Abstract
Background
Little is known about whether tolerability and adherence to treatment can be influenced by weather and temperature conditions. The objective of this study was to assess monthly and seasonal adherence to and safety of sc IFN-β1a (Rebif®, Merck) in relapsing-remitting multiple sclerosis (RRMS) patients using the RebiSmart® electronic autoinjector.
Methods
A multicentre, prospective observational study in Greece in adult RRMS patients with EDSS < 6, under Rebif®/RebiSmart® treatment for ≤6 weeks before enrollment. The primary endpoint was monthly, seasonal and annual adherence over 12 months (defined in text). Secondary endpoints included number of relapses, disability, adverse events.
Results
Sixty four patients enrolled and 47 completed all study visits (Per Protocol Set - PPS). Mean annual adherence was 97.93% ± 5.704 with no significant monthly or seasonal variations. Mean relapses in the pre- and post- treatment 12-months were 1.1 ± 0.47 and 0.2 ± 0.54 (p < 0.0001, PPS). 10 patients (22%) showed 3-month disability progression, 19 (40%) stabilization and 18 (38%) improvement. EDSS was not correlated to pre- (r = 0.024, p = 0.87) or post-treatment relapses (r = 0.022, p = 0.88).
Conclusion
High adherence with no significant seasonal or weather variation was observed over 12 months. While the efficacy on relapses was consistent with published studies, we could not identify a relationship between relapses and disability.
Trial registration
Greek registry of non-interventional clinical trials ID: 200136, date of registration: February 18th, 2013.
Keywords: Multiple sclerosis, Interferons, Rebif, Rebismart, Treatment adherence and compliance, Clinical efficacy
Background
Adherence to treatment in Multiple Sclerosis (MS) is an important determinant of long-term outcomes, as suggested by the World Health Organization [1] and evidenced by several published studies [2–4]. However, the need for long-term treatment and the frequently debilitating nature of the disease make treatment adherence particularly challenging. This may impact disease progression, as on the one hand up 72% of patients do not adhere to disease-modifying MS treatments according to published studies [2, 5, 6], while on the other poor adherence has been associated with a higher rate of relapse [6].
The interferons (beta-1a and beta-1b) are among the first Disease Modifying Drugs (DMDs) that were approved for MS. These platform therapies are frequently associated with flu-like syndrome and injection-site reactions, which are among the reasons of non-adherence according to some studies [7]. Taking into account that the flu-like syndrome comprises a constellation of symptoms some of which may be more difficult to tolerate when the weather is hot, such as fever, chills and headache, we asked whether seasonal variation of weather conditions affects adherence to interferon treatment. As higher temperatures are typically observed in the Mediterranean countries, especially during the summer period, any effects of seasonal variation on adherence would be expected to be more pronounced in these countries. We, therefore, studied the seasonal variation of the adherence to sc IFN-β1a tiw (Rebif®), administered through the RebiSmart® autoinjector device, for a 12-month period, in patients with Relapsing-Remitting Multiple Sclerosis (RRMS) in Greece. Rebif® safety, including the occurrence of flu-like, was also studied.
Methods
The GEPAT-SMART study (Greece Epidemiological Project on Adherence and Temperature Using RebiSMART®) was a multicentre, prospective, observational study carried out at 9 sites in Greece (Greek registry of non-interventional clinical trials id: 200136, date of registration: February 18th, 2013 [8]). The recruitment period lasted from February 2013 to February 2014. The last patient follow-up ended on April 2015. The study was carried out in accordance with the Declaration of Helsinki and applicable national regulatory requirements and was approved by local ethics committees at each study site [ethics committee of the Papageorgiou Hospital of Thessaloniki (reference number 161/20.9.2012), ethics committee of the AHEPA Hospital (reference number 32/5.12.2012), ethics committee of the University Hospital of Ioannina (reference number 754/12.11.2012), ethics committee of the University Hospital of Patras (reference number 83/7.2.2013), ethics committee of the 401 Army Hospital of Athens (reference number 15/2012), ethics committee of the University Hospital of Larissa (reference number 19/13.11.2012), ethics committee of the Papanikolaou Hospital of Thessaloniki (reference number 11/3.10.2012), ethics committee of the Evangelismos Hospital (reference number 345/13.12.12), ethics committee of the Attiko Hospital (reference number 10/5.10.2012)]. Patients were enrolled after written informed consent had been obtained.
Participants
Inclusion criteria were 1) RRMS diagnosis (revised McDonald criteria (2010)), 2) Rebif® multi-dose injected by RebiSmart® prescribed according to the approved Summary of Product Characteristics (SmPC) within six (6) weeks prior to their enrolment into the study, 3) capable to handle RebiSmart®, 4) willing and capable to comply will all study requirements and procedures, 5) 18 to 65 years old and 6) Expanded Disability Scale Score (EDSS) < 6 at enrollment.
Exclusion criteria were 1) presence of any contraindication mentioned in the locally approved SmPC, 2) severe relapse within 30 days before study treatment commencement, 3) visual or physical impairment precluding them from self-injecting with RebiSmart®, 4) MS therapy within 6 months prior to study, 5) current or past (within the last 2 years prior to study enrolment) history of alcohol or drug abuse, 6) participation in another clinical trial during the last 30 days prior to study treatment commencement. Female subjects who were pregnant or breast-feeding were also excluded. Female patients with childbearing potential had to utilize a highly effective method of contraception for the duration of the study.
Administration of the study drug
All patients were provided with a RebiSmart® device (Merck, Darmstadt, Germany) for self-administration of serum-free Rebif® 44 μg or 22 μg sc three times weekly (tiw) for 12 months or until early discontinuation (ED). RebiSmart® is a CE-certified medical device. The dose of Rebif® was titrated over the first 4 weeks in accordance with the drug labeling information; the final dose was at the discretion of the treating physician and based on the recommendations in the drug labeling information.
