Abstract
Objective
To investigate the expression of tumor suppressor protein ASK1-interacting protein-1 (AIP1) in human esophageal squamous cell carcinoma (ESCC) and its role in tumor progression, angiogenesis, and prognosis.
Methods
A total of 117 biopsy samples were obtained from ESCC patients. None of the patients had distant metastasis before surgery, and did not receive preoperative chemotherapy or radiotherapy. Immunohistochemistry was used to detect the expression of AIP1 protein and vascular endothelial growth factor receptor 2 (VEGFR2) in ESCC specimens collected from 117 patients who underwent esophageal cancer radical surgery. Microvessel density (MVD) was evaluated by immunohistochemical staining of vascular endothelial CD34. The correlation between AIP1 protein and clinicopathological characteristics, tumor angiogenesis, and prognosis was analyzed.
Results
The downregulation of AIP1 protein in esophageal carcinoma tissues was detected in 63 cases. This downregulation significantly correlated with lymph node metastasis, clinicopathological staging, and tumor MVD (P<0.05). Survival analysis showed that ESCC patients with a low expression of AIP1, a high expression of VEGFR2, and a high level of MVD had a lower 5-year survival rate (P<0.05). Multivariate analysis confirmed that the downregulation of AIP1 significantly affected patient survival.
Conclusion
The downregulation of AIP1 correlated with ESCC progression, tumor angiogenesis, and poor prognosis. AIP1 could be a promising biomarker for predicting ESCC prognosis and a potential target for anti-angiogenic therapy.
Keywords: esophageal squamous cell carcinoma, angiogenesis, ASK1-interacting protein-1, microvessel density
Introduction
Human esophageal squamous cell carcinoma (ESCC) is a major disease threatening human life and health, which is prevalent worldwide.1 Owing to insufficient screening for early esophageal cancer, most patients are diagnosed in advanced stages. The overall 5-year survival rate of esophageal cancer is less than 20% because of the local invasion, metastasis, recurrence, and limitation of current treatments of advanced esophageal neoplasms.2,3 Patients who are in the same pathological stage and receive similar surgery and other treatments often have a completely different prognosis.4,5 The commonly used TNM staging system often fails to accurately predict the patient’s prognosis.6 Studies showed that the expressions of some genes in tumor tissues were closely associated with tumor differentiation, angiogenesis, metastasis, and prognosis. The interaction of multiple genes can lead to the oncogenesis and development of esophageal cancer. Novel biomarkers targeting these genes are valuable in the diagnosis and prognostic prediction of esophageal cancer. Investigation into their mechanisms will be helpful in preventing and detecting the disease earlier, with the potential to develop new therapeutic targets.7
ASK1-interacting protein-1 (AIP1) is a newly discovered signal scaffold protein, which is a member of the Ras-GTPase activating protein (Ras-GAP) family.8 The expression of AIP1 in tumor cells is inhibited by histone-lysine N-methyltransferase EZH2 protein,9,10 which is highly expressed in various tumor tissues and functions to promote the proliferation and spread of tumor cells.11 AIP1 expression is decreased in human prostate cancer, where it is known to be a suppressor gene,12 and in breast cancer.9,13 The activation or inhibition of the AIP1 gene can affect a variety of tumor-related signaling pathways. For example, inhibiting Ras, PI3K/Akt, GSK-3/β-catenin, and NF-κB signal pathways by modulating AIP1 activity can suppress tumor cell proliferation, epithelial-mesenchymal transition (EMT), and metastasis.14–16 Vascular endothelial growth factor receptor 2 (VEGFR2) is a receptor of VEGF, an important factor that regulates angiogenesis. The binding of VEGF to VEGFR2 not only directly promotes angiogenesis but is also involved in the tumor infiltration and spread.17,18 Recent studies suggested that AIP1 inhibited tumor angiogenesis and the formation of pre-metastasis microenvironment by inactivating VEGFR2-dependent signaling in the tumor microenvironment through directly binding to the residual tyrosine kinases activated by VEGFR2. Loss of AIP1 expression in vascular vessels directly stimulates tumor neovascularization, and also enhances the vascular endothelial cell VEGFR2 signaling system, thus facilitating tumor angiogenesis and metastasis.19
However, as far as we know, the correlation between AIP1 and angiogenesis in ESCC has not been elucidated. In addition, the potential value of AIP1 as an independent prognostic factor in ESCC has not been systematically studied.
