Abstract
Bone formation, for example during bone remodelling or fracture repair, requires mature osteoblasts to deposit bone with remarkable spatial precision. As osteoblast precursors derive either from circulation or resident stem cell pools, they and their progeny are required to migrate within the three-dimensional bone space and to navigate to their destination, i.e. to the site of bone formation. An understanding of this process is emerging based on in vitro and in vivo studies of several vertebrate species. Receptors on the osteoblast surface mediate cell adhesion and polarization, which induces osteoblast migration. Osteoblast migration is then facilitated along gradients of chemoattractants. The latter are secreted or released proteolytically by several cell types interacting with osteoblasts, including osteoclasts and vascular endothelial cells. The positions of these cellular sources of chemoattractants in relation to the position of the osteoblasts provide the migrating osteoblasts with tracks to their destination, and osteoblasts possess the means to follow a track marked by multiple chemoattractant gradients. In addition to chemotactic cues, osteoblasts sense other classes of signals and utilize them as landmarks for navigation. The composition of the osseous surface guides adhesion and hence migration efficiency and can also provide steering through haptotaxis. Further, it is likely that signals received from surface interactions modulate chemotaxis. Besides the nature of the surface, mechanical signals such as fluid flow may also serve as navigation signals for osteoblasts. Alterations in osteoblast migration and navigation might play a role in metabolic bone diseases such as osteoporosis.
Keywords: bone, osteoblasts, cell migration, chemotaxis, mineralized surfaces, fluid flow
I. INTRODUCTION
Bone formation is an essential part of bone remodelling, the physiological restoration process of the mature skeleton (Seeman, 2009). In this process, bone designated for overhaul is first removed and then replaced with newly formed bone. Remodelling in both cancellous and cortical bone takes place in a defined compartment referred to as the basic multicellular unit (BMU) (Jilka, 2003; Sims & Martin, 2014). How a BMU is initiated remains under investigation, but recent findings showed that osteocytes, which reside in the mineralized matrix of bone (Bonewald, 2017), play a role early in BMU formation, particularly after fatigue damage of bone (Schaffler et al., 2014).
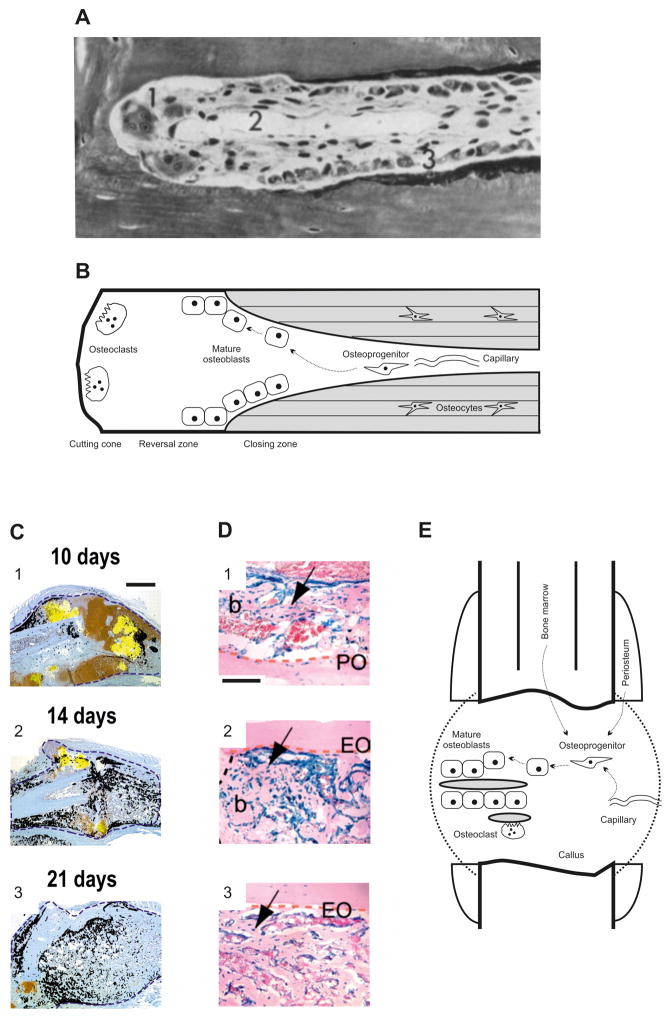
Upon initiation, BMU formation, for example in cortical bone, is driven by osteoclastic bone resorption, which carves out a bone trench (Fig. 1A, B). Subsequent osteoblastic bone formation occurs in a temporally and spatially controlled manner at a distance from resorption (Fig. 1A, B). In a highly organized way collectives of mature osteoblasts deposit a non-mineralized, organic matrix referred to as osteoid, thereby completely or partly filling the trench cross section and forming the lamellae that then undergo mineralization. A limited number of mature osteoblasts remain at discrete positions within the newly formed bone and undergo terminal differentiation into osteocytes. In a cortical BMU, both bone resorption and formation are dependent on vascular invasion as it permits infiltration of progenitor cells that give rise to osteoclasts and osteoblasts. Upon exiting a capillary, osteoblast precursors of likely subendothelial perivascular origin are required to migrate to designated areas of bone formation within the at least several hundred micrometer large space (Buenzli et al., 2012) of the BMU. As the BMU displays considerable cellularity (Fig. 1A, B), migration of osteoblasts is expected to occur on (1) the extracellular matrix produced by BMU cells, including osteoblast precursors and endothelial cells, and (2) the osseous surfaces, i.e. the resorbed and newly formed bone, produced by osteoclasts and osteoblasts, respectively.
Fig. 1.
Bone formation and the requirement for osteoblast migration in bone remodelling and endochondral fracture healing. (A) Goldner stain of a cortical basic morphometric unit (BMU) in dog radius. 1, osteoclasts; 2, central capillary; 3, osteoblasts. Reprinted with permission from (Schenk & Willenegger, 1964). Magnification 320:1. (B) Model of the structure and cellular organization of a cortical BMU. Schematic derived from histological assessments such as that in A. Newly formed bone is shaded grey. The dashed arrows denote potential migration paths of osteoblasts. (C) Closed, non-stabilized tibial fracture in mouse. Transcripts for the osteoblast marker osteocalcin (black) and the hypertrophic chondrocyte marker collagen type X (yellow) were superimposed onto adjacent tissue sections stained with safranin-O/fast green. 1, on day 10, osteoblasts deposit bone mainly on the periosteal surface. 2, day 14 is characterized by a large number of individual areas of bone formation throughout the entire callus. 3, after 21 days, osteoblast activity remains prominent in the callus. Blue dotted lines represent the boundary of the callus. Scale bar for images 1–3, 1 mm. Reprinted with permission from Colnot (2003). (D) Bone grafts derived from lacZ reporter gene mice were used to track cells during healing of non-stabilized tibial fractures. The following three tissue sources were tracked: 1, periosteum; 2, endosteum; 3, endosteum and bone marrow. Images show X-gal stainings on day 14. Osteoblasts were found to derive from all three tissue sources. Abbreviations: b, bone; EO, endosteal surface; PO, periosteal surface. The arrow denotes graft-derived osteoblasts/osteocytes. Dotted orange and black lines delimit the bone graft and bone/cartilage junction, respectively. Scale bar for images 1–3: 100 μm. Reprinted with permission from Colnot et al. (2009). (E) Model of the structure and cellular organization of a typical fracture callus. Representative areas of newly formed bone are shaded grey. The dashed arrows denote potential migration paths of osteoblasts.
Besides its fundamental role in skeletal physiology, bone formation is also indispensable for skeletal repair, including endochondral fracture healing. For example, in long bone fractures, massive, callus-wide bone formation is typically observed between days 10 and 21 post insult (Fig. 1C); osteoblasts required for this bone formation originate primarily from periosteal osteoprogenitor pools but can also be of bone-marrow or perivascular origin (Fig. 1D). Thus, osteoblast populations are likely to migrate and navigate from these pools to areas of bone deposition and might even cover distances in the millimeter range (Fig. 1E). Alternately, cells from these pools or perivascular sources may utilize the vascular network to reach their destination within the callus. This is particularly relevant upon vascular invasion of the mineralized cartilaginous matrix. Upon extravasation, osteoprogenitors are required to migrate along osseous surfaces and navigate to their designated areas of bone formation (Fig. 1E). Together, osteoprogenitors and their progeny migrate within the three-dimensional bone space and navigate to their destination, i.e. the site of bone formation. Herein, we discuss the migration mechanisms of osteoblasts. Our discussion is limited to osteoblasts, largely excluding data from related cell types such as odontoblasts or transformed, i.e. malignant, osteoblastic cells. Although we focus on osteoblasts in post-natal bone, we occasionally draw insightful data from embryonic development. We note that, for the sake of simplicity, the term osteoblasts in this review encompasses osteoprogenitors, immature osteoblasts, and mature osteoblasts.
II. OSTEOBLAST MIGRATION
The migratory potential of cells of the osteoblast lineage was described in detail first by Jones & Boyde (1977). Examining rat (Rattus norvegicus) flat bone explants, an in vitro scratch assay was employed to stimulate migration of densely packed osteoblasts. Detection of migration on the osseous surface was carried out histologically using electron microscopy (Fig. 2A). Observations from the study led to the following conclusions: (1) osteoblasts migrate on bone; (2) osteoblasts migrate as single cells as well as in close proximity to each other; (3) migrating osteoblasts display an elongated morphology; (4) particularly at higher density and under hormonal stimulation, osteoblasts prefer a path along collagen fibrils; and (5) osteoblasts also migrate on exogenous surfaces, such as glass, yet with altered morphology and migratory behaviour.
Fig. 2.