Patient assessments
Following a pre-study evaluation visit, patients attended the study site at Study Day 1 (baseline), Month 6, and Month 12. At the baseline visit, all patients provided written informed consent and information on demographics, medical history, concomitant diseases, and MS history, including the number and characteristics of relapses in the past 12 months, was collected. At each post-baseline visit, the investigators recovered adherence data from the autoinjector. Reasons for missed injections were recorded in a patient diary. Relapse assessment, EDSS score, MS-related concomitant medication, vital signs, on-going therapy with Rebif® (including dose), and adverse events (AEs) were also recorded.
Study endpoints
The primary endpoint was the monthly, seasonal and annual adherence rate over the 12-month study treatment. Adherence rate was defined as 100 × number of injections actually administered divided by the expected number of injections over the defined time period (month, season, year), as captured by RebiSmart®. Secondary endpoints were: 1) reasons for missed injections, 2) proportion of patients free of relapses at month 12, 3) mean number of relapses at 12 months, 4) proportion of patients without progression of 3-month confirmed disability, at 12 months. Disability progression was defined as worsening by at least 0.5 EDSS points from baseline, 5) proportion of patients who discontinued prematurely the study treatment and the reasons for discontinuation. All (Serious) Adverse Events [(S)AEs] and Adverse Drug Reactions [(S)ADRs] were also recorded, 6) Patient evaluation of RebiSmart® based on a Convenience Questionnaire.
The following criteria were to be met for establishing an MS relapse: 1) Neurological abnormality, either newly appearing or re-appearing, at least 30 days after the onset of a preceding clinical event, with > = 24 h duration, 2) absence of fever (temperature > 37.5C) or known infection and 3) objective neurological impairment, correlating with the patient’s reported symptoms, defined as either increase in at least one of the functional systems of the EDSS domain or increase of the total EDSS score. Severity of relapses was described as mild, moderate, or severe according to the Activities of Daily Living criteria [9]. AEs were classified according to MedDRA v14.0 [10].
Sample size
The calculation of the sample size was based on the primary endpoint of the study. Due to the lack of literature data regarding the seasonal and monthly adherence, the adherence over the 12-month treatment period was used. According to available data the expected adherence during the study period was expected to be approximately 70% and the standard deviation 15% [11]. Therefore, 70 patients would be required to estimate the mean adherence rate with accuracy of less than ± 4%.
Statistical analyses
This manuscript was development according to the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guideline for reporting observational studies [12]. Descriptive statistics were calculated for all study variables. Summary statistics for categorical variables were presented as the number and percent of subjects in each category.
Seasonal and monthly variance of the adherence level was analyzed by One Way Analysis of Variance (ANOVA). Pre- and post-treatment relapse rate was compared by the Wilcoxon signed-rank test. Pearson’s r was used to study correlation between variables. The level of significance was set to 5% (two-sided). Descriptive statistics were used for AEs and SAEs. Adverse events where handled according to the study protocol.
Statistical analyses sets were performed in the following sets:
Full analysis set (FAS): all recruited subjects who fulfilled the inclusion/exclusion criteria.
Per-protocol set (PPS): all FAS subjects who completed all study visits.
Safety set: all study patients who actually received at least one dose of treatment for MS following informed consent.
No replacement policy existed in this study for drop-out patients and missing data.
Results
Patient demographics
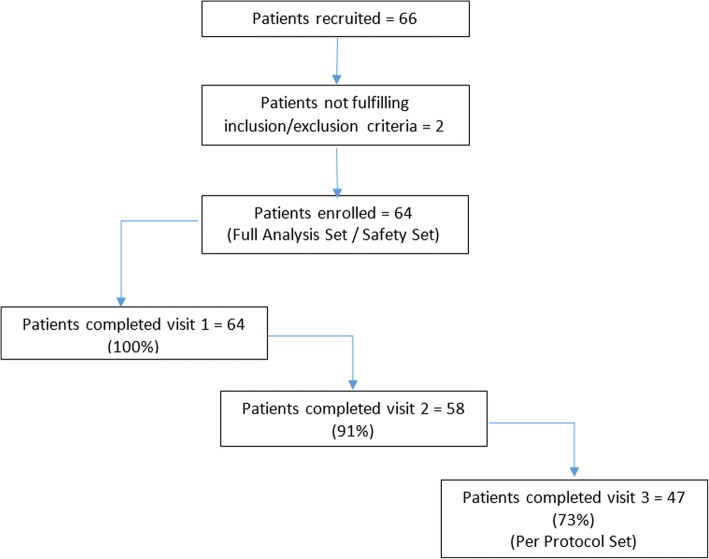
Sixty four of the 66 patients that started documentation received at least one dose of Rebif® and were included in the Safety Set and FAS, while the remaining two did not fulfill the inclusion/exclusion criteria and were not enrolled. Of these, 58 patients (87.9%) completed the month-6 visit, and 47 (71.2%) completed the month-12 visit. Patient disposition is shown in Fig. 1 and demographics are shown in Table 1. Baseline MS characteristics are shown in Table 2.
Fig. 1.
Patient Disposition
Table 1.