In this study, we examined the expression of AIP1 protein in 117 surgically resected specimens from patients with ESCC by immunohistochemical methods. We analyzed the correlation between AIP1 protein expression and different clinicopathological features and prognoses, as well as the correlation between AIP1 protein and VEGFR2 and microvessel density (MVD). We further explored the potential mechanisms of AIP1 in inhibiting angiogenesis, and evaluated the value of AIP1 as an independent prognostic factor to predict long-term survival of ESCC patients.
Materials and methods
Patients
The study subjects included 117 patients, who were pathologically diagnosed with ESCC by two senior pathologists and underwent subtotal esophagectomy and regional lymph node dissection surgery at the Qianfoshan Hospital, Shandong Province, China, between January 2011 and December 2012. Tissue samples from patients with ESCC and adjacent histologically normal tissue samples were acquired from these patients. Among the 117 ESCC patients, 46 were females and 71 males with age ranging from 54 to 76 years. The pathological and clinical data, as well as follow-up information of all patients, were complete. Exclusion criteria were defined as following: 1) distant metastasis before surgery by positron emission tomography–computed tomography (PET-CT) scan or with chest and abdominal enhanced CT scans; 2) reception of preoperative chemotherapy or radiotherapy; and 3) patients who underwent palliative resection. TNM staging was conducted based on the International Union Against Cancer (UICC) standards.20 This study was approved by the ethics committee of Qianfoshan Hospital, and written informed consent was obtained from all the patients.
Cancer staging categories for cancer of the esophagus
The pathologic TNM (pTNM) staging was analyzed according to the eighth edition staging primer of esophagus cancer as following: The lymph node metastasis (N) category: NX, regional lymph nodes cannot be assessed; N0, no regional lymph node metastasis; N1, metastasis in 1–2 regional lymph nodes; N2, metastasis in 3–6 regional lymph nodes; and N3, metastasis in ≥7 regional lymph nodes. The squamous cell carcinoma differentiation (G) category: GX, differentiation cannot be assessed; G1, well-differentiated, with prominent keratinization with pearl formation and a minor component of nonkeratinizing basal-like cells, tumor cells arranged in sheets, and mitotic counts low; G2, moderately differentiated, with variable histologic features ranging from parakeratotic to poorly keratinizing lesions and pearl formation generally absent; and G3, poorly differentiated, consisting predominantly of basal-like cells forming large and small nests with frequent central necrosis and with the nests consisting of sheets or pavement-like arrangements of tumor cells that are occasionally punctuated by small numbers of parakeratotic or keratinizing cells.
Immunohistochemical staining for AIP1, VEGFR2, and CD34
ESCC tissues were fixed overnight in 10% neutral buffered formalin and embedded in paraffin. Tissue sections were cut at 3 µm and stained with H&E. Immunostaining was performed on paraffin sections using a microwave-based antigen-retrieval technique. The antibodies used in this study were those against AIP1 (ab87811; Santa Cruz Biotechnology, Santa Cruz, CA, USA), CD34 (sc-19621; Santa Cruz Biotechnology), and VEGFR2 (Cell Signaling Technology, Beverly, MA, USA). Sections were treated with the Envision + DAB kit (Dako, Glostrup, Denmark), according to the manufacturer’s instructions. A human kidney cancer specimen known to express AIP1 protein was used as the positive control. For negative controls, sections were incubated with PBS instead of the primary antibodies.
Analysis of AIP1 protein expression and MVD
A semi-quantitative method was used to measure the staining intensity and the proportion of stained cells for AIP1 and VEGFR2. Ten visual fields were observed at 400× magnification. Each field counted 100 tumor cells. The positive cell scoring was as follows: the proportion of positive cells <5% was 0 point; 5%–25% was 1 point; 25%–50% was 2 points; 50%–75% was 3 points; and >75% was 4 points. The staining intensity scoring was as follows: non-staining was 0 point; light yellow was 1 point; yellow brown was 2 points; and dark brown was 3 points. The final score was the sum of the positive cell score and the staining intensity score, and was further graded as follows: (−), 0–1; (+), 2–3; (++), 4–6; and (+++), 8–12. A final score ≥4 points defined a high expression of AIP1 and VEGFR2.