Osteoblast migration. (A) The osteoblast layer on the endocranial aspect of rat parietal bones was mechanically disrupted and the subsequent migration of osteoblasts onto the cleared bone matrix observed using scanning electron microscopy (SEM) at several time points. 1, SEM at 420 μm field width acquired 8 h post cell clearance. Osteoblasts had partially repopulated the cleared surface. The first-rank cells, i.e. the migration front (marked by arrow) had moved away from their neighbours so that their cell bodies were no longer close to other osteoblasts, although other osteoblasts migrated in a densely packed formation. 2, SEM at 160 μm field width acquired 24 h post cell clearance. Some of the spaced cells of the most forward ranks had migrated with apparent disregard for the pattern of the collagen they had traversed. Reprinted with permission from Jones & Boyde (1977). (B) Dynamic, non-invasive molecular imaging of osteoblast migration in vivo. Still images from confocal time-lapse microscopy of migrating photoconverted Kaede-labelled zebrafish (Danio rerio) osteoblasts (red) in close proximity to the fracture. Protrusions of one cell are highlighted in yellow, its leading edge with an arrowhead. Abbreviations: BF, bright field; dpi, day post injury; entpd5, ectonucleoside triphosphate diphosphohydrolase 5. Scale bar, 20 μm. Reprinted with permission from Geurtzen et al. (2014).
These and other observations led to the general view that osteoblasts exhibit migratory potential (Dirckx, Van Hul & Maes, 2013). It was, however, not until recently that the spatial and temporal in vivo dynamics of migration of endogenous osteoblasts were recorded (Fig. 2B). Geurtzen et al. (2014) investigated osteoblast dedifferentiation in a bone fracture model in the zebrafish (Danio rerio). To allow in vivo time-lapse microscopy of migrating osteoblasts they utilized the ectonucleoside triphosphate diphosphohydrolase 5 promoter to drive osteoblast-restricted expression of the photoactivatable fluorescent protein Kaede. In pulse-chase experiments, controlled photoactivation of Kaede permitted tracking of osteoblasts. Their experiments demonstrated that osteoblasts start to move towards the fracture site within a single day after bone damage and that movement was due to active migration rather than passive displacement caused by cell proliferation. In further studies they used Cre-mediated osterix (Osx) promoter-based labelling to follow undifferentiated osteoblast populations in pulse-chase experiments, and found considerable migration potential in this cell population. These observations are in line with previous findings by Maes et al. (2010) showing that in mouse (Mus musculus) fracture callus, which is expected to harbour migrating osteoblasts, Osx-expressing cell populations are found, particularly in proximity to invading vasculature. The same in vivo study also examined mouse embryonic bone development, comparing the migratory potential of undifferentiated and differentiated osteoblasts in lineage-tracing studies between embryonic day 12.5 (E12.5) and E16.5. Cells were marked in an inducible fashion using utilizing elegant Cre-mediated Osx or 3.2 kb collagen Ia1 (Col1) promoter strategies. Histological analyses revealed the temporal patterns of the marked cells and demonstrated that Osx-expressing rather than 3.2 kb Col1-expressing cells moved from the perichondrium and gave rise to trabecular osteoblasts in the bone cavity. Interestingly, a subset of migrating osteoblasts showed pericytic localization onto the vascular endothelium. Together, these findings demonstrated that in comparison to differentiated, mature osteoblasts undifferentiated osteoblasts migrated more efficiently.
Migration of osteoblasts is likely not to be restricted to single-cell movement but also to occur as collective cell movement. Histological evidence for collective osteoblast orientation can be found in the coordinated alignment of mature osteoblasts, for example, during bone remodelling (Schenk & Willenegger, 1964) (Fig. 1A). Jones & Boyde (1977) also noted collective cell migration (Fig. 2A). They observed that with the exception of the osteoblasts in the first rank, i.e. the migration front, all other osteoblasts moved with minimal spacing and, in particular on a glass surface, showed a sheet-like appearance during migration (Jones & Boyde, 1977). These findings are consistent with the proposal that collective cell migration contributes to the formation of complex yet highly organized tissues, such as bone, through controlled cell distribution and coordinated cell activities (Rorth, 2009). Notably, epithelial cells, which often show collective cell movement, prefer single-cell migration after undergoing epithelial-to-mesenchymal cell transition (Baum, Settleman & Quinlan, 2008). Therefore, we speculate that during differentiation of osteoprogenitors, which have mesenchymal characteristics, into mature osteoblasts, which during active bone formation display epithelial-like features such as formation of a cell layer and cell–cell adherence, the capacity for collective cell movement increases. At least partially, the formation of osteoblast collectives is likely to be mediated through gap junctions composed of six connexin 43 proteins (Zappitelli & Aubin, 2014).
Lastly, an alternative mode of cell locomotion utilizes protrusions that are rapidly produced and retracted from the plasma membrane, referred to as blebs (Fackler & Grosse, 2008). In in vitro studies, Panupinthu et al. (2007) and Baldini et al. (2008) found P2X7 nucleotide receptor-mediated blebbing and the associated cytoskeletal reorganization in osteoblasts, but a role of blebbing in osteoblast migration needs to be clarified. All together, these data demonstrated that (1) osteoblasts have migration capacity, (2) the migration capacity is greater in undifferentiated compared to differentiated osteoblasts, and (3) osteoblast migration is at least partially related to vascular invasion.
(1) Osteoblast migration: chemotaxis and navigation along gradients of chemoattractants
The sequence of molecular events that many cell types utilize to facilitate movement has been well described and we refer readers to the excellent summaries of this topic (Petrie, Doyle & Yamada, 2009; Ridley et al., 2003). In brief, for an adhering cell to move, it polarizes to gain an axis of movement, thereby defining the front and rear of the cell. The leading edge at the front of the cell forms cytoskeletal projections, such as lamellipodia and filopodia, and novel adhesive contacts, and initiates directional cell movement through protusion, along with actin formation. Concomitantly, the rear of the cell retracts through actin disassembly and detachment of adhesions. During forward movement, integrin receptors become endocytosed into early endosomes, recycled, and inserted into the membrane at the cell front (Ulrich & Heisenberg, 2009).
The cell polarization observed in migrating cells is controlled by the asymmetrical distribution of surface receptors that initiate migration upon ligand binding (Sullivan, Daukas & Zigmond, 1984). Uniform concentrations of ligands provoke a random non-directional migratory response in cells, referred to as chemokinesis. By contrast, gradients of ligands lead to directed cell migration, referred to as chemotaxis. Ligands that attract cells along a gradient are referred to as chemoattractants. Generally, gradients of chemoattractants have a source and sink in which the chemoattractant is produced and consumed, respectively (Majumdar, Sixt & Parent, 2014). Cells have the ability to adjust their migration direction along the direction of the chemotactic gradient through endocytosis of the ligand–receptor complex, degradation of the ligand, and recycling of the empty receptor to the surface (Ulrich & Heisenberg, 2009).
(a) Osteoblast chemoattractants and repellents
A straightforward method for the identification of chemoattractants of osteoblast-lineage cells is measurement of the migratory response they potentially elicit. This is most frequently done using osteoblasts cultured from bone in combination with migration assays such as the Boyden chamber. A total of 27 chemoattractants have been reported (Table 1), all of which were discovered utilizing in vitro experiments. Only three of the known factors are classical chemokines of the immune system. This might reflect an intrinsic separation between cell migration in the blood and skeletal compartment which are in close spatial proximity. In studies discussed elsewhere additional chemoattractants were reported for mesenchymal stem cells (MSCs) (Vanden Berg-Foels, 2014). In accordance with the use of the term MSC in the data summarized herein, MSCs refer to a heterogenous population of cells originally derived from bone marrow which can contain osteoprogenitor cells (Bianco, 2014).
Table 1.
Osteoblast chemoattractants. Studies addressing malignant skeletal cell types were not included.
| Class | Factor | Source1 | Cell types | Surface (assay) | Comments2 | References |
|---|---|---|---|---|---|---|
| GF | BMP-2 | rh, n.r. | bOB, hmOB, hdOB, hOB | n.r., PC, PC+COL I, PC+gelatin (Boyden)Glass (Dunn, time-lapse) | 1 ng/ml | Fiedler et al. (2002); Li et al. (2005a); Lind et al. (1996); Mayr-Wohlfart et al. (2002); Midy & Plouet (1994) |
| GF | BMP-4 | rx | hdOB, hOB | PC (Boyden) | n.a. | Fiedler et al. (2002) |
| GF | BMP-7 | rh | E1 | TC-Glass (time-lapse) | n.a. | Lee et al. (2006) |
| GF | bFGF | rh | hOB | PC+gelatin (Boyden) | 1 ng/ml | Mayr-Wohlfart et al. (2002) |
| GF | IGF-1 | rh, rm | mOB, rOB, hOB, E1 | PC, PC+COL I, PETE+Fibronectin (Boyden)TC-plastic (gap closure) | PI3K signalling | Hengartner et al. (2013); Lind et al. (1995a); Nakasaki et al. (2008); Panagakos (1993) |
| GF | IGF-2 | rh | rOB, hOB | PC, PC+COL I (Boyden) | n.a. | Lind et al. (1995a); Nakasaki et al. (2008); Panagakos (1993) |
| GF | PDGF-AA | rh | rOB, hdOB, hOB | PC, PC+COL I, PC+gelatin (Boyden) | Effect OB<MSC | Colciago et al. (2009); Fiedler et al. (2004); Hughes et al. (1992); Lind et al. (1995a) |
| GF | PDGF-AB | rh | rOB, hdOB, hOB | PC, PC+COL I (Boyden) | Effect OB≤MSC | Fiedler et al. (2004); Hughes et al. (1992) |
| GF | PDGF-BB | pr, rh | E1, mOB, rOB, hdOB, hOB | PC, PC+COL I, PC+gelatin (Boyden)COL I-TC-plastic (gap closure) | 10–25 ng/ml; effect OB<MSCPI3K & p38 MAP kinase signalling | Colciago et al. (2009); Fiedler et al. (2004, 2002); Godwin & Soltoff (1997); Hengartner et al. (2013); Hughes et al. (1992); Lind et al. (1995a); Mehrotra et al. (2004); Sanchez-Fernandez et al. (2008); Tsukamoto et al. (1991) |
| GF | PIGF-1 | rh | hOB | PC+gelatin (Boyden) | 30 ng/ml | Mayr-Wohlfart et al. (2002) |
| GF | PTN | rh | hOB | Glass (Dunn, time-lapse) | n.a. | Li et al. (2005a) |
| GF | TGF-α | rOB | hOB | PC (Boyden) | n.a. | Panagakos (1994) |
| GF | TGF-β1 | ph, rh | mOB, rOB, hOB | PC+COL I, NC (Boyden)/TC-plastic (gap closure) | 100 pg/ml | Hughes et al. (1992); Lind et al. (1995a); Ota et al. (2013); Pfeilschifter et al. (1990); Yee et al. (1993) |
| GF | TGF-β2 | ph | rOB | PC+COL I, NC (Boyden) | 5–15 pg/ml | Hughes et al. (1992); Pfeilschifter et al. (1990) |
| GF | VEGF-A | rh | dhOB, bOB, hOB | n.r., PC, PC+gelatin (Boyden)/glass (Dunn, time-lapse) | 25 pg/ml; effect OB≤MSC | Fiedler et al. (2005); Hengartner et al. (2013); Li et al. (2005a); Mayr-Wohlfart et al. (2002); Midy & Plouet (1994) |
| ECM | CCN1 | r | dhOB | PC+gelatin (Boyden) | n.a. | Schutze et al. (2007) |
| ECM | CCN6 | r | dhOB | PC+gelatin (Boyden) | n.a. | Schutze et al. (2007) |
| ChK | CCL5 | rm | E1, rOB | PC (Boyden) | PI3K signalling | Yano et al. (2005) |
| ChK | CXCL16 | rm | mOB | PC (Boyden) | n.a. | Ota et al. (2013) |
| CK | IL-4 | rh | hOB | PC+COL I (Boyden) | IL-10 has no effect | Lind et al. (1995b) |
| CK | IL-13 | rm | hOB | PC+COL I (Boyden) | n.a. | Lind et al. (1995b) |
| CK | sRANKL | n.r. | hOB | PC+fibronectin/TC-plastic (gap closure) | PI3K & NF-κB signalling;inhibited by ERK signalling | Golden et al. (2015) |
| Hormone | PTH | n.r. | mOB | TC-plastic (gap closure) | n.a. | Hughes et al. (2012) |
| Other | Ca5 | rh | hOB | PC (Boyden) | n.a. | Hengartner et al. (2013); Ignatius et al. (2011) |
| Other | Ca2+ | n.a. | E1 | PC (Boyden) | PLC signalling | Godwin & Soltoff (1997) |
| Other | LPA | n.a. | E1 | PC+gelatin (Boyden), PC (gap closure & time-lapse) | GPCR (LPA1) signalling | Masiello et al. (2006) |
| Other | TP508 | n.r. | hOB | Glass (Dunn, time-lapse) | n.a. | Li et al. (2005a) |
Source of factor studied in assay.
