosphatase (IL-24-AP) (Wang, Tan, Zhang, Kotenko, & Liang, 2002). They observed that MDA-7/IL-24 utilized two heterodimeric receptors, IL-22R1/IL-20R2 and IL20-R1/IL-20R2, to activate downstream signaling (Wang et al., 2002). Dumoutier and colleagues utilized ligand-dependent STAT (signal transducer and activator of transcription) activation as readout for receptor activation and independently identified these same receptors (Dumoutier, Leemans, Lejeune, Kotenko, & Renauld, 2001). More recent studies by Dash and colleagues in the Fisher laboratory demonstrated that MDA-7/IL-24 can also signal and induce growth suppression and apoptosis in a cancer-selective manner using the IL-20R1/IL22-R1 heterodimeric receptors (Dash et al., 2014; Pradhan et al., 2017). However, the mechanism through which these two R1 receptor dimers to promote signaling after interacting with MDA-7/IL-24 remains to be determined.
3. PHYSIOLOGICAL ROLE OF MDA-7/IL-24
Extensive studies were performed to understand the role of MDA-7/IL-24 in cancer, however, our understanding of the physiological role of MDA-7/IL-24 is fairly limited. In the following section, we discuss the cellular source of MDA-7/IL-24 and its functions in normal physiology.
3.1. Naturally Occurring Cellular Source of MDA-7/IL-24
MDA-7/IL-24 can be produced by immune cells (myeloid cells and lymphoid cells and monocytes) in response to treatment with lipopolysaccha-rides or specific cytokines (Buzas, Oppenheim, & Zack Howard, 2011). Physiological levels of MDA-7/IL-24 are induced in Th2 lymphocytes by stimulation with phorbol myristate acetate and ionomycin and in T cells, especially CD4+ naïve and memory cells activated by anti-CD3 monoclonal antibody (Sahoo et al., 2011; Schaefer, Venkataraman, & Schindler, 2001). In monocytes, MDA-7/IL-24 is induced by antigenic stimulation with lipopolysaccharide, concanavalin A, and cytokines (Caudell et al., 2002; Wolk et al., 2004). B-cell receptor signaling also triggers MDA-7/IL-24 expression in B-lymphocytes (Maarof et al., 2010). Nonlymphoid cells can also produce physiological levels of MDA-7/IL-24 in response to cytokines secreted by immune cells (Persaud et al., 2016). Several in vitro and in vivo studies established that epithelial cells when stimulated with cytokines can secret MDA-7/IL-24 (Buzas et al., 2011; Persaud et al., 2016; Whitaker, Filippov, & Duerksen-Hughes, 2012). Additionally, IL-1 can stimulate MDA-7/IL-24 expression in keratinocytes and human colon cells (Andoh et al., 2009). Basal expression of MDA-7/IL-24 at physiological levels is found in melanocytes and expression gradually decreases as the melanocytes begin to transform into metastatic melanoma (Ekmekcioglu et al., 2001; Ellerhorst et al., 2002; Jiang et al., 1995).
3.2. MDA-7/IL-24 Function Under Physiological Conditions
MDA-7/IL-24 is produced by various immune cells and exerts a range of immune functions (Persaud et al., 2016). At lower physiological concentrations, MDA-7/IL-24 mainly functions as a cytokine. MDA-7/IL-24, when secreted, interacts with distinct sets of receptors including IL-20R1/IL-20R2, IL-22R1/IL-20R2, or IL-22R1/IL-20R1 receptor complexes (Dash et al., 2014; Dumoutier et al., 2001; Wang & Liang, 2005; Wang et al., 2002). Most immune cells lack the cognate pairs of receptors and chiefly express IL-20R2. One study by Caudell and colleagues assessed the secretion profile of peripheral blood mononuclear cells treated with MDA-7/IL-24 protein, which showed increased secretion of immune modulatory cytokines such as IL-6, IL-1β, IFN-γ, TNFα, IL-12, and GM-CSF (Caudell et al., 2002). The enhanced secretion of IFN-γ in turn upregulates IL-22R1 expression in keratinocytes, which facilitates formation of IL-22R1/IL-20R2 receptor pairs and induces innate immunity responses (Wolk et al., 2004). In addition to immune functions, MDA-7/IL-24 also induces several additional changes in normal skin cells. He and colleagues developed transgenic mice, which overexpress MDA-7/IL-24 specifically in skin (He & Liang, 2010). This genetically modified mouse is embryonic lethal and exhibits epidermal hyperplasia and abnormal keratinocyte differentiation. In contrast, treatment of human keratinocytes with MDA-7/IL-24 in a wound-healing model results in suppression of keratinocyte proliferation, suggesting a potential therapeutic role of this cytokine in proliferating skin lesions (Liang et al., 2011; Poindexter et al., 2010). MDA-7/IL-24 also impedes B-cell maturation to plasma cells by regulating several transcription factors, which are important for plasma cell differentiation (Maarof et al., 2010). Additionally, MDA-7/IL-24 plays a diverse role in proinflammatory, infectious, and autoimmune skin diseases, which is discussed in further detail below (Persaud et al., 2016).
Apart from these immune and dermatologic functions, several studies have also reported other biological functions of MDA-7/IL-24 in vascular diseases and inflammatory bowel disease (IBD) (Persaud et al., 2016). MDA-7/IL-24 is also expressed in normal cultured fetal membranes, suggesting a potential role in normal pregnancy (Nace, Fortunato, Maul, & Menon, 2010).
4. FUNCTIONAL ROLE OF MDA-7/IL-24 IN CANCER
The role of mda-7/IL-24 has been extensively studied in cancer. In this section, we describe briefly some of the important findings.
4.1. Stem Cells and Differentiation
Tumors are comprised of heterogeneous cell populations with diverse biological properties. Cancer stem cells are immortal cells within tumors which display the property of self-renewal. They can divide and differentiate to give rise to a heterogeneous cell population, in which subsets of cells can form distant tumors (Talukdar, Emdad, Das, Sarkar, & Fisher, 2016). Stem cells detach from the primary tumor, migrate, and generate tumors at distant sites. Cancer stem cells can relapse and metastasize making the need for specific therapies against them essential (Eyler & Rich, 2008; Talukdar et al., 2016). They are also resistant to conventional therapies and divide more rapidly (Morrison, Morris, & Steel, 2013).
mda-7/IL-24 inhibits the growth of breast cancer stem cells. Specifically, infection of Ad.mda-7 decreased proliferation of breast cancer initiating cells without harming normal stem cells (Bhutia, Das, et al., 2013). Over-expression of mda-7/IL-24 induces apoptosis and endoplasmic reticulum (ER) stress in sorted stem cell populations of breast cancer cells, which is similar to what is observed in unsorted breast cancer cells (Bhutia, Das, et al., 2013). Overexpression of mda-7/IL-24 also decreases the self-renewal capabilities of cancer stem cells. mda-7/IL-24 suppresses β-catenin/Wnt signaling (Chada et al., 2005; Sieger et al., 2004) and regulates the proliferation of stem cells. The Wnt/β catenin pathway is one of the key signaling pathways that promote self-renewal of stem cells (Xu et al., 2016). Wnt proteins interact with Frizzled and LRP receptors to signal β-catenin to activate Wnt target genes (MacDonald & He, 2012). It can also signal through ROR/RYK receptors as an alternative pathway (Green, Nusse, & van Amerongen, 2014). In cancer, these are dynamically expressed and this causes an imbalance in the proliferation and differentiation of cancer stem cells. Alteration of the β-catenin signaling pathway increases the survival of stem cells. This suggests that mda-7/IL-24-mediated blockage in proliferation of stem cells is facilitated through the β-catenin pathway. In a subcutaneous human tumor xenograft nude mouse model, injection of Ad.mda-7 inhibited the growth of subcutaneous tumors. Tumor growth inhibition is associated with inhibition in cellular proliferation and angiogenesis (Bhutia, Das, et al., 2013).
Overexpression of mda-7/IL-24 by an adenoviral system increased the expression of tumor suppressors including PTEN, E-cadherin, GSK-3β, and APC and downregulated protooncogenes involved in β-catenin and PI3K signaling (Gupta, Su, et al., 2006). β-Catenin translocates to the plasma membrane from the nucleus upon mda-7/IL-24 treatment, which reduces the transcriptional activity of TCF/LEF (Mhashilkar et al., 2003). This upregulates the expression of E-cadherin–β-catenin adhesion in a cancer-selective manner. In lung and breast cancer, mda-7/IL-24 regulates cell–cell adhesion by modulating these signaling cascades (Mhashilkar et al., 2003). These effects are not common in normal cells and are specific for cancer cells.
Ad.mda-7 downregulates the tendency of breast cancer cells to form mammospheres and also inhibits the formation of distant tumors (Bhutia, Das, et al., 2013). MDA-7/IL-24 regulates the PI3K/Akt pathway, decreases β-catenin phosphorylation, and proteosomal degradation pathways (Bhutia, Das, et al., 2013; Mhashilkar et al., 2003). Stem cells also display over-expression of Akt, Bcl-2, and Bcl-xL (Wang & Scadden, 2015). mda-7/IL-24 can induce apoptosis by downregulating Akt, Bcl-2, and Bcl-xL as described earlier (Fig. 3).
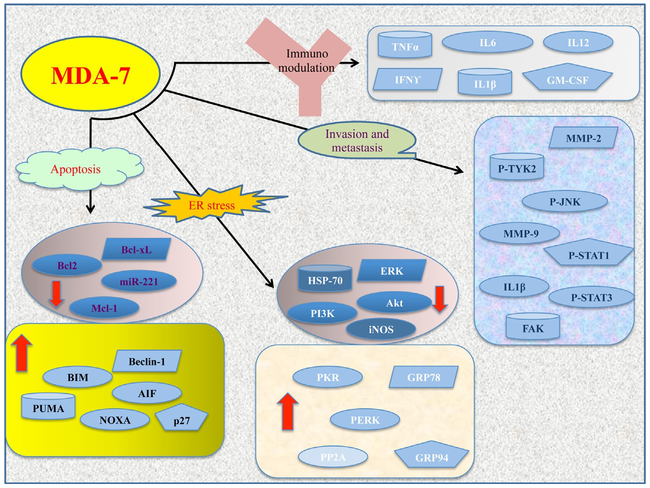
Fig. 3.
Schematic representation of the pathways regulated by MDA-7/IL-24. MDA-7/IL-24 regulates both pro- and antiapoptotic molecules to induce tumor-specific cell death. This involves a series of signaling events including downregulation of Mcl-1 and Bcl-xL and activation of tumor suppressors, i.e., SARI, PUMA, AIF, PERK, and others as shown in the figure. Also the cytokine induces ER stress and regulates a number of genes/proteins to block invasion and metastasis. MDA-7/IL-24 also modulates the immune pathways by deregulating a number of cytokines, which in turn activates the immune system to induce cytotoxic cell death.
4.2. Apoptosis
Programmed cell death or apoptosis plays a pivotal therapeutic role in cancer drug sensitivity (Naik, Karrim, & Hanahan, 1996). One of the hallmarks of cancer is apoptosis (Hanahan & Weinberg, 2000). It involves a series of signaling events that are disrupted in cancer. Cancer cells bypass the apoptotic signaling pathway and evade this mechanism of cell death (Fernald & Kurokawa, 2013). Much of the research focusing on cancer therapeutics involves the ability of the therapy to induce apoptosis specifically in cancer cells (Lebedeva, Sarkar, et al., 2003). Understanding the mechanism by which cancer cells evade the general apoptotic pathways is critical to develop new therapies against cancer.
mda-7/IL-24 regulates ER stress and the mitochondrial apoptotic pathway (Fisher, 2005; Gopalkrishnan, Sauane, & Fisher, 2004; Lebedeva, Sauane, et al., 2005; Lebedeva, Su, et al., 2005; Lebedeva, Su, Sarkar, Kitada, et al., 2003; Sauane et al., 2008; Sieger et al., 2004). Overexpression of mda-7/IL-24 has been shown to induce apoptosis in different cancer cells without any harmful effect to normal cells (reviewed in Fisher, 2005). This cancer cell-specific death is both time- and dose-dependent. The p38MAPK or mitogen protein kinase pathway is altered due to overexpression of mda-7/IL-24 (Sarkar et al., 2002b). SB203580, an inhibitor of the p38MAPK pathway, inhibits Ad.mda-7-induced apoptosis. This induces growth arrest and DNA damage genes leading to cell cycle arrest and cell death (Sarkar et al., 2002b). AIF-mediated apoptosis by mda-7/IL-24 has recently been demonstrated to occur uniquely in neuroblastoma (Bhoopathi et al., 2016). A recent study from our group showed that mda-7/IL-24 regulates a subset of microRNAs (Pradhan et al., 2017). One microRNA, miR-221, was downregulated following treatment with mda-7/IL-24. miR-221 targets PUMA, a proapoptotic gene, blocking apoptosis (Pradhan et al., 2017). mda-7/IL-24 downregulates miR-221, which in turn upregulates PUMA inducing cell death (Pradhan et al., 2017). mda-7/IL-24 downregulates the expression of antiapoptotic proteins Mcl-1, Bcl-xL, and Bcl-2, while inducing proapoptotic proteins such as Bid, Bim, Bax, and Bak (Menezes et al., 2014). In so doing, mda-7/IL-24 increases the Bax/Bcl-2 ratio (Pei et al., 2012). Previous studies also demonstrated a role of PERK in mda-7/IL-24-mediated cell death (Park et al., 2008).