Intratumoral microvessels were measured by counting CD34-positive cells. Although CD34 is not a specific marker in order to evaluate angiogenesis, positive CD34 staining referred to clear brown-yellow or dark brown particles in the cytoplasm and/or cell membrane, which might include stained microvessels, individual endothelial cells, or the cell plexus. Isolated or multiple closely aligned endothelial cell masses, if they had a clear boundary with adjacent tumor cells and surrounding connective tissues regardless of vascular lumen, were counted as one microvessel.21 With reference to the Chalkley counting method,22 two pathologists blinded to patient information first scanned whole slices at low magnification to find three “hot spots,” which were the areas where positively stained vessels were highly concentrated; then the number of microvessels was counted at high-power field (HPF). Vessels with lumen diameters greater than erythrocytes, or when smooth muscles were observed in the wall of the vessels, were excluded. The average value of microvessel number of five visual fields was the MVD of the specimen. Re-counting was required if the difference of the counts from the two pathologists was more than 10%.23
Patient follow-up
The patients were followed every 3 months during 2 years after discharge, and then every 6 months until death or termination of the study. The follow-up items included physical examination, gastrointestinal barium meal test, blood analysis, CT scans, ultrasonography, and gastrointestinal endoscopy. Recurrence was determined according to clinical, radiological, or histological examinations. The location and time of tumor recurrence were recorded. Follow-up was completed in December 2016, with a median follow-up of 31 months (5–72 months).
Statistical analysis
SPSS, version 13.0 software (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Chi-square tests were adopted for analysis of correlations between AIP1 protein, MVD, VEGFR2, and various clinicopathological factors. For categorical analysis, the median value of MVD was used as a cut-off point to dichotomize the continuous variables. The Mann–Whitney nonparametric test was used to compare the density of immunostaining results between ESCC groups. Spearman’s rank correlation method was used to evaluate the correlations between the density of AIP1, MVD, and VEGFR2 expression. Survival curves were estimated by the Kaplan–Meier method. Multivariate Cox regression analysis was used to identify significant independent prognostic factors. P-values <0.05 were considered statistically significant.
Results
Expression of AIP1 protein and the correlation of AIP1 density with ESCC clinicopathological factors
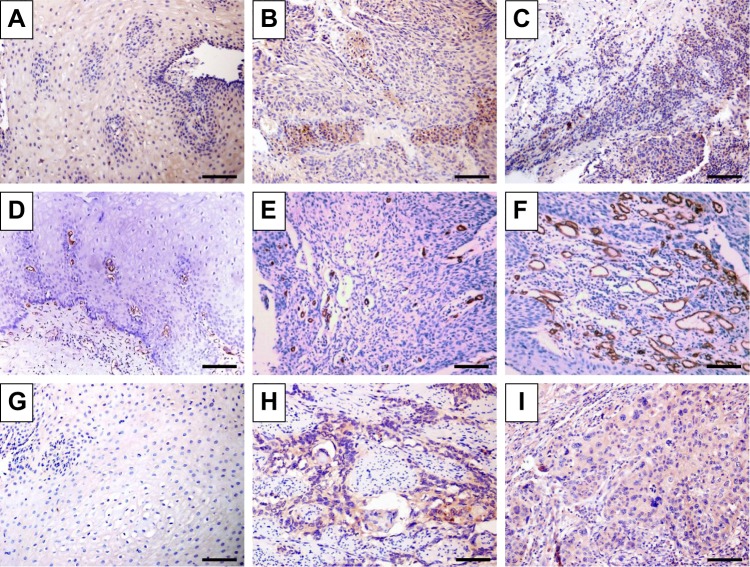
Immunohistochemical staining showed that AIP1 protein was expressed in the tumor cell membrane and cytoplasm (Figure 1), and the expression of AIP1 protein in ESCC tissues was significantly lower than that in adjacent normal tissues (Figure 1A–C, P<0.01). The staining intensity of AIP1 was not the same in different patients’ ESCC tissues. We detected 63 cases with a low expression of AIP1 in all 117 patients according to the standard defined in the methods. In order to understand the role of AIP1 in esophageal cancer, we investigated the correlation of AIP1 with clinicopathological features such as age, sex, tumor location, histological grades, invasion depth, lymph node metastasis stage (TNM), and clinical stage. We found that a low expression of AIP1 in cancer tissues was significantly correlated with some malignant phenotypes including lymph node metastasis and late clinical stages (chi-squared test, P<0.05), but did not show a significant correlation with sex, age, tumor size, invasion depth, and histological grade (Table 1).
Figure 1.
Immunohistochemical staining of normal and esophageal cancer specimens in which antibodies to AIP1 (A–C), CD34 (D–F), and VEGFR2 (G–I) were used.
