Abstract
Objectives
During the period from December 2007 to November 2012, the epidemiology of diarrhea caused by Shigella was studied among children <5 years of age residing in Manhiça District, Southern Mozambique.
Materials and methods
Children from 0 to 5 years with moderate-to-severe diarrhea (MSD) and less severe diarrhea (LSD) were enrolled along with matched controls (by age, gender, and neighborhood). Age-stratified logistic regression analyses were conducted to identify clinical features and risk factors associated with Shigella positivity in cases of diarrhea. The impact of antibiotic treatment was assessed for patients with known outcome.
Results
A total of 916 cases of MSD and 1979 matched controls, and 431 cases of LSD with equal number of controls were enrolled. Shigella was identified as significant pathogen in both cases of MSD and LSD compared to their respective controls. Shigella was detected in 3.9% (17/431) of LSD compared to 0.5% (2/431) in controls (P=0.001) and in 6.1% (56/916) of MSD cases compared to 0.2% (4/1979) in controls (P<0.0001), with an attributable fraction of 8.55% (95% CI: 7.86–9.24) among children aged 12–23 months. Clinical symptoms associated to Shigella among MSD cases included dysentery, fever, and rectal prolapse. Water availability, giving stored water to child, washing hands before preparing baby’s food, and mother as caretaker were the protective factors against acquiring diarrhea caused by Shigella. Antibiotic treatment on admission was associated with a positive children outcome.
Conclusion
Shigella remains a common pathogen associated with childhood diarrhea in Mozambique, with dysentery being a significant clinical feature of shigellosis. Adherence to the basic hygiene rules and the use of antibiotic treatment could contribute to the prevention of most of diarrhea due to Shigella.
Keywords: Shigella, moderate-to-severe diarrhea, less severe diarrhea, epidemiology
Introduction
Diarrhea is the leading cause of mortality in children <5 years with its long-term impact on growth and cognitive development.1 Although the burden is greater in low-income populations, acute infectious diarrhea is also a common cause of outpatient visits and hospital admissions in high-income regions and is an important health problem globally.2 Associated risk factors for infection include poor living standards, overcrowding, inadequate sanitation, and poor hand hygiene. These factors may result in a significant disease burden and economic effect due to direct medical costs, loss of work, lower quality of life, and mortality.3
The high burden of the disease is also associated with the wide range of recognized enteric pathogens such as virus, bacteria, and parasites that may cause diarrhea.4 Among them, Shigella infections remain a global public health concern, in resource-limited countries where the disease may cause as many as 163 million episodes of diarrhea and over a million deaths annually with the majority (60%) occurring in children under 5.5 Due to the high disease burden and endemicity of Shigella in resource-limited countries, the WHO has set the development of candidate vaccines against Shigella as priority.6 Thus, considering the efficacy of Shigella vaccine, it is necessary to have reliable estimates of the burden and epidemiology of disease in targeted endemic areas, including age-specific incidence data and risk factors associated with Shigella infections.
Primarily, shigellosis is transmitted from person to person through the fecal–oral route, but it may also spread indirectly by fecal contamination of water or food.7 Signs/symptoms of individuals who are infected with Shigella expand fever, painful bloody diarrhea, and stomach cramps.8 WHO recommends that all episodes of diarrhea with blood in the stool must be treated with antibiotics.9 Antimicrobial therapies reduce the period and intensity of disease symptoms, decrease excretion of bacteria, and prevent potentially lethal complications. Decreasing the bacterial load excreted by a child with dysentery also reduces the probability of fecal–oral transmission to close contacts, such as neighbors, friends, or members of the child’s household.10
In Mozambique, epidemiologic data on diarrhea caused by Shigella remain scarce and existing data are limited to few studies.11,12 Moreover, the contribution of Shigella to less severe diarrhea (LSD), the risk factors, and the impact of antibiotic treatment on patient outcome remain unknown. We hereby report the epidemiology of diarrhea due to Shigella among children <5 years of age enrolled between 2007 and 2012 in Manhiça district as part of the Global Enteric Multicenter Study.13
Materials and methods
Study area and population
The study was conducted by the Manhiça Health Research Centre (Centro de Investigação em Saúde de Manhiça – CISM) in Manhiça district, a rural area of Maputo province in southern Mozambique. The climate is subtropical with warm and rainy season from October to April and a fresh and dry season for the rest of the year. A round-the-clock morbidity surveillance system, covering pediatric inpatient and outpatient visits, was established in 1998 in the Manhiça District Hospital in a joint collaboration with CISM. Clinical data are routinely collected from all children under 15 years seeking health care. HIV infection is among the highest worldwide, with prevalence rates in women in childbearing age as high as 40% in the district.14 Diarrhea is the third leading cause of hospital admission among children aged 0–14 years and the fourth cause of death in children from 12 to 59 months.15 Additionally, since 1996, CISM has been running a Health Demographic Surveillance System (HDSS) for vital events and migrations in the population living within the study area covering approximately 95,000 inhabitants during the study period. In 2014, the study area was expanded to the whole district, and currently, 183,000 inhabitants are under DSS follow-up. Each person living within the DSS study area is issued a unique Permanent Identification Number that describes the geographic location, household number, and personal number within the household. A full description of the geographic and sociodemographic characteristics of the study community has been detailed elsewhere.16
Study design
The present analysis is part of a prospective case–control study of diarrhea conducted in Manhiça district from December 2007 to November 2012 to estimate the burden and etiology of moderate-to-severe diarrhea (MSD) in children <5 years of age to guide future interventions. To assess the role of Shigella in LSD, cases of LSD were enrolled from November 3rd 2011 to November 2nd 2012. Age in months was stratified in three strata (0–11, 12–23, and 24–59). Clinical and epidemiologic data were collected over the study period. Cases of diarrhea included in the study corresponded to children <5 years of age living within the HDSS area presenting to Manhiça district hospital with symptoms of diarrhea, defined as three or more loose stools in the last 24 hours. Clinicians assessed each child with diarrhea for eligibility; to be included, the episode had to be new (onset after ≥7 diarrhea-free days), acute (onset within the previous 7 days), and fulfill at least one of the following criteria for MSD: sunken eyes (confirmed by parent or caretaker as more than normal); loss of skin turgor (abdominal skin pinch with slow [≤2 seconds] or very slow [≥2 seconds] recoil); intravenous hydration administered or prescribed; dysentery (visible blood in loose stools); or admission to hospital with diarrhea or dysentery.17 For each child with diarrhea, one to three healthy control children (no story of diarrhea in the previous 7 days, matched by age, sex, and neighborhood) were randomly selected using the HDSS database and enrolled within 14 days of presentation of the corresponding index case.
Sample collection and laboratory analysis
Fecal samples from cases were collected within 12 hours of registration of the diarrheal episode, and control samples within 14 days after case enrolment. Once collected, samples were kept in a cool box until processed. Each fecal specimen comprised a whole stool specimen (in screw top fecal specimen cups carried in Styrofoam boxes with cold packs), a fecal swab in Modified Cary Blair medium in a plastic screw top test tube, and a fecal swab in buffered glycerol saline in a screw top test tube.13 Additionally, if antibiotics were to be given to patients before the production of stool sample, two rectal swabs were obtained for bacterial culture pending passage of the whole stool for the remaining assays. Stool samples were tested for the presence of bacterial, viral, and parasitic pathogens by culture, ELISA, and PCR as appropriate.18 In the case of Shigella, species were identified by colonies morphology upon culturing in MacConkey and XLD followed by slide agglutination with specific antisera (Denka Seiken Co., Ltd., Tokyo, Japan).
Data analysis
The software for analysis was Stata/SE version 14.1 (STATA Corporation, College Station, TX, USA) and the package coxphf from R version 3.2.2. All analyses were stratified by age. The frequency of Shigella isolation was calculated dividing the number of Shigella positivity by the total number of children enrolled over the study period with known culture result for Shigella. The pathogenicity of Shigella was assessed comparing the isolation rate between cases of diarrhea and control group. Logistic regression models were used to evaluate associations. All models were estimated with the penalized likelihood according to the Firth’s approach.19,20 Multivariable models were estimated by forward-stepwise selection from covariates with a P-value<0.20 in the crude models and no more than 5% of missing values. Significant levels for removal and addition in the multivariable models were 0.10 and 0.05, respectively, by Wald test. The association between MSD and Shigella was assessed at crude level for all pathogens and for Shigella adjusted for other significant pathogens and pairwise interactions between each one of them with Shigella. Analysis of sign/symptoms and sociodemographic factors associated with Shigella-MSD vs other types of MSD was performed among MSD cases. Interactions between significant covariates in the multivariable model were also assessed.