Enhanced expression of mda-7/IL-24 induces the production of reactive oxygen species (ROS), which regulates multiple signaling cascades deregulating the mitochondrial integrity and cell death (Dent et al., 2010; Lebedeva, Su, et al., 2005; Lebedeva, Su, Sarkar, Kitada, et al., 2003). The role of ROS in mda-7/IL-24-mediated cell death is well established. N-acetyl cysteine (Nace et al., 2010) inhibits cell death mediated by mda-7/IL-24 (Lebedeva, Sauane, et al., 2005). Simultaneously, ROS inducers enhance cell death mediated by mda-7/IL-24 (Lebedeva, Su, et al., 2005; Sauane et al., 2008). These results confirm the role of ROS and mitochondrial membrane potential as an important component in cell death promoted in cancer cells by the cytokine MDA-7/IL-24.
mda-7/IL-24 also upregulates SARI, a tumor suppressor, which is cancer specific (Dash et al., 2014). Ectopic expression of mda-7/IL-24 induces SARI mRNA and protein in a broad panel of cancer cells (Dash et al., 2014). SARI expression is required for the antitumor effects of mda-7/IL-24. Recombinant MDA-7/IL-24 protein also induces SARI expression through binding to its cognate receptors, IL-22R1/IL-20R2 or IL20-R1/IL-20R2 (Dash et al., 2014).
The FasL signaling pathway is another pathway activated by Ad. mda-7, which results in cancer cell-selective apoptosis (Gopalan et al., 2005). Ad.mda-7 induces activation of the transcription factors c-Jun and activating transcription factor 2 which then activates downstream target, FasL-Fas (Gopalan et al., 2005). siRNA targeting Fas decreased mda-7/IL-24-induced cell death in ovarian cancer cells (Gopalan et al., 2005). This work reveals a role of mda-7/IL-24 in regulating the Fas-FasL signaling cascade to induce cancer cell death.
mda-7/IL-24 upregulates PKR (serine/threonine protein kinase) in non-small cell lung cancer, which is independent of p53 expression (Mhashilkar et al., 2003). The regulation of PKR by mda-7/IL-24 is posttranscriptional (Gupta, Su, et al., 2006). Exogenous recombinant mda-7/IL-24 also induces PKR and mda-7/IL-24 interacts with PKR in cancer cells (Pataer et al., 2005).
Apoptosis mediated by mda-7/IL-24 is independent of p53 mutations and functions (Gupta, Su, et al., 2006; Su et al., 2003). It is established that mda-7/IL-24 induces apoptosis in diverse breast cancer cells, i.e., MCF7 (p53-wt), MDA-MB-231 (mutant p53), MDA-MB-453 (mutant p53), and T47D (mutant p53) (Chada et al., 2006). Based on the different genetic backgrounds, these results indicate that cell death induction by mda-7/IL-24 is also independent of ER/PR/HER2 status in breast cancer cells. In these contexts, mda-7/IL-24-induced apoptosis is distinct from other identified tumor suppressors.
Secreted MDA-7/IL-24 protein binds to its cognate receptor pairs and induces phosphorylation and nuclear translocation of STAT3 (Chada, Mhashilkar, et al., 2004). This receptor interaction induces BAX protein leading to cell death (Gupta, Su, et al., 2006). This process is STAT3-independent as other interleukins (IL-10, IL-19, IL-20, and IL-22) also activate STAT3 without promoting cell death (Mosser & Zhang, 2008). mda-7/IL-24 binds IL-20/IL-22 receptor complexes resulting in activation of the JAK/STAT cascade. Studies have shown that mda-7/IL-24 induces apoptosis of cancer cells independent of the JAK/STAT pathway (Sauane, Gopalkrishnan, Lebedeva, et al., 2003). Specifically, inhibitors of JAK/STAT pathway do not inhibit apoptosis mediated by mda-7/IL-24 (Sauane, Gopalkrishnan, Lebedeva, et al., 2003). These results demonstrate that mda-7/IL-24 functions independent of tyrosine kinase activation.
4.3. Autophagy
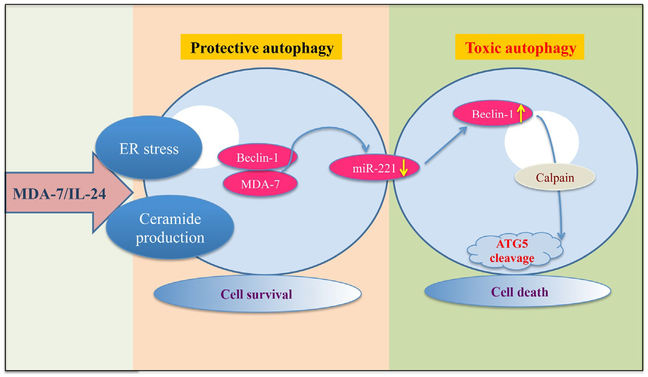
Autophagy is the process of degradation of organelles located in the cytoplasm. This process is complex due to its differential context dependent role. “Is autophagy good or bad for life and cancer?” is a difficult question to answer (Bhutia, Mukhopadhyay, et al., 2013). Sometimes it is protective, helping cancer cells to survive adverse conditions but it can also be toxic toward cancer cells (Bhutia, Mukhopadhyay, et al., 2013; Liu & Debnath, 2016) (Fig. 4). Small molecules that can control autophagy may in certain contexts provide therapeutic benefit. mda-7/IL-24 induces autophagy, which is mediated by PERK (Park et al., 2008) and Beclin-1 (Bhutia et al., 2010). mda-7/IL-24 regulates a subset of microRNAs, including the oncogenic microRNA, miR-221 (Pradhan et al., 2017). Beclin-1 was identified as a new transcriptional target of miR-221 (Pradhan et al., 2017). mda-7/IL-24 downregulates miR-221, which in turn induces beclin-1, leading to autophagy (Pradhan et al., 2017). Cleavage of LC3, a marker of autophagy, is also observed. In renal and ovarian cancers, CD95 is an important regulatory molecule in the induction of autophagy mediated by mda-7/IL-24 (Park et al., 2009).
Fig. 4.
Model depicting the molecular mechanism of MDA-7/IL-24-mediated autophagy induction. MDA-7/IL-24 regulates autophagy mediated through ER stress and ceramide production. Also MDA-7/IL-24 downregulates miR-221, which in turn upregulates Beclin-1 to induce toxic autophagy leading to cell death. The transition of protective to toxic autophagy is mediated by the cleavage of ATG5 by Calpain.
4.4. Angiogenesis
Cancer and metastatic spread depends on an adequate supply of nutrients and oxygen to cells (Welch & Fisher, 2016). Additionally, removal of waste products also requires new blood and lymph vessels. The process of formation of new blood vessels is called angiogenesis, which represents another hallmark of cancer (Hanahan & Weinberg, 2011). Angiogenesis is regulated by a number of activator and inhibitor molecules. Although not as effective as anticipated when used as a single agent, angiogenesis inhibitors combined with other therapeutic agents are showing promise in the treatment of various cancers.
Overexpression of mda-7/IL-24 in human umbilical vascular endothelial cells inhibits endothelial cell differentiation (Dash et al., 2010; Wang, Wang, Chen, & Lv, 2016). Similarly, treatment of tumor xenografts with mda-7/IL-24 reduces expression of angiogenesis markers (Bhutia et al., 2012). Vascular endothelial growth factor and basic fibroblast growth factor, which induce angiogenesis, are inhibited by MDA-7/IL-24 protein (Nishikawa, Ramesh, Munshi, Chada, & Meyn, 2004). The PI3K/Akt pathway is another signaling cascade known to regulate angiogenesis (Karar & Maity, 2011), and mda-7/IL-24 downregulates phospho Akt and can therefore negatively modulate angiogenesis (Dash et al., 2010).
4.5. Invasion and Metastasis
mda-7/IL-24 has been shown to impede the migration of cancer cells (Ramesh et al., 2004). Also, overexpression of mda-7/IL-24 results in a decrease in the in vitro invasion of an array of different cancer cell types (Ramesh et al., 2004). Lung cancer cells showed an inhibition in migration and invasion by modulating a number of signaling cascades following over-expression of mda-7/IL-24 (Panneerselvam et al., 2015). Focal adhesion kinase (FAK) and matrix metalloproteinases (MMPs) play a critical role in migration and invasion of cells (Hauck, Hsia, & Schlaepfer, 2002; Lin et al., 2000). mda-7/IL-24 downregulates FAK and MMP-2/MMP-9 protein, which indirectly inhibits migration and invasion of cancer cells (Menezes et al., 2014; Ramesh et al., 2004). mda-7/IL-24 has been shown to promote potent antiinvasive activity in lung cancer cells, cervical cancer cells, and liver cancer cells (Emdad et al., 2009; Lebedeva et al., 2007). mda-7/IL-24 regulates a number of molecules related to metastasis, i.e., cyclin-B1, TGF-β, Survinin, Twist, ICAM-1, and CD44 (Huo, Li, Zhu, Bao, & An, 2013). Also, E-cadherin, NF-κB, and PERK are regulated by mda-7/IL-24 (Panneerselvam, Munshi, & Ramesh, 2013). mda-7/IL-24-mediated inhibition in invasion and metastasis is both receptor-dependent and receptor-independent (Menezes et al., 2014).
4.6. Synergistic Effects
Cancer is a complex process that is mediated by multiple genetic and epigenetic changes that impact directly and indirectly on a number of pivotal signaling pathways involved in cell growth, survival, resistance to apoptosis, and additional physiologically relevant processes (Hanahan & Weinberg, 2000, 2011). Considering this complexity, it is not surprising that a single targeting molecule fails to provide complete therapy resulting in a cure in most cancers. Conversely, a combinatorial approach using multiple target-selective agents directed toward specific signaling abnormalities in defined cancers has shown promise in cancer therapy.
Based on Phase I/II clinical studies, mda-7/IL-24 has been shown to have a therapeutic role in cancer (Cunningham et al., 2005; Fisher et al., 2003, 2007; Tong et al., 2005). Also, preclinical studies have confirmed synergistic therapeutic responses when mda-7/IL-24 is combined with existing therapies, including radiation, chemotherapy, antibody-based therapies, small molecule, and immunotherapies (Table 1). The mechanisms underlying this synergy include the regulation of similar pathways as well as different pathways by mda-7/IL-24. This is tabulated in Table 1.
Table 1.