Notes: Representative immunostaining images of (A, D, G) normal adjacent tissues and (B, C, E, F, H, I) ESCC tumor tissues. (B and C) Distribution of AIP1 in ESCC tumor tissues revealed diffuse staining of membranes and cytoplasm of ESCC tumor tissues. (C) Low density of AIP1 located in ESCC tissues. (D–F) Immunohistochemical staining of CD34, which was used to mark endothelial cells and to evaluate MVD in different tissues. (E) Low MVD in ESCC tissues. (F) High MVD in ESCC tissues. (H and I) Different distribution of VEGFR2 in ESCC tumor tissues. Scale bar=100 µm.
Abbreviations: AIP1, ASK1-interacting protein-1; ESCC, esophageal squamous cell carcinoma; MVD, microvessel density; VEGFR2, vascular endothelial growth factor receptor 2.
Table 1.
The correlation of clinicopathological variables with AIP1 in primary tumors
| Variables | Cases (N) | AIP1 protein immunoreactivity | χ2 | P-value | |
|---|---|---|---|---|---|
| Low | High | ||||
| Age, years | 2.716 | 0.099 | |||
| ≤ median (58) | 68 | 41 | 27 | ||
| > median (58) | 49 | 22 | 27 | ||
| Sex | 1.105 | 0.293 | |||
| Male | 71 | 41 | 30 | ||
| Female | 46 | 22 | 24 | ||
| Tumor length (cm) | 1.022 | 0.600 | |||
| <3 | 38 | 20 | 18 | ||
| 3–5 | 57 | 33 | 24 | ||
| >5 | 22 | 10 | 12 | ||
| Differentiation | 0.606 | 0.738 | |||
| Well | 31 | 18 | 13 | ||
| Moderate | 67 | 34 | 33 | ||
| Poor | 19 | 11 | 8 | ||
| Depth of invasion | 2.511 | 0.113 | |||
| T1+T2 | 76 | 45 | 31 | ||
| T3+T4 | 41 | 18 | 23 | ||
| Lymph node metastasis | 3.976 | 0.046* | |||
| pN− | 45 | 19 | 26 | ||
| pN+ | 72 | 44 | 28 | ||
| Stage | 6.825 | 0.009* | |||
| I+II | 65 | 28 | 37 | ||
| III | 52 | 35 | 17 | ||
Note:
P<0.05.
Abbreviations: AIP1, ASK1-interacting protein-1; pN−, no lymph node metastasis; pN+, lymph node metastasis.
Distributions of microvessels in ESCCs and the correlation of MVD with ESCC clinicopathological factors
In this study, CD34 immunohistochemical staining was used to measure MVD in tumor tissues. In positive endothelial cells, CD34 was diffusely stained in the cytoplasm and membrane (Figure 1D–F). The average MVD in ESCC was 28 per HPF with a range of 12–57. We divided patients into a high MVD group (≥28) and a low MVD group (<28) with mean MVD as the cutoff value. We investigated the correlation between MVD and clinicopathological factors and found that a high MVD was significantly associated with invasion depth, lymph node metastasis, and pathological stage (Table 2).
Table 2.
The correlation of clinicopathological variables with VEGFR2 and intratumoral MVD in primary tumors
| Variables | Cases (N) | VEGFR2 immunoreactivity | Intratumoral MVD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Low | High | χ2 | P-value | Low ≤28 | High >28 | χ2 | P-value | ||
| Age, years | 1.770 | 0.183 | 0.068 | 0.794 | |||||
| ≤58 | 68 | 29 | 39 | 28 | 40 | ||||
| >58 | 49 | 27 | 22 | 19 | 30 | ||||
| Sex | 0.139 | 0.710 | 0.948 | 0.330 | |||||
| Male | 71 | 33 | 38 | 26 | 45 | ||||
| Female | 46 | 23 | 23 | 21 | 25 | ||||
| Tumor length (cm) | 0.513 | 0.774 | 1.215 | 0.545 | |||||
| <3 | 38 | 20 | 18 | 18 | 20 | ||||
| 3–5 | 57 | 26 | 31 | 21 | 36 | ||||
| >5 | 22 | 10 | 12 | 8 | 14 | ||||
| Differentiation | 1.437 | 0.487 | 1.428 | 0.490 | |||||
| Well | 31 | 12 | 19 | 15 | 16 | ||||
| Moderate | 67 | 34 | 33 | 24 | 43 | ||||
| Poor | 19 | 10 | 9 | 8 | 11 | ||||
| Depth of invasion | 3.217 | 0.073 | 6.540 | 0.011* | |||||
| T1+T2 | 76 | 41 | 35 | 37 | 39 | ||||
| T3+T4 | 41 | 15 | 26 | 10 | 31 | ||||
| Lymph node metastasis | 6.042 | 0.014* | 11.963 | 0.001* | |||||
| pN− | 45 | 28 | 17 | 27 | 18 | ||||
| pN+ | 72 | 28 | 44 | 20 | 52 | ||||
| Stage | 4.811 | 0.028* | 6.835 | 0.009* | |||||
| I+II | 65 | 37 | 28 | 33 | 32 | ||||
| III | 52 | 19 | 33 | 14 | 38 | ||||
Note:
P<0.05.