Ethical approval
The study is part of the Global Enteric Multi-Center Study (GEMS), which was approved by the Institutional Review Board at the University of Maryland School of Medicine, USA and the National Bioethics Committee for Health of Mozambique and Hospital Clinic, University of Barcelona, Spain. A written informed consent was obtained from children’s mothers or caretakers for participation in the study.
Results
Shigella and association with diarrhea
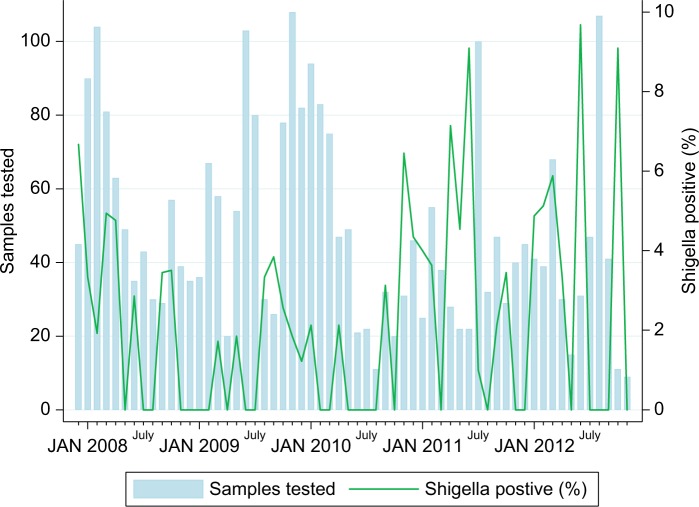
During the study period, a total of 916 cases and 1979 controls for MSD (December 9, 2007 to November 2, 2012), and 431 LSD cases and their respective 431 matched controls (November 3, 2011 to November 2, 2012) were enrolled. Shigella was isolated in 6.1% (56/916) of MSD cases compared to 0.2% (4/1979) in controls (P<0.0001) and in 3.9% (17/431) of LSD compared to 0.5% (2/431) in control group (Table 1). In addition, Shigella infection was predominantly detected during rainy season (October–March) compared to dry season (Figure 1). The incidence rate of Shigella-associated MSD was 0.61 per 100 child-year (95% CI: 0.48–0.74) with an attributable fraction of 8.55 (95% CI: 7.86–9.24) in children aged 12–23 months. A strong association of Shigella and MSD was found by both crude and pathogen-adjusted multivariate analysis for all age groups especially in children aged 24–59 months where adjusted odds ratio (aOR) of 190.92 (95% CI: 23.80–24565.84; P<0.0001) was observed as shown in Table 2.
Table 1.
Detection rate of Shigella among children 0–59 months of age with diarrhea (MSD and LSD) and their matched controls in Manhiça district, December 2007 to November 2012
| Age groups | Case/control
|
P-value | |
|---|---|---|---|
| Controls, n (%) | Cases, n (%) | ||
|
| |||
| MSD | |||
| 0–11 months | 1/1047 (0.1) | 6/495 (1.2) | 0.002 |
| 12–23 months | 2/629 (0.3) | 21/276 (7.6) | <0.0001 |
| 24–59 months | 1/303(0.3) | 29/145 (20) | <0.0001 |
| Total | 4/1979 (0.2) | 56/916 (6.1) | <0.0001 |
| LSD | |||
| 0–11 months | 0/155 (0) | 1/155 (0.7) | 0.317 |
| 12–23 months | 1/175 (0.6) | 10/175 (5.7) | 0.006 |
| 24–59 months | 1/101 (1) | 6/101 (5.9) | 0.054 |
| Total | 2/431 (0.5) | 17/431 (3.9) | 0.001 |
Abbreviations: LSD, less severe diarrhea; MSD, moderate-to-severe diarrhea.
Figure 1.
Seasonal distribution of Shigella infection in children aged 0–59 months in Manhiça District (December 2007–November 2012).
Table 2.
Crude and pathogen-adjusted multivariate analysis of the association of Shigella spp. with moderate-to-severe diarrhea among children under 5 years (December 2007–November 2012)
| Variables | Group
|
Crude analysis
|
Adjusted analysis
|
|||
|---|---|---|---|---|---|---|
| Controls, n (%) | Cases, n (%) | OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | |
|
| ||||||
| 0–11 months | ||||||
| Shigella spp. | 1/1047 (0) | 6/495 (1) | 22.01 (2.44–2912.20) | 0.0028 | 42 (93.96–5717.57) | 0.0008 |
| Cryptosporidium | 96/1046 (9) | 98/495 (20) | 2.89 (2.07–4.06) | <0.0001 | 3.71 (2.54–5.50) | <0.0001 |
| Giardia | 177/1046 (17) | 45/495 (9) | 0.48 (0.33–0.68) | <0.0001 | 0.47 (0.31–0.69) | 0.0001 |
| Rotavirus | 160/1046 (15) | 217/495 (44) | 5.91 (4.42–8.01) | <0.0001 | 6.37 (4.69–8.79) | <0.0001 |
| Adenovírus (40/41) | 9/1044 (1) | 12/493 (2) | 3.05 (1.25–7.75) | 0.0144 | 4.43 (1.62–12.57) | 0.0038 |
| Nontyphoidal Salmonella | 6/1047 (1) | 6/495 (1) | 2.53 (0.81–7.85) | 0.108 | ||
| Campylobacter | 52/1047 (5) | 30/495 (6) | 1.45 (0.88–2.38) | 0.1456 | ||
| Sapovírus | 29/1047 (3) | 9/495 (2) | 0.61 (0.26–1.28) | 0.1967 | ||
| Enteroaggregative E. coli (aaic only) | 59/1047 (6) | 40/495 (8) | 1.38 (0.89–2.12) | 0.1527 | ||
| 12–23 months | ||||||
| Shigella spp. | 2/629 (0) | 21/276 (8) | 20.75 (6.61–103.57) | <0.0001 | 20.6 (6.30–105.59) | <0.0001 |
| Cryptosporidium | 60/628 (10) | 51/275 (19) | 2.63 (1.70–4.08) | <0.0001 | 2.61 (1.62–4.23) | <0.0001 |
| Giardia | 278/628 (44) | 74/275 (27) | 0.44 (0.32–0.61) | <0.0001 | 045 (0.32–0.63) | <0.0001 |
| Rotavirus | 102/628 (16) | 62/275 (23) | 1.87 (1.28–2.75) | 0.0014 | 2.07 (1.36–3.16) | 0.0007 |
| EAEC (aatA only) | 30/629 (5) | 30/276 (11) | 2.14 (1.22–3.80) | 0.0079 | 2.12 (1.15–3.96) | 0.0168 |
| Aeromonas spp. | 2/629 (0) | 3/276 (1) | 4.20 (0.82–25.21) | 0.0837 | ||
| Vibrio cholerae 01 | 1/629 (0) | 4/276 (1) | 3.97 (0.69–41.39) | 0.1255 | ||
| Nontyphoidal Salmonella | 0/629 (0) | 2/276 (1) | 15.00 (1.22–2068.84) | 0.0336 | ||
| Adenovírus (40/41) | 12/628 (2) | 4/272 (1) | 1.08 (0.33–2.97) | 0.1416 | ||
| 24–59 months | ||||||
| Shigella spp. | 1/303 (0) | 29/145 (20) | 126.56 (17.67–16063.77) | <0.0001 | 191 (23.80–24565.84) | <0.0001 |
| Giardia | 155/303 (51) | 50/145 (34) | 0.51 (0.32–0.78) | 0.0022 | 0.43 (0.25–0.72) | 0.001 |
| EAEC (aatA/aaic) | 10/303 (3) | 1/145 (1) | 0.25 (0.03–1.13) | 0.0743 | 0.07 (0.00–0.81) | 0.0284 |
| V. cholerae | 0/303 (0) | 9/145 (6) | 23.41 (2.87–3044.31) | 0.0008 | 19.6 (2.48–2523.83) | 0.0015 |
| Cryptosporidium | 22/303 (7) | 11/145 (8) | 1.35 (0.60–2.94) | 0.4577 | ||
| Norovírus | 15/303 (5) | 5/145 (3) | 0.46 (0.16–1.18) | 0.1091 | ||
| Adenovírus (non-40/41) | 8/303 (3) | 0/145 (0) | 0.11 (0.00–1.01) | 0.0519 | ||
Clinical presentation
The clinical characteristics (signs and symptoms) of children infected with Shigella were compared with non-MSD Shigella using crude and multivariate analysis and presented in Table 3. The odds of having Shigella infections were associated with significant clinical features according to age, and the results from adjusted analysis are highlighted here. Dysentery, a classic symptom of shigellosis, was one of the most important features of diarrhea caused by Shigella compared to non-Shigella-MSD in both children aged 12–23 months (aOR =3.99; 95% CI: 153–10.39; P=0.0046) and 24–59 months (aOR =5.29; 95% CI: 2.05–13.66; P=0.0006). Moreover, the proportion of Shigella isolation was high among bloody diarrheal cases (20.1%; 32/158) compared to only 3.2% (24/758) for watery diarrhea, with the highest rate in children aged 24–59 months accounting with approximately one-third of all bloody diarrheal cases (Table 4). Fever was also an important symptom of Shigella-MSD in children from 12 to 23 months (aOR =3.02; 1.15–7.94; P=0.025) while rectal prolapse in 24–59 months (aOR =32.08; 1.18–869.71; P=0.0394). Irritability or restless attitude was an important sign for infants by crude analysis (OR =4.93; 1.10–22.9; P=0.0372).