Factor concentrations (if based on titration experiments) and signalling pathways where reported in the cited publications.
Abbreviations: bFGF, basic fibroblast growth factor; BMP-2, -4, -7, bone morphogenetic protein-2, -4, -7; bOB, bovine osteoblasts; Ca5, complement component 5a; CCL5, chemokine (C-C motif) ligand 5; ChK, chemokine; CK, cytokine; CCN1, -6; cysteine-rich 61/connective tissue growth factor/nephroblastoma overexpressed protein 1, -6; COL, collagen; CXCL16, chemokine (C-X-C motif) ligand 16; dhOB, differentiated human osteoblasts; E1, mouse osteoblastic cell line MC3T3-E1; ECM, extracellular matrix; ERK, extracellular signal-regulated kinase; GF, growth factor; GPCR, G protein-coupled receptor; hdOB, human differentiated osteoblasts; hmOB, human marrow-derived OB; hOB, human osteoblasts; IGF-1, -2, insulin-like growth factor-1, -2; IL-4, -10, -13, interleukin-4, -10, -13; LPA, lysophosphatidic acid; MAP, p38 mitogen-activated protein kinase; mOB, mouse osteoblasts; MSC, mesenchymal stem cell; n.a., not applicable; NC, nitrocellulose; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; n.r., not reported; OB, osteoblast; PC, polycarbonate; PDGF-AA, -AB, -BB, platelet-derived growth factor-AA, -AB, -BB; PETE, polyethylene terephthalate; PI3K, phosphoinositide 3-kinase; PIGF-1, placental growth factor 1; ph, purified human; PLC, phospholipase C; pr, purified rat; PTH, parathyroid hormone; PTN, pleiotrophin; r, recombinant; rh, recombinant human; rm, recombinant mouse; rOB, rat osteoblasts; rx, recombinant xenopus; sRANKL, soluble receptor activator of nuclear factor kappa-B ligand; TC, tissue culture-treated; TGF-α, -β1, -β2, transforming growth factor-α, -β1, -β2; TP508, thrombine peptide 508; VEGF-A, vascular endothelial growth factor-A.
Some chemoattractants may display specificity for osteoblasts when compared to MSCs. For example, in vitro treatment with transforming growth factor β1 (TGF-β1) stimulates migration of osteoblasts but not MSCs, while bone morphogenetic protein 4 stimulates migration of MSCs but not of osteoblasts (Fiedler et al., 2002). However, in vivo studies challenge this observation by showing TGF-β1-mediated recruitment of MSCs to the BMU (Tang et al., 2009). Assuming comparable MSC populations in the in vitro and in vivo studies, this suggests that the chemotactic signals elicited by growth factors with multiple skeletal functions, such as TGF-β1, may vary dependent on the osseous milieu. Notably, activity of some of the osteoblast chemoattractants appears to be regulated by inhibitors such as leukaemia inhibitory factor (LIF) which in vitro blocks migration towards TGF-β1 (Ota et al., 2013). Another example is suppression of migration by interleukin-1β (IL-1β) (Hengartner et al., 2013). These inhibitors are factors which do not directly repel osteoblasts but rather inhibit migration in response to a specific chemoattractant, and are termed chemorepellents. Thus far, three such chemorepellents have been identified (Table 2).
Table 2.
Osteoblast chemorepellents.
| Class | Factor | Source1 | Cell types | Surface (assay) | Comments | References |
|---|---|---|---|---|---|---|
| CK | IL-1β | rh | hOB | PC (Boyden) | Inhibits IGF-1-, PDGF-BB-, VEGF-A-, and Ca5-induced migration; ERK1/2 and JNK signalling; no effect on hMSC | Hengartner et al. (2013) |
| CK | LIF | n.a | mOB | PC (Boyden) | Inhibits TGF-β1-induced migration; purified LIF was not studied | Ota et al. (2013) |
| Other | Sema3e | rm | mOB | TC-plastic (scratch) | Inhibits serum-induced migration | Hughes et al. (2012) |
Source of factor studied in assay.
Abbreviations: Ca5, complement component 5a; CK, cytokine; ERK1/2, extracellular signal-regulated kinase 1/2; hMSC, human mesenchymal stem cells; hOB, human osteoblast; IGF-1, insulin-like growth factor-1; IL-1β, interleukin-1β; JNK, c-Jun N-terminal kinase; LIF, leukaemia inhibitory factor; mOB, mouse osteoblast; n.a., not applicable; PC, polycarbonate; PGDF-BB, platelet-derived growth factor-BB; rh, recombinant human; rm, recombinant mouse; Sema3e, semaphorin-3e; TC, tissue culture-treated; TGF-β1, transforming growth factor β1; VEGF-A, vascular endothelial growth factor-A.
In comparison to the number of known chemoattractants and repellents, much less is known about the signalling pathways activated in response to them. The clearest information is for platelet-derived growth factor (PDGF) signalling. Recent in vitro work by Schmidt et al. (2015) demonstrated that micro ribonucleic acid 126 (miRNA-126) functions as an indirect regulator of PDGF receptor α expression. In gain- and loss-of-function studies they showed that miRNA-126 can act as positive and negative regulator of osteoblast migration while not affecting proliferation or differentiation (Schmidt et al., 2015). Because co-cultivation of osteoblasts with vascular endothelial cells leads to an upregulation of miRNA-126 in osteoblasts, miRNA-126 might serve as a stop signal for PDGF-controlled osteoblast migration in close proximity to blood vessels. With respect to signalling downstream of the PDGF receptor, two studies reported phosphoinositide 3-kinase (PI3K) and p38 mitogen-activated protein kinase (p38 MAP kinase) signalling during PDGF-BB-stimulated migration of mouse osteoblasts in vitro (Godwin & Soltoff, 1997; Mehrotra et al., 2004), in agreement with known signalling pathways involved in cell migration (see Section III). Further, in vitro work by Ren et al. (2012) revealed that phosphorylation of tyrosine 321 of G protein-coupled receptor (GPCR) kinase interacting protein-1 (GIT-1) is essential for proper downstream activity of focal adhesion kinase (FAK) in PDGF-stimulated osteoblast migration. This suggests that in osteoblasts FAK, in concert with the regulator GIT-1, acts upstream of Rho GTPase signalling by integrating signals from tyrosine kinases and integrin receptors. Together, these findings show that osteoblasts orchestrate a specific migratory response based on multi-facetted, generic signalling pathways. Further, they substantiate the intimate relationship between migrating osteoblasts and vascular endothelial cells observed by Maes et al. (2010) and discussed above (Section II).
Although the above studies highlight the pivotal role that chemoattractants play in osteoblast migration, an observation that challenges the idea that they have entire responsibility for the control of osteoblast migration is the fact that few, if any, of the known growth factors and cytokines appear to be specific to osteoblast migration (Tables 1 and 2). In fact, all discussed chemoattractants exhibit multiple physiological functions in osteoblasts, i.e. guiding differentiation and proliferation (Dai & Rabie, 2007; Janssens et al., 2005; Minuto et al., 2005) that often occur in temporal–spatial proximity to migration. This strongly suggests that osteoblasts have unknown ways of integrating growth factor or cytokine signals to refine their response, i.e. differentiation or migration. Intriguingly, osteoblast chemoattractants can also provide concurrent physiological signals. In vitro work has reported that parathyroid hormone (PTH) induced loss of the mature phenotype in osteocytes along with increased migratory potential in these cells (Prideaux et al., 2015), a change likely to be a result of osteocyte dedifferentiation (Torreggiani et al., 2013).