Combinatorial Enhancement of Therapy by Combining mda-7/IL-24 With Other Therapeutic Modalities
| Therapeutic Agent | Cancer Types | References |
|---|---|---|
| Trastuzumab | Breast cancer | McKenzie et al., (2004) |
| Bevacizumab | Lung cancer | Inoue et al. (2007) |
| Erlotinib | Melanoma | Deng, Kwon, Ekmekcioglu, Poindexter, and Grimm (2011) |
| Gefitinib | NSCLC | Emdad, Lebedeva, Su, Gupta, et al. (2007) |
| Temozolamide | Glioblastoma | Hamed, Yacoub, Park, Eulitt, Dash, et al. (2010) |
| Tarceva | NSCLC | Gupta et al. (2008) |
| Arsenic trioxide | Renal carcinoma | Yacoub et al. (2003) |
| Cisplatin | Liver, colorectal | Wu et al. (2009) |
| Sabutoclax (Mcl-1 inhibitor) | Prostate | Dash et al. (2011) |
| Sabutoclax (Mcl-1 inhibitor) | Colorectal | Azab et al. (2012) |
| BI-97D6 (Mcl-1 inhibitor) | Prostate | Sarkar, Quinn, Shen, Dash, et al. (2015) |
| Grpl70 | Prostate | Gao et al. (2008) |
| Radiation | Prostate | Su et al. (2006) |
| BI-69A11 | Colon | Pal et al. (2014) |
| 5-FU | Esophageal | Ma et al. (2014) |
| HSP90 inhibitors | Pancreatic | Zhang et al. (2013) |
| HDAC inhibitors | Renal carcinoma | Hamed, Das, et al. (2013) |
| HDAC inhibitors | Glioblastoma | Hamed, Yacoub, et al. (2013) |
| 5-FU, doxorubicin | Colon | Xu et al. (2013) |
| Sorafenib | Renal carcinoma | Eulitt et al. (2010) |
| Doxorubicin | Hepatocellular carcinoma | Wang et al. (2010) |
| Dichloroacetate | Hepatocellular carcinoma | Xiao et al. (2010) |
| osu-03012 | Glioblastoma | Hamed, Yacoub, Park, Eulitt, Sarkar, et al. (2010) |
| Perillyl alcohol | Pancreatic cancer | Lebedeva et al. (2008) |
| CDDP, Epirubicin, VCR | B cell lymphoma | Ma, Zhao, et al. (2016) |
| Doxorubicin | Colorectal | Emdad, Lebedeva, Su, Sarkar, et al. (2007) |
| Vitamin E succinate | Ovarian cancer | Shanker et al. (2007) |
| Geldanamycin | Lung cancer | Pataer et al. (2007) |
| Radiation | Ovarian | Emdad et al. (2006) |
| Celecoxib | Breast cancer | Suh et al. (2005) |
| Sulindac | Lung cancer | Oida et al. (2005) |
4.7. Bystander Activity
Evidence of bystander activity of mda-7/IL-24 (Su et al., 2005) was shown in vivo in animal studies, where tumor cells were injected in both flanks of nude mice (Pradhan et al., 2017; Sarkar et al., 2007, 2008, 2005; Su et al., 2005). A tumor on one flank was treated while the tumor on the other flank was left untreated. Tumor measurements showed a decrease in tumor size in the treated as well as the untreated tumor. The inhibitory action on distant tumors can be explained by the antitumor “bystander” activity of the secreted mda-7/IL-24 cytokine and its ability to induce apoptosis and promote production of MDA-7/IL-24 through dimeric receptor pairs in the untreated tumor (Menezes et al., 2014; Sauane et al., 2008). Additionally, in a syngeneic model this distant antitumor effect can also be explained by the activation of immune pathways, i.e., cytotoxic T cells and NK cells by administration of mda-7/IL-24 (Gao et al., 2008; Menezes et al., 2015; Miyahara et al., 2006). Overexpression of mda-7/IL-24 gene results in production of MDA-7/IL-24 protein which is secreted as a glycosylated protein (Dash et al., 2010; Fuson et al., 2009; Sauane et al., 2006). Infection of Ad. mda-7, which is dependent on coxsackie and adenovirus viral receptors on cells, or treatment with GST-MDA-7 is not dependent on the IL-22R1/IL-20R2 or IL20-R1/IL-20R2 receptors (Dent et al., 2010; Sauane et al., 2004). In contrast, to provoke a signaling and biological effect, secreted MDA-7/IL-24 requires a complete set of dimeric cell surface receptors (Dash et al., 2014; Dumoutier et al., 2001; Wang et al., 2002). Secreted MDA-7/IL-24 binds to the dimeric receptor pair and induces cancer cell death (Dash et al., 2014; Menezes et al., 2014). By the use of IL-20R1/IL-20R2 antibodies, it has been demonstrated that mda-7/IL-24-mediated cell death is receptor dependent (Chada, Mhashilkar, et al., 2004). Zheng and colleagues described the role of IL-20R1/IL-20R2 receptor pair in mda-7/IL-24-mediated cell death and its independence of STAT3 phosphor-ylation (Zheng, Bocangel, et al., 2007). Biological activity of mda-7/IL-24 was also shown to be independent of JAK/STAT signaling using inhibitors and various receptor mutant cells (Sauane, Gopalkrishnan, Lebedeva, et al., 2003).
Normal cells also promote “bystander” activity after exposure to mda-7/IL-24, which results in production and secretion of MDA-7/IL-24 without inducing toxicity or cell death. Infection of normal primary or immortal human cells, such primary human fetal astrocytes, FM-516 or P69, results in secretion of MDA-7/IL-24. Addition of supernatant from normal cells infected with Ad.mda-7 to cancer cells results in suppression of their growth and induction of apoptosis. Since Ad.mda-7 will result in MDA-7/IL-24 protein production in normal and cancer cells, this can result in a robust “bystander” effect that is observed both in preclinical animal models and in a Phase I/II clinical trial in patients with advanced cancers (Dash et al., 2010).
Activation of the immune system provides another important mechanism underlying the “bystander” activity of mda-7/IL-24. MDA-7/IL-24 induces IL-6, TNFα, IFN-γ, IL-1β, and IL-12, which are potent immunoregulatory molecules (Caudell et al., 2002; Deng et al., 2011; Menezes et al., 2014). Also, these immunoregulatory molecules can regulate APCs to present tumor antigens to trigger immune response (Caudell et al., 2002). In addition to immune-mediated effects, the “bystander” antitumor activity of MDA-7/IL-24 is also elicited through its direct proapoptotic and antiangiogenic activity (Dash et al., 2010).
5. ROLE OF MDA-7/IL-24 IN OTHER DISEASES
MDA-7/IL-24 has been extensively studied in cancer. In addition to its function as a tumor suppressor and apoptosis-toxic autophagy inducing cytokine in cancer, MDA-7/IL-24 has also been reported to play a significant role in inflammation, cardiovascular disease, autoimmune diseases, and viral replication.
5.1. Inflammation
The skin is the largest organ in the body and plays an essential role in promoting immunity and defense against pathogenic microorganisms. However, dysregulated immune reactions can cause chronic inflammatory skin diseases. Extensive crosstalk between the different cellular and microbial components of the skin regulates local immune responses to ensure efficient host defense, to maintain and restore homeostasis, and to prevent chronic disease. In this section, we briefly discuss recent findings that highlight a role of MDA-7/IL-24 in inflammation. IL-19 and MDA-7/IL-24 belong to the IL-20 subfamily and are known to be involved in host defense against bacteria and fungi, tissue remodeling, and wound healing (Fonseca-Camarillo, Furuzawa-Carballeda, Granados, & Yamamoto-Furusho, 2014). These groups of cytokines are involved in protecting the epithelial tissue from damage that is a consequence of bacterial and viral infections. MDA-7/IL-24 may be a member of a complex cascade of cytokines involved in inflammation as MDA-7/IL-24 can induce expression of many cytokines, including TNFα, IL-6, and IFN-γ (Wang & Liang, 2005). MDA-7/IL-24 and its receptor expression pattern support a major physiological function related to epidermal functions, such as wound healing, and abnormalities may be part of the cause of pathological skin conditions such as psoriasis.
5.2. Inflammatory Bowel Disease (IBD)
Chronic inflammation of all parts of the digestive tract may bring about IBD. This includes primarily ulcerative colitis (UC) and Crohn’s disease (CD). The symptoms for both of these conditions include severe diarrhea, pain, fatigue, and weight loss. Genomic abnormalities and environmental factors can trigger IBD. Andoh and colleagues assessed the expression of MDA-7/IL-24 in inflamed mucosa of IBD patients and determined the molecular mechanism that resulted in MDA-7/IL-24 expression in colonic subepithelial myofibroblasts (Andoh et al., 2009). They demonstrated that MDA-7/IL-24 expression is enhanced in the inflamed mucosa of active IBD patients. Their data suggest that MDA-7/IL-24 targets epithelial cells and play antiinflammatory and protective roles in the intestinal mucosa. This elevated expression of MDA-7/IL-24 leads to increased Jak/Stat pathway signals leading to increased expression of different MUC genes in the mucosa. MUC genes are the primary component of the mucin barrier that divides the intestinal microbiota and the intestinal epithelium. MUC genes also play an important role in the pathogenesis of IBD. This study showed that MDA-7/IL-24 expression is elevated in inflamed mucosa of IBD patients compared to control patients. Work done by other researchers show that the IL-10 subfamily of cytokines is involved in immune regulation and inflammatory responses. To obtain an enhanced understanding of this group of cytokines for potential therapeutic applications, more focus is required on mechanism; some of them may in the future reduce adverse side effects and/or increase the efficacy typically observed in IL-10 therapy for IBD. In active IBD, MDA-7/IL-24 is synthesized by peripheral B cells, CD4+ T cells, CD8+ T cells, and monocytes. Overall, MDA-7/IL-24 can promote a suppressive inflammatory effect on colonic epithelial cells and mucosal inflammation in IBD.
Studies by Fonseca-Camarillo and colleagues explored the role of MDA-7/IL-24 in Mexican matzo patients with IBD (Fonseca-Camarillo et al., 2014). The authors studied a total of 113 patients that included 77 patients with UC and 36 patients with CD. This study also included 33 patients as control. They compared the gene expression profiles of IL-19 and MDA-7/IL-24 in these patients. The study found that IL-19 and MDA-7/IL-24 levels were elevated significantly with active IBD disease compared with inactive IBD at both a transcriptional and translational levels. Additionally, they showed that when compared with active UC and noninflammatory tissue an increase in IL-19 and MDA-7/IL-24 producing cells were observed in active CD. This study indicates that in patients with active IBD, circulating B cells and monocytes produce IL-19 and peripheral B cells, CD4+ T cells, CD8+ T cells and monocytes produce MDA-7/IL-24.
5.3. Psoriasis
Psoriasis is a common chronic inflammatory skin disease resulting from a complex interplay among the immune system, keratinocytes, susceptibility genes, and environmental factors with a prevalence of 2% in the Caucasian population. Kumari and colleagues observed the presence of MDA-7/IL-24 as well as IL-19 and IL-20 in psoriatic skin lesions (Kumari et al., 2013). Results from these studies showed that MDA-7/IL-24 was elevated in psoriatic skin compared to normal skin. It is also reported that MDA-7/IL-24 can induce different psoriasis-associated factors, which can promote inflammation and epidermal hyperplasia (Kumari et al., 2013).
The IL-10 family of cytokines including MDA-7/IL-24 has been implicated in the pathogenesis of psoriasis (Kunz et al., 2006; Leng et al., 2011; Romer et al., 2003; Weiss et al., 2004; Wolk et al., 2009). These reports also showed an increased expression of MDA-7/IL-24 in psoriatic skin compared to normal skin. MDA-7/IL-24 was mainly produced by keratinocytes, myeloid cells, and T cells (Conti et al., 2003; Kunz et al., 2006; Zheng, Danilenko, et al., 2007). High expression of MDA-7/IL-24 receptors are also found in keratinocytes and they signal by activating STAT3 (Dumoutier et al., 2001; Kunz et al., 2006; Parrish-Novak et al., 2002). STAT3 overexpression is also observed in psoriatic skin conditions and the expression of constitutively active STAT3 in epidermal keratinocytes also caused psoriasis-like skin inflammation in mice (Sano et al., 2005), which suggests an important role for epidermal STAT3 signaling in psoriasis (Kumari et al., 2013).
Kumari and colleagues report that epidermis-specific NF-κB inhibition increased MDA-7/IL-24 and STAT3 expression in keratinocytes in a TNFR1-dependent manner in psoriasis-like skin inflammation. In the psoriasis epidermis, MDA-7/IL-24 expression was elevated and inhibition of NF-κB increased MDA-7/IL-24 expression in TNF-stimulated human primary keratinocytes. This suggests the importance of this molecular pathway in human psoriasis. They also showed a new keratinocyte-intrinsic mechanism that linked TNFR1, NF-κB, ERK, MDA-7/IL-24, IL-22R1, and STAT3 signaling to disease initiation in psoriasis pathogenesis. The authors also show that skin inflammation requires both TNFR1 signaling in IKK2-deficient epidermal keratinocytes and also identified skin epithelial cells as the major cellular target of this model. This manuscript also demonstrates that in keratinocytes, TNFR1-induced, ROS-, and ERK-dependent expression of MDA-7/IL-24 is a key early event in skin inflammation. In the inflammatory process, epidermis-specific inhibition of NF-κB activates Stat3 and increases MDA-7/IL-24 expression in primary keratinocytes (Persaud et al., 2016). Taken together, the studies on MDA-7/IL-24 in psoriasis indicate a significant role in the expression of proinflammatory mediators thereby resulting in psoriatic skin lesions. The studies also provide evidence suggesting that MDA-7/IL-24 may play a key role in psoriasis initiation.