Table 3.
Clinical presentation of Shigella-associated MSD by crude and pathogen-adjusted analysis
| Viables | Type of MSD crude analysis
|
Adjusted analysis
|
||||
|---|---|---|---|---|---|---|
| Non-Shigella-MSD, n (%) | Shigella-MSD, n (%) | OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | |
|
| ||||||
| 0–11 months of age | ||||||
| Dysentery | 35/489 (7) | 1/6 (17) | 3.49 (0.56–21.93) | 0.1823 | ||
| Sunken eyes | 279/489 (57) | 3/6 (50) | 0.75 (0.17–3.35) | 0.7096 | ||
| Loss of skin turgor | 172/489 (35) | 2/6 (33) | 1.02 (0.22–4.85) | 0.9776 | ||
| Intravenous rehydration | 259/489 (53) | 3/6 (50) | 0.89 (0.20–3.95) | 0.8763 | ||
| Hospitalized | 344/489 (70) | 5/6 (83) | 1.55 (0.25–9.52) | 0.6369 | ||
| Vomiting 3 or more times per daya | 266/489 (54) | 2/6 (33) | 0.47 (0.10–2.21) | 0.3361 | ||
| Very thirstya | 345/487 (71) | 4/6 (67) | 0.74 (0.16–3.53) | 0.7079 | ||
| Drank much less than usuala | 70/489 (14) | 1/6 (17) | 1.62 (0.26–10.05) | 0.6027 | ||
| Unable to drinka | 43/489 (9) | 0/6 (0) | 0.79 (0.04–14.25) | 0.8728 | ||
| Belly paina | 129/489 (26) | 2/6 (33) | 1.55 (0.33–7.35) | 0.5836 | ||
| Fevera | 170/489 (35) | 2/6 (33) | 1.04 (0.22–4.94) | 0.9596 | ||
| Irritability or restless attitudea | 82/488 (17) | 3/6 (50) | 4.93 (1.10–22.09) | 0.0372 | ||
| Decreased activity or lethargya | 238/488 (49) | 1/6 (17) | 0.29 (0.05–1.76) | 0.1768 | ||
| Loss of consciousnessa | 8/489 (2) | 0/6 (0) | 4.36 (0.23–83.70) | 0.3290 | ||
| Rectal straininga | 24/489 (5) | 0/6 (0) | 1.46 (0.08–26.70) | 0.7979 | ||
| Rectal prolapsea | 2/489 (0) | 0/5 (0) | 17.73 (0.76–413.90) | 0.0737 | ||
| Cougha | 331/489 (68) | 3/6 (50) | 0.48 (0.11–2.13) | 0.3329 | ||
| Difficulty breathinga | 54/489 (11) | 1/6 (17) | 2.18 (0.35–13.55) | 0.4034 | ||
| Convulsiona | 13/489 (3) | 0/6 (0) | 2.72 (0.15–50.69) | 0.5036 | ||
| Very thirstyb | 349/487 (72) | 4/6 (67) | 0.71 (0.15–3.39) | 0.6709 | ||
| Drinks poorlyb | 60/488 (12) | 1/6 (17) | 1.93 (0.31–11.99) | 0.4796 | ||
| Sunken eyesb | 268/488 (55) | 3/6 (50) | 0.82 (0.18–3.65) | 0.7959 | ||
| Wrinkled skinb | 165/488 (34) | 1/6 (17) | 0.53 (0.09–3.27) | 0.4970 | ||
| Irritable or restlessb | 78/489 (16) | 2/6 (33) | 2.91 (0.61–13.93) | 0.1806 | ||
| Lethargy or loss of consciousnessb | 238/489 (49) | 1/6 (17) | 0.29 (0.05–1.76) | 0.1782 | ||
| Dry mouthb | 216/488 (44) | 1/6 (17) | 0.34 (0.06–2.11) | 0.2480 | ||
| Fast breathingb | 148/489 (30) | 1/6 (17) | 0.63 (0.10–3.85) | 0.6145 | ||
| Undernutrition | 48/489 (10) | 0/6 (0) | 0.70 (0.04–12.62) | 0.8092 | ||
| Low or very low skin pinch | 144/450 (32) | 1/5 (20) | 0.71 (0.11–4.53) | 0.7147 | ||
| 12–23 months of age | ||||||
| Dysentery | 45/255 (18) | 9/21 (43) | 3.52 (1.43–8.67) | 0.0063 | 3.99 (1.53–10.39) | 0.0046 |
| Sunken eyes | 121/255 (47) | 6/21 (29) | 0.46 (0.18–1.20) | 0.1127 | ||
| Loss of skin turgor | 66/255 (26) | 4/21 (19) | 0.73 (0.25–2.14) | 0.5700 | ||
| Intravenous rehydration | 113/255 (44) | 7/21 (33) | 0.65 (0.26–1.62) | 0.3553 | ||
| Hospitalized | 172/255 (67) | 12/21 (57) | 0.64 (0.26–1.54) | 0.3168 | ||
| Vomiting 3 or more times per daya | 124/255 (49) | 7/21 (33) | 0.55 (0.22–1.36) | 0.1953 | ||
| Very thirstya | 208/255 (82) | 15/20 (75) | 0.64 (0.23–1.79) | 0.3957 | ||
| Drank much less than usuala | 14/255 (5) | 1/21 (5) | 1.22 (0.21–6.95) | 0.8238 | ||
| Unable to drinka | 16/255 (6) | 1/21 (5) | 1.06 (0.19–6.00) | 0.9456 | ||
| Belly paina | 68/255 (27) | 6/21 (29) | 1.15 (0.44–2.99) | 0.7776 | ||
| Fevera | 84/255 (33) | 10/21 (48) | 1.85 (0.77–4.45) | 0.1676 | 3.02 (1.15–7.94) | 0.025 |
| Irritability or restless attitudea | 40/255 (16) | 1/21 (5) | 0.39 (0.07–2.11) | 0.2744 | ||
| Decreased activity or lethargya | 135/255 (53) | 9/21 (43) | 0.68 (0.28–1.63) | 0.3821 | ||
| Loss of consciousnessa | 5/255 (2) | 0/21 (0) | 1.06 (0.06–19.80) | 0.9693 | ||
| Rectal straininga | 12/254 (5) | 1/21 (5) | 1.42 (0.25–8.19) | 0.6952 | ||
| Rectal prolapsea | 0/255 (0) | 0/21 (0) | – | – | ||
| Cougha | 159/255 (62) | 7/21 (33) | 0.31 (0.13–0.78) | 0.0130 | 0.27 (0.10–0.72) | 0.0085 |
| Difficulty breathinga | 21/255 (8) | 0/21 (0) | 0.25 (0.01–4.33) | 0.3435 | ||
| Convulsiona | 12/255 (5) | 1/21 (5) | 1.43 (0.25–8.22) | 0.6918 | ||
| Very thirstyb | 209/255 (82) | 15/21 (71) | 0.53 (0.20–1.40) | 0.1983 | ||
| Drinks poorlyb | 15/254 (6) | 1/21 (5) | 1.13 (0.20–6.41) | 0.8897 | ||
| Sunken eyesb | 124/255 (49) | 6/21 (29) | 0.44 (0.17–1.14) | 0.0923 | ||
| Wrinkled skinb | 62/255 (24) | 2/21 (10) | 0.40 (0.10–1.53) | 0.1788 | ||