Patient demographic characteristics (Full Analysis Set)
| n (%) | ||
|---|---|---|
| Gender | Males | 14 (21.9) |
| Females | 50 (78.1) | |
| Age (yrs) | n, mean ± sd | 64, 36.2 ± 11.22 |
| min-max | 18.3–68.8 | |
| Weight (kg) | n, mean ± sd | 64, 69.9 ± 15.2 |
| min-max | 47–127 | |
| Height (cm) | n, mean ± sd | 64, 166.7± 9.24 |
| min-max | 116–186 | |
| BMI (kg/m2) | n, mean ± sd | 64, 25.16± 5.16 |
| min-max | 18.40–46.2 | |
| Race | Caucasian | 64 (100) |
| Place of Residence | Urban | 46 (71.9) |
| Semi urban | 6 (9.4) | |
| Rural | 12 (18.8) | |
| Region | Attica | 17 (26.6) |
| Peloponnese | 10 (15.6) | |
| Epirus | 2 (3.1) | |
| Central Greece | 2 (3.1) | |
| Central Macedonia | 17 (26.6) | |
| Western Macedonia | 7 (10.9) | |
| Eastern Macedonia / Thrace | 1 (1.6) | |
| Crete | 6 (9.4) | |
| Thessaly | 2 (3.1) | |
| Ionian Islands | 7 (10.9) | |
| North Aegean islands | 0 (0.0) | |
| South Aegean Islands | 0 (0.0) | |
| Marital Status | Not married | 29 (45.3) |
| Married | 32 (50) | |
| Widow/er | 2 (3.1) | |
| Divorced | 0 (0.0) | |
| Separated | 1 (1.6) | |
| Educational Status | 0 yrs | 0 (0.0) |
| Elementary (1–6 yrs) | 4 (6.3) | |
| High School/Lyceum (7–12 yrs) | 32 (50.0) | |
| University (> 12 yrs) | 28 (43.8) | |
| Working status | Private Sector Employee | 18 (28.1) |
| Public Sector Employee | 12 (18.8) | |
| Retired | 2 (3.1) | |
| Free lancer | 8 (12.5) | |
| Student | 6 (9.4) | |
| Unemployed | 18 (28.1) | |
Table 2.
Summary of MS History (Full Analysis Set)
| n | mean ± sd | median | min-max | |
|---|---|---|---|---|
| Years since MS diagnosis | 64 | 2.1 ± 4.00 | 0.2 | 0.04–14.3 |
| Mean number of relapses within the last 24 months prior to Rebif® Rebismart™ initiation | 62 | 1.5 ± 0.76 | 1.0 | 0–4 |
| Mean number of relapses in which corticosteroids were usedwere used were used | 62 | 0.9 ± 0.71 | 1.0 | 0–3 |
| Mean number of relapses within the last 12 months prior to Rebif® Rebismart™ initiation | 63 | 1.1 ± 0.47 | 1.0 | 0–2 |
| Mean number of relapses in which corticosteroids were used | 62 | 0.9 ± 0.57 | 1.0 | 0–2 |
Primary endpoint: 12-month and seasonal adherence
Mean adherence to Rebif®, administered through RebiSmart®, was 97.93% (±5.704) in FAS and 98.32% (±2.628) in PPS respectively (Table 3). No significant variations in monthly and seasonal adherence were noted (one-way ANOVA). Adherence did not vary significantly among different subgroups of the various demographic factors (Table 4).
Table 3.
12-month, seasonal and monthly adherence
| n | mean ± sd | median | min-max | |
|---|---|---|---|---|
| 12 month adherence to Rebif® - Rebismart® (Per Protocol Set) | 46 | 98.32 ± 2.628 | 99.09 | 90.30–100 |
| Study adherence to Rebif® - Rebismart® (Full Analysis Set) | 62 | 97.93 ± 5.704 | 100 | 90.30–100 |
| Seasonal adherence | ||||
| Jan-Mar | 61 | 98.02 ± 6.879 | 100 | 57.97–100 |
| Apr-Jun | 57 | 98.36 ± 5.678 | 100 | 60.94–100 |
| Jul-Sep | 55 | 98.58 ± 3.276 | 100 | 81.63–100 |
| Oct-Dec | 56 | 97.91 ± 6.837 | 100 | 52.0–100 |
| Monthly adherence | ||||
| Jan | 60 | 97.54 ± 10.409 | 100 | 33.33–100 |
| Feb | 60 | 97.56 ± 8.513 | 100 | 54.55–100 |
| March | 59 | 98.34 ± 7.192 | 100 | 54.17–100 |
| April | 57 | 98.60 ± 6.826 | 100 | 50.00–100 |
| May | 57 | 98.67 ± 6.795 | 100 | 52.00–100 |
| June | 53 | 98.21 ± 5.560 | 100 | 65.00–100 |
| July | 52 | 98.45 ± 5.777 | 100 | 60.87–100 |
| August | 49 | 98.873 ± 2.935 | 100 | 86.67–100 |
| September | 52 | 98.46 ± 4.073 | 100 | 81.25–100 |
| October | 53 | 99.01 ± 2.963 | 100 | 86.67–100 |
| November | 52 | 97.933 ± 6.282 | 100 | 68.42–100 |
| December | 59 | 98.14 ± 6.721 | 100 | 52.00–100 |
Annual adherence: 100 × (total no of injections in 12 months) / expected no of infections in the respective months
Seasonal adherence: 100 × (total no of injections in a 3 month-period) / expected no of infections during the same period
Monthly adherence: 100 × (total no of injections in specific month) / expected no of infections
Table 4.