5.4. Cardiovascular Disease
Vascular calcification is a symptom of cardiovascular disease. Wang and colleagues showed that low concentration of H2O2 treatment induced abnormal proliferation of vascular endothelial cells and MDA-7/IL-24 inhibited this proliferation (Wang et al., 2016). They also showed that MDA-7/IL-24 could inhibit apoptosis by inhibiting ROS production in vascular endothelial cells. MDA-7/IL-24 is also involved in the downregulation of several genes that regulate cardiovascular disease. The authors concluded that MDA-7/IL-24 can provide a basic therapeutic strategy for treating vascular disease and cancer by inhibiting ROS production in vascular cells. Lower levels of MDA-7/IL-24 were observed in hypertensive rats compared to controls, and antihypertensive therapy increased MDA-7/IL-24 levels. Hypertension is also a hallmark of cardiovascular disease. MDA-7/IL-24 was identified as 1 of the 16 differentially regulated genes in spontaneously hypertensive rats. MDA-7/IL-24 also regulates the expression of inflammation- and hypertension-related genes in a H2O2-treated mouse vascular smooth muscle cell line, MOVAS. This study showed that MDA-7/IL-24 attenuates H2O2-induced activation of PI3K/Akt and Erk. Studies by Ki-Mo Lee and colleagues also suggests that MDA-7/IL-24 can inhibit ROS production by regulating mitochondrial ROS release mediated by PI3K/Akt and Erk pathway in H2O2-treated vascular smooth muscle cells (VSMCs) (Lee et al., 2012). This inhibition of ROS in VSMC leads to reduced cell growth and migration. Another study by Chen and colleagues indicated that adenovirus-mediated expression of MDA-7/IL-24 could inhibit pulmonary arterial smooth muscle cell line (PAC1-SMC) migration and proliferation, leading to reduced intimal hyperplasia (Chen, Chada, Mhashilkar, & Miano, 2003). This study also emphasizes the role of MDA-7/IL-24 in cancer-specific cell death as the authors validated the inhibition of proliferation and induction of apoptosis in PAC1-SMCs (these cells have tumorigenic potential) compared to normal human coronary artery SMC and rat aortic SMC. Based on this data, MDA-7/IL-24 could be used as a therapeutic option for vascular proliferative disorders. Taken together, these studies suggest that MDA-7/IL-24 may be a novel therapeutic target for cardiovascular disease and/or hypertension.
Another study showed that MDA-7/IL-24 inhibits β-GP-induced VSMC calcification. Activation of the Wnt/β-catenin pathway by β-GP is inhibited by MDA-7/IL-24, which indicates that the inhibitory effect of MDA-7/IL-24 on VSMC calcification correlates with the inactivation of the Wnt/β-catenin pathway. This inhibition by MDA-7/IL-24 correlates with suppression of apoptosis, and the expression of osteoblast markers and calcification by downregulation of BMP-2 and the Wnt/β-catenin pathway. They also showed that β-GP increased the expression of calcification and osteoblastic markers in VSMCs (Persaud et al., 2016). This effect is specifically inhibited by MDA-7/IL-24 suggesting that MDA-7/IL-24 suppresses downstream molecules by inhibiting BMP-2 expression. The inhibitory effect MDA-7/IL-24 on VSMC calcification is mediated at least in part through antiapoptotic activity. The effect of MDA-7/IL-24 on VSMC calcification is similar to statins, which are hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. These results explain the role of MDA-7/IL-24 in pathophysiology of vascular calcification.
Vargas-Alarcon and colleagues showed in a case–control association study that individuals with premature coronary artery disease (CAD), sub-clinical atherosclerosis (SA), and healthy controls who had several metabolic and cardiovascular risk factors were associated with MDA-7/IL-24 polymorphisms (Vargas-Alarcon et al., 2014). The authors used an informatics approach and showed that the rs1150253 and rs1150258 polymorphisms in MDA-7/IL-24 had a functional effect generating DNA binding sites for transcription factors. In Mexican populations, these polymorphisms can be used as risk factors for cardiovascular disease, hypertension, diabetes, and increased levels of lipids. The authors concluded that the association of MDA-7/IL-24 polymorphisms with metabolic parameters and cardiovascular risk factors was due to characteristic genetic background with important differences in Mexican populations compared to other populations.
Based on the available literature, MDA-7/IL-24 appears to play a distinct role in cardiovascular disease. MDA-7/IL-24 can promote the growth of VSMCs by suppressing calcification and osteoblast marker expression, which is associated with atherosclerosis pathogenesis. MDA-7/IL-24 also may provide benefit in the treatment of vascular disorders since it selectively inhibits rat pulmonary arterial smooth muscle cell growth and migration. Polymorphisms in the MDA-7/IL-24 gene also correlate with cardiovascular and metabolic risk factors, further supporting a relationship between MDA-7/IL-24 and cardiovascular diseases.
5.5. Rheumatoid Arthritis (RA)
RA is an inflammatory autoimmune disease that can lead to progressive joint damage and disability. Cytokines including IL-1, IL-6, IL-8, IL-10, monocyte chemoattractant protein 1 (CCL2/MCP-1), and tumor necrosis factor (TNFα) play an important role in RA. A study in RA and spondyloarthropathy (SpA) patients with osteoarthritis (OA) patients as controls, analyzed the role of IL-20 and MDA-7/IL-24 by measuring levels of expression, cellular sources, and targets and effects on cytokine production. This study indicated increased levels of IL-20 and MDA-7/IL-24 in RA and SpA patients as compared with inflammatory disease controls and normal controls. They also found that MDA-7/IL-24 levels were almost 10-times greater in these samples as compared to IL-20 levels in synovial fluid, demonstrating the dominant role of MDA-7/IL-24 locally in the joints, because these two cytokines share the same receptors. This study also showed that IL-20R1 and IL-22R are expressed in granulocytes from the RA and SpA patients’ synovial fluid. This indicates that these two cytokines could be involved in neutrophil chemotaxis in arthritis. This study also showed that IL-20 and MDA-7/IL-24 are not involved directly in TNFα and IL-6 production in arthritis, whereas increased expression of CCL2/MCP-1 in SFMC cultures was evident indicating a positive correlation in RA and SpA patients. Taken together, this study demonstrates the association of IL-20 and MDA-7/IL-24 to the synovium of RA and SpA. It also implicates the importance of IL-20 and MDA-7/IL-24 in endothelial cell function and recruitment of granulocytes and mononuclear cells to the synovial joint (Kragstrup et al., 2008).
Kragstrup and colleagues observed an increased plasma concentration of IL-20 and MDA-7/IL-24 in early RA patients as compared to normal healthy controls, and with conventional or antiinflammatory treatment these levels decreased (Kragstrup et al., 2008). Radiographic progression of the disease and the association of IL-20 and MDA-7/IL-24 suggest an involvement of these cytokines in bone destruction. These two cytokines link RA-associated autoantibodies and radiographic progression of IL-22R1. By showing the relationship between IL-20 and MDA-7/IL-24 and RA-associated immune complexes and osteoclasts stimulation via IL-22R1, the investigators demonstrate a correlation between the IL-20R axis and they also provide evidence for a relationship between the IL-20R axis and progression of structural damage. This study showed that targeting the IL-20R axis could be a viable treatment option for bone destruction in rheumatic disease. It also suggests that the dual inhibition of IL-20 and MDA-7/IL-24 or inhibition of IL-22R1 could be helpful in seropositive RA. These changes in this IL-20R axis provide a promising treatment modality for RA. These studies show a clear association of MDA-7/IL-24 and RA, though additional research is vital to fully understand the potential role of MDA-7/IL-24 in RA.
5.6. Tuberculosis
Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis in humans. Although the lungs are the primary organs altered by TB infection, other parts of the body can also be affected. Wu and colleagues reported that active TB patients had decreased expression of MDA-7/IL-24 compared to individuals with latent TB infection. This observation led them to investigate the role of MDA-7/IL-24 in pulmonary TB patients. Since IFN-γ plays an important role in TB infection, and the levels of IFN-γ were similar to MDA-7/IL-24 levels in these patients, they investigated the role of MDA-7/IL-24 on IFN-γ expression. PBMCs isolated from these individuals were stimulated with M. tuberculosis early secreted Ag of 6kDa (EAST-6) to determine the levels of gene expression. Exogenous MDA-7/IL-24 in the presence of EAST-6 stimulation in PBMCs increased IFN-γ levels and neutralizing MDA-7/IL-24 decreased IFN-γ. This upregulation of IFN-γ with exogenous MDA-7/IL-24 was due to increased levels of IL-12α, IL-12β, IL-23α, and IL-27. Taken together, these results show that MDA-7/IL-24 regulates IFN-γ in TB patients and targeting MDA-7/IL-24 might be a treatment option for these patients. Another study by Kumar and colleagues indicated significantly lower levels of MDA-7/IL-24 in TB patients (Kumar et al., 2015; Ma et al., 2011). Additional research is required in this area to decipher molecular mechanism of MDA-7/IL-24 in the pathophysiology of TB in patients.
5.7. Influenza Virus Replication
Influenza infection also known as flu, is associated with mild to severe symptoms including fever, headaches, runny nose, and fatigue. Weiss and colleagues studied the role of MDA-7/IL-24 in influenza A virus replication, as MDA-7/IL-24 is known to influence TLR3-mediated apoptosis and influenza virus can stimulate the TLR3 receptor (Weiss et al., 2015). In this study, the investigators demonstrated that the expression of MDA-7/IL-24 could decrease influenza A virus subtypes replication by inducing apoptosis. The reduction of viral replication by MDA-7/IL-24 could be independent of type I interferon. MDA-7/IL-24 could inhibit Mcl1 and induce caspase 3 cleavage due to initiation of TLR3-mediated apoptosis. This was further demonstrated by TLR3 knockdown or by treating cells with a Pan-Caspase inhibitor. Inhibition of antiapoptotic proteins Bcl-2, Bax, and Bcl-xL was also observed following MDA-7/IL-24 expression. They established that Mcl1 is the key factor in MDA-7/IL-24-mediated inhibition of influenza A virus replication. They also showed that MDA-7/IL-24 expressed by influenza A virus vector does not have any toxicity in mice. Another study by Seong and colleagues also showed that MDA-7/IL-24 expression decreased influenza viral replication (Seong, Choi, & Shin, 2016). MDA-7/IL-24 decreased the transcript level of the viral nucleoprotein (NP) gene following influenza virus infection as compared to viral infection alone, confirming an inhibitory role of MDA-7/IL-24 in viral replication. Furthermore, an MDA-7/IL-24 expressing recombinant adenovirus did not induce toxicity as compared to a wild-type adenovirus, suggesting that MDA-7/IL-24 can specifically target virus-infected cells. Taken together, these studies suggest that MDA-7/IL-24 exerts potent inhibitory activity of influenza viral replication and can be used as a promising novel approach to suppress viral infections (Seong et al., 2016; Weiss et al., 2015).
6. IMMUNOLOGICAL EFFECTS OF MDA-7/IL-24
The role of MDA-7/IL-24 in normal physiology and disease pathology is quite diverse and depends principally on the source of production/secretion and the target tissue. As a cytokine, MDA-7/IL-24 exerts immune-modulatory functions in diverse autoimmune, infectious, and immunopathological diseases including RA, psoriasis, IBDs and others, as discussed in detail earlier (also reviewed in Persaud et al., 2016). MDA-7/IL-24 also plays a prominent role in host defense by inducing innate immune response in epithelial tissue during infection and inflammation by induction of chemokines and recruitment/activation of leukocytes (Jin, Choi, Chun, & Noh, 2014; Tamai et al., 2012).