| Irritable or restlessb | 41/255 (16) | 2/21 (10) | 0.66 (0.17–2.58) | 0.5526 | ||
| Lethargy or loss of consciousnessb | 125/253 (49) | 9/21 (43) | 0.78 (0.32–1.87) | 0.5759 | ||
| Dry mouthb | 112/255 (44) | 8/21 (38) | 0.80 (0.33–1.96) | 0.6304 | ||
| Fast breathingb | 53/253 (21) | 2/21 (10) | 0.48 (0.12–1.85) | 0.2875 | ||
| Undernutrition | 69/255 (27) | 4/21 (19) | 0.69 (0.24–2.02) | 0.4975 | ||
| Low or very low skin pinch | 50/237 (21) | 4/19 (21) | 1.08 (0.36–3.22) | 0.8931 | ||
| 24–59 months of age | ||||||
| Dysentery | 46/116 (40) | 22/29 (76) | 4.55 (1.84–11.25) | 0.0010 | 5.29 (2.05–13.66) | 0.0006 |
| Sunken eyes | 43/116 (37) | 8/29 (28) | 0.67 (0.28–1.61) | 0.3679 | ||
| Loss of skin turgor | 20/116 (17) | 2/29 (7) | 0.43 (0.11–1.70) | 0.2279 | ||
| Intravenous rehydration | 34/116 (29) | 5/29 (17) | 0.54 (0.20–1.47) | 0.2258 | ||
| Hospitalized | 59/116 (51) | 5/29 (17) | 0.22 (0.08–0.59) | 0.0026 | ||
| Vomiting 3 or more times per daya | 38/116 (33) | 3/29 (10) | 0.27 (0.08–0.88) | 0.0293 | ||
| Very thirstya | 93/116 (80) | 22/29 (76) | 0.75 (0.29–1.93) | 0.5569 | ||
| Drank much less than usuala | 11/116 (9) | 1/29 (3) | 0.48 (0.08–2.78) | 0.4151 | ||
| Unable to drinka | 9/116 (8) | 3/29 (10) | 1.49 (0.41–5.47) | 0.5438 | ||
| Belly paina | 23/116 (20) | 10/29 (34) | 2.14 (0.89–5.14) | 0.0883 | ||
| Fevera | 40/115 (35) | 11/29 (38) | 1.16 (0.51–2.66) | 0.7275 | ||
| Irritable or restlessa | 9/115 (8) | 2/29 (7) | 1.02 (0.24–4.37) | 0.9796 | ||
| Decreased activity or lethargya | 58/115 (50) | 10/29 (34) | 0.53 (0.23–1.22) | 0.1348 | ||
| Loss of consciousnessa | 2/116 (2) | 1/29 (3) | 2.41 (0.31–19.02) | 0.4038 | ||
| Rectal straininga | 9/115 (8) | 5/29 (17) | 2.52 (0.81–7.86) | 0.1120 | ||
| Rectal prolapsea | 0/116 (0) | 1/29 (3) | 12.26 (0.49–308.99) | 0.1279 | 32.08 (1.18–869.71) | 0.0394 |
| Cougha | 52/116 (45) | 13/29 (45) | 1.01 (0.45–2.25) | 0.9900 | ||
| Difficulty breathinga | 2/115 (2) | 0/29 (0) | 0.77 (0.04–16.46) | 0.8669 | ||
| Convulsiona | 8/116 (7) | 1/29 (3) | 0.67 (0.11–4.00) | 0.6621 | ||
| Very thirstyb | 94/116 (81) | 22/29 (76) | 0.71 (0.28–1.84) | 0.4856 | ||
| Drinks poorlyb | 15/116 (13) | 1/29 (3) | 0.34 (0.06–1.94) | 0.2266 | ||
| Sunken eyesb | 45/116 (39) | 8/29 (28) | 0.62 (0.26–1.49) | 0.2873 | ||
| Wrinkled skinb | 19/116 (16) | 2/29 (7) | 0.45 (0.11–1.81) | 0.2638 | ||
| Irritability or restless attitudeb | 3/116 (3) | 2/29 (7) | 2.95 (0.55–15.75) | 0.2060 | ||
| Lethargy or loss of consciousnessb | 52/116 (45) | 9/29 (31) | 0.57 (0.24–1.33) | 0.1946 | ||
| Dry mouthb | 35/116 (30) | 5/29 (17) | 0.52 (0.19–1.41) | 0.1962 | ||
| Fast breathingb | 8/116 (7) | 0/29 (0) | 0.22 (0.01–3.86) | 0.2977 | ||
| Undernutrition | 8/116 (7) | 0/29 (0) | 0.22 (0.01–3.86) | 0.2977 | ||
| Low or very low skin pinch | 16/108 (15) | 1/28 (4) | 0.31 (0.05–1.72) | 0.1782 | ||
Notes:
Since began;
current.
Abbreviation: MSD, moderate-to-severe diarrhea.
Table 4.
Crude analysis of Shigella-associated diarrhea by severity (bloody diarrhea and watery diarrhea) in the different age groups (December 2007–November 2012)
| Age groups | Watery diarrhea, n (%) | Bloody diarrhea, n (%) | OR (95% CI) | P-value |
|---|---|---|---|---|
|
| ||||
| 0–11 months | 5/459 (1) | 1/36 (3) | 3.49 (0.56–21.93) | 0.1823 |
| 12–23 months | 12/222 (5) | 9/54 (17) | 3.52 (1.43–8.67) | 0.0063 |
| 24–59 months | 7/77 (9) | 22/68 (32) | 4.55 (1.84–11.25) | 0.001 |
Risk factors
The role of different risk factors for MSD was age-dependent, although most of the studied socioeconomic or hygienic explanatory variables were not statistically significant (Table 5). Partial or exclusive breastfeeding was a remarkable protective factor against Shigella in younger children aged 0–11 months with MSD by crude analysis (OR =0.10; 0.02–0.68; P=0.0184). Protective factors in children aged 12–23 months included partial or exclusive breastfeeding (OR =0.12; 0.04–0.40; P=0.0006), mother as primary child caretaker (aOR =0.28; 0.09–0.87; P=0.0281), and giving stored water to child (aOR =0.17; 0.04–0.82; P=0.0267). In 24–59 months, water availability (not always per day) (aOR =0.32; 0.12–0.81; P=0.0167) and washing hands before preparing baby’s food (aOR =0.28; 0.08–0.94; P=0.0394) were protective against shigellosis. Among controls (Table 6), relevant protective factors for acquiring Shigella included child primary caretaker for children aged 0–11 months (aOR =0.03; 0.00–0.87; P=0.0408), and washing hands before eating in children aged 12–23 months (aOR =0.05; 0.01, 0.52; P=0.0119).
Table 5.