Comparison of adherence in different subgroups, according to demographic factors
| 12 months Compliance to | |||||
|---|---|---|---|---|---|
| n | mean ± sd | min-max | p-value | ||
| Gender, | Males | 13 | 98.55 ± 2.501 | 90.67–100 | 0.712 |
| Females | 33 | 98.23 ± 2.708 | 90.30–100 | ||
| Age (yrs) | < 65 | 45 | 98.28 ± 2.645 | 90.30–100 | NA |
| ≥ 65 | 1 | 100.00 | 100–100 | ||
| Race | Caucasian | 46 | 98.32 ± 2.623 | 90.30–100 | NA |
| African | (−) | ||||
| Asian | (−) | ||||
| Other | (−) | ||||
| Place of Residence | Urban | 33 | 98.42 ± 2.600 | 90.30–100 | 0.322 |
| Semi urban | 4 | 99.66 ± 0.676 | 98.65–100 | ||
| Rural | 9 | 97.35 ± 3.105 | 90.67–100 | ||
| Region | Attica | 10 | 98.05 ± 3.099 | 90.30–100 | NA |
| Peloponnese | 6 | 97.89 ± 3.485 | 90.91--100 | ||
| Epirus | 2 | 98.81 ± 0.025 | 98.80–98-83 | ||
| Central Greece | 2 | 100 ± 0 | 100–100 | ||
| Central Macedonia | 14 | 98.68 ± 1.721 | 93.85–100 | ||
| Western Macedonia | 5 | 97.73 ± 4.041 | 90.67–100 | ||
| Eastern Macedonia | (−) | ||||
| Thrace | (−) | ||||
| Crete | (−) | ||||
| Thessaly | 5 | 97.42 ± 3.108 | 92.09–100 | ||
| Ionian Islands | 2 | 100 ± 0 | 100–100 | ||
| Northern/Southern Aeg. islands | (−) | ||||
| Marital Status | Unmarried | 22 | 98.36 ± 2.914 | 90.30–100 | 0.988 |
| Married | 22 | 98.26 ± 2.465 | 90.67–100 | ||
| Widow/er | 2 | 98.48 ± 2.143 | 96.97–100 | ||
| Divorced | (−) | ||||
| Separated | (−) | ||||
| Educational Status | 0 yrs | (−) | |||
| Elementary (1–6 yrs) | 4 | 96.64 ± 3.150 | 92.09–98.80 | 0.243 | |
| High School/Lyceum (7–12 yrs) | 22 | 98.08 ± 2.823 | 90.67–100 | ||
| University (> 12 yrs) | 20 | 98.92 ± 2.222 | 90.30–100 | ||
| Working status | Private sector employee | 14 | 98.35 ± 2.619 | 90.30–100 | 0.993 |
| Public sector employee | 9 | 98.73 ± 3.061 | 90.67–100 | ||
| Retired | 2 | 98.63 ± 0 | 98.63–98.63 | ||
| Free lancer | 5 | 98.12 ± 0.896 | 96.89–98.83 | ||
| Student | 4 | 98.46 ± 3.073 | 93.85–100 | ||
| Unemployed | 12 | 97.96 ± 3.185 | 90.91–100 | ||
Thirty-one patients missed at least one dose of the study treatment. The main reasons for non-adherence were forgotten dose and other (12 subjects each, 18.8%), followed by presence of viral infection (flu, 15.6%) and absence from home (10.9%, Table 5).
Table 5.
Reasons for non-adherence (Full Analysis Set)
| n (%) | Period of no injections | Events | Subject’s Location | Events | |
|---|---|---|---|---|---|
| Subjects that missedat least oneinjection | 31 (48.4) | ||||
| Reasons for missing the injections | |||||
| 1. They forgot the injection | 12 (18.8) | Week days | 22 | Home Area | 25 |
| Bank Holidays | 3 | Out of Residence | 2 | ||
| Holidays | 2 | ||||
| Total Events | 27 | ||||
| 2. They were not willing to inject for cosmetic reasons | 0 | ||||
| 3. Absence from home | 7 (10.9) | Week days | 13 | Home Area | 11 |
| Bank Holidays | 2 | Out of Residence | 6 | ||
| Holidays | 3 | ||||
| Total Events | 17 | ||||
| 4. Other reasons | 12 (18.8) | Week days | 55 | Home Area | 54 |
| Bank Holidays | 2 | Out of Residence | 3 | ||
| Holidays | 0 | ||||
| Total Events | 57 | ||||
| 5. Pain reaction at injection site | 0 | ||||
| 6. Flu-like illness | 10 (15.6) | Week days | 55 | Home Area | 53 |
| Bank Holidays | 0 | Out of Residence | 2 | ||
| Holidays | 0 | ||||
| Total Events | 55 | ||||
Secondary endpoints
Efficacy
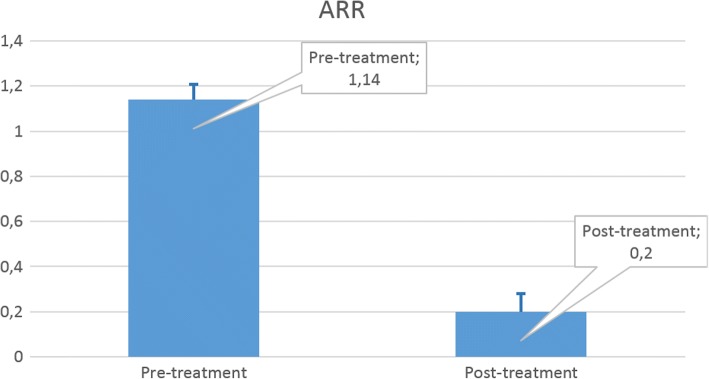
Among the 47 patients that completed all study visits (PPS), 6 (12.8%) relapsed with a mean number of relapses 1.3 ± 0.8 and 41 (87.2%) did not relapse. In the FAS 10 patients relapsed (15.6%) with a mean number of relapses 1.3 ± 0.6 and 54 (84.4%) did not relapse. Annual mean number of relapses in the PPS was 0.2 ± 0.54. This value was significantly lower compared to the mean number of relapses in the 12-month pre-Rebif® period (1.1 ± 0.47, p < 10− 15, Wilcoxon rank-sum test) (Fig. 2).