Apart from these immune-modulatory roles in diverse biological diseases, MDA-7/IL-24 also exerts a profound immune stimulatory effect in the context of cancer. Forced expression of MDA-7/IL-24 induces IFN-γ and IL-6 secretion from melanoma cells and displays potent antitumor functions (Caudell et al., 2002; Chada, Sutton, et al., 2004). Transduction of MDA-7/IL-24 via an adenoviral vector resulted in a significant increase in the CD3+ and CD8+ population, thereby facilitating immune activation and antitumor immunity. In one recent study, Ma et al. evaluated the efficacy of MDA-7/IL-24 in inhibiting colon cancer progression in murine models with an intact immune system and explored the immune-modulatory role of MDA-7/IL-24 in colon cancer progression (Ma, Ren, et al., 2016). The investigators found that MDA-7/IL-24 promoted CD4 + CD8+ T cells to secrete IFN-γ and facilitated the cytotoxicity of CD8 + T cells. In another recent study, Menezes and colleagues in the Fisher laboratory assessed the relevance of immune response in MDA-7/IL-24-mediated tumor suppression in a transgenic murine mouse model of breast cancer with an intact immune system (Menezes et al., 2015). The investigators found that intratumoral injection of Ad.5-CTV (replication competent cancer-selective adenovirus expressing MDA-7/IL-24; a cancer terminator virus) resulted in a marked increased IFN-γ expression and intratumoral CD8+ T cell infiltration. Interestingly, a significant increase in infiltrating CD8+ T cells, along with increased IFN-γ and granzyme B expression was also observed in nontreated tumors derived from MMTV-PyMT transgenic mice that received Ad.5-CTV suggesting that MDA-7/IL-24 is capable of inducing a systemic immune response in an intact immune microenvironment (Menezes et al., 2015). Another study by the Wang and Fisher laboratories evaluated the therapeutic efficacy of Ad.mda-7 in combination with an ER resident chaperone grp170 (Ad. sgrp170) in a prostate cancer model (Gao, Sun, Chen, Subjeck, & Wang, 2009; Gao et al., 2008). The investigators demonstrated that the combination treatment of MDA-7/IL-24 and grp170 was more effective in inhibiting TRAMP-C2 prostate tumor growth as compared to a single agent. The combination treatment resulted in increased IFN-γ production and cytolytic activity suggesting an antigen and tumor-specific T-cell response. Interestingly, the combination treatment was able to reduce distant tumor burden suggesting induction of profound “bystander” systemic antitumor immunity (Gao et al., 2009, 2008). Additionally, a vaccine effect was evident with subsequent tumor challenge experiments associated with a significant increase in the CD3+ and CD8+ cell populations. All of these studies in diverse cancer models strongly support an anticancer immune modulatory role of MDA-7/IL-24.
Evidence of immune activation was also evident in a Phase I/II clinical trial of Ad.mda-7 (INGN-241) in patients with advanced cancers (Cunningham et al., 2005; Dash et al., 2010; Fisher et al., 2007; Sarkar et al., 2007; Tong et al., 2005). A majority of the patients receiving intermediate- or high-dose injections of Ad.mda-7 (INGN-241) showed a marked increase in CD3+ and CD8+ T cells at day 15 following injection as well as transient increases in circulating cytokines, such as IL-6, IL-10, and TNFα (Cunningham et al., 2005; Tong et al., 2005). A few patients showed elevated levels of GM-CSF and IL-2 as well. These immune and cytokine profiles following injection of Ad.mda-7 (INGN-241) in patients mimic a TH-1 type immune response and strongly support an immune stimulatory function of MDA-7/IL-24 in eliciting an antitumor response.
7. CONCLUSIONS AND FUTURE PERSPECTIVES
As described in this review, MDA-7/IL-24 plays significant roles in a number of different human diseases. When initially identified, MDA-7/IL-24 was primarily recognized for its role as a tumor suppressor in cancer. However, as more information regarding the role of MDA-7/IL-24 became available our understanding of its relevance in other diseases has also increased. A detailed understanding of the molecular mechanisms defining the function of MDA-7/IL-24 has helped develop several preclinical therapeutic options as well as therapeutic targets against cancer. As mentioned previously, MDA-7/IL-24 has already been tested in clinical trials for cancer and a Phase I clinical trial with MDA-7/IL-24 (INGN 241) showed promising results (Cunningham et al., 2005; Tong et al., 2005). Currently, research is focused on developing novel approaches to enhance MDA-7/IL-24 potency and tumor-specific delivery. The search for new molecules and compounds that can enhance or stabilize MDA-7/IL-24 protein are also ongoing. Finally, combination therapies that would enhance MDA-7/IL-24-mediated tumor cell killing and prevent tumor growth and metastasis are also being identified and tested preclinically (Menezes et al., 2014). As new MDA-7/IL-24 therapeutic options are developed in one disease indication, they will also be valuable against other human diseases with MDA-7/IL-24 involvement. Further information gained regarding the role of MDA-7/IL-24 in diseases where MDA-7/IL-24 is overexpressed will allow researchers and clinicians to develop newer approaches to manage these conditions. Given the currently known functions of MDA-7/IL-24, it is likely that MDA-7/IL-24 will also be implicated in other disease indications. Such information will be critical for understanding the multifaceted role of MDA-7/IL-24 in human physiology.
ACKNOWLEDGMENTS
The research reported in this review was supported in part by National Institutes of Health, National Cancer Institute grants R01 CA097318, R01 CA108520, P01 CA104177, P30 CA16059, and P50 CA058236; DOD grant W81XWH-14-1-0409; the Samuel Waxman Cancer Research Foundation; and the National Foundation for Cancer Research (NFCR). Support from the Human and Molecular Genetics Enhancement Fund was provided to S.K.D. and L.E. P.B.F. is the holder of the Thelma Newmeyer Corman Chair in Cancer Research in the VCU Massey Cancer Center. D.S. is the holder of the Harrison Endowed Chair.
Footnotes
CONFLICT OF INTEREST
P.B.F. is a cofounder and owns stock in Cancer Targeting Systems (CTS). Virginia Commonwealth University, Johns Hopkins University, and Columbia University own stock in CTS.
REFERENCES
- Allen M, Pratscher B, Krepler C, Frei K, Schofer C, Pehamberger H, et al. (2005). Alternative splicing of IL-24 in melanocytes by deletion of exons 3 and 5. International Journal of Immunogenetics, 32, 375–378. [DOI] [PubMed] [Google Scholar]
- Allen M, Pratscher B, Roka F, Krepler C, Wacheck V, Schofer C, et al. (2004). Loss of novel mda-7 splice variant (mda-7s) expression is associated with metastatic melanoma. The Journal of Investigative Dermatology, 123, 583–588. [DOI] [PubMed] [Google Scholar]
- Andoh A, Shioya M, Nishida A, Bamba S, Tsujikawa T, Kim-Mitsuyama S, et al. (2009). Expression of IL-24, an activator of the JAK1/STAT3/SOCS3 cascade, is enhanced in inflammatory bowel disease. Journal of Immunology, 183, 687–695. [DOI] [PubMed] [Google Scholar]
- Azab B, Dash R, Das SK, Bhutia SK, Shen XN, Quinn BA, et al. (2012).Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) in combination with the apogossypol derivative BI-97C1 (Sabutoclax) improves therapeutic efficacy in low CAR colorectal cancer cells. Journal of Cellular Physiology, 227, 2145–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhoopathi P, Lee N, Pradhan AK, Shen XN, Das SK, Sarkar D, et al. (2016). mda-7/IL-24 induces cell death in neuroblastoma through a novel mechanism involving AIF and ATM. Cancer Research, 76, 3572–3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutia SK, Das SK, Azab B, Menezes ME, Dent P, Wang XY, et al. (2013).Targeting breast cancer-initiating/stem cells with melanoma differentiation-associated gene-7/interleukin-24. International Journal of Cancer, 133, 2726–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutia SK, Das SK, Kegelman TP, Azab B, Dash R, Su ZZ, et al. (2012). mda-7/IL-24 differentially regulates soluble and nuclear clusterin in prostate cancer. Journal of Cellular Physiology, 227, 1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutia SK, Dash R, Das SK, Azab B, Su ZZ, Lee SG, et al. (2010). Mechanism of autophagy to apoptosis switch triggered in prostate cancer cells by antitumor cytokine melanoma differentiation-associated gene 7/interleukin-24. Cancer Research, 70, 3667–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutia SK, Mukhopadhyay S, Sinha N, Das DN, Panda PK, Patra SK, et al. (2013). Autophagy: Cancer’s friend or foe? Advances in Cancer Research, 118, 61–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzas K, Oppenheim JJ, & Zack Howard OM (2011). Myeloid cells migrate in response to IL-24. Cytokine, 55, 429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudell EG, Mumm JB, Poindexter N, Ekmekcioglu S, Mhashilkar AM, Yang XH, et al. (2002). The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. Journal of Immunology, 168, 6041–6046. [DOI] [PubMed] [Google Scholar]
- Chada S, Bocangel D, Ramesh R, Grimm EA, Mumm JB, Mhashilkar AM, et al. (2005). mda-7/IL24 kills pancreatic cancer cells by inhibition of the Wnt/PI3K signaling pathways: Identification of IL-20 receptor-mediated bystander activity against pancreatic cancer. Molecular Therapy, 11, 724–733. [DOI] [PubMed] [Google Scholar]
- Chada S, Mhashilkar AM, Liu Y, Nishikawa T, Bocangel D, Zheng M, et al. (2006). mda-7 gene transfer sensitizes breast carcinoma cells to chemotherapy, biologic therapies and radiotherapy: Correlation with expression of bcl-2 family members. Cancer Gene Therapy, 13, 490–502. [DOI] [PubMed] [Google Scholar]
- Chada S, Mhashilkar AM, Ramesh R, Mumm JB, Sutton RB, Bocangel D, et al. (2004). Bystander activity of Ad-mda7: Human MDA-7 protein kills melanoma cells via an IL-20 receptor-dependent but STAT3-independent mechanism. Molecular Therapy, 10, 1085–1095. [DOI] [PubMed] [Google Scholar]
- Chada S, Sutton RB, Ekmekcioglu S, Ellerhorst J, Mumm JB, Leitner WW, et al. (2004). MDA-7/IL-24 is a unique cytokine—Tumor suppressor in the IL-10 family. International Immunopharmacology, 4, 649–667. [DOI] [PubMed] [Google Scholar]
- Chen J, Chada S, Mhashilkar A, & Miano JM (2003). Tumor suppressor MDA-7/IL-24 selectively inhibits vascular smooth muscle cell growth and migration. Molecular Therapy, 8, 220–229. [DOI] [PubMed] [Google Scholar]
- Conti P, Kempuraj D, Frydas S, Kandere K, Boucher W, Letourneau R, et al. (2003). IL-10 subfamily members: IL-19, IL-20, IL-22, IL-24 and IL-26. Immunology Letters, 88, 171–174. [DOI] [PubMed] [Google Scholar]
- Cunningham CC, Chada S, Merritt JA, Tong A, Senzer N, Zhang Y, et al. (2005).Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: A phase I study. Molecular Therapy, 11, 149–159. [DOI] [PubMed] [Google Scholar]
- Dash R, Azab B, Quinn BA, Shen X, Wang XY, Das SK, et al. (2011). Apogossypol derivative BI-97C1 (Sabutoclax) targeting Mcl-1 sensitizes prostate cancer cells to mda-7/IL-24-mediated toxicity. Proceedings of the National Academy of Sciences of the United States of America, 108, 8785–8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash R, Bhoopathi P, Das SK, Sarkar S, Emdad L, Dasgupta S, et al. (2014). Novel mechanism of MDA-7/IL-24 cancer-specific apoptosis through SARI induction. Cancer Research, 74, 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash R, Bhutia SK, Azab B, Su ZZ, Quinn BA, Kegelmen TP, et al. (2010). mda-7/IL-24: A unique member of the IL-10 gene family promoting cancer-targeted toxicity. Cytokine & Growth Factor Reviews, 21, 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng WG, Kwon J, Ekmekcioglu S, Poindexter NJ, & Grimm EA (2011). IL-24 gene transfer sensitizes melanoma cells to erlotinib through modulation of the Apaf-1 and Akt signaling pathways. Melanoma Research, 21, 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent P, Yacoub A, Hamed HA, Park MA, Dash R, Bhutia SK, et al. (2010). MDA-7/IL-24 as a cancer therapeutic: From bench to bedside. Anti-Cancer Drugs, 21, 725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoutier L, Leemans C, Lejeune D, Kotenko SV, & Renauld JC (2001). Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types. Journal of Immunology, 167, 3545–3549. [DOI] [PubMed] [Google Scholar]