Risk factors of Shigella-associated moderate-to-severe diarrhea by crude and adjusted analysis
| Variables |
Shigella spp.
|
Crude analysis
|
Adjusted analysis
|
|||
|---|---|---|---|---|---|---|
| Negative, n (%) | Positive, n (%) | OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | |
|
| ||||||
| 0–11 months | ||||||
| >6 months of age | 259/489 (53) | 5/6 (83) | 3.26 (0.53–19.98) | 0.2021 | ||
| Child sex (male) | 294/489 (60) | 4/6 (67) | 1.19 (0.25–5.67) | 0.8226 | ||
| Child primary caretaker (mother) | 473/489 (97) | 6/6 (100) | 0.45 (0.02–8.38) | 0.5948 | ||
| Caretaker formal education | 135/487 (28) | 3/6 (50) | 2.60 (0.58–11.60) | 0.2100 | ||
| Animals in compound | 411/489 (84) | 4/6 (67) | 0.34 (0.07–1.64) | 0.1806 | ||
| Water availability (not always per day) | 256/488 (52) | 4/6 (67) | 1.63 (0.34–7.73) | 0.5375 | ||
| Access to improved water | 411/489 (84) | 4/6 (67) | 0.34 (0.07–1.64) | 0.1806 | ||
| Give stored water to child | 450/489 (92) | 6/6 (100) | 1.14 (0.06–20.61) | 0.9294 | ||
| Treating water habit | 54/489 (11) | 1/6 (17) | 2.18 (0.35–13.55) | 0.4034 | ||
| Facility to dispose child’s stool | 248/486 (51) | 2/6 (33) | 0.53 (0.11–2.53) | 0.4284 | ||
| Improved facility for household stool | 43/488 (9) | 0/6 (0) | 0.79 (0.04–14.22) | 0.8716 | ||
| Wash hands before eating | 463/489 (95) | 5/6 (83) | 0.21 (0.03–1.33) | 0.0974 | ||
| Wash hands before cooking | 304/489 (62) | 3/6 (50) | 0.61 (0.14–2.71) | 0.5152 | ||
| Wash hands before preparing baby’s food | 129/489 (26) | 3/6 (50) | 2.78 (0.62–12.42) | 0.1796 | ||
| Wash hands after defecating | 413/489 (84) | 6/6 (100) | 2.41 (0.13–43.14) | 0.5513 | ||
| Wash hands after handling animals | 12/489 (2) | 0/6 (0) | 2.94 (0.16–55.07) | 0.4710 | ||
| Wash hands after cleaning child feces | 127/489 (26) | 2/6 (33) | 1.58 (0.33–7.51) | 0.5655 | ||
| Partial or exclusive breastfeeding | 413/424 (97) | 5/6 (83) | 0.10 (0.02–0.68) | 0.0184 | ||
| Lowest quintile of wealth index | 110/484 (23) | 2/6 (33) | 1.88 (0.40–8.96) | 0.4268 | ||
| 12–23 months | ||||||
| >18 months of age | 75/255 (29) | 10/21 (48) | 2.18 (0.91–5.26) | 0.0816 | ||
| Child sex (male) | 147/255 (58) | 10/21 (48) | 0.67 (0.28–1.61) | 0.3713 | ||
| Child primary caretaker (mother) | 237/255 (93) | 17/21 (81) | 0.30 (0.10–0.95) | 0.0398 | 0.28 (0.09–0.87) | 0.0281 |
| Caretaker formal education | 58/253 (23) | 4/21 (19) | 0.86 (0.29–2.52) | 0.7825 | ||
| Animals in compound | 213/255 (84) | 16/21 (76) | 0.60 (0.22–1.66) | 0.3217 | ||
| Water availability (not always per day) | 140/255 (55) | 11/21 (52) | 0.90 (0.38–2.15) | 0.8135 | ||
| Access to improved water | 214/255 (84) | 16/21 (76) | 0.58 (0.21–1.61) | 0.2962 | ||
| Give stored water to child | 249/255 (98) | 19/21 (90) | 0.20 (0.04–0.94) | 0.0412 | 0.17 (0.04–0.82) | 0.0267 |
| Treating water habit | 14/255 (5) | 2/21 (10) | 2.14 (0.52–8.83) | 0.2948 | ||
| Facility to dispose child’s stool | 207/253 (82) | 18/21 (86) | 1.18 (0.36–3.88) | 0.7796 | ||
| Improved facility for household stool | 15/255 (6) | 0/21 (0) | 0.36 (0.02–6.24) | 0.4834 | ||
| Wash hands before eating | 225/255 (88) | 19/21 (90) | 1.05 (0.27–4.15) | 0.9390 | ||
| Wash hands before cooking | 152/255 (60) | 11/21 (52) | 0.74 (0.31–1.78) | 0.5055 | ||
| Wash hands before preparing baby’s food | 78/255 (31) | 4/21 (19) | 0.58 (0.20–1.69) | 0.3203 | ||
| Wash hands after defecating | 219/255 (86) | 19/21 (90) | 1.30 (0.33–5.07) | 0.7083 | ||
| Wash hands after handling animals | 4/255 (2) | 0/21 (0) | 1.30 (0.07–24.95) | 0.8619 | ||
| Wash hands after cleaning child feces | 59/255 (23) | 7/21 (33) | 1.71 (0.68–4.32) | 0.2581 | ||
| Partial or exclusive breastfeeding | 139/214 (65) | 3/18 (17) | 0.12 (0.04–0.40) | 0.0006 | ||
| Lowest quintile of wealth index | 55/255 (22) | 2/21 (10) | 0.46 (0.12–1.79) | 0.2637 | ||
| 24–59 months | ||||||
| >42 months of age | 25/116 (22) | 3/29 (10) | 0.47 (0.14–1.57) | 0.2218 | ||
| Child sex (male) | 68/116 (59) | 19/29 (66) | 1.31 (0.57–3.03) | 0.5208 | ||
| Child primary caretaker (mother) | 100/116 (86) | 28/29 (97) | 3.12 (0.56–17.46) | 0.1955 | ||
| Caretaker formal education | 23/115 (20) | 11/29 (38) | 2.45 (1.03–5.81) | 0.0424 | ||
| Animals in compound | 95/116 (82) | 24/29 (83) | 1.00 (0.36–2.83) | 0.9957 | ||
| Water availability (not always per day) | 60/116 (52) | 7/29 (24) | 0.31 (0.13–0.77) | 0.0113 | 0.32 (0.12–0.81) | 0.0167 |
| Access to improved water | 95/116 (82) | 25/29 (86) | 1.28 (0.42–3.86) | 0.6661 | ||
| Give stored water to child | 109/115 (95) | 27/29 (93) | 0.65 (0.14–2.98) | 0.5819 | ||
| Treating water habit | 5/116 (4) | 3/29 (10) | 2.68 (0.66–10.92) | 0.1696 | ||
| Facility to dispose child’s stool | 110/116 (95) | 28/29 (97) | 1.12 (0.18–6.92) | 0.9048 | ||
| Improved facility for household stool | 13/116 (11) | 4/29 (14) | 1.35 (0.43–4.28) | 0.6069 | ||
| Wash hands before eating | 106/116 (91) | 27/29 (93) | 1.08 (0.26–4.58) | 0.9122 | ||
| Wash hands before cooking | 70/116 (60) | 19/29 (66) | 1.22 (0.53–2.83) | 0.6346 | ||
| Wash hands before preparing baby’s food | 34/116 (29) | 3/29 (10) | 0.32 (0.10–1.03) | 0.0563 | 0.28 (0.08–0.94) | 0.0394 |
| Wash hands after defecating | 101/116 (87) | 24/29 (83) | 0.68 (0.23–1.98) | 0.4796 | ||
| Wash hands after handling animals | 4/116 (3) | 1/29 (3) | 1.32 (0.20–8.74) | 0.7763 | ||
| Wash hands after cleaning child feces | 23/116 (20) | 6/29 (21) | 1.10 (0.41–2.93) | 0.8480 | ||
| Partial or exclusive breastfeeding | 18/100 (18) | 4/20 (20) | 1.22 (0.38–3.87) | 0.7404 | ||
| Lowest quintile of wealth index | 31/115 (27) | 7/29 (24) | 0.89 (0.36–2.25) | 0.8121 | ||
Table 6.