Abbreviations: MVD, microvessel density; pN−, no lymph node metastasis; pN+, lymph node metastasis; VEGFR2, vascular endothelial growth factor receptor 2.
Expression of VEGFR2 and its correlation with ESCC clinicopathological factors
Immunohistochemical staining showed that VEGFR2 was mainly located in the cytoplasm and membrane of tumor cells as well as vascular endothelial cells in the tumor matrix. VEGFR2 was not expressed in normal esophageal mucosa tissue (Figure 1G–I). VEGFR2 expression was associated with lymph node metastasis (P<0.05) and later clinical stage (P<0.05) but not with other pathological factors (Table 2).
Correlations between the density of AIP1, MVD, and VEGFR2 expression in ESCC
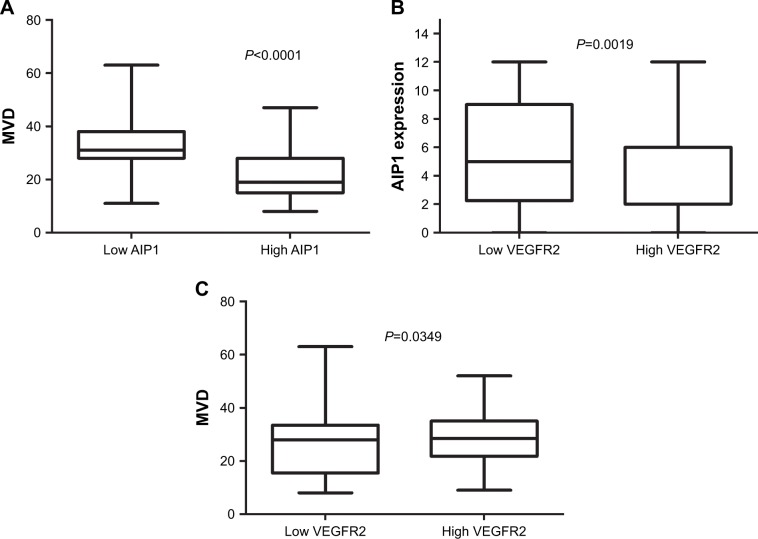
To understand whether AIP1 inhibited the invasion and metastasis of ESCC by suppressing angiogenesis, we investigated the relationship between AIP1 expression and MVD in ESCC. We detected MVD by CD34 immunohistochemical staining. In 63 cases, in which AIP1 was of low expression, 42 cases had a high MVD (66.7%). Spearman’s rank correlation showed AIP1 expression was significantly negatively correlated with MVD (P<0.0001; Figure 2A). The proliferation and migration of tumor vascular endothelial cells were regulated by VEGFR2; we thereby analyzed the correlation between AIP1 and VEGFR2. Among 117 ESCC cases, 61 cases showed high expression of VEGFR2, in which 39 cases showed low expression of AIP1 (63.9%). AIP1 expression was significantly negatively correlated with VEGFR2 expression (P=0.0019; Figure 2B). We also found that VEGFR2 expression was positively correlated with MVD (P=0.0349; Figure 2C).
Figure 2.
Cross-correlation analyses revealed strong relationships among the expressions of AIP1 and VEGFR2 and MVD in ESCC.
Notes: (A) Significant correlation was observed between the low density of AIP1 and high MVD in ESCC tumor tissues (P<0.0001). (B) Significant correlation was observed between the high VEGFR2 in ESCC tumor tissues and low density of AIP1 (P<0.01). (C) Significant correlation was observed between the density of the expression of VEGFR2 and MVD in ESCC tumor tissues (P=0.0349).
Abbreviations: AIP1, ASK1-interacting protein-1; ESCC, esophageal squamous cell carcinoma; MVD, microvessel density; VEGFR2, vascular endothelial growth factor receptor 2.