- Ekmekcioglu S, Ellerhorst J, Mhashilkar AM, Sahin AA, Read CM, Prieto VG, et al. (2001). Down-regulated melanoma differentiation associated gene (mda-7) expression in human melanomas. International Journal of Cancer, 94, 54–59. [DOI] [PubMed] [Google Scholar]
- Ellerhorst JA, Prieto VG, Ekmekcioglu S, Broemeling L, Yekell S, Chada S, et al. (2002). Loss of MDA-7 expression with progression of melanoma. Journal of Clinical Oncology, 20, 1069–1074. [DOI] [PubMed] [Google Scholar]
- Emdad L, Lebedeva IV, Su ZZ, Gupta P, Sarkar D, Settleman J, et al. (2007). Combinatorial treatment of non-small-cell lung cancers with gefitinib and Ad.mda-7 enhances apoptosis-induction and reverses resistance to a single therapy. Journal of Cellular Physiology, 210, 549–559. [DOI] [PubMed] [Google Scholar]
- Emdad L, Lebedeva IV, Su ZZ, Gupta P, Sauane M, Dash R, et al. (2009). Historical perspective and recent insights into our understanding of the molecular and biochemical basis of the antitumor properties of mda-7/IL-24. Cancer Biology & Therapy, 8, 391–400. [DOI] [PubMed] [Google Scholar]
- Emdad L, Lebedeva IV, Su ZZ, Sarkar D, Dent P, Curiel DT, et al. (2007). Melanoma differentiation associated gene-7/interleukin-24 reverses multidrug resistance in human colorectal cancer cells. Molecular Cancer Therapeutics, 6, 2985–2994. [DOI] [PubMed] [Google Scholar]
- Emdad L, Sarkar D, Lebedeva IV, Su ZZ, Gupta P, Mahasreshti PJ, et al. (2006). Ionizing radiation enhances adenoviral vector expressing mda-7/IL-24-mediated apoptosis in human ovarian cancer. Journal of Cellular Physiology, 208, 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulitt PJ, Park MA, Hossein H, Cruikshanks N, Yang C, Dmitriev IP, et al. (2010). Enhancing mda-7/IL-24 therapy in renal carcinoma cells by inhibiting multiple protective signaling pathways using sorafenib and by Ad.5/3 gene delivery. Cancer Biology & Therapy, 10, 1290–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler CE, & Rich JN (2008). Survival of the fittest: Cancer stem cells in therapeutic resistance and angiogenesis. Journal of Clinical Oncology, 26, 2839–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald K, & Kurokawa M (2013). Evading apoptosis in cancer. Trends in Cell Biology, 23,620–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov V, Schmidt EL, Filippova M, & Duerksen-Hughes PJ (2008). Splicing and splice factor SRp55 participate in the response to DNA damage by changing isoform ratios of target genes. Gene, 420, 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PB (2005). Is mda-7/IL-24 a “magic bullet” for cancer? Cancer Research, 65,10128–10138. [DOI] [PubMed] [Google Scholar]
- Fisher PB, Gopalkrishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR, et al. (2003). mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: From the laboratory into the clinic. Cancer Biology & Therapy, 2, S23–37. [PubMed] [Google Scholar]
- Fisher PB, Sarkar D, Lebedeva IV, Emdad L, Gupta P, Sauane M, et al. (2007).Melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24): Novel gene therapeutic for metastatic melanoma. Toxicology and Applied Pharmacology, 224, 300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca-Camarillo G, Furuzawa-Carballeda J, Granados J, & Yamamoto-Furusho JK(2014). Expression of interleukin (IL)-19 and IL-24 in inflammatory bowel disease patients: A cross-sectional study. Clinical and Experimental Immunology, 177, 64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuson KL, Zheng M, Craxton M, Pataer A, Ramesh R, Chada S, et al. (2009). Structural mapping of post-translational modifications in human interleukin-24: Role of N-linked glycosylation and disulfide bonds in secretion and activity. Journal of Biological Chemistry, 284, 30526–30533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Sun X, Chen X, Subjeck J, & Wang XY (2009). Secretion of stress protein grp170 promotes immune-mediated inhibition of murine prostate tumor. Cancer Immunology, Immunotherapy, 58, 1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Sun X, Chen X, Wang Y, Foster BA, Subjeck J, et al. (2008). Secretable chaperone Grp170 enhances therapeutic activity of a novel tumor suppressor, mda-7/IL-24. Cancer Research, 68, 3890–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalan B, Litvak A, Sharma S, Mhashilkar AM, Chada S, & Ramesh R (2005). Activation of the Fas-FasL signaling pathway by MDA-7/IL-24 kills human ovarian cancer cells. Cancer Research, 65, 3017–3024. [DOI] [PubMed] [Google Scholar]
- Gopalkrishnan RV, Sauane M, & Fisher PB (2004). Cytokine and tumor cell apoptosis inducing activity of mda-7/IL-24. International Immunopharmacology, 4, 635–647. [DOI] [PubMed] [Google Scholar]
- Greco A, Di Benedetto A, Howard CM, Kelly S, Nande R, Dementieva Y, et al. (2010). Eradication of therapy-resistant human prostate tumors using an ultrasound-guided site-specific cancer terminator virus delivery approach. Molecular Therapy, 18, 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Nusse R, & van Amerongen R (2014). The role of Ryk and Ror receptor tyrosine kinases in Wnt signal transduction. Cold Spring Harbor Perspectives in Biology, 6, a009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Emdad L, Lebedeva IV, Sarkar D, Dent P, Curiel DT, et al. (2008). Targeted combinatorial therapy of non-small cell lung carcinoma using a GST-fusion protein of full-length or truncated MDA-7/IL-24 with Tarceva. Journal of Cellular Physiology, 215, 827–836. [DOI] [PubMed] [Google Scholar]
- Gupta P, Su ZZ, Lebedeva IV, Sarkar D, Sauane M, Emdad L, et al. (2006). mda-7/IL-24: Multifunctional cancer-specific apoptosis-inducing cytokine. Pharmacology and Therapeutics, 111, 596–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Walter MR, Su ZZ, Lebedeva IV, Emdad L, Randolph A, et al. (2006). BiP/GRP78 is an intracellular target for MDA-7/IL-24 induction of cancer-specific apoptosis. Cancer Research, 66, 8182–8191. [DOI] [PubMed] [Google Scholar]
- Hamed HA, Das SK, Sokhi UK, Park MA, Cruickshanks N, Archer K, et al. (2013). Combining histone deacetylase inhibitors with MDA-7/IL-24 enhances killing of renal carcinoma cells. Cancer Biology & Therapy, 14, 1039–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed HA, Yacoub A, Park MA, Archer K, Das SK, Sarkar D, et al. (2013). Histone deacetylase inhibitors interact with melanoma differentiation associated-7/interleukin-24 to kill primary human glioblastoma cells. Molecular Pharmacology, 84, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed HA, Yacoub A, Park MA, Eulitt PJ, Dash R, Sarkar D, et al. (2010). Inhibition of multiple protective signaling pathways and Ad.5/3 delivery enhances mda-7/IL-24 therapy of malignant glioma. Molecular Therapy, 18, 1130–1142. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hamed HA, Yacoub A, Park MA, Eulitt P, Sarkar D, Dmitriev IP, et al. (2010). OSU-03012 enhances Ad.7-induced GBM cell killing via ER stress and autophagy and by decreasing expression of mitochondrial protective proteins. Cancer Biology & Therapy, 9, 526–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, & Weinberg RA (2000). The hallmarks of cancer. Cell, 100, 57–70. [DOI] [PubMed] [Google Scholar]
- Hanahan D, & Weinberg RA (2011). Hallmarks of cancer: The next generation. Cell,144, 646–674. [DOI] [PubMed] [Google Scholar]
- Hauck CR, Hsia DA, & Schlaepfer DD (2002). The focal adhesion kinase—A regulator of cell migration and invasion. IUBMB Life, 53, 115–119. [DOI] [PubMed] [Google Scholar]
- He M, & Liang P (2010). IL-24 transgenic mice: In vivo evidence of overlapping functions for IL-20, IL-22, and IL-24 in the epidermis. Journal of Immunology, 184, 1793–1798. [DOI] [PubMed] [Google Scholar]
- Huang EY, Madireddi MT, Gopalkrishnan RV, Leszczyniecka M, Su Z, Lebedeva IV, et al. (2001). Genomic structure, chromosomal localization and expression profile of a novel melanoma differentiation associated (mda-7) gene with cancer specific growth suppressing and apoptosis inducing properties. Oncogene, 20, 7051–7063. [DOI] [PubMed] [Google Scholar]
- Huo W, Li ZM, Zhu XM, Bao YM, & An LJ (2013). MDA-7/IL-24 suppresses tumor adhesion and invasive potential in hepatocellular carcinoma cell lines. Oncology Reports, 30, 986–992. [DOI] [PubMed] [Google Scholar]
- Inoue S, Hartman A, Branch CD, Bucana CD, Bekele BN, Stephens LC, et al. (2007). mda-7 In combination with bevacizumab treatment produces a synergistic and complete inhibitory effect on lung tumor xenograft. Molecular Therapy, 15, 287–294. [DOI] [PubMed] [Google Scholar]
- Jiang H, & Fisher PB (1993). Use of a sensitive and efficient subtraction hybridization protocol for the identification of genes differentially regulated during the induction of differentiation in human melanoma cells. Molecular and Cellular Differentiation, 1(3), 285–299. [Google Scholar]
- Jiang H, Lin JJ, Su ZZ, Goldstein NI, & Fisher PB (1995). Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene, 11, 2477–2486. [PubMed] [Google Scholar]
- Jiang H, Su ZZ, Lin JJ, Goldstein NI, Young CS, & Fisher PB (1996). The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proceedings of the National Academy of Sciences of the United States of America, 93, 9160–9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SH, Choi D, Chun YJ, & Noh M (2014). Keratinocyte-derived IL-24 plays a role in the positive feedback regulation of epidermal inflammation in response to environmental and endogenous toxic stressors. Toxicology and Applied Pharmacology, 280, 199–206. [DOI] [PubMed] [Google Scholar]
- Karar J, & Maity A (2011). PI3K/AKT/mTOR pathway in angiogenesis. Frontiers inMolecular Neuroscience, 4, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragstrup TW, Otkjaer K, Holm C, Jorgensen A, Hokland M, Iversen L, et al. (2008). The expression of IL-20 and IL-24 and their shared receptors are increased in rheumatoid arthritis and spondyloarthropathy. Cytokine, 41, 16–23. [DOI] [PubMed] [Google Scholar]
- Kumar NP, Banurekha VV, Nair D, Kumaran P, Dolla CK, & Babu S (2015). Type 2 diabetes—Tuberculosis co-morbidity is associated with diminished circulating levels of IL-20 subfamily of cytokines. Tuberculosis (Edinburgh, Scotland), 95, 707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S, Bonnet MC, Ulvmar MH, Wolk K, Karagianni N, Witte E, et al. (2013). Tumor necrosis factor receptor signaling in keratinocytes triggers interleukin-24-dependent psoriasis-like skin inflammation in mice. Immunity, 39, 899–911. [DOI] [PubMed] [Google Scholar]
- Kunz S, Wolk K, Witte E, Witte K, Doecke WD, Volk HD, et al. (2006). Interleukin (IL)-19, IL-20 and IL-24 are produced by and act on keratinocytes and are distinct from classical ILs. Experimental Dermatology, 15, 991–1004. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Emdad L, Su ZZ, Gupta P, Sauane M, Sarkar D, et al. (2007). mda-7/IL-24, novel anticancer cytokine: Focus on bystander antitumor, radiosensitization and antiangiogenic properties and overview of the phase I clinical experience (Review). International Journal of Oncology, 31, 985–1007. [PubMed] [Google Scholar]
- Lebedeva IV, Sarkar D, Su ZZ, Kitada S, Dent P, Stein CA, et al. (2003). Bcl-2 and Bcl-x(L) differentially protect human prostate cancer cells from induction of apoptosis by melanoma differentiation associated gene-7, mda-7/IL-24. Oncogene, 22, 8758–8773. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Sauane M, Gopalkrishnan RV, Sarkar D, Su ZZ, Gupta P, et al. (2005). mda-7/IL-24: Exploiting cancer’s Achilles’ heel. Molecular Therapy, 11, 4–18. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Su ZZ, Chang Y, Kitada S, Reed JC, & Fisher PB (2002). The cancer growth suppressing gene mda-7 induces apoptosis selectively in human melanoma cells. Oncogene, 21, 708–718. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Su ZZ, Sarkar D, & Fisher PB (2003). Restoring apoptosis as a strategy for cancer gene therapy: Focus on p53 and mda-7. Seminars in Cancer Biology, 13, 169–178. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Su ZZ, Sarkar D, Gopalkrishnan RV, Waxman S, Yacoub A, et al. (2005). Induction of reactive oxygen species renders mutant and wild-type K-ras pancreatic carcinoma cells susceptible to Ad.mda-7-induced apoptosis. Oncogene, 24, 585–596. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Su ZZ, Sarkar D, Kitada S, Dent P, Waxman S, et al. (2003). Melanoma differentiation associated gene-7, mda-7/interleukin-24, induces apoptosis in prostate cancer cells by promoting mitochondrial dysfunction and inducing reactive oxygen species. Cancer Research, 63, 8138–8144. [PubMed] [Google Scholar]