(b) Sources of osteoblast chemoattractants and repellents
All known osteoblast chemoattractants and repellents (Tables 1 and 2) can be released from cells. Within a particular skeletal space, such as the BMU or fracture site, the cell types in sensory reach of osteoblasts vary. For example, in the cortical BMU, cells of the osteoblast lineage interact primarily with other osteoblasts, osteoclasts, vascular endothelial cells and haematopoietic cells (Fig. 1A), although the latter has not been well described and quantitative cell-type composition over time is dynamic and subject to change. In tissues such as the fracture callus, other cell types, including cells of the haematopoietic and cartilage linages provide additional cellular sources. To assign chemoattractants definitively to particular cell types, it would be desirable to detect and measure the factor secretion of individual cells accurately in vivo in a temporal–spatial manner. However, current methodology does not permit such measurements. Therefore, it will be important to begin to extend in vitro migration studies of purified chemoattractants and repellents to studies assessing the migration of osteoblasts during two-, three-, or four-party cell interactions with osteoclasts and vascular endothelial cells.
Another important cell type associated with the BMU is the osteocyte. Mature osteocytes are located at the periphery of a BMU as well as formed de novo within the BMU as part of the newly synthesized bone tissue. It is known that osteocytes produce a range of osteoblast chemoattractants (Bonewald, 2017; Goldring, 2015), including receptor activator of nuclear factor kappa-B ligand (RANKL), PTH, insulin-like growth factor-1 (IGF-1) and vascular endothelial growth factor-A (VEGF-A), and hence they provide chemotaxis signals to osteoblasts. In addition to the secretion of chemoattractants, it should be considered that osteocyte death, which initiates bone remodelling after fatigue damage (Goldring, 2015; Schaffler et al., 2014), provides additional signals either through release of factors during osteocyte apoptosis or due to signal termination in the absence of osteocytes. The role of osteocytes in osteoblast navigation is likely to be broader. For example, bone formation during adaptive modelling, a load-dependent process that shapes bones, does not require initial bone resorption or vascular invasion (Seeman, 2009), and thus requires directed osteoblast migration in an environment with limited cellular sources of chemoattractants. It can be postulated that in this environment the osteocyte constitutes the primary cellular source of osteoblast navigation signals. The interaction between osteocytes and migrating osteoblasts is not limited to chemoattractants. More recent studies have shown that osteocytes respond to load with decreased production of the wingless-related integration site (Wnt) signalling inhibitors sclerostin and Dickkopf Wnt signalling pathway inhibitor 1 (Robling et al., 2008). Further, during embryonic development, loss of Wnt signalling typically results in severe skeletal deformities due to disturbed patterning (Maupin, Droscha & Williams, 2013), a process intimately related to cell migration. Considering that canonical Wnt signalling is an important regulator of osteoblast function (Macsai, Foster & Xian, 2008) and that adenomatous polyposis coli (APC), which participates in Wnt signalling, is an important regulator of cell migration (Etienne-Manneville, 2009), the concept of osteocyte-mediated control of osteoblast migration via blockage of Wnt signalling warrants further investigation.
In skeletal tissues, an alternative to the direct release of chemoattractants and repellents from cells exists. Because cells such as osteoblasts or chondroblasts deposit an array of biological factors within the matrix during its formation, the matrix serves as a reservoir for chemoattractants and repellents. Release from the matrix is facilitated by proteolytic degradation. For example, Oursler (1994) showed in vitro that avian osteoclasts secrete proteases into the extracellular milieu and that this facilitates the release of the chemoattractant TGF-β from its latent precursor. More importantly, in vivo and in vitro studies by Tang et al. (2009) demonstrated that an osteoclast-mediated release of TGF-β1 from the matrix is not only important as a migration signal but also facilitates proper coupling of bone resorption to formation, suggesting a functional relationship between osteoblast migration and bone remodelling. It should, however, be noted that (1) proteolytic release of chemoattractants from the matrix might be rather unspecific, and (2) it likely leads to concurrent release of several chemoattractants. How cells migrate in such environments is discussed in Section II.1c.
(c) Migration of osteoblasts in multiple chemotactic gradients
Osteoblasts can be exposed to more than one chemotactic gradient at a time, raising the question of how they respond to such an environment. The principal behaviour of cells, likely including osteoblasts, to multiple chemotactic gradients was established in in vitro work (Foxman, Campbell & Butcher, 1997; Foxman, Kunkel & Butcher, 1999). These authors made four important observations. First, there is hierarchy in the chemoattractive response because at identical conditions of migratory stimulation, chemoattractants exhibit different potency. Recent work has shown that specific silencing of PDGF-BB in osteoclasts is sufficient to reduce osteoblast migration by 50%, while suppression of VEGF-A or LIF had no effect (Sanchez-Fernandez et al., 2008). Second, the migration path follows the vector sum, assuming that individual gradients can be described as vectors. Third, migration along a chemoattractant gradient can be abolished, for example by saturation of a receptor with chemoattractant or by receptor desensitization. This, for example, permits cells to ignore a near gradient while responding to a far gradient. GPCRs are particularly well understood in this respect, and have been shown to undergo ligand-dependent receptor desensitization, in which ligand-bound receptors are turned off after a transient period of signalling (Lin & Butcher, 2008). Fourth, there are dominant chemoattractants which, at defined concentrations, can induce a migratory stop. However, cells are released from this in response to a different chemotactic gradient. At present, we anticipate that above behaviour also applies to osteoblasts as the reported optimum concentrations of osteoblast chemoattractants support this proposal (Table 1).
III. OSSEOUS SURFACES AS OSTEOBLAST NAVIGATION SIGNALS
With the exception of circulating osteogenic precursors (Pignolo & Shore, 2010), osteoblasts migrate on surfaces and hence adhesion to a surface typically occurs prior to movement. Similar to other cell types, osteoblasts adhere to surfaces via focal adhesions and in vitro work by Biggs et al. (2008) investigated the relationship between focal adhesion formation and surface topography (Fig. 3). They examined grooved biomaterials with variable width and a fixed depth of approximately 300 nm, a size dimension found in tropocollagen. A groove width of 25 μm led to the formation of adhesions predominantly on the raised ridge areas (Fig. 3A), while osteoblasts on 10 μm grooves aligned with groove direction. By contrast, a groove width of 100 μm (Fig. 3B) did not affect adhesion formation compared to a flat surface, despite this groove width previously being shown to facilitate osteoblast aggregation (Zinger et al., 2005). We speculate that osteoblasts utilize surface recognition features as landmarks, for example to differentiate elevated and lowered surface structures such as osteoid and resorption pits, respectively. However, the above studies were carried out on biomaterials and validation of these observations on true osseous surfaces is necessary.
Fig. 3.
Topography recognition of osteoblasts. Scanning electron microscope (SEM) images of osteoblasts on experimental substrates with a fixed groove depth of 330 nm. Cells were immunolabelled using an antibody against vinculin, a marker of adhesion complexes. (A) Osteoblasts on 25 μm grooves formed adhesions predominantly on the raised ridge areas (indicated in insert). (B) Diffuse 100 μm grooves did not affect adhesion formation. Scale bars are shown. Reprinted with permission from Biggs et al. (2008).
Osteoblasts utilize the focal adhesions to facilitate surface recognition and binding through the extracellular domains of cell-membrane-spanning receptors, namely receptors of the integrin family. In situ and in vitro studies show that in osteoblasts the β1 integrin subunit is predominant, while the β2 and β4 subunits are not detectable (Helfrich et al., 2008). The β1 subunit in osteoblasts forms a number of heterodimeric receptors, including α1–5β1 and αvβ1, 3, 5 (Gronthos et al., 1997; Hughes et al., 1993), however, both the in vivo expression pattern and the specific role of each of these heterodimeric integrin receptors has not yet been fully established. The functional relevance of the β1 integrin subunit has been demonstrated in mouse models in vivo. Osteoblast-specific overexpression of a dominant negative β1 integrin subunit resulted in decreased osteoblast adhesion, altered bone formation, and decreased bone mass (Zimmerman et al., 2000), while global deletion of integrin cytoplasmic domain-associated protein-1, an inhibitor of β1 integrin downstream signalling, shows enhanced osteoblast adhesion along with altered bone formation (Bouvard et al., 2007). These findings not only demonstrate that altered integrin function is associated with bone abnormalities but also that integrins tune the adhesion strength of osteoblasts to surfaces. This is of considerable relevance to osteoblast migration, because the efficiency of cell movement on a given surface is a function of the level of adhesion, and optimal movement is typically observed at an intermediate adhesion level (Huttenlocher, Sandborg & Horwitz, 1995).
The importance of integrin-mediated adhesion to osteoblast migration can be appreciated in embryonic development. Formation of the sclerotome during dorsoventral patterning is guided by gradients of Wnt and Sonic hedgehog (Brent & Tabin, 2002), and in vitro studies using neural crest cells, an embryonic osteoblast precursor cell type derived from avian embryos at a 20–25 somite stage, demonstrated that Sonic hedgehog directly inhibited migration of neural crest cells (Testaz et al., 2001). Inhibition was concentration-dependent and data suggested that Sonic hedgehog acted as a competitor for integrin ligand binding. Thus, independent of integrin receptor expression, the availability of the free receptor binding sites can regulate spatial migration of embryonic osteoblast precursors.
The interplay between osseous surfaces and osteoblast migration is likely to be multifaceted. We begin our discussion by outlining the major surface components recognized by adhering osteoblasts. The heterodimeric integrin receptors on osteoblasts are capable of binding to the major components of bone matrix, such as type I collagen and non-collagenous matrix components including bone sialoprotein, osteopontin, vitronectin, osteoactivin, osteonectin, thrombospondin(s) and fibronectin (Helfrich et al., 2008). Binding, in particular to bone sialoprotein, osteopontin and vitronectin, is mediated by the tripeptide Arg-Gly-Asp (RGD) in these proteins (Grzesik & Robey, 1994). Functionally, the heterodimeric integrin receptors serve as links between the extracellular matrix, where they bind to immobilized ligands including collagen and RGD, and the intracellular cytoskeleton, where they not only connect to the actin network but also facilitate signal transduction. For example, Popov et al. (2011) demonstrated that in MSCs a deficiency in the α2 or α11 integrin subunit led to disturbed FAK and v-Akt murine thymoma viral oncogene (Akt) signalling accompanied by inhibited migration, reduced osteogenic differentiation, and increased apoptosis. Conversely, the composition of integrin ligands in an osseous surface modulates cellular responses including migration, an observation frequently exploited in bone tissue engineering (Meyer et al., 2005). That the composition of integrin ligands presented on an osseous surface modulates cellular responses is of relevance because the composition of the osseous surface is not uniform. For instance, Boskey et al. (2015) showed heterogeneity of collagen maturity in human (Homo sapiens) bone. Even more importantly, Carter, Sloan & Aaron (1991) found a heterogeneous distribution of matrix components in different types of bone tissue of the human femur and reported that fibronectin, although considered a principal non-collagenous component of bone matrix, is absent from mature lamellar bone. Further, in vitro work by McKee et al. (1993) demonstrated that the RGD-containing matrix components osteocalcin and osteopontin were not localized homogenously but restricted to patches of mineralized bone and mineralization loci of osteoid.