Association of AIP1 with ESCC survival time
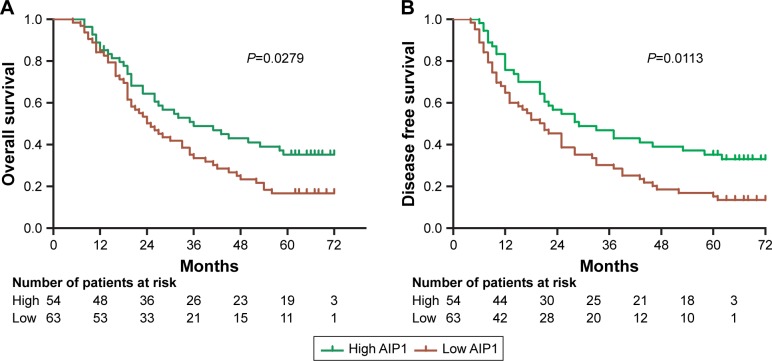
We next investigated the effect of AIP1 on patient prognosis. According to the staining intensity of AIP1 in tumor tissues, we divided the patients into the AIP high-expression group and low-expression group. The Kaplan–Meier survival curve was used to demonstrate the effect of AIP1 expression on overall survival (OS) and disease-free survival (DFS) (Figure 3). During the follow-up period, four cases were excluded. Up to the end of the 60-month follow-up, 85 patients died and four patients survived with tumor. In 63 patients with low expression of AIP1, 51 patients died; while in 54 patients with high expression of AIP1, 34 patients died. The overall 5-year survival rate was 27.78%. The univariate Cox proportional hazard model suggested that lymph node metastasis (HR=3.433), late clinical stage (HR =2.632), and low expression of AIP1 (HR =1.614) were the risk factors for poor prognosis of ESCC patients (P<0.001; Table 3). In addition, the multivariate Cox model showed that low expression of AIP1 was an independent prognostic factor for ESCC (HR =5.464, P<0.05; Table 3).
Figure 3.
Kaplan–Meier survival curves of patients stratified according to AIP1 expression (A and B). Patients with low density of AIP1 in tumor tissues had a poor overall survival (P<0.05).
Abbreviation: AIP1, ASK1-interacting protein-1.
Table 3.
Univariate and multivariate analyses of prognostic variables
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age (≤58 vs >58) | 0.263 | |||||
| Sex (male vs female) | 0.809 | |||||
| Tumor length | ||||||
| (<3 cm and 3–5 cm vs >5 cm) | 1.346 | 0.973–1.863 | 0.003 | 1.170 | 0.773–1.770 | 0.458 |
| Differentiation | ||||||
| (Well and moderate vs poor) | 1.213 | 0.833–1.765 | 0.001 | 1.285 | 0.845–1.955 | 0.324 |
| pT factor (T1+2 vs T3+4) | 0.125 | |||||
| pN factor (N− vs N+) | 3.433 | 2.106–5.595 | 0.000* | 2.820 | 1.560–5.096 | 0.001* |
| Stage (I+II vs III+IV) | 2.632 | 1.704–4.065 | 0.000* | 1.373 | 0.731–2.575 | 0.324 |
| AIP1 expression (high vs low) | 1.614 | 1.044–2.497 | 0.028* | 1.766 | 1.136–2.746 | 0.011* |
Note:
P<0.05.
Abbreviations: pN−, no lymph node metastasis; pN+, lymph node metastasis.
Discussion
Esophageal cancer is a fatal disease in African and Asian countries and responsible for approximately 406,800 deaths each year.24 Despite the development of surgical techniques, new drugs, and radiotherapy, the survival rate of esophageal cancer is still not optimal. Clinically, although many patients belong to the T1–3N0M0 stage, their long-term survival is still seriously affected by postoperative recurrence and metastasis.4,25 Even referring to the latest staging standards of the UICC (version 8.0),20 the OS and DFS of individual patients cannot yet be accurately predicted. TNM staging is unable to predict recurrence and metastasis in many cases; therefore, many researchers are committed to finding new prognostic biomarkers as supplements for TNM staging.26–28 Unfortunately, currently there are no confirmed biomarkers to be used in the clinic. Our study explored the possibility of AIP1 as a prognostic indicator for ESCC. We found AIP1 protein was downregulated in ESCC tissues and was closely associated with invasion depth, lymph node metastasis, and late tumor stage, suggesting AIP1 may act as a tumor suppressor protein.