- Lebedeva IV, Su ZZ, Vozhilla N, Chatman L, Sarkar D, Dent P, et al. (2008). Mechanism of in vitro pancreatic cancer cell growth inhibition by melanoma differentiation-associated gene-7/interleukin-24 and perillyl alcohol. Cancer Research, 68, 7439–7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Kang HA, Park M, Lee HY, Song MJ, Ko K, et al. (2012). Interleukin-24 suppresses the growth of vascular smooth muscle cells by inhibiting H(2)O(2)-induced reactive oxygen species production. Pharmacology, 90, 332–341. [DOI] [PubMed] [Google Scholar]
- Leng RX, Pan HF, Tao JH, & Ye DQ (2011). IL-19, IL-20 and IL-24: Potential therapeutic targets for autoimmune diseases. Expert Opinion on Therapeutic Targets, 15, 119–126. [DOI] [PubMed] [Google Scholar]
- Liang J, Huang RL, Huang Q, Peng Z, Zhang PH, & Wu ZX (2011). Adenovirus-mediated human interleukin 24 (MDA-7/IL-24) selectively suppresses proliferation and induces apoptosis in keloid fibroblasts. Annals of Plastic Surgery, 66, 660–666. [DOI] [PubMed] [Google Scholar]
- Lin SW, Lee MT, Ke FC, Lee PP, Huang CJ, Ip MM, et al. (2000). TGFbeta1 stimulates the secretion of matrix metalloproteinase 2 (MMP2) and the invasive behavior in human ovarian cancer cells, which is suppressed by MMP inhibitor BB3103. Clinical and Experimental Metastasis, 18, 493–499. [DOI] [PubMed] [Google Scholar]
- Liu J, & Debnath J (2016). The evolving, multifaceted roles of autophagy in cancer.Advances in Cancer Research, 130, 1–53. [DOI] [PubMed] [Google Scholar]
- Lv C, Su Q, Liang Y, Hu J, & Yuan S (2016). Oncolytic vaccine virus harbouring the IL-24 gene suppresses the growth of lung cancer by inducing apoptosis. Biochemical and Biophysical Research Communications, 476, 21–28. [DOI] [PubMed] [Google Scholar]
- Ma Y, Chen H, Wang Q, Luo F, Yan J, & Zhang XL (2009). IL-24 protects against Salmonella typhimurium infection by stimulating early neutrophil Th1 cytokine production, which in turn activates CD8+ T cells. European Journal of Immunology, 39, 3357–3368. [DOI] [PubMed] [Google Scholar]
- Ma Y, Chen HD, Wang Y, Wang Q, Li Y, Zhao Y, et al. (2011). Interleukin 24 as a novel potential cytokine immunotherapy for the treatment of Mycobacterium tuberculosis infection. Microbes and Infection, 13, 1099–1110. [DOI] [PubMed] [Google Scholar]
- Ma Q, Jin B, Zhang Y, Shi Y, Zhang C, Luo D, et al. (2016). Secreted recombinant human IL-24 protein inhibits the proliferation of esophageal squamous cell carcinoma Eca-109 cells in vitro and in vivo. Oncology Reports, 35, 2681–2690. [DOI] [PubMed] [Google Scholar]
- Ma G, Kawamura K, Shan Y, Okamoto S, Li Q, Namba M, et al. (2014). Combination of adenoviruses expressing melanoma differentiation-associated gene-7 and chemotherapeutic agents produces enhanced cytotoxicity on esophageal carcinoma. Cancer Gene Therapy, 21, 31–37. [DOI] [PubMed] [Google Scholar]
- Ma YF, Ren Y, Wu CJ, Zhao XH, Xu H, Wu DZ, et al. (2016). Interleukin(IL)-24 transforms the tumor microenvironment and induces anticancer immunity in a murine model of colon cancer. Molecular Immunology, 75, 11–20. [DOI] [PubMed] [Google Scholar]
- Ma M, Zhao L, Sun G, Zhang C, Liu L, Du Y, et al. (2016). Mda-7/IL-24 enhances sensitivity of B cell lymphoma to chemotherapy drugs. Oncology Reports, 35, 3122–3130. [DOI] [PubMed] [Google Scholar]
- Maarof G, Bouchet-Delbos L, Gary-Gouy H, Durand-Gasselin I, Krzysiek R, & Dalloul A (2010). Interleukin-24 inhibits the plasma cell differentiation program in human germinal center B cells. Blood, 115, 1718–1726. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, & He X (2012). Frizzled and LRP5/6 receptors for Wnt/beta-catenin signaling. Cold Spring Harbor Perspectives in Biology, 4, a007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie T, Liu Y, Fanale M, Swisher SG, Chada S, & Hunt KK (2004). Combination therapy of Ad-mda7 and trastuzumab increases cell death in Her-2/neuoverexpressing breast cancer cells. Surgery, 136, 437–442. [DOI] [PubMed] [Google Scholar]
- Menezes ME, Bhatia S, Bhoopathi P, Das SK, Emdad L, Dasgupta S, et al. (2014). MDA-7/IL-24: Multifunctional cancer killing cytokine. Advances in Experimental Medicine and Biology, 818, 127–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes ME, Shen XN, Das SK, Emdad L, Guo C, Yuan F, et al. (2015). MDA-7/IL-24 functions as a tumor suppressor gene in vivo in transgenic mouse models of breast cancer. Oncotarget, 6, 36928–36942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhashilkar AM, Stewart AL, Sieger K, Yang HY, Khimani AH, Ito I, et al. (2003). MDA-7 negatively regulates the beta-catenin and PI3K signaling pathways in breast and lung tumor cells. Molecular Therapy, 8, 207–219. [DOI] [PubMed] [Google Scholar]
- Miyahara R, Banerjee S, Kawano K, Efferson C, Tsuda N, Miyahara Y, et al. (2006). Melanoma differentiation-associated gene-7 (mda-7)/interleukin (IL)-24 induces anti-cancer immunity in a syngeneic murine model. Cancer Gene Therapy, 13, 753–761. [DOI] [PubMed] [Google Scholar]
- Morrison BJ, Morris JC, & Steel JC (2013). Lung cancer-initiating cells: A novel target for cancer therapy. Targeted Oncology, 8, 159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, & Zhang X (2008). Interleukin-10: New perspectives on an old cytokine.Immunological Reviews, 226, 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm JB, Ekmekcioglu S, Poindexter NJ, Chada S, & Grimm EA (2006). Soluble human MDA-7/IL-24: Characterization of the molecular form(s) inhibiting tumor growth and stimulating monocytes. Journal of Interferon & Cytokine Research, 26, 877–886. [DOI] [PubMed] [Google Scholar]
- Nace J, Fortunato SJ, Maul H, & Menon R (2010). The expression pattern of two novel cytokines (IL-24 and IL-29) in human fetal membranes. Journal of Perinatal Medicine, 38, 665–670. [DOI] [PubMed] [Google Scholar]
- Naik P, Karrim J, & Hanahan D (1996). The rise and fall of apoptosis during multistage tumorigenesis: Down-modulation contributes to tumor progression from angiogenic progenitors. Genes and Development, 10, 2105–2116. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Ramesh R, Munshi A, Chada S, & Meyn RE (2004). Adenovirusmediated mda-7 (IL24) gene therapy suppresses angiogenesis and sensitizes NSCLC xenograft tumors to radiation. Molecular Therapy, 9, 818–828. [DOI] [PubMed] [Google Scholar]
- Oida Y, Gopalan B, Miyahara R, Inoue S, Branch CD, Mhashilkar AM, et al. (2005). Sulindac enhances adenoviral vector expressing mda-7/IL-24-mediated apoptosis in human lung cancer. Molecular Cancer Therapeutics, 4, 291–304. [PubMed] [Google Scholar]
- Pal I, Sarkar S, Rajput S, Dey KK, Chakraborty S, Dash R, et al. (2014). BI-69A11 enhances susceptibility of colon cancer cells to mda-7/IL-24-induced growth inhibition by targeting Akt. British Journal of Cancer, 111, 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneerselvam J, Jin J, Shanker M, Lauderdale J, Bates J, Wang Q, et al. (2015). IL-24 inhibits lung cancer cell migration and invasion by disrupting the SDF-1/CXCR4 signaling axis. PLoS One, 10, e0122439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneerselvam J, Munshi A, & Ramesh R (2013). Molecular targets and signaling pathways regulated by interleukin (IL)-24 in mediating its antitumor activities. Journal of Molecular Signaling, 8, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MA,Walker T,Martin AP,Allegood J,Vozhilla N,Emdad L,et al. (2009).MDA-7/IL-24-induced cell killing in malignant renal carcinoma cells occurs by a ceramide/CD95/PERK-dependent mechanism. Molecular Cancer Therapeutics, 8, 1280–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MA, Yacoub A, Sarkar D, Emdad L, Rahmani M, Spiegel S, et al. (2008). PERK-dependent regulation of MDA-7/IL-24-induced autophagy in primary human glioma cells. Autophagy, 4, 513–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish-Novak J, Xu W, Brender T, Yao L, Jones C, West J, et al. (2002). Interleukins 19, 20, and 24 signal through two distinct receptor complexes. Differences in receptor–ligand interactions mediate unique biological functions. Journal of Biological Chemistry, 277, 47517–47523. [DOI] [PubMed] [Google Scholar]
- Pasparakis M, Haase I, & Nestle FO (2014). Mechanisms regulating skin immunity and inflammation. Nature Reviews. Immunology, 14, 289–301. [DOI] [PubMed] [Google Scholar]
- Pataer A, Bocangel D, Chada S, Roth JA, Hunt KK, & Swisher SG (2007). Enhancement of adenoviral MDA-7-mediated cell killing in human lung cancer cells by geldanamycin and its 17-allyl- amino-17-demethoxy analogue. Cancer Gene Therapy, 14, 12–18. [DOI] [PubMed] [Google Scholar]
- Pataer A, Vorburger SA, Chada S, Balachandran S, Barber GN, Roth JA, et al. (2005). Melanoma differentiation-associated gene-7 protein physically associates with the double-stranded RNA-activated protein kinase PKR. Molecular Therapy, 11, 717–723. [DOI] [PubMed] [Google Scholar]
- Pei DS, Yang ZX, Zhang BF, Yin XX, Li LT, Li HZ, et al. (2012). Enhanced apoptosis-inducing function of MDA-7/IL-24 RGD mutant via the increased adhesion to tumor cells. Journal of Interferon & Cytokine Research, 32, 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud L, De Jesus D, Brannigan O, Richiez-Paredes M, Huaman J, Alvarado G, et al. (2016). Mechanism of action and applications of interleukin 24 in immunotherapy. International Journal of Molecular Sciences, 17, E869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poindexter NJ, Williams RR, Powis G, Jen E, Caudle AS, Chada S, et al. (2010). IL-24 is expressed during wound repair and inhibits TGFalpha-induced migration and proliferation of keratinocytes. Experimental Dermatology, 19, 714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AK, Talukdar S, Bhoopathi P, Shen XN, Emdad L, Das SK, et al. (2017). mda-7/IL-24 mediates cancer cell-specific death via regulation of miR-221 and the beclin-1 axis. Cancer Research, 77, 949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani M, Mayo M, Dash R, Sokhi UK, Dmitriev IP, Sarkar D, et al. (2010). Melanoma differentiation associated gene-7/interleukin-24 potently induces apoptosis in human myeloid leukemia cells through a process regulated by endoplasmic reticulum stress. Molecular Pharmacology, 78, 1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh R, Ito I, Gopalan B, Saito Y, Mhashilkar AM, & Chada S (2004). Ectopic production of MDA-7/IL-24 inhibits invasion and migration of human lung cancer cells. Molecular Therapy, 9, 510–518. [DOI] [PubMed] [Google Scholar]
- Romer J, Hasselager E, Norby PL, Steiniche T, Thorn Clausen J, & Kragballe K (2003). Epidermal overexpression of interleukin-19 and −20 mRNA in psoriatic skin disappears after short-term treatment with cyclosporine a or calcipotriol. Journal of Investigative Dermatology, 121, 1306–1311. [DOI] [PubMed] [Google Scholar]
- Sahoo A, Lee CG, Jash A, Son JS, Kim G, Kwon HK, et al. (2011). Stat6 and c-Jun mediate Th2 cell-specific IL-24 gene expression. Journal of Immunology, 186, 4098–4109. [DOI] [PubMed] [Google Scholar]