Fig. 2.
Mean number of relapses in the pre- and post-treatment 12 months
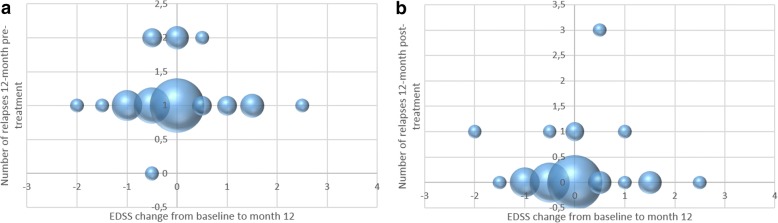
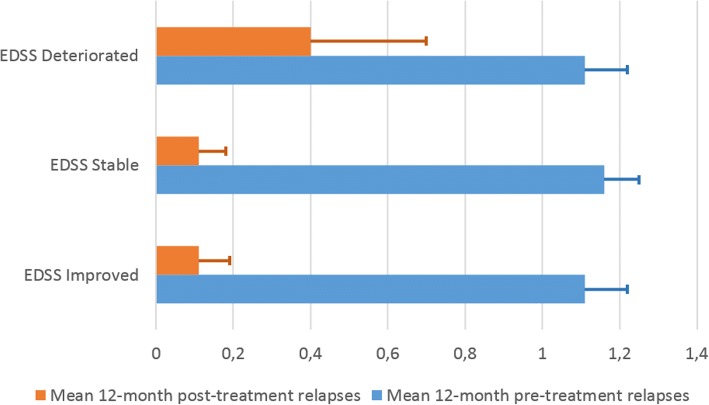
3-month confirmed disability progression at the end of the study period was observed in 10 patients (21%), while in 19 (40%) patients EDSS remained stable and improved in 18 (39%). Median EDSS progression was 1 point (range 0.5–2.5) in the former group, while median improvement was 0.5 points (range 0.5–2) in the latter group. Overall, in the PPS mean EDSS change was not significantly different from zero with a mean of 0.17 ± 1.13 points (median 0). EDSS was related neither to the 12-month pre-treatment number of relapses (r = 0.024, p = 0.87), nor to the 12-month post-treatment number of relapses (r = 0.022, p = 0.88) (Fig. 3). Furthermore, mean 12-month relapses pre- and post-treatment were not significantly different among those in whom EDSS improved, remained stable or deteriorated (one-way ANOVA, p = 0.94 and 0.24 respectively, Fig. 4).
Fig. 3.
Change of EDSS between baseline and visit 3, at 12 months vs number of 12-month pre-treatment relapses (n = 47) (a) and 12-month post-treatment relapses (n = 47) (b). The size of the bubbles represents the number of observations at each point on the graph
Fig. 4.
The mean number of relapses in the 12-month pre-treatment period did not differ significantly among patients in whom EDSS at the end of the trial was improved, stable or had worsened (one way anova, p = 0.94). Similarly, there were no statistically significant differences among these groups in the mean number of relapses in the 12-month post-treatment period (one way anova, p = 0.24). This result is in support of a dissociation between disability progression and relapses
Safety
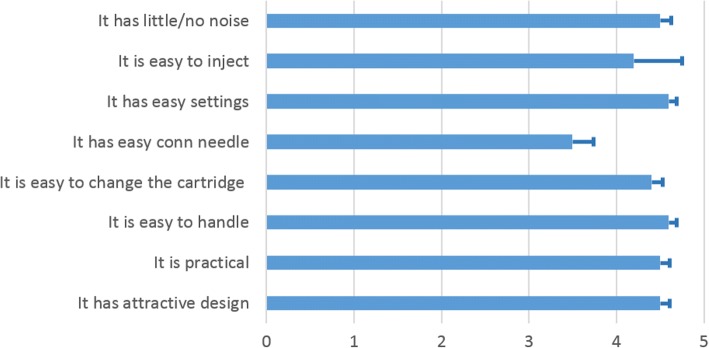
Of the 64 patients in the FAS, 58 (90%) assessed RebiSmart®. Median score was 5 (highest) for all questionnaire items, while mean values are shown in Fig. 5. ED was documented for 19 patients (29% of the Safety Population). The most common reason was ‘patient’s decision to quit treatment’ (8/64, 12.5%), followed by ‘adverse event’ (4/64, 6.2%). Among the four cases where the drug was discontinued due to AEs, pregnancy was the reason in one, while in the other three cases the reasons were fatigue, malaise, anorexia, pyrexia and infections.
Fig. 5.
Responses to convenience questionnaire (n = 58). Mean scores and standard error bars are shown
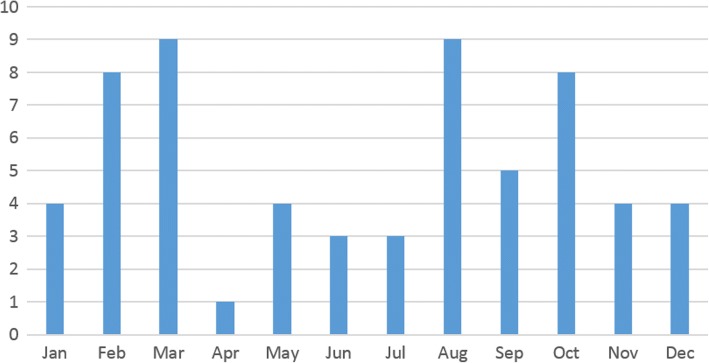
Treatment with Rebif® using RebiSmart® was well tolerated. No new safety signals were detected through this study. Sixty two reports of flu-like syndrome and of related symptoms (headache, malaise, myalgia) were obtained. Figure 6 shows the monthly distribution of these reports. While the distribution is not even throughout the year (p < 0.001, χ2 test), peaks are observed in the spring, summer and autumn; this speaks against an effect of hot weather on the frequency and gravity of flu-like syndrome. Furthermore, monthly reports of flu-like syndrome did not correlate with monthly adherence (r = 0.14, p = 0.66).