The mechanisms by which osseous surfaces control osteoblast migration are sophisticated. For example, matrix components such as type I collagen or fibronectin, although immobilized within a ridged matrix, can be recognized by osteoblasts as chemoattractants and induce haptotaxis, i.e. migration along a gradient of cellular adhesion sites or substrate-bound chemoattractants. Sotobori et al. (2006) found that immobilized fibronectin and in particular immobilized type I collagen elicit marked osteoblast migration as compared to laminin-1 and that the response was concentration dependent. Further, they observed that bone morphometric protein-2 (BMP-2) increased the haptotactic osteoblast migration, possibly through modulation of integrin β1 presentation in membrane lipid rafts (Sotobori et al., 2006). Hence, osteoblasts may also migrate along haptotactic gradients of immobilized matrix components. A potential receptor mediating the haptotactic response of osteoblasts on resorbed bone, i.e. a catabolic matrix (Abdelgawad et al., 2014; Everts et al., 2002) has already been reported by Abdelgawad et al. (2014). They found that, in particular, reversal cells express the endocytotic collagen receptor urokinase-type plasminogen activator receptor-associated protein (uPARAP) and that blockage of the receptor reduced haptotaxis towards type I collagen (Abdelgawad et al., 2014). As uPARAP is engaged in the reversal phase between bone resorption and formation, its function in the BMU might be more relevant to osteoblast migration as compared to other collagen receptors.
The emerging evidence that the matrix may play an important part in controlling osteoblast migration on osseous surfaces led us to propose that the mineral portion of bone may also be involved in these processes. This suggestion is based on work showing that the collagen fibrils in bone contain mineral both inside and on their surface (Hassenkam et al., 2004). Thus, bone mineral could partially mask RGD motifs, thereby decreasing adhesion and permitting increased migration. Such a relationship would predict increased migration of mineralized surfaces and decreased migration on osteoid. For instance, in the BMU (Fig. 1A), bone apposition by osteoblasts occurs while the cells adhere to osteoid resulting in slow collective osteoblast movement, thus permitting sufficient time for proper apposition of the complex yet highly organized matrix. As the BMU constitutes several types of bone tissue including mature bone, resorbed bone, osteoid and immature bone, it is possible that the osseous surface the osteoblasts migrate upon offers important signals, a possibility that warrants experimental investigation.
In skeletal disease, specifically osteoporosis, the pathophysiological relevance of an altered osseous surface composition has been investigated by applying Fourier-transform infrared microspectroscopy techniques to in vitro studies comparing matrix and mineral properties between human osteoporotic and healthy bone. Paschalis et al. (2004) found a decrease in collagen flexibility, reflected by increased cross-linking, while Boskey et al. (2005) reported a larger size of the mineral crystals. Therefore, alterations in both matrix and mineral properties of osteoporotic bone exist. Another important aspect relates to surface modifications through pharmacological intervention; in osteoporosis intervention, the anti-resorptive bisphosphonates target the osseous surface. Consistent with a toxic effect of bisphosphonate on osteoblasts (Basso et al., 2013), zoledronate was shown in vitro to halt osteoblast migration (Koch et al., 2011). In the future, it will be particularly important to determine if surface alterations or pharmaceutical treatments influence migratory behaviour of osteoblasts in patients afflicted by osteoporosis. Since osteoblastic activity is thought to be relatively decreased in osteoporosis (Biggs et al., 2008), this seems a likely scenario.
Both alterations in the surface composition and in the molecular pathways of the migrating osteoblastic cells may contribute to abnormal migration of osteoblastic cells in skeletal pathologies including osteoporosis. Haasters et al. (2014) investigated differences in the migratory behaviour of human osteoprogenitors derived from normal and osteoporotic bone using a horizontal cell-migration system in vitro. Their data showed that cells from osteoporotic bone exhibited a reduction in migration towards chemotactic gradients of BMP-2 and -7 on a type IV collagen substrate (Haasters et al., 2014). Additional gene-expression analysis revealed a pronounced reduction in integrin α2 expression, potentially attributing to the observed migration defect (Haasters et al., 2014).
IV. OSSEOUS FLUID FLOW AS AN OSTEOBLAST NAVIGATION LANDMARK
It has long been recognized that mechanical loading is essential for proper bone function, including maintaining the structural integrity of the skeleton and fracture healing (Balogh et al., 2012; Klein-Nulend et al., 2013). In an excellent review, Thompson, Rubin & Rubin (2012) indicated that the entire osteoblast lineage, as well as osteoclasts, participate in the response to mechanical signals such as strain, pressure or shear stress induced by fluid flow. Based on previously published reports, they delineated four classes of mechanotransducer systems. Firstly, and perhaps most importantly, the integrin–actin axis acts as principal transducer of mechanical signals. Second, cell–cell connections, including connexin 43, function as mechanotransducers. Third, calcium channels and purinergic receptors, such as the purinergic receptor P2X7 which has been shown to mediate fluid shear stress in osteoblasts (Li et al., 2005b), serve as signal transducers following mechanical stimulation. Indeed, Jorgenson et al. (2012) recently reported that in patients, a loss-of-function Arg307Gln amino acid substitution in the P2X7 receptor is associated with decreased bone mass. Fourth, primary cilia transduce mechanical signals and osteoblasts possess cilia (Xiao et al., 2006). It is striking that the first three classes have also been implicated in the migration of osteoblasts, as discussed above. In concordance with this overlap in transducers of mechanical and migration signals, the cellular responses to these signals share key signalling pathways, such as β1 integrin-FAK-signalling, growth factor receptor signalling via PI3K or Ras, GPCR and MAP kinase signalling (Liedert et al., 2006; Papachroni et al., 2009). These data point to mechanical signals, such as fluid flow, as potential landmarks for osteoblast migration and navigation. Recent in vitro work by Riehl et al. (2015) demonstrated in mouse C3H10T1/2 cells, which can undergo osteogenic differentiation in vitro, that fluid flow guides these cells in the flow direction and that their migration accelerates with increasing shear stress. These effects were dependent on FAK signalling. In another intriguing contribution, Smit, Burger & Huyghe (2002) modelled strain-induced fluid flow in the BMU and concluded that during compression a fluid influx along the reversal and closing zones and a fluid efflux along the cutting cone occurs. With potential pathophysiological relevance, altered fluid flow around osteocytes in the lacunar–canalicular network of oestrogen-deficient mice suggested a role of abnormal fluid flow in osteoporosis (Ciani et al., 2014; Sharma et al., 2012). Another type of signal produced by mechanical load in bone is a time-varying electric field (Otter, McLeod & Rubin, 1998). In conjunction with similar fields elicited by contracting muscle and steady direct currents produced by cell metabolism, these cues are likely to result in electric field variations in bone. Such fields appear to have the potential to guide osteoblast motility and the Na+/H+ exchanger, and intracellular Ca2+ signalling seems to play a role in this (Ozkucur et al., 2009; Perike et al., 2014). Altogether, it is evident that mechanical signals modulate osteoblast migration and navigation and we feel that the relationship between mechanical signals and osteoblast migration deserves further investigation.
V. CONCLUSIONS
Since the first detailed description of osteoblast migration 30 years ago, components of the molecular and cellular machinery that drive osteoblast migration have been identified. Osteoblasts utilize integrins to adhere to osseous surfaces, thereby using the strength of adhesion to control their motility. However, the integrin-mediated bond of osteoblasts to the osseous surface is not restricted to physical adherence. Rather osteoblasts use integrins to survey the surface for ligand composition, in turn informing the osteoblast via intracellular signalling pathways about navigation signals provided by the surface composition. Further, a spatial change in the surface composition can be recorded and processed to help to determine the osteoblast’s migratory path. In addition, integrins can integrate migration signals from the surface with navigation signals received through mechanical force. The multifaceted navigation signals that guide osteoblasts to their destination are summarized in a novel working model (Fig. 4).
The migratory potential of osteoblasts is higher in undifferentiated osteoblasts than in differentiated, bone-forming osteoblasts. Thus, osteoblast differentiation and migration are inversely related. However, there is no evidence that even mature osteoblasts lose their ability to navigate precisely during active bone formation.
The osteoblasts discussed in this review encompass osteoprogenitors, immature osteoblasts, and mature osteoblasts from several species, and the cells were isolated and characterized using a number of techniques. This variability in cell populations poses limitations to comparisons of results from different studies. Thus, forthcoming migration studies might benefit from the use of more-standardized cell-isolation techniques and additional validated osteoblastic markers. Similarly, migration studies are often based on surfaces with limited relevance to the osseous surface in vivo and it will be important to define signals better from such surfaces to allow us to understand, for example, how woven and lamellar bone affect osteoblast movement. The discovery of different classes of navigation signals will allow us to understand better how osteoblasts process and respond to multiple signals. Finally, much of the work discussed herein was derived from in vitro studies and, despite considerable technical challenges, it will be of great importance to begin to validate current concepts in vivo.
Fig. 4.
Working model osteoblast navigation. Osteoprogenitor cells originate from vascular structures or stem cell pools. The migration direction of movement of osteoprogenitors and their progeny is governed by gradients of chemoattractants (shaded grey) which are released either proteolytically from matrix through osteoclastic resorption or are secreted by neighbouring cell types. Osteoblasts can also sense mechanical signals, for example from fluid flow, that can guide the tracks of osteoblasts. Generally, migration of osteoblasts occurs on surfaces such as resorbed bone or newly formed osteoid and these surfaces control, for example, cell adhesion or receptors for chemotaxis; they can also provide substrate-bound chemoattractants for haptotaxis. Abbreviations: IL-1β, interleukin β1; TGF-β1, transforming growth factor-β1.
Acknowledgments
This work was funded by the German Research Foundation (DFG MA 2188/5-1) and the National Institutes of Health (NIH grant AR041325).