Risk factors for Shigella infection in controls by crude and adjusted analysis
| Variables |
Shigella spp.
|
Crude analysis
|
Adjusted analysis
|
|||
|---|---|---|---|---|---|---|
| Negative, n (%) | Positive, n (%) | OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | |
|
| ||||||
| 0–11 months | ||||||
| >6 months of age | 595/1046 (57) | 1/1 (100) | 2.27 (0.09–55.97) | 0.6151 | ||
| Child sex (male) | 629/1046 (60) | 1/1 (100) | 1.99 (0.08–48.96) | 0.6738 | ||
| Child primary caretaker (mother) | 1035/1046 (99) | 1/1 (100) | 0.03 (0.00–0.86) | 0.0404 | 0.03 (0.00–0.87) | 0.0408 |
| Caretaker formal education | 313/1044 (30) | 1/1 (100) | 7.00 (0.28–172.30) | 0.2338 | ||
| Animals in compound | 897/1045 (86) | 1/1 (100) | 0.50 (0.02–12.24) | 0.6684 | ||
| Water availability (not always per day) | 333/1046 (32) | 0/1 (0) | 0.71 (0.03–17.55) | 0.8361 | ||
| Access to improved water | 888/1046 (85) | 0/1 (0) | 0.06 (0.00–1.47) | 0.0844 | ||
| Give stored water to child | 829/1046 (79) | 0/1 (0) | 0.09 (0.00–2.15) | 0.1360 | ||
| Treating water habit | 66/1046 (6) | 0/1 (0) | 4.91 (0.20–121.81) | 0.3310 | ||
| Facility to dispose child’s stool | 656/1046 (63) | 1/1 (100) | 1.78 (0.07–43.91) | 0.7231 | ||
| Improved facility for household stool | 79/1046 (8) | 0/1 (0) | 4.06 (0.16–100.39) | 0.3924 | ||
| Wash hands before eating | 980/1046 (94) | 1/1 (100) | 0.20 (0.01–5.04) | 0.3310 | ||
| Wash hands before cooking | 855/1046 (82) | 0/1 (0) | 0.07 (0.00–1.84) | 0.1124 | ||
| Wash hands before preparing baby’s food | 627/1046 (60) | 1/1 (100) | 2.01 (0.08–49.35) | 0.6702 | ||
| Wash hands after defecating | 765/1046 (73) | 1/1 (100) | 1.10 (0.04–27.16) | 0.9521 | ||
| Wash hands after handling animals | 421/1046 (40) | 0/1 (0) | 0.49 (0.02–12.17) | 0.6667 | ||
| Wash hands after cleaning child feces | 642/1046 (61) | 1/1 (100) | 1.89 (0.08–46.48) | 0.6972 | ||
| Partial or exclusive breastfeeding | 1018/1039 (98) | 1/1 (100) | 0.06 (0.00–1.60) | 0.0939 | ||
| Lowest quintile of wealth index | 194/1045 (19) | 0/1 (0) | 1.46 (0.06–35.96) | 0.8172 | ||
| 12–23 months | ||||||
| >18 months of age | 200/627 (32) | 0/2 (0) | 0.43 (0.02–8.92) | 0.5828 | ||
| Child sex (male) | 373/627 (59) | 2/2 (100) | 3.41 (0.16–71.26) | 0.4294 | ||
| Child primary caretaker (mother) | 598/627 (95) | 2/2 (100) | 0.25 (0.01–5.25) | 0.3695 | ||
| Caretaker formal education | 172/625 (28) | 1/2 (50) | 2.63 (0.27–25.45) | 0.4039 | ||
| Animals in compound | 555/627 (89) | 2/2 (100) | 0.65 (0.03–13.73) | 0.7836 | ||
| Water availability (not always per day) | 216/627 (34) | 2/2 (100) | 9.50 (0.45–198.84) | 0.1467 | ||
| Access to improved water | 521/627 (83) | 1/2 (50) | 0.20 (0.02–1.98) | 0.1707 | ||
| Give stored water to child | 583/625 (93) | 2/2 (100) | 0.36 (0.02–7.71) | 0.5166 | ||
| Treating water habit | 26/627 (4) | 0/2 (0) | 4.54 (0.21–96.93) | 0.3327 | ||
| Facility to dispose child’s stool | 600/626 (96) | 2/2 (100) | 0.22 (0.01–4.71) | 0.3333 | ||
| Improved facility for household stool | 27/627 (4) | 0/2 (0) | 4.37 (0.20–93.17) | 0.3451 | ||
| Wash hands before eating | 596/627 (95) | 1/2 (50) | 0.05 (0.01–0.52) | 0.0119 | 0.05 (0.01–0.52) | 0.0119 |
| Wash hands before cooking | 522/627 (83) | 2/2 (100) | 1.01 (0.05–21.18) | 0.9951 | ||
| Wash hands before preparing baby’s food | 386/627 (62) | 0/2 (0) | 0.12 (0.01–2.61) | 0.1801 | ||
| Wash hands after defecating | 454/627 (72) | 2/2 (100) | 1.91 (0.09–39.96) | 0.6770 | ||
| Wash hands after handling animals | 254/627 (41) | 0/2 (0) | 0.29 (0.01–6.14) | 0.4294 | ||
| Wash hands after cleaning child feces | 367/627 (59) | 0/2 (0) | 0.14 (0.01–2.97) | 0.2079 | ||
| Partial or exclusive breastfeeding | 443/621 (71) | 2/2 (100) | 2.01 (0.10–42.13) | 0.6522 | ||
| Lowest quintile of wealth index | 135/627 (22) | 1/2 (50) | 3.63 (0.38–35.22) | 0.2654 | ||
| 24–59 months | ||||||
| >42 months of age | 51/302 (17) | 0/1 (0) | 1.63 (0.07–40.52) | 0.7664 | ||
| Child sex (male) | 201/302 (67) | 0/1 (0) | 0.17 (0.01–4.16) | 0.2759 | ||
| Child primary caretaker (mother) | 268/302 (89) | 1/1 (100) | 0.39 (0.02–9.65) | 0.5618 | ||
| Caretaker formal education | 74/302 (25) | 0/1 (0) | 1.02 (0.04–25.37) | 0.9892 | ||
| Animals in compound | 252/302 (83) | 1/1 (100) | 0.60 (0.02–14.94) | 0.7555 | ||
| Water availability (not always per day) | 103/302 (34) | 0/1 (0) | 0.64 (0.03–15.91) | 0.7870 | ||
| Access to improved water | 254/302 (84) | 1/1 (100) | 0.57 (0.02–14.24) | 0.7332 | ||
| Give stored water to child | 277/302 (92) | 1/1 (100) | 0.28 (0.01–6.94) | 0.4337 | ||
| Treating water habit | 9/302 (3) | 0/1 (0) | 10.30 (0.39–269.66) | 0.1616 | ||
| Facility to dispose child’s stool | 298/301 (99) | 1/1 (100) | 0.04 (0.00–1.02) | 0.0515 | ||
| Improved facility for household stool | 24/302 (8) | 0/1 (0) | 3.79 (0.15–95.51) | 0.4185 | ||
| Wash hands before eating | 286/302 (95) | 1/1 (100) | 0.17 (0.01–4.41) | 0.2880 | ||
| Wash hands before cooking | 232/302 (77) | 1/1 (100) | 0.91 (0.04–22.58) | 0.9539 | ||
| Wash hands before preparing baby’s food | 156/302 (52) | 0/1 (0) | 0.31 (0.01–7.72) | 0.4768 | ||
| Wash hands after defecating | 210/302 (70) | 0/1 (0) | 0.15 (0.01–3.63) | 0.2408 | ||
| Wash hands after handling animals | 122/302 (40) | 1/1 (100) | 4.42 (0.18–109.40) | 0.3640 | ||
| Wash hands after cleaning child feces | 161/302 (53) | 1/1 (100) | 2.63 (0.11–65.04) | 0.5550 | ||
| Partial or exclusive breastfeeding | 8/279 (3) | 0/1 (0) | 10.65 (0.40–280.87) | 0.1566 | ||
| Lowest quintile of wealth index | 53/302 (18) | 0/1 (0) | 1.55 (0.06–38.68) | 0.7879 | ||
Impact of antibiotic treatment on patient outcome
The impact of antibiotic treatment on patient outcome was assessed among children with known outcome (Table 7). Patient outcome was available for all of Shigella-diarrheal children while antibiotic treatment was administered in only 30.1% (22/73). The most administered antibiotics were ampicillin and gentamicin (12.3%; 9/73), followed by trimethoprim-sulfametoxazole (9.6%; 7/73), chloramphenicol (8.2%; 6/73), and nalidixic acid (6.9%; 5/73). Although high rates of resistance were found, patient improvement at discharge was much higher in children that received antibiotic treatment (63.0%; 17/27) compared to 37.0% (10/27), for those without any treatment (P<0.0001).