Fig. 6.
Monthly variation of reports of flu-like syndrome and of related symptoms (headache, malaise, myalgias) (n = 62)
RebiSmart® was evaluated by study participants as easy to use and convenient, giving an average score of 4.5 or above in most items of the convenience questionnaire (Fig. 5). The lower score (average 3.5 ± 1.79) was given to the item “it has easy connection needle”.
Discussion
To our knowledge, this is the first prospective study to assess yearly, seasonal and monthly adherence to, and efficacy, safety, and tolerability of Rebif® for RRMS administered with an electronic autoinjector. In a previous study seasonal adherence to the interferons and glatiramer acetate had been studied retrospectively, by means of patient-administered questionnaire [7]. Here, adherence data was objectively captured by the autoinjector electronically and therefore not subject to patient reporting errors [2, 13].
In our study cumulative 12-month adherence to Rebif® was very high (97.93 ± 5.704, FAS), confirming the findings of a previous 12-month and of two 12-week user trials with the same autoinjector [2, 11, 14], (97.0 ± 7.3% cumulative 12-month adherence [2], 90.3% of patients with > 90% adherence [14], and 88.2% of patients having administered ≥80% of scheduled injections, with 67% administering all scheduled injections [11] respectively). The use of an intramuscular IFN b-1a autoinjector in another study resulted in monthly compliance rates of 87.5–96.2%, supporting the notion that an autoinjector may contribute to high compliance [15].
Interestingly, the COMPLIANCE study investigators [7], which took place in Spain, where weather conditions are similar to Greece’s, reported findings seemingly opposite to ours, namely that seasons had a considerable impact on adherence. The authors comment in the discussion that they found a correlation between summer months and non-adherence, however they acknowledge that this association was not statistically significant. Furthermore, 81% of their patients reported that seasons did not affect their adherence. Hence, the data in the COMPLIANCE study support our finding that seasons do not affect adherence.
Thirty one patients (48%) missed at least one injection during the study period. The main reasons for non-adherence (Table 5) are in agreement with previous reports [2, 5, 16]. RebiSmart® was evaluated by study participants as easy to use and convenient (Fig. 5). The lower score was given to the item “it has easy connection needle” and this might be an aspect of the device that can be improved.
Treatment with Rebif® was efficacious; 87% of the per-protocol population were relapse free at month 12, which compares favorably with the rates of 66.8% at 48 weeks and 53.3% at 96 weeks with the same serum-free Rebif® formulation administered manually or with a mechanical autoinjector [16, 17]. Mean number of relapses was significantly lower at month 12 compared to the pre-treatment year. These numbers are consistent with those recently reported for RebiSmart® [2], yet lower compared to the ARR obtained for Rebif® in a series of recent clinical trials where the latter was used a comparator (Rebif® vs Alemtuzumab in CARE-MS-I and CARE-MS-II where ARR for Rebif® was 0.39 ± 0.907 and 0.52 ± 1.01 respectively [18, 19] and Rebif® vs Ocrelizumab in OPERA-I and OPERA-II where ARR for Rebif® was 0.29 ± 0.72 and 0.29 ± 0.73 respectively [20]. The mean number of relapses in the 12 months pre-treatment was also higher in these studies: 1.33 ± 0.64 and 1.34 ± 0.73 in OPERA-I and II [20], 1.8 ± 0.8 and 1.5 ± 0.75 in CARE_MS I and II respectively [18, 19]. These differences in the study populations, as well as in the design of the trials, might account for the lower post-treatment relapse rate that we have observed. On the other hand, in the SMART trial, which recruited a similar patient population in terms of pre-treatment relapses, the one-year pre- and post- treatment ARR was comparable [2].
3-month confirmed disability progression at the end of the study period was observed in 10 patients (21%), while in 19 (40%) patients EDSS remained stable and in 18 (39%) it improved. Overall, in the PPS mean EDSS change of 0.17 ± 1.13 was not significant. It is notable that the change in EDSS from baseline was not related to the 12-month either pre- or post-treatment relapses (Fig. 3), while the mean number of relapses in the 12-month pre- and post- treatment period did not differ significantly among patients in whom EDSS had progressed, remained stable or improved at the end of the study (Fig. 4). Albeit there was a trend towards a higher mean number of relapses in the 12-month post-treatment period in those with EDSS progression, this difference did not reach statistical significance. It should be noted that these correlation analyses are post-hoc and should be treated with caution, as they are statistically under-powered.