The abnormal methylation in the AIP1 promoter can lead to the downregulation of the gene and the loss of its tumor-suppressing function. Studies have found AIP1 to play a key role in the development of kidney cancer, breast cancer, gastric cancer, colorectal cancer, and lung cancer.29–31 AIP1 is known to mediate the apoptosis of endothelial cells induced by cytokines such as tumor necrosis factor-α.16,32,33 In the inflammatory tumor microenvironment, the death or proliferation of endothelial cells has a critical impact on tumor angiogenesis and metastasis.34–36 Moreover, studies have found that AIP1 limits tumor metastasis by inhibiting the EMT, tumor angiogenesis, and the formation of the pre-metastatic niche.17 However, few studies have explored the role of AIP1 in ESCC and its value as a predictive biomarker. Xu et al demonstrated that AIP1 is under the control of miR-889 in ESCC, which is significantly associated with clinicopathological features of ESCC patients. miR-889 negatively regulates AIP1 and both miR-889 and DAB2IP may serve as promising biomarkers and therapeutic targets in patients with ESCC.37 Such conclusion is inconsistent with ours. The present study, for the first time, investigated the expression of AIP1 in ESCC and revealed a correlation between AIP1 expression and tumor microvessel formation, as well as patient prognosis.
Most researchers believe that angiogenesis is a prerequisite for solid tumor growth and metastasis.38,39 The speed of tumor growth and patient prognosis are associated with the microvascular growth in tumors. Tumor blood vessels provide a material basis for the rapid division of tumor cells; at the same time, the nascent tumor vessels have abnormalities in their function and structure, increasing the migration of tumor cells and making tumor cells susceptible to invasion and distant metastasis.17 In addition, the enhancement of angiogenesis also increases the chance of tumor cells invading the accompanying lymphatic vessels, thus leading to lymph node metastasis. MVD is a recognized factor that affects tumor progression and prognosis of cancer patients.40,41 In this study, we used CD34 immunohistochemical staining to label vascular endothelial cells to measure MVD in ESCC tissue. We found that MVD in tumors was significantly higher than that in adjacent tissues. MVD in tumors was associated with more malignant phenotypes, including lymph node metastasis and late clinical stages. Analyses of the correlation between AIP1 expression and MVD found that the increase of MVD was closely related to the decrease of AIP1 expression. Some studies have shown that the absence of AIP1 in vascular endothelial cells is sufficient to enhance tumor growth and metastasis of subcutaneous melanoma and in situ in breast cancer models. Active angiogenesis and metastasis have also been found in AIP1 knockout mice.17 Therefore, a decrease or deletion of AIP1 expression may be an important factor that promotes angiogenesis in ESCC, and the subsequent tumor growth and metastasis. Recovery of AIP1 expression by the non-viral delivery system may be a highly potential way of therapeutic ESCC treatments.42 Combining inorganic nanoparticles (such as calcium phosphate, gold nanoparticles, silica nanoparticles, iron oxide, and hydroxyapatite nanoparticles) with AIP1 would efficiently deliver AIP1 package to target tissues and inhibit tumor growth and metastasis. Therefore, AIP1 could become a novel target for anti-angiogenic treatment, although its precise mechanism needs further study.
Studies have shown that low expression of AIP1 in a variety of tumor tissues is associated with worse OS; the abnormal decrease of AIP1 predicted a poorer prognosis.43,44 In our study, univariate regression analyses demonstrated that low expression of AIP1 was associated with a lower 5-year survival rate. The Cox multivariate model suggested that AIP1 expression and pathological stage were the independent prognostic factors for ESCC.
In conclusion, our study supports the role of AIP1 as a biomarker to indicate the progression and metastasis of ESCC. The deficiency of its expression was associated with tumor angiogenesis and poor prognosis. AIP1 seems to be an independent prognostic factor and a promising therapeutic target for ESCC.