Footnotes
This article is dedicated to the late Dr. Adele Boskey.
VII. REFERENCES
- Abdelgawad ME, Soe K, Andersen TL, Merrild DM, Christiansen P, Kjaersgaard-Andersen P, Delaisse JM. Does collagen trigger the recruitment of osteoblasts into vacated bone resorption lacunae during bone remodeling? Bone. 2014;67:181–188. doi: 10.1016/j.bone.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Baldini G, Ponti C, Bortul R, Narducci P, Grill V, Martelli AM. Sparc localizes to the blebs of hobit cells and human primary osteoblasts. Journal of Cellular Biochemistry. 2008;104:2310–2323. doi: 10.1002/jcb.21789. [DOI] [PubMed] [Google Scholar]
- Balogh ZJ, Reumann MK, Gruen RL, Mayer-Kuckuk P, Schuetz MA, Harris IA, Gabbe BJ, Bhandari M. Advances and future directions for management of trauma patients with musculoskeletal injuries. Lancet. 2012;380:1109–1119. doi: 10.1016/S0140-6736(12)60991-X. [DOI] [PubMed] [Google Scholar]
- Basso FG, Silveira Turrioni AP, Hebling J, de Souza Costa CA. Zoledronic acid inhibits human osteoblast activities. Gerontology. 2013;59:534–541. doi: 10.1159/000351194. [DOI] [PubMed] [Google Scholar]
- Baum B, Settleman J, Quinlan MP. Transitions between epithelial and mesenchymal states in development and disease. Seminars in Cell & Developmental Biology. 2008;19:294–308. doi: 10.1016/j.semcdb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Bianco P. “Mesenchymal” stem cells. Annual Review of Cell and Developmental Biology. 2014;30:677–704. doi: 10.1146/annurev-cellbio-100913-013132. [DOI] [PubMed] [Google Scholar]
- Biggs MJ, Richards RG, McFarlane S, Wilkinson CD, Oreffo RO, Dalby MJ. Adhesion formation of primary human osteoblasts and the functional response of mesenchymal stem cells to 330nm deep microgrooves. Journal of the Royal Society Interface. 2008;5:1231–1242. doi: 10.1098/rsif.2008.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonewald LF. The role of the osteocyte in bone and nonbone disease. Endocrinology and Metabolism Clinics of North America. 2017;46:1–18. doi: 10.1016/j.ecl.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey AL, DiCarlo E, Paschalis E, West P, Mendelsohn R. Comparison of mineral quality and quantity in iliac crest biopsies from high- and low-turnover osteoporosis: an FT-IR microspectroscopic investigation. Osteoporosis International. 2005;16:2031–2038. doi: 10.1007/s00198-005-1992-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey AL, Donnelly E, Boskey E, Spevak L, Ma Y, Zhang W, Lappe J, Recker RR. Examining the relationships between bone tissue composition, compositional heterogeneity and fragility fracture: a matched case controlled FTIRI study. Journal of Bone and Mineral Research. 2015;31:1070–1081. doi: 10.1002/jbmr.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvard D, Aszodi A, Kostka G, Block MR, Albiges-Rizo C, Fassler R. Defective osteoblast function in ICAP-1-deficient mice. Development. 2007;134:2615–2625. doi: 10.1242/dev.000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent AE, Tabin CJ. Developmental regulation of somite derivatives: muscle, cartilage and tendon. Current Opinion in Genetics and Development. 2002;12:548–557. doi: 10.1016/s0959-437x(02)00339-8. [DOI] [PubMed] [Google Scholar]
- Buenzli PR, Jeon J, Pivonka P, Smith DW, Cummings PT. Investigation of bone resorption within a cortical basic multicellular unit using a lattice-based computational model. Bone. 2012;50:378–389. doi: 10.1016/j.bone.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter DH, Sloan P, Aaron JE. Immunolocalization of collagen types I and III, tenascin, and fibronectin in intramembranous bone. Journal of Histochemistry and Cytochemistry. 1991;39:599–606. doi: 10.1177/39.5.1707904. [DOI] [PubMed] [Google Scholar]
- Ciani C, Sharma D, Doty SB, Fritton SP. Ovariectomy enhances mechanical load-induced solute transport around osteocytes in rat cancellous bone. Bone. 2014;59:229–234. doi: 10.1016/j.bone.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colciago A, Celotti F, Casati L, Giancola R, Castano SM, Antonini G, Sacchi MC, Negri-Cesi P. In Vitro Effects of PDGF Isoforms (AA, BB, AB and CC) on Migration and Proliferation of SaOS-2 Osteoblasts and on Migration of Human Osteoblasts. International Journal of Biomedical Science. 2009;5:380–389. [PMC free article] [PubMed] [Google Scholar]
- Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. Journal of Bone and Mineral Research. 2009;24:274–282. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colnot C, Thompson Z, Miclau T, Werb Z, Helms JA. Altered fracture repair in the absence of MMP9. Development. 2003;130:4123–4133. doi: 10.1242/dev.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Rabie AB. VEGF: an essential mediator of both angiogenesis and endochondral ossification. Journal of Dental Research. 2007;86:937–950. doi: 10.1177/154405910708601006. [DOI] [PubMed] [Google Scholar]
- Dirckx N, Van Hul M, Maes C. Osteoblast recruitment to sites of bone formation in skeletal development, homeostasis, and regeneration. Birth Defects Res C Embryo Today. 2013;99:170–191. doi: 10.1002/bdrc.21047. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S. APC in cell migration. Advances in Experimental Medicine and Biology. 2009;656:30–40. doi: 10.1007/978-1-4419-1145-2_3. [DOI] [PubMed] [Google Scholar]
- Everts V, Delaisse JM, Korper W, Jansen DC, Tigchelaar-Gutter W, Saftig P, Beertsen W. The bone lining cell: its role in cleaning Howship’s lacunae and initiating bone formation. Journal of Bone and Mineral Research. 2002;17:77–90. doi: 10.1359/jbmr.2002.17.1.77. [DOI] [PubMed] [Google Scholar]
- Fackler OT, Grosse R. Cell motility through plasma membrane blebbing. Journal of Cell Biology. 2008;181:879–884. doi: 10.1083/jcb.200802081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler J, Etzel N, Brenner RE. To go or not to go: Migration of human mesenchymal progenitor cells stimulated by isoforms of PDGF. Journal of Cellular Biochemistry. 2004;93:990–998. doi: 10.1002/jcb.20219. [DOI] [PubMed] [Google Scholar]
- Fiedler J, Leucht F, Waltenberger J, Dehio C, Brenner RE. VEGF-A and PlGF-1 stimulate chemotactic migration of human mesenchymal progenitor cells. Biochemical and Biophysical Research Communications. 2005;334:561–568. doi: 10.1016/j.bbrc.2005.06.116. [DOI] [PubMed] [Google Scholar]
- Fiedler J, Roderer G, Gunther KP, Brenner RE. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. Journal of Cellular Biochemistry. 2002;87:305–312. doi: 10.1002/jcb.10309. [DOI] [PubMed] [Google Scholar]
- Foxman EF, Campbell JJ, Butcher EC. Multistep navigation and the combinatorial control of leukocyte chemotaxis. Journal of Cell Biology. 1997;139:1349–1360. doi: 10.1083/jcb.139.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman EF, Kunkel EJ, Butcher EC. Integrating conflicting chemotactic signals. The role of memory in leukocyte navigation. Journal of Cell Biology. 1999;147:577–588. doi: 10.1083/jcb.147.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurtzen K, Knopf F, Wehner D, Huitema LF, Schulte-Merker S, Weidinger G. Mature osteoblasts dedifferentiate in response to traumatic bone injury in the zebrafish fin and skull. Development. 2014;141:2225–2234. doi: 10.1242/dev.105817. [DOI] [PubMed] [Google Scholar]
- Godwin SL, Soltoff SP. Extracellular calcium and platelet-derived growth factor promote receptor-mediated chemotaxis in osteoblasts through different signaling pathways. Journal of Biological Chemistry. 1997;272:11307–11312. doi: 10.1074/jbc.272.17.11307. [DOI] [PubMed] [Google Scholar]
- Golden D, Saria EA, Hansen MF. Regulation of osteoblast migration involving receptor activator of nuclear factor (NF)-kappa B (RANK) signaling. Journal of Cellular Physiology. 2015;230:2951–2960. doi: 10.1002/jcp.25024. [DOI] [PubMed] [Google Scholar]
- Goldring SR. The osteocyte: key player in regulating bone turnover. RMD Open. 2015;1:e000049. doi: 10.1136/rmdopen-2015-000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Stewart K, Graves SE, Hay S, Simmons PJ. Integrin expression and function on human osteoblast-like cells. Journal of Bone and Mineral Research. 1997;12:1189–1197. doi: 10.1359/jbmr.1997.12.8.1189. [DOI] [PubMed] [Google Scholar]
- Grzesik WJ, Robey PG. Bone matrix RGD glycoproteins: immunolocalization and interaction with human primary osteoblastic bone cells in vitro. Journal of Bone and Mineral Research. 1994;9:487–496. doi: 10.1002/jbmr.5650090408. [DOI] [PubMed] [Google Scholar]
- Haasters F, Docheva D, Gassner C, Popov C, Bocker W, Mutschler W, Schieker M, Prall WC. Mesenchymal stem cells from osteoporotic patients reveal reduced migration and invasion upon stimulation with BMP-2 or BMP-7. Biochemical and Biophysical Research Communications. 2014;452:118–123. doi: 10.1016/j.bbrc.2014.08.055. [DOI] [PubMed] [Google Scholar]
- Hassenkam T, Fantner GE, Cutroni JA, Weaver JC, Morse DE, Hansma PK. High-resolution AFM imaging of intact and fractured trabecular bone. Bone. 2004;35:4–10. doi: 10.1016/j.bone.2004.02.024. [DOI] [PubMed] [Google Scholar]
- Helfrich MH, Stenbeck G, Nesbitt SA, Horton MA. Integrins and Other Cell Surface Attachment Molecules of Bone Cells. In: Bilezikian JP, Raisz LG, Martin TJ, editors. Principles of Bone Biology. Vol. 1. Academic Press; San Diego: 2008. pp. 385–424. [Google Scholar]