Table 7.
Impact of antibiotic treatment for Shigella-diarrhea in children outcome when leaving the hospital from December 2007 to November 2012
| Outcomes | Antibiotic treatment, n (%)
|
|||
|---|---|---|---|---|
| No | Yes | Total | P-value | |
|
| ||||
| Improved | 10/27 (37) | 17/27 (63) | 27 | <0.0001 |
| Not improved | 41/46 (89.1) | 5/46 (10.8) | 46 | |
| Total | 51/73 (69.8) | 22/73 (30.1) | 73 | |
|
| ||||
| Antibiotics administered | Proportion, n (%) | Resistance, n (%) | ||
|
| ||||
| Trimethoprim-sulfametoxazole | 7/73 (9.6) | 62/67 (92.5) | ||
| Gentamicin | 9/73 (12.3) | 0 | ||
| Chloramphenicol | 6/73 (8.2) | 36/67 (53.7) | ||
| Amoxicillin clavulanic acid | 4/73 (5.5) | 3/67 (4.5) | ||
| Ampicillin | 9/73 (12.3) | 34/67 (50.7) | ||
| Nalidixic acid | 5/73 (6.9) | 0 | ||
| Ciprofloxacin | 1/73 (1.4) | 0 | ||
Discussion
In this prospective, case–control study, we demonstrated the association of Shigella spp. with MSD and LSD among children under 5 years in Manhiça District, a rural area of southern Mozambique. Its low detection rate in children without diarrhea and in cases of LSD confirms the high probability that whenever present, this pathogen causes a virulent disease, which could be explained in part by the low infectious dose of the bacterium (10–100 cells).21 While an association between Enterobacteriaceae and Giardia has been reported,22 in this study we showed that the prevalence of Giardia was independent of Shigella by adjusted analysis. In addition, Giardia was more common in controls while Shigella was mostly isolated in cases. The proportion of cases presenting with Shigella was similar with those reported in our previous study12 and in other African countries.3,23,24 Overall, the detection rate of Shigella was high during rainy season, which is similar to other studies.25 As expected, the classical signs/symptoms of Shigella-MSD were mostly of dysentery accompanied with fever or rectal prolapse.8 Irritability or restless attitude was also an importance feature in infants. The observation that Shigella may also be associated with less severe cases is a matter of concern, because the disease is mostly considered severe and frequently associated with dysentery. These findings expand the spectrum of clinical features associated with Shigella infection and demonstrate the importance of active surveillance for disease prevention. Another explanation could be related to health-seeking behavior, meaning that mothers or child caretakers are looking for assistance earlier before the disease becomes severe, as demonstrated in our community study about health care utilization and attitudes in cases of MSD.26
The finding that children who received antibiotic treatment was associated with a good outcome at the discharge, reinforcing the importance of antibiotic therapy for the management of shigellosis. Similar reports have shown that, with an effective antibiotic therapy, clinical improvement occurs within 48 hours,27 resulting in a decreased risk of serious complications and death, shorter duration of symptoms, the elimination of Shigella from the stool, and subsequently decreased infection transmission.
MSD caused by Shigella was associated with a high OR compared to other significant pathogens in adjusted analysis, which may suggest its high pathogenicity as observed by the low detection rate in healthy children compared to other significant pathogens. In accordance with previous reports,28 we found a low risk of Shigella-associated diarrhea in infancy and a steady increase through the second year of life. One likely explanation is that the partial or exclusive breastfeeding, which was present in 97% of infants (<12 months) of age and in 61% of children under 2 years in our study, neutralized the infectious inoculum and prevented disease. The strong association between MSD caused by Shigella and children over 2 years of age has been attributed to a decreased exposure to breast milk and an increased exposure to sources of Shigella infection such as hands contact in the environment as children become more active.29,30 In addition, the benefits of human milk for infant health have been recognized as an important source of bacteria that may contribute to neonatal gastrointestinal colonization leading to immune response development and possible infection prevention. Thus, the microbiota of breast-fed infants is considered the gold standard in terms of a healthy infant gastrointestinal microbiota.31
In addition, as children wean, they are exposed to an increased array of food and water that could serve as sources of Shigella, thus increasing the probability of getting infected.32 Therefore, this study also sought to quantify the effects of several socioeconomic indicators on the relative increased risk for acquiring Shigella in children presenting MSD. Overall, the role of the assessed risk factors for Shigella-MSD was age-related, possibly due to differences in the degree of exposure of the studied population. The presence of broader protective factors for diarrhea caused by Shigella in the present study could be attributed to an improved health-seeking behavior among the cases and improvement in accessibility of health services over the years. The population leaving in Manhiça district is under monitoring through HDSS since 1996, and is thus exposed to several interventions that contribute to health education that may influence the health-seeking behavior. This may explain why socioeconomic characteristics such as formal education were less relevant in the present study. Considering these variations, the importance of children primary caretaker (mother), giving stored water to child, water availability (not always per day), and washing hands before preparing baby’s food in preventing diarrhea caused by Shigella is highlighted.
The importance of hand-washing practices in preventing diarrheal illness is supported by a number of studies.33,34 A systematic review of several studies estimated that appropriate hand washing with soap could reduce the risks of severe intestinal infections and shigellosis up to 48% and 59%, respectively.34 The fact that stored water consumption was protective for Shigella infection leads us to believe that the hygienic conditions for water transportation and the containers where the water is stored are adequate or properly cleaned, because water contamination can occur at any stage from the source to the point of use. Conversely, unavailability of water throughout the day was protective for Shigella infection and may reflect the rational use of scarce water at the household level, or be linked to the hypothesis that the lack of water can interrupt the chain of transmission of some waterborne transmitted enteropathogens like Shigella as families are less exposed due to the limited amount of water that they have. The prevalence of malnutrition in both Shigella-associated MSD and in non-Shigella MSD highlights the vicious cycle of malnutrition and diarrhea. While malnutrition makes children more vulnerable to diarrheal infections due to its negative effect on the barrier protection leading to deterioration of the immune system, chronic infections can contribute to long-term gut damage and preventing nutrient absorption.35
Conclusion
Shigella is a significant pathogen associated with MSD in Mozambican children from 1 to 5 years. The presence of cases of LSD caused by Shigella is a matter of concern and may suggest the need to expand the clinical feature of the disease. The presence of dysentery remains an important clinical feature of Shigella-associated diarrhea. The study reinforces the recommendations from the WHO on the use of antibiotic treatment for the management of shigellosis while vaccines to prevent disease are not available. As demonstrated in this study, promoting awareness of good personal hygiene and a safe water storage is of great importance in prevention and control of the disease. Moreover, continuous surveillance studies are needed to track changes over time.
Acknowledgments
The authors thank all children and their parents for participating in the surveillance. Thanks to clinicians, nurses, and other staff from CISM and Manhiça District Hospital for collecting and processing the data. Thanks to the district health authorities and the Ministry of Health for their collaboration in the research activities ongoing in Manhiça District.