Despite the relatively short observation period, these findings add to the on-going debate on the relation between relapses and disability in MS. Relapses and disability progression are two important clinical characteristics of MS. Relapses are the clinical expression of inflammatory insults localized at different parts of the central nervous system, whereas disability progression is the phenotypic expression of ongoing demyelination, axonal loss and gliosis [21]. In an earlier study of 1844 patients who had MS for 11 ± 10 years, it was found that once a certain clinical threshold is reached (namely, 4 on the EDSS), the progression of disability is not further affected by relapses. This, according to the authors, suggests that there is a dissociation between the pathophysiological mechanisms underpinning relapses and disability progression [21]. A more recent observational study of 162 MS patients treated with interferon beta for at least 2 years, found that compared to patients with no relapses in the first 2 years, those with 1 or ≥ 2 relapses were more likely to exhibit early sustained disability progression (Hazard Ratio for 1 relapse: 3.4, p = 0.05; Hazard Ratio for ≥2 relapses: 4.3, p < 0.001). However, there was no statistically significant difference between patients that had 1 or ≥ 2 relapses [22]. Finally, a real world evidence study comparing alemtuzumab, interferon beta, fingolimod, or natalizumab in terms of relapses and disability progression showed that despite the fact that alemtuzumab was associated with a lower ARR than Rebif® (0.19 [95% CI 0.14–0.23] vs 0.53 [0.46–0.61], p < 0.0001), it was associated with similar probabilities of both disability accumulation (hazard ratio 0.66 [95% CI 0.36–1.22], p = 0.37) and disability improvement as Rebif® (0.98 [0.65–1.49], p = 0.93) at 5 years [23]. Our results favor those studies that support a dissociation between relapses and disability progression, calling for more research on the pathophysiological mechanisms underpinning this phenomenon and on the appropriate treatment strategies.
A limitation of our study is its relatively small size. However, it is the only study that we are aware of, in which seasonal adherence to interferon treatment for MS is evaluated by means of an autoinjector, rather than by patient-administered questionnaires. This increases the objectivity of the measurements. Furthermore, the sample size for this study was calculated based on the primary endpoint, namely 12-month adherence, due to the lack of published data regarding the seasonal and monthly adherence. As, according to our findings, 12-month, seasonal and monthly adherence are similar and have higher mean values and lower standard deviations than what was assumed during sample size calculations, the recruited number of patients was adequate to also estimate seasonal and monthly adherence.
The very high adherence rate that we have observed could have been confounded by factors such as higher educational level, occupation or willingness to participate in a clinical study. While the latter is a common factor in all studies, our sample was balanced in terms of educational level (high school – university), social status (married or not) and occupation (public/private sector). Furthermore, we did not observe any discrepancies in the adherence rates, which was high in all these subgroups.
While the observational design of the study and its 12-month duration are suitable for evaluating adherence (primary endpoint), they are less relevant to the efficacy measurements (relapse rate and disability progression), which were however secondary endpoints and should be interpreted with caution. Nevertheless, the results that we have obtained on relapse rate are consistent with those published in the literature, while our finding that pre- and post-treatment relapses are not related to disability progression or improvement at 12-months adds to the on-going discussion on the matter.
Conclusions
In conclusion, treatment with Rebif® using RebiSmart® was well tolerated and adherence exceeded 97% in a real world setting. There was no association of adherence with specific time periods of the year or geographical areas of Greece, which implies that weather conditions are not among its important determinants. Our data shows that Rebif® is effective in decreasing annual relapse rates, however there no correlation between ARR and disability progression.
Acknowledgements
None.
Funding
This study was funded by Merck Hellas S.A., who participated in the design of the study, in the analysis and interpretation of the data and in the writing of this manuscript.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available according to company policies but are available from the corresponding author on reasonable request.
Abbreviations
- ADR
Adverse Drug Reaction
- AE
Adverse Event
- ANOVA
Analysis of Variance
- DMD
Disease Modifying Drug
- ED
Early Discontinuation
- EDSS
Expanded Disability Scale Score
- FAS
Full Analysis Set
- MS
Multiple Sclerosis
- PPS
Per Protocol Set
- RRMS
Relapsing Remitting Multiple Sclerosis
- SADR
Serious Adverse Drug Reaction
- SAE
Serious Adverse Event
- scIFN-β1a
Subcutaneous Interferon β1a
- SmPC
Summary of Product Characteristics
- STROBE
STrengthening the Reporting of OBservational studies in Epidemiology
Authors’ contributions
SND analyzed and interpreted the patient data and was the main contributor in writing the manuscript. EvK, EfK, AK, PP, NF, VT, NV, AT and KV evaluated patients and were major contributors in writing the manuscript. MA, DS and FDL analyzed and interpreted the patient data and were major contributors in writing the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was carried out in accordance with the Declaration of Helsinki and applicable national regulatory requirements and was approved by local ethics committees at each study site [ethics committee of the Papageorgiou Hospital of Thessaloniki (reference number 161/20.9.2012), ethics committee of the AHEPA Hospital (reference number 32/5.12.2012), ethics committee of the University Hospital of Ioannina (reference number 754/12.11.2012), ethics committee of the University Hospital of Patras (reference number 83/7.2.2013), ethics committee of the 401 Army Hospital of Athens (reference number 15/2012), ethics committee of the University Hospital of Larissa (reference number 19/13.11.2012), ethics committee of the Papanikolaou Hospital of Thessaloniki (reference number 11/3.10.2012), ethics committee of the Evangelismos Hospital (reference number 345/13.12.12), ethics committee of the Attiko Hospital (reference number 10/5.10.2012)]. Patients were enrolled after written informed consent had been obtained.
Consent for publication
Not Applicable.
Competing interests
Spyros N Deftereos, Dimitrios Sakellariou and Filippo DeLorenzo are employees of Merck Hellas S.A. Michalis Arvanitis was an employee of Merck Hellas S.A when the study was conducted.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Spyros N. Deftereos, Phone: +306936110334, Email: spyros.deftereos@external.merckgroup.gr
Evangelos Koutlas, Email: koutlasevangelos@gmail.com.
Efrosini Koutsouraki, Email: efrosink@gmail.com.
Athanassios Kyritsis, Email: thkyrits@uoi.gr.
Panagiotis Papathanassopoulos, Email: papat@med.upatras.gr.
Nikolaos Fakas, Email: drfakas@yahoo.gr.
Vaia Tsimourtou, Email: vana.tsimourtou@gmail.com.