- Hengartner NE, Fiedler J, Ignatius A, Brenner RE. IL-1beta inhibits human osteoblast migration. Molecular Medicine. 2013;19:36–42. doi: 10.2119/molmed.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A, Kleine-Albers J, Helfrich MH, Ralston SH, Rogers MJ. A class III semaphorin (Sema3e) inhibits mouse osteoblast migration and decreases osteoclast formation in vitro. Calcified Tissue International. 2012;90:151–162. doi: 10.1007/s00223-011-9560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DE, Salter DM, Dedhar S, Simpson R. Integrin expression in human bone. Journal of Bone and Mineral Research. 1993;8:527–533. doi: 10.1002/jbmr.5650080503. [DOI] [PubMed] [Google Scholar]
- Hughes FJ, Aubin JE, Heersche JN. Differential chemotactic responses of different populations of fetal rat calvaria cells to platelet-derived growth factor and transforming growth factor beta. Bone and Mineral. 1992;19:63–74. doi: 10.1016/0169-6009(92)90844-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, Sandborg RR, Horwitz AF. Adhesion in cell migration. Current Opinion in Cell Biology. 1995;7:697–706. doi: 10.1016/0955-0674(95)80112-x. [DOI] [PubMed] [Google Scholar]
- Ignatius A, Ehrnthaller C, Brenner RE, Kreja L, Schoengraf P, Lisson P, Blakytny R, Recknagel S, Claes L, Gebhard F, Lambris JD, Huber-Lang M. The anaphylatoxin receptor C5aR is present during fracture healing in rats and mediates osteoblast migration in vitro. Journal of Trauma. 2011;71:952–960. doi: 10.1097/TA.0b013e3181f8aa2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens K, ten Dijke P, Janssens S, Van Hul W. Transforming growth factor-beta1 to the bone. Endocrine Reviews. 2005;26:743–774. doi: 10.1210/er.2004-0001. [DOI] [PubMed] [Google Scholar]
- Jilka RL. Biology of the basic multicellular unit and the pathophysiology of osteoporosis. Medical and Pediatric Oncology. 2003;41:182–185. doi: 10.1002/mpo.10334. [DOI] [PubMed] [Google Scholar]
- Jones SJ, Boyde A. The migration of osteoblasts. Cell and Tissue Research. 1977;184:179–193. doi: 10.1007/BF00223067. [DOI] [PubMed] [Google Scholar]
- Jorgensen NR, Husted LB, Skarratt KK, Stokes L, Tofteng CL, Kvist T, Jensen JE, Eiken P, Brixen K, Fuller S, Clifton-Bligh R, Gartland A, Schwarz P, Langdahl BL, Wiley JS. Single-nucleotide polymorphisms in the P2X7 receptor gene are associated with post-menopausal bone loss and vertebral fractures. European Journal of Human Genetics. 2012;20:675–681. doi: 10.1038/ejhg.2011.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Nulend J, Bakker AD, Bacabac RG, Vatsa A, Weinbaum S. Mechanosensation and transduction in osteocytes. Bone. 2013;54:182–190. doi: 10.1016/j.bone.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Koch FP, Wunsch A, Merkel C, Ziebart T, Pabst A, Yekta SS, Blessmann M, Smeets R. The influence of bisphosphonates on human osteoblast migration and integrin aVb3/tenascin C gene expression in vitro. Head & Face Medicine. 2011;7:4. doi: 10.1186/1746-160X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Park BJ, Lee MS, Lee JW, Kim JK, Yang HC, Park JC. Chemotactic migration of human mesenchymal stem cells and MC3T3-E1 osteoblast-like cells induced by COS-7 cell line expressing rhBMP-7. Tissue Engineering. 2006;12:1577–1586. doi: 10.1089/ten.2006.12.1577. [DOI] [PubMed] [Google Scholar]
- Li G, Cui Y, McIlmurray L, Allen WE, Wang H. rhBMP-2, rhVEGF(165), rhPTN and thrombin-related peptide, TP508 induce chemotaxis of human osteoblasts and microvascular endothelial cells. Journal of Orthopaedic Research. 2005a;23:680–685. doi: 10.1016/j.orthres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Li J, Liu D, Ke HZ, Duncan RL, Turner CH. The P2X7 nucleotide receptor mediates skeletal mechanotransduction. Journal of Biological Chemistry. 2005b;280:42952–42959. doi: 10.1074/jbc.M506415200. [DOI] [PubMed] [Google Scholar]
- Liedert A, Kaspar D, Blakytny R, Claes L, Ignatius A. Signal transduction pathways involved in mechanotransduction in bone cells. Biochemical and Biophysical Research Communications. 2006;349:1–5. doi: 10.1016/j.bbrc.2006.07.214. [DOI] [PubMed] [Google Scholar]
- Lin F, Butcher EC. Modeling the role of homologous receptor desensitization in cell gradient sensing. Journal of Immunology. 2008;181:8335–8343. doi: 10.4049/jimmunol.181.12.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind M, Deleuran B, Thestrup-Pedersen K, Soballe K, Eriksen EF, Bunger C. Chemotaxis of human osteoblasts. Effects of osteotropic growth factors. APMIS. 1995a;103:140–146. [PubMed] [Google Scholar]
- Lind M, Deleuran B, Yssel H, Fink-Eriksen E, Thestrup-Pedersen K. IL-4 and IL-13, but not IL-10, are chemotactic factors for human osteoblasts. Cytokine. 1995b;7:78–82. doi: 10.1006/cyto.1995.1010. [DOI] [PubMed] [Google Scholar]
- Lind M, Eriksen EF, Bunger C. Bone morphogenetic protein-2 but not bone morphogenetic protein-4 and -6 stimulates chemotactic migration of human osteoblasts, human marrow osteoblasts, and U2-OS cells. Bone. 1996;18:53–57. doi: 10.1016/8756-3282(95)00423-8. [DOI] [PubMed] [Google Scholar]
- Macsai CE, Foster BK, Xian CJ. Roles of Wnt signalling in bone growth, remodelling, skeletal disorders and fracture repair. Journal of Cellular Physiology. 2008;215:578–587. doi: 10.1002/jcp.21342. [DOI] [PubMed] [Google Scholar]
- Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Developmental Cell. 2010;19:329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar R, Sixt M, Parent CA. New paradigms in the establishment and maintenance of gradients during directed cell migration. Current Opinion in Cell Biology. 2014;30:33–40. doi: 10.1016/j.ceb.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiello LM, Fotos JS, Galileo DS, Karin NJ. Lysophosphatidic acid induces chemotaxis in MC3T3-E1 osteoblastic cells. Bone. 2006;39:72–82. doi: 10.1016/j.bone.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Maupin KA, Droscha CJ, Williams BO. A comprehensive overview of skeletal phenotypes associated with alterations in Wnt/beta-catenin signaling in humans and mice. Bone Research. 2013;1:27–71. doi: 10.4248/BR201301004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr-Wohlfart U, Waltenberger J, Hausser H, Kessler S, Gunther KP, Dehio C, Puhl W, Brenner RE. Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone. 2002;30:472–477. doi: 10.1016/s8756-3282(01)00690-1. [DOI] [PubMed] [Google Scholar]
- McKee MD, Farach-Carson MC, Butler WT, Hauschka PV, Nanci A. Ultrastructural immunolocalization of noncollagenous (osteopontin and osteocalcin) and plasma (albumin and alpha 2HS-glycoprotein) proteins in rat bone. Journal of Bone and Mineral Research. 1993;8:485–496. doi: 10.1002/jbmr.5650080413. [DOI] [PubMed] [Google Scholar]
- Mehrotra M, Krane SM, Walters K, Pilbeam C. Differential regulation of platelet-derived growth factor stimulated migration and proliferation in osteoblastic cells. Journal of Cellular Biochemistry. 2004;93:741–752. doi: 10.1002/jcb.20138. [DOI] [PubMed] [Google Scholar]
- Meyer U, Buchter A, Wiesmann HP, Joos U, Jones DB. Basic reactions of osteoblasts on structured material surfaces. European Cells & Materials. 2005;9:39–49. doi: 10.22203/ecm.v009a06. [DOI] [PubMed] [Google Scholar]
- Midy V, Plouet J. Vasculotropin/vascular endothelial growth factor induces differentiation in cultured osteoblasts. Biochemical and Biophysical Research Communications. 1994;199:380–386. doi: 10.1006/bbrc.1994.1240. [DOI] [PubMed] [Google Scholar]
- Minuto F, Palermo C, Arvigo M, Barreca AM. The IGF system and bone. Journal of Endocrinological Investigation. 2005;28:8–10. [PubMed] [Google Scholar]
- Nakasaki M, Yoshioka K, Miyamoto Y, Sasaki T, Yoshikawa H, Itoh K. IGF-I secreted by osteoblasts acts as a potent chemotactic factor for osteoblasts. Bone. 2008;43:869–879. doi: 10.1016/j.bone.2008.07.241. [DOI] [PubMed] [Google Scholar]
- Ota K, Quint P, Weivoda MM, Ruan M, Pederson L, Westendorf JJ, Khosla S, Oursler MJ. Transforming growth factor beta 1 induces CXCL16 and leukemia inhibitory factor expression in osteoclasts to modulate migration of osteoblast progenitors. Bone. 2013;57:68–75. doi: 10.1016/j.bone.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter MW, McLeod KJ, Rubin CT. Effects of electromagnetic fields in experimental fracture repair. Clinical Orthopaedics and Related Research. 1998:S90–104. doi: 10.1097/00003086-199810001-00011. [DOI] [PubMed] [Google Scholar]
- Oursler MJ. Osteoclast synthesis and secretion and activation of latent transforming growth factor beta. Journal of Bone and Mineral Research. 1994;9:443–452. doi: 10.1002/jbmr.5650090402. [DOI] [PubMed] [Google Scholar]
- Ozkucur N, Monsees TK, Perike S, Do HQ, Funk RH. Local calcium elevation and cell elongation initiate guided motility in electrically stimulated osteoblast-like cells. PloS One. 2009;4:e6131. doi: 10.1371/journal.pone.0006131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagakos FS. Insulin-like growth factors-I and -II stimulate chemotaxis of osteoblasts isolated from fetal rat calvaria. Biochimie. 1993;75:991–994. doi: 10.1016/0300-9084(93)90150-q. [DOI] [PubMed] [Google Scholar]
- Panagakos FS. Transforming growth factor--alpha stimulates chemotaxis of osteoblasts and osteoblast-like cells in vitro. Biochemistry and Molecular Biology International. 1994;33:643–650. [PubMed] [Google Scholar]