This study was part the GEMS study funded by the Bill and Melinda Gates Foundation. CISM receives core funds from Spanish Agency for International Cooperation and Development (AECID). Delfino Vubil received a fellowship from Fundação Calouste Gulbenkian – Programa Gulbenkian Parcerias para o Desenvolvimento (www.gulbenkian.pt).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Das JK, Tripathi A, Ali A, Hassan A, Dojosoeandy C, Bhutta ZA. Vaccines for the prevention of diarrhea due to cholera, shigella, ETEC and rotavirus. BMC Public Health. 2013;13(Suppl 3):S11. doi: 10.1186/1471-2458-13-S3-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine MM, Kotloff KL, Nataro JP, Muhsen K. The Global Enteric Multicenter Study (GEMS): impetus, rationale, and genesis. Clin Infect Dis. 2012;55(Suppl 4):S215–S224. doi: 10.1093/cid/cis761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonkoungou IJ, Haukka K, Österblad M, et al. Bacterial and viral etiology of childhood diarrhea in Ouagadougou, Burkina Faso. BMC Pediatr. 2013;13:36. doi: 10.1186/1471-2431-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian L, Zhu X, Chen Z, et al. Characteristics of bacterial pathogens associated with acute diarrhea in children under 5 years of age: a hospital-based cross-sectional study. BMC Infect Dis. 2016;16(1):1–8. doi: 10.1186/s12879-016-1603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotloff KL, Winickoff JP, Ivanoff B, et al. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ. 1999;77(8):651–666. [PMC free article] [PubMed] [Google Scholar]
- 6.Bohles N, Busch K, Hensel M. Vaccines against human diarrheal pathogens: current status and perspectives. Hum Vaccines Immunother. 2014;10:1522–1535. doi: 10.4161/hv.29241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aragón TJ, Vugia DJ, Shallow S, et al. Case-control study of shigellosis in San Francisco: the role of sexual transmission and HIV infection. Clin Infect Dis. 2007;44(3):327–334. doi: 10.1086/510593. [DOI] [PubMed] [Google Scholar]
- 8.Sur D, Ramamurthy T, Deen J, Bhattacharya SK. Shigellosis: challenges & management issues. Indian J Med Res. 2004;120(5):454–462. [PubMed] [Google Scholar]
- 9.Traa BS, Walker CL, Munos M, Black RE. Antibiotics for the treatment of dysentery in children. Int J Epidemiol. 2010;39(Suppl 1):i70–i74. doi: 10.1093/ije/dyq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Bushra HE, Bin Saeed AA. Intrafamilial person-to-person spread of bacillary dysentery due to Shigella dysenteriae in southwestern Saudi Arabia. East Afr Med J. 1999;76(5):255–259. [PubMed] [Google Scholar]
- 11.Nhampossa T, Mandomando I, Acacio S, et al. Diarrheal disease in rural Mozambique: burden, risk factors and etiology of diarrheal disease among children aged 0-59 months seeking care at health facilities. PLoS One. 2015;10(5):e0119824. doi: 10.1371/journal.pone.0119824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandomando I, Sigaúque B, Vallès X, et al. Epidemiology and clinical presentation of shigellosis in children less than five years of age in rural Mozambique. Pediatr Infect Dis J. 2007;26(11):1059–1061. doi: 10.1097/INF.0b013e31812e55a2. [DOI] [PubMed] [Google Scholar]
- 13.Kotloff KL, Blackwelder WC, Nasrin D, et al. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study. Clin Infect Dis. 2012;55(Suppl 4):S232–S245. doi: 10.1093/cid/cis753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González R, Munguambe K, Aponte J, et al. High HIV prevalence in a southern semi-rural area of Mozambique: a community-based survey. HIV Med. 2012;13(10):581–588. doi: 10.1111/j.1468-1293.2012.01018.x. [DOI] [PubMed] [Google Scholar]
- 15.Sacarlal J, Nhacolo AQ, Sigaúque B. A 10 year study of the cause of death in children under 15 years in Manhiça, Mozambique. BMC Public Health. 2006;3002:1–10. doi: 10.1186/1471-2458-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sacoor C, Nhacolo A, Nhalungo D, et al. Profile: Manhiça Health Research Centre (Manhiça HDSS) Int J Epidemiol. 2013;42(5):1309–1318. doi: 10.1093/ije/dyt148. [DOI] [PubMed] [Google Scholar]
- 17.Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 18.Panchalingam S, Antonio M, Hossain A, et al. Diagnostic micro-biologic methods in the GEMS-1 case/control study. Clin Infect Dis. 2012;55(Suppl 4):S294–S302. doi: 10.1093/cid/cis754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- 20.Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med. 2002;21(16):2409–2419. doi: 10.1002/sim.1047. [DOI] [PubMed] [Google Scholar]
- 21.Mattock E, Blocker AJ. How do the virulence factors of Shigella work together to cause disease? Front Cell Infect Microbiol. 2017;7:1–24. doi: 10.3389/fcimb.2017.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zerpa R, Huicho L. Intestinal coinfection with numerous Giardia trophozoites and Vibrio cholerae in hospitalized children with watery diarrhea. Wilderness Environ Med. 1995;6(2):167–172. doi: 10.1580/1080-6032(1995)006[0167:icwngt]2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Nitiema LW, Nordgren J, Ouermi D, et al. Burden of rotavirus and other enteropathogens among children with diarrhea in Burkina Faso. Int J Infect Dis. 2011;15(9):e646–e652. doi: 10.1016/j.ijid.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Moyo SJ, Gro N, Matee MI, et al. Age specific aetiological agents of diarrhoea in hospitalized children aged less than five years in Dares Salaam, Tanzania. BMC Pediatr. 2011;11:19. doi: 10.1186/1471-2431-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HS, Ha Hoang TT, Pham-Duc P, et al. Seasonal and geographical distribution of bacillary dysentery (shigellosis) and associated climate risk factors in Kon Tum Province in Vietnam from 1999 to 2013. Infect Dis Poverty. 2017;6(1):1–11. doi: 10.1186/s40249-017-0325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nhampossa T, Mandomando I, Acacio S, et al. Health care utilization and attitudes survey in cases of moderate-to-severe diarrhea among children ages 0-59 months in the District of Manhica, southern Mozambique. Am J Trop Med Hyg. 2013;89(1 Suppl):41–48. doi: 10.4269/ajtmh.12-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organisation . Guidelines for the control of shigellosis, including epidemics due to Shigella dysenteriae type 1. Geneva: World Health Organization; 2005. [Accessed October 3, 2018]. Available from: http://apps.who.int/iris/bitstream/10665/43252/1/924159330X.pd. [Google Scholar]
- 28.Abu-Elyazeed RR, Wierzba TF, Frenck RW, et al. Epidemiology of Shigella-associated diarrhea in rural Egyptian children. Am J Trop Med Hyg. 2004;71(3):367–372. [PubMed] [Google Scholar]
- 29.Clemens JD, Stanton B, Stoll B, Shahid NS, Banu H, Chowdhury AK. Breast feeding as a determinant of severity in shigellosis. Evidence for protection throughout the first three years of life in Bangladeshi children. Am J Epidemiol. 1986;123(4):710–720. doi: 10.1093/oxfordjournals.aje.a114291. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed F, Clemens JD, Rao MR, Sack DA, Khan MR, Haque E. Community-based evaluation of the effect of breast-feeding on the risk of microbiologically confirmed or clinically presumptive shigellosis in Bangladeshi children. Pediatrics. 1992;90(3):406–411. [PubMed] [Google Scholar]
- 31.Murphy K, Curley D, O’Callaghan TF, et al. The composition of human milk and infant faecal microbiota over the first three months of life: a pilot study. Sci Rep. 2017;7:40597. doi: 10.1038/srep40597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindsay B, Saha D, Sanogo D, et al. Association between shigella infection and diarrhea varies based on location and age of children. Am J Trop Med Hyg. 2015;93(5):918–924. doi: 10.4269/ajtmh.14-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maponga BA, Chirundu D, Gombe NT, Tshimanga M, Shambira G, Takundwa L. Risk factors for contracting watery diarrhoea in Kadoma City, Zimbabwe, 2011: a case control study. BMC Infect Dis. 2013;13:567. doi: 10.1186/1471-2334-13-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curtis V, Cairncross S. Effect of washing hands with soap on diarrhoea risk in the community: a systematic review. Lancet Infect Dis. 2003;3(5):275–281. doi: 10.1016/s1473-3099(03)00606-6. [DOI] [PubMed] [Google Scholar]
- 35.Rodríguez L, Cervantes E, Ortiz R. Malnutrition and gastrointestinal and respiratory infections in children: a public health problem. Int J Environ Res Public Health. 2011;8(4):1174–1205. doi: 10.3390/ijerph8041174. [DOI] [PMC free article] [PubMed] [Google Scholar]