Nikolaos Vlaikidis, Email: vlaikid@auth.gr.
Antonios Tavernarakis, Email: antontavernie@gmail.com.
Konstantinos Voumvourakis, Email: cvoumvou@otenet.gr.
Michalis Arvanitis, Email: info@drarvanitis.gr.
Dimitrios Sakellariou, Email: dimitris.sakellariou@merckgroup.com.
Filippo DeLorenzo, Email: filippo.de-lorenzo@merckgroup.com.
References
- 1.Sabate EE. Adherence to long-term therapies: evidence for action. General: World Health Organization; 2003. [Google Scholar]
- 2.Bayas A, Ouallet JC, Kallmann B, et al. Adherence to, and effectiveness of, subcutaneous interferon β-1a administered by RebiSmart® in patients with relapsing multiple sclerosis: results of the 1-year, observational SMART study. Expert Opin Drug Deliv. 2015;12:1239–1250. doi: 10.1517/17425247.2015.1057567. [DOI] [PubMed] [Google Scholar]
- 3.Kappos L, Kuhle J, Multanen J, et al. Factors influencing long-term outcomes in relapsing-remitting multiple sclerosis: PRISMS-15. J Neurol Neurosurg Psychiatry. 2015;86:1202–1207. doi: 10.1136/jnnp-2014-310024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kappos L, Edan G, Freedman MS, et al. The 11-year long-term follow-up study from the randomized BENEFIT CIS trial. Neurology. 2016;87:978–987. doi: 10.1212/WNL.0000000000003078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Treadaway K, Cutter G, Salter A, et al. Factors that influence adherence with disease-modifying therapy in MS. J Neurol. 2009;256:568–576. doi: 10.1007/s00415-009-0096-y. [DOI] [PubMed] [Google Scholar]
- 6.Menzin J, Caon C, Nichols C, et al. Narrative review of the literature on adherence to disease-modifying therapies among patients with multiple sclerosis. J Manag Care Pharm. 2013;19(1 Suppl A):S24–S40. doi: 10.18553/jmcp.2013.19.s1.S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saiz A, Mora S, Blanco J. Therapeutic compliance of first line disease-modifying therapies in patients with multiple sclerosis. COMPLIANCE Study. Neurologia. 2015;30:214–222. doi: 10.1016/j.nrl.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Hellenic Association of Pharmaceutical Companies. Registry of non-interventional studies. https://www.dilon.sfee.gr/studiesp_d.php?meleti_id=200136. Accessed 25 Sept 2017.
- 9.Costello K, Kennedy P, Scanzillo J. Recognizing nonadherence in patients with multiple sclerosis and maintaining treatment adherence in the long term. Medscape J Med. 2008;10:225. [PMC free article] [PubMed] [Google Scholar]
- 10.Medical Dictionary for Regulatory Activities. https://www.meddra.org/. Accessed 25 Sept 2017.
- 11.Lugaresi A, Florio C, Brescia-Morra V, et al. Patient adherence to and tolerability of self-administered interferon β-1a using an electronic autoinjection device: a multicentre, open-label, phase IV study. BMC Neurol. 2012;12:7. doi: 10.1186/1471-2377-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, et al. The Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blaschke TF, Osterberg L, Vrijens B, et al. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annu Rev Pharmacol Toxicol. 2012;52:275–301. doi: 10.1146/annurev-pharmtox-011711-113247. [DOI] [PubMed] [Google Scholar]
- 14.Singer B, Wray S, Miller T, et al. Patient-rated ease of use and functional reliability of an electronic autoinjector for self-injection of subcutaneous interferon beta-1a for relapsing multiple sclerosis. Mult Scler Relat Disord. 2012;1:87–94. doi: 10.1016/j.msard.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Hupperts R, Becker V, Friedrich J, et al. Multiple sclerosis patients treated with intramuscular IFN-β-1a autoinjector in a real-world setting: prospective evaluation of treatment persistence, adherence, quality of life and satisfaction. Expert Opin Drug Deliv. 2015;12:15–25. doi: 10.1517/17425247.2015.989209. [DOI] [PubMed] [Google Scholar]
- 16.Devonshire V, Lapierre Y, Macdonell R, et al. The global adherence project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol. 2011;18:69–77. doi: 10.1111/j.1468-1331.2010.03110.x. [DOI] [PubMed] [Google Scholar]
- 17.Giovannoni G, Barbarash O, Casset-Semanaz F, et al. Immunogenicity and tolerability of an investigational formulation of interferon-beta1a: 24- and 48-week interim analyses of a 2-year, single-arm, historically controlled, phase IIIb study in adults with multiple sclerosis. Clin Ther. 2007;29:1128–1145. doi: 10.1016/j.clinthera.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380:1819–1828. doi: 10.1016/S0140-6736(12)61769-3. [DOI] [PubMed] [Google Scholar]
- 19.Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380:1829–1839. doi: 10.1016/S0140-6736(12)61768-1. [DOI] [PubMed] [Google Scholar]
- 20.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon Beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 21.Confavreux C, Vukusic S, Moreau T, et al. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000;343:1430–1438. doi: 10.1056/NEJM200011163432001. [DOI] [PubMed] [Google Scholar]
- 22.Bosca I, Coret F, Valero C, et al. Effect of relapses over early progression of disability in multiple sclerosis patients treated with beta-interferon. Mult Scler. 2008;14:636–639. doi: 10.1177/1352458507086666. [DOI] [PubMed] [Google Scholar]
- 23.Kalincik T, Brown JWL, Robertson N, et al. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study. Lancet Neurol. 2017;16:271–281. doi: 10.1016/S1474-4422(17)30007-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available according to company policies but are available from the corresponding author on reasonable request.