- Panupinthu N, Zhao L, Possmayer F, Ke HZ, Sims SM, Dixon SJ. P2X7 nucleotide receptors mediate blebbing in osteoblasts through a pathway involving lysophosphatidic acid. Journal of Biological Chemistry. 2007;282:3403–3412. doi: 10.1074/jbc.M605620200. [DOI] [PubMed] [Google Scholar]
- Papachroni KK, Karatzas DN, Papavassiliou KA, Basdra EK, Papavassiliou AG. Mechanotransduction in osteoblast regulation and bone disease. Trends in Molecular Medicine. 2009;15:208–216. doi: 10.1016/j.molmed.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Paschalis EP, Shane E, Lyritis G, Skarantavos G, Mendelsohn R, Boskey AL. Bone fragility and collagen cross-links. Journal of Bone and Mineral Research. 2004;19:2000–2004. doi: 10.1359/JBMR.040820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perike S, Ozkucur N, Sharma P, Staroske W, Blasche R, Barth K, Funk RH. Phospho-NHE3 forms membrane patches and interacts with beta-actin to sense and maintain constant direction during cell migration. Experimental Cell Research. 2014;324:13–29. doi: 10.1016/j.yexcr.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nature Reviews: Molecular Cell Biology. 2009;10:538–549. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J, Wolf O, Naumann A, Minne HW, Mundy GR, Ziegler R. Chemotactic response of osteoblastlike cells to transforming growth factor beta. Journal of Bone and Mineral Research. 1990;5:825–830. doi: 10.1002/jbmr.5650050805. [DOI] [PubMed] [Google Scholar]
- Pignolo RJ, Shore EM. Circulating osteogenic precursor cells. Critical Reviews in Eukaryotic Gene Expression. 2010;20:171–180. doi: 10.1615/critreveukargeneexpr.v20.i2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov C, Radic T, Haasters F, Prall WC, Aszodi A, Gullberg D, Schieker M, Docheva D. Integrins alpha2beta1 and alpha11beta1 regulate the survival of mesenchymal stem cells on collagen I. Cell Death & Disease. 2011;2:e186. doi: 10.1038/cddis.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prideaux M, Dallas SL, Zhao N, Johnsrud ED, Veno PA, Guo D, Mishina Y, Harris SE, Bonewald LF. Parathyroid hormone induces bone cell motility and loss of mature osteocyte phenotype through L-calcium channel dependent and independent mechanisms. PloS One. 2015;10:e0125731. doi: 10.1371/journal.pone.0125731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Yu L, Fan J, Rui Z, Hua Z, Zhang Z, Zhang N, Yin G. Phosphorylation of GIT1 tyrosine 321 is required for association with FAK at focal adhesions and for PDGF-activated migration of osteoblasts. Molecular and Cellular Biochemistry. 2012;365:109–118. doi: 10.1007/s11010-012-1249-3. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Riehl BD, Lee JS, Ha L, Lim JY. Fluid-flow-induced mesenchymal stem cell migration: role of focal adhesion kinase and RhoA kinase sensors. Journal of The Royal Society Interface. 2015;12:20141351. doi: 10.1098/rsif.2014.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. Journal of Biological Chemistry. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- Rorth P. Collective cell migration. Annual Review of Cell and Developmental Biology. 2009;25:407–429. doi: 10.1146/annurev.cellbio.042308.113231. [DOI] [PubMed] [Google Scholar]
- Sanchez-Fernandez MA, Gallois A, Riedl T, Jurdic P, Hoflack B. Osteoclasts control osteoblast chemotaxis via PDGF-BB/PDGF receptor beta signaling. PloS One. 2008;3:e3537. doi: 10.1371/journal.pone.0003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffler MB, Cheung WY, Majeska R, Kennedy O. Osteocytes: master orchestrators of bone. Calcified Tissue International. 2014;94:5–24. doi: 10.1007/s00223-013-9790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk R, Willenegger H. on the Histology of Primary Bone Healing. Langenbecks Archiv für klinische Chirurgie, vereinigt mit Deutsche Zeitschrift für Chirurgie. 1964;308:440–452. [PubMed] [Google Scholar]
- Schmidt Y, Simunovic F, Strassburg S, Pfeifer D, Stark GB, Finkenzeller G. miR-126 regulates platelet-derived growth factor receptor-alpha expression and migration of primary human osteoblasts. Biological Chemistry. 2015;396:61–70. doi: 10.1515/hsz-2014-0168. [DOI] [PubMed] [Google Scholar]
- Schutze N, Schenk R, Fiedler J, Mattes T, Jakob F, Brenner RE. CYR61/CCN1 and WISP3/CCN6 are chemoattractive ligands for human multipotent mesenchymal stroma cells. BMC Cell Biology. 2007;8:45. doi: 10.1186/1471-2121-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman E. Bone modeling and remodeling. Critical Reviews in Eukaryotic Gene Expression. 2009;19:219–233. doi: 10.1615/critreveukargeneexpr.v19.i3.40. [DOI] [PubMed] [Google Scholar]
- Sharma D, Ciani C, Marin PA, Levy JD, Doty SB, Fritton SP. Alterations in the osteocyte lacunar-canalicular microenvironment due to estrogen deficiency. Bone. 2012;51:488–497. doi: 10.1016/j.bone.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims NA, Martin TJ. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey Rep. 2014;3:481. doi: 10.1038/bonekey.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit TH, Burger EH, Huyghe JM. A case for strain-induced fluid flow as a regulator of BMU-coupling and osteonal alignment. Journal of Bone and Mineral Research. 2002;17:2021–2029. doi: 10.1359/jbmr.2002.17.11.2021. [DOI] [PubMed] [Google Scholar]
- Sotobori T, Ueda T, Myoui A, Yoshioka K, Nakasaki M, Yoshikawa H, Itoh K. Bone morphogenetic protein-2 promotes the haptotactic migration of murine osteoblastic and osteosarcoma cells by enhancing incorporation of integrin beta1 into lipid rafts. Experimental Cell Research. 2006;312:3927–3938. doi: 10.1016/j.yexcr.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Sullivan SJ, Daukas G, Zigmond SH. Asymmetric distribution of the chemotactic peptide receptor on polymorphonuclear leukocytes. Journal of Cell Biology. 1984;99:1461–1467. doi: 10.1083/jcb.99.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, Hu J, Feng X, Van Hul W, Wan M, Cao X. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nature Medicine. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testaz S, Jarov A, Williams KP, Ling LE, Koteliansky VE, Fournier-Thibault C, Duband JL. Sonic hedgehog restricts adhesion and migration of neural crest cells independently of the Patched- Smoothened-Gli signaling pathway. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12521–12526. doi: 10.1073/pnas.221108698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WR, Rubin CT, Rubin J. Mechanical regulation of signaling pathways in bone. Gene. 2012;503:179–193. doi: 10.1016/j.gene.2012.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torreggiani E, Matthews BG, Pejda S, Matic I, Horowitz MC, Grcevic D, Kalajzic I. Preosteocytes/osteocytes have the potential to dedifferentiate becoming a source of osteoblasts. PloS One. 2013;8:e75204. doi: 10.1371/journal.pone.0075204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto T, Matsui T, Fukase M, Fujita T. Platelet-derived growth factor B chain homodimer enhances chemotaxis and DNA synthesis in normal osteoblast-like cells (MC3T3-E1) Biochemical and Biophysical Research Communications. 1991;175:745–751. doi: 10.1016/0006-291x(91)91629-q. [DOI] [PubMed] [Google Scholar]
- Ulrich F, Heisenberg CP. Trafficking and cell migration. Traffic. 2009;10:811–818. doi: 10.1111/j.1600-0854.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- Vanden Berg-Foels WS. In situ tissue regeneration: chemoattractants for endogenous stem cell recruitment. Tissue Engineering Part B: Reviews. 2014;20:28–39. doi: 10.1089/ten.teb.2013.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Zhang S, Mahlios J, Zhou G, Magenheimer BS, Guo D, Dallas SL, Maser R, Calvet JP, Bonewald L, Quarles LD. Cilia-like structures and polycystin-1 in osteoblasts/osteocytes and associated abnormalities in skeletogenesis and Runx2 expression. Journal of Biological Chemistry. 2006;281:30884–30895. doi: 10.1074/jbc.M604772200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano S, Mentaverri R, Kanuparthi D, Bandyopadhyay S, Rivera A, Brown EM, Chattopadhyay N. Functional expression of beta-chemokine receptors in osteoblasts: role of regulated upon activation, normal T cell expressed and secreted (RANTES) in osteoblasts and regulation of its secretion by osteoblasts and osteoclasts. Endocrinology. 2005;146:2324–2335. doi: 10.1210/en.2005-0065. [DOI] [PubMed] [Google Scholar]
- Yee JA, Yan L, Dominguez JC, Allan EH, Martin TJ. Plasminogen-dependent activation of latent transforming growth factor beta (TGF beta) by growing cultures of osteoblast-like cells. Journal of Cellular Physiology. 1993;157:528–534. doi: 10.1002/jcp.1041570312. [DOI] [PubMed] [Google Scholar]
- Zappitelli T, Aubin JE. The “connexin” between bone cells and skeletal functions. Journal of Cellular Biochemistry. 2014;115:1646–1658. doi: 10.1002/jcb.24836. [DOI] [PubMed] [Google Scholar]
- Zimmerman D, Jin F, Leboy P, Hardy S, Damsky C. Impaired bone formation in transgenic mice resulting from altered integrin function in osteoblasts. Developmental Biology. 2000;220:2–15. doi: 10.1006/dbio.2000.9633. [DOI] [PubMed] [Google Scholar]
- Zinger O, Zhao G, Schwartz Z, Simpson J, Wieland M, Landolt D, Boyan B. Differential regulation of osteoblasts by substrate microstructural features. Biomaterials. 2005;26:1837–1847. doi: 10.1016/j.biomaterials.2004.06.035. [DOI] [PubMed] [Google Scholar]