- Sano S, Chan KS, Carbajal S, Clifford J, Peavey M, Kiguchi K, et al. (2005). Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nature Medicine, 11, 43–49. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Lebedeva IV, Gupta P, Emdad L, Sauane M, Dent P, et al. (2007). Melanoma differentiation associated gene-7 (mda-7)/IL-24: A ‘magic bullet’ for cancer therapy? Expert Opinion on Biological Therapy, 7, 577–586. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Quinn BA, Shen XN, Dash R, Das SK, Emdad L, et al. (2015). Therapy of prostate cancer using a novel cancer terminator virus and a small molecule BH-3 mimetic. Oncotarget, 6, 10712–10727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Quinn BA, Shen X, Dent P, Das SK, Emdad L, et al. (2015). Reversing translational suppression and induction of toxicity in pancreatic cancer cells using a chemoprevention gene therapy approach. Molecular Pharmacology, 87, 286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Dent P, et al. (2002a). mda-7 (IL-24): Signaling and functional roles. BioTechniques, (Suppl), 30–39. [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Valerie K, et al. (2002b). mda-7 (IL-24) mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proceedings of the National Academy of Sciences of the United States of America, 99, 10054–10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Park ES, Vozhilla N, Dent P, Curiel DT, et al. (2008). A cancer terminator virus eradicates both primary and distant human melanomas. Cancer Gene Therapy, 15, 293–302. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Vozhilla N, Park ES, Gupta P, & Fisher PB (2005). Dual cancer specific targeting strategy cures primary and distant breast carcinomas in nude mice. Proceedings of the National Academy of Sciences of the United States of America, 102, 14034–14039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauane M, Gopalkrishnan RV, Choo HT, Gupta P, Lebedeva IV, Yacoub A, et al. (2004). Mechanistic aspects of mda-7/IL-24 cancer cell selectivity analyzed via a bacterial fusion protein. Oncogene, 23, 7679–7690. [DOI] [PubMed] [Google Scholar]
- Sauane M, Gopalkrishnan RV, Lebedeva I, Mei MX, Sarkar D, Su ZZ, et al. (2003). Mda-7/IL-24 induces apoptosis of diverse cancer cell lines through JAK/STAT-independent pathways. Journal of Cellular Physiology, 196, 334–345. [DOI] [PubMed] [Google Scholar]
- Sauane M, Gopalkrishnan RV, Sarkar D, Su ZZ, Lebedeva IV, Dent P, et al. (2003). MDA-7/IL-24: Novel cancer growth suppressing and apoptosis inducing cytokine. Cytokine & Growth Factor Reviews, 14, 35–51. [DOI] [PubMed] [Google Scholar]
- Sauane M, Gupta P, Lebedeva IV, Su ZZ, Sarkar D, Randolph A, et al. (2006). N-glycosylation of MDA-7/IL-24 is dispensable for tumor cell-specific apoptosis and “bystander” antitumor activity. Cancer Research, 66, 11869–11877. [DOI] [PubMed] [Google Scholar]
- Sauane M, Su ZZ, Gupta P, Lebedeva IV, Dent P, Sarkar D, et al. (2008). Autocrine regulation of mda-7/IL-24 mediates cancer-specific apoptosis. Proceedings of the National Academy of Sciences of the United States of America, 105, 9763–9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer G, Venkataraman C, & Schindler U (2001). Cutting edge: FISP (IL-4-induced secreted protein), a novel cytokine-like molecule secreted by Th2 cells. Journal of Immunology, 166, 5859–5863. [DOI] [PubMed] [Google Scholar]
- Seong RK, Choi YK, & Shin OS (2016). MDA7/IL-24 is an anti-viral factor that inhibits influenza virus replication. Journal of Microbiology, 54, 695–700. [DOI] [PubMed] [Google Scholar]
- Shanker M, Gopalan B, Patel S, Bocangel D, Chada S, & Ramesh R (2007). Vitamin E succinate in combination with mda-7 results in enhanced human ovarian tumor cell killing through modulation of extrinsic and intrinsic apoptotic pathways. Cancer Letters, 254, 217–226. [DOI] [PubMed] [Google Scholar]
- Shapiro BA, Vu NT, Shultz MD, Shultz JC, Mietla JA, Gouda MM, et al. (2016). Melanoma differentiation-associated gene 7/IL-24 exerts cytotoxic effects by altering the alternative splicing of Bcl-x pre-mRNA via the SRC/PKCδ signaling axis. Journal of Biological Chemistry, 291, 21669–21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieger KA, Mhashilkar AM, Stewart A, Sutton RB, Strube RW, Chen SY, et al. (2004). The tumor suppressor activity of MDA-7/IL-24 is mediated by intracellular protein expression in NSCLC cells. Molecular Therapy, 9, 355–367. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Emdad L, Sauane M, Lebedeva IV, Sarkar D, Gupta P, et al. (2005). Unique aspects of mda-7/IL-24 antitumor bystander activity: Establishing a role for secretion of MDA-7/IL-24 protein by normal cells. Oncogene, 24, 7552–7566. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Lebedeva IV, Sarkar D, Emdad L, Gupta P, Kitada S, et al. (2006). Ionizing radiation enhances therapeutic activity of mda-7/IL-24: Overcoming radiation- and mda-7/IL-24-resistance in prostate cancer cells overexpressing the antiapoptotic proteins bcl-xL or bcl-2. Oncogene, 25, 2339–2348. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Lebedeva IV, Sarkar D, Gopalkrishnan RV, Sauane M, Sigmon C, et al. (2003). Melanoma differentiation associated gene-7, mda-7/IL-24, selectively induces growth suppression, apoptosis and radiosensitization in malignant gliomas in a p53-independent manner. Oncogene, 22, 1164–1180. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Madireddi MT, Lin JJ, Young CS, Kitada S, Reed JC, et al. (1998). The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proceedings of the National Academy of Sciences of the United States of America, 95, 14400–14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh YJ, Chada S, McKenzie T, Liu Y, Swisher SG, Lucci A, et al. (2005). Synergistic tumoricidal effect between celecoxib and adenoviral-mediated delivery of mda-7 in human breast cancer cells. Surgery, 138, 422–430. [DOI] [PubMed] [Google Scholar]
- Talukdar S, Emdad L, Das SK, Sarkar D, & Fisher PB (2016). Evolving strategies for therapeutically targeting cancer stem cells. Advances in Cancer Research, 131, 159–191. [DOI] [PubMed] [Google Scholar]
- Tamai H, Miyake K, Yamaguchi H, Takatori M, Dan K, Inokuchi K, et al. (2012). AAV8 vector expressing IL24 efficiently suppresses tumor growth mediated by specific mechanisms in MLL/AF4-positive ALL model mice. Blood, 119, 64–71. [DOI] [PubMed] [Google Scholar]
- Tian H, Li L, Zhang B, Di J, Chen F, Li H, et al. (2012). Critical role of lysine 123 in the ubiquitin-mediated degradation of MDA-7/IL-24. Journal of Interferon & Cytokine Research, 32, 575–582. [DOI] [PubMed] [Google Scholar]
- Tong AW, Nemunaitis J, Su D, Zhang Y, Cunningham C, Senzer N, et al. (2005). Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): Biologic outcome in advanced cancer patients. Molecular Therapy, 11, 160–172. [DOI] [PubMed] [Google Scholar]
- Vargas-Alarcon G, Posadas-Romero C, Villarreal-Molina T, Alvarez-Leon E, Angeles-Martinez J, Posadas-Sanchez R, et al. (2014). IL-24 gene polymorphisms are associated with cardiometabolic parameters and cardiovascular risk factors but not with premature coronary artery disease: The genetics of atherosclerotic disease Mexican study. Journal of Interferon & Cytokine Research, 34, 659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, & Liang P (2005). Interleukin-24 and its receptors. Immunology, 114, 166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, & Scadden DT (2015). Harnessing the apoptotic programs in cancer stem-like cells. EMBO Reports, 16, 1084–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Tan Z, Zhang R, Kotenko SV, & Liang P (2002). Interleukin 24 (MDA-7/MOB-5) signals through two heterodimeric receptors, IL-22R1/IL-20R2 and IL-20R1/IL-20R2. Journal of Biological Chemistry, 277, 7341–7347. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang Y, Chen Y, & Lv J (2016). The IL-24 gene protects human umbilical vein endothelial cells against H(2)O(2)-induced injury and may be useful as a treatment for cardiovascular disease. International Journal of Molecular Medicine, 37, 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ye Z, Zhong J, Xiang J, & Yang J (2007). Adenovirus-mediated Il-24 expression suppresses hepatocellular carcinoma growth via induction of cell apoptosis and cycling arrest and reduction of angiogenesis. Cancer Biotherapy & Radiopharmaceuticals, 22, 56–63. [DOI] [PubMed] [Google Scholar]
- Wang CJ, Zhang H, Chen K, Zheng JW, Xiao CW, Ji WW, et al. (2010). Ad. mda-7 (IL-24) selectively induces apoptosis in hepatocellular carcinoma cell lines, suppresses metastasis, and enhances the effect of doxorubicin on xenograft tumors. Oncology Research, 18, 561–574. [DOI] [PubMed] [Google Scholar]
- Weiss R, Laengle J, Sachet M, Shurygina AP, Kiselev O, Egorov A, et al. (2015). Interleukin-24 inhibits influenza A virus replication in vitro through induction of toll-like receptor 3 dependent apoptosis. Antiviral Research, 123, 93–104. [DOI] [PubMed] [Google Scholar]
- Weiss B, Wolk K, Grunberg BH, Volk HD, Sterry W, Asadullah K, et al. (2004). Cloning of murine IL-22 receptor alpha 2 and comparison with its human counterpart. Genes Immunology, 5, 330–336. [DOI] [PubMed] [Google Scholar]
- Welch DR, & Fisher PB (2016). Preface. Advances in Cancer Research, 132, xi–xiv. [DOI] [PubMed] [Google Scholar]
- Whitaker EL, Filippov VA, & Duerksen-Hughes PJ (2012). Interleukin 24: Mechanisms and therapeutic potential of an anti-cancer gene. Cytokine and Growth Factor Reviews, 23, 323–331. [DOI] [PubMed] [Google Scholar]
- Whitaker EL, Filippov V, Filippova M, Guerrero-Juarez CF, & Duerksen-Hughes PJ (2011). Splice variants of mda-7/IL-24 differentially affect survival and induce apoptosis in U2OS cells. Cytokine, 56, 272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, et al. (2009). IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. Journal of Molecular Medicine (Berlin), 87, 523–536. [DOI] [PubMed] [Google Scholar]
- Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, & Sabat R (2004). IL-22 increases the innate immunity of tissues. Immunity, 21, 241–254. [DOI] [PubMed] [Google Scholar]
- Wu YM, Zhang KJ, Yue XT, Wang YQ, Yang Y, Li GC, et al. (2009). Enhancement of tumor cell death by combining cisplatin with an oncolytic adenovirus carrying MDA-7/IL-24. Acta Pharmacologica Sinica, 30, 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Li X, Niu N, Qian J, Xie G, & Wang Y (2010). Dichloroacetate (DCA) enhances tumor cell death in combination with oncolytic adenovirus armed with MDA-7/IL-24. Molecular and Cellular Biochemistry, 340, 31–40. [DOI] [PubMed] [Google Scholar]
- Xu J, Mo Y, Wang X, Liu J, Zhang X, Wang J, et al. (2013). Conditionally replicative adenovirus-based mda-7/IL-24 expression enhances sensitivity of colon cancer cells to 5-fluorouracil and doxorubicin. Journal of Gastroenterology, 48, 203–213. [DOI] [PubMed] [Google Scholar]
- Xu Z, Robitaille AM, Berndt JD, Davidson KC, Fischer KA, Mathieu J, et al. (2016). Wnt/beta-catenin signaling promotes self-renewal and inhibits the primed state transition in naive human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America, 113, E6382–E6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub A, Mitchell C, Brannon J, Rosenberg E, Qiao L, McKinstry R, et al. (2003). MDA-7 (interleukin-24) inhibits the proliferation of renal carcinoma cells and interacts with free radicals to promote cell death and loss of reproductive capacity. Molecular Cancer Therapeutics, 2, 623–632. [PubMed] [Google Scholar]
- Zhang Z, Kawamura K, Jiang Y, Shingyoji M, Ma G, Li Q, et al. (2013). Heat-shock protein 90 inhibitors synergistically enhance melanoma differentiation-associated gene-7-mediated cell killing of human pancreatic carcinoma. Cancer Gene Therapy, 20, 663–670. [DOI] [PubMed] [Google Scholar]
- Zheng M, Bocangel D, Doneske B, Mhashilkar A, Ramesh R, Hunt KK, et al. (2007). Human interleukin 24 (MDA-7/IL-24) protein kills breast cancer cells via the IL-20 receptor and is antagonized by IL-10. Cancer Immunology, Immunotherapy, 56, 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. (2007). Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature, 445, 648–651. [DOI] [PubMed] [Google Scholar]
- Zhuo B, Shi Y, Qin H, Sun Q, Li Z, Zhang F, et al. (2017). Interleukin-24 inhibits osteosarcoma cell migration and invasion via the JNK/c-Jun signaling pathways. Oncology Letters, 13, 4505–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]