Acknowledgments
This work was supported by the National Science Foundation of China (81401933) and Shanghai Science and Technology Commitment (13ZR1461300).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wang M, Smith JS, Wei WQ. Tissue protein biomarker candidates to predict progression of esophageal squamous cell carcinoma and precancerous lesions. Ann N Y Acad Sci. 2018 doi: 10.1111/nyas.13863. [DOI] [PubMed] [Google Scholar]
- 2.Ren Y, Cao B, Law S, et al. Hepatocyte growth factor promotes cancer cell migration and angiogenic factors expression: a prognostic marker of human esophageal squamous cell carcinomas. Clin Cancer Res. 2005;11(17):6190–6197. doi: 10.1158/1078-0432.CCR-04-2553. [DOI] [PubMed] [Google Scholar]
- 3.Mariette C, Balon JM, Piessen G, Fabre S, van Seuningen I, Triboulet JP. Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer. 2003;97(7):1616–1623. doi: 10.1002/cncr.11228. [DOI] [PubMed] [Google Scholar]
- 4.Law S, Kwong DL, Kwok KF, et al. Improvement in treatment results and long-term survival of patients with esophageal cancer: impact of chemoradiation and change in treatment strategy. Ann Surg. 2003;238(3):339–347. doi: 10.1097/01.sla.0000086545.45918.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okawa T, Naomoto Y, Nobuhisa T, et al. Heparanase is involved in angiogenesis in esophageal cancer through induction of cyclooxygenase-2. Clin Cancer Res. 2005;11(22):7995–8005. doi: 10.1158/1078-0432.CCR-05-1103. [DOI] [PubMed] [Google Scholar]
- 6.Zheng Y, Fu S, He T, et al. Predicting prognosis in resected esophageal squamous cell carcinoma using a clinical nomogram and recursive partitioning analysis. Eur J Surg Oncol. 2018;44(8):1199–1204. doi: 10.1016/j.ejso.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Salem ME, Puccini A, Xiu J, et al. Comparative Molecular Analyses of Esophageal Squamous Cell Carcinoma, Esophageal Adenocarcinoma, and Gastric Adenocarcinoma. Oncologist. 2018 doi: 10.1634/theoncologist.2018-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H, Pong RC, Wang Z, Hsieh JT. Differential regulation of the human gene DAB2IP in normal and malignant prostatic epithelia: cloning and characterization. Genomics. 2002;79(4):573–581. doi: 10.1006/geno.2002.6739. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Toyooka S, Gazdar AF, Hsieh JT. Epigenetic regulation of a novel tumor suppressor gene (hDAB2IP) in prostate cancer cell lines. J Biol Chem. 2003;278(5):3121–3130. doi: 10.1074/jbc.M208230200. [DOI] [PubMed] [Google Scholar]
- 10.Min J, Zaslavsky A, Fedele G, et al. An oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-kappaB. Nat Med. 2010;16(3):286–294. doi: 10.1038/nm.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie D, Gore C, Zhou J, et al. DAB2IP coordinates both PI3K-Akt and ASK1 pathways for cell survival and apoptosis. Proc Natl Acad Sci U S A. 2009;106(47):19878–19883. doi: 10.1073/pnas.0908458106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duggan D, Zheng SL, Knowlton M, et al. Two genome-wide association studies of aggressive prostate cancer implicate putative prostate tumor suppressor gene DAB2IP. J Natl Cancer Inst. 2007;99(24):1836–1844. doi: 10.1093/jnci/djm250. [DOI] [PubMed] [Google Scholar]
- 13.Dote H, Toyooka S, Tsukuda K, et al. Aberrant promoter methylation in human DAB2 interactive protein (hDAB2IP) gene in breast cancer. Clin Cancer Res. 2004;10(6):2082–2089. doi: 10.1158/1078-0432.ccr-03-0236. [DOI] [PubMed] [Google Scholar]
- 14.Min J, Zaslavsky A, Fedele G, et al. An oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-kappaB. Nat Med. 2010;16(3):286–294. doi: 10.1038/nm.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Zhang R, Luo Y, D’Alessio A, Pober JS, Min W. AIP1/DAB2IP, a novel member of the Ras-GAP family, transduces TRAF2-induced ASK1-JNK activation. J Biol Chem. 2004;279(43):44955–44965. doi: 10.1074/jbc.M407617200. [DOI] [PubMed] [Google Scholar]
- 16.Xie D, Gore C, Liu J, et al. Role of DAB2IP in modulating epithelial-to-mesenchymal transition and prostate cancer metastasis. Proc Natl Acad Sci U S A. 2010;107(6):2485–2490. doi: 10.1073/pnas.0908133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji W, Li Y, He Y, et al. AIP1 expression in tumor niche suppresses tumor progression and metastasis. Cancer Res. 2015;75(17):3492–3504. doi: 10.1158/0008-5472.CAN-15-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vallböhmer D, Lenz HJ. Predictive and prognostic molecular markers in outcome of esophageal cancer. Dis Esophagus. 2006;19(6):425–432. doi: 10.1111/j.1442-2050.2006.00622.x. [DOI] [PubMed] [Google Scholar]
- 19.Shimada H, Matsushita K, Tagawa M. Recent advances in esophageal cancer gene therapy. Ann Thorac Cardiovasc Surg. 2008;14(1):3–8. [PubMed] [Google Scholar]
- 20.Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. J Thorac Oncol. 2017;12(1):36–42. doi: 10.1016/j.jtho.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan A, Yu CJ, Shun CT, et al. Total cyclooxygenase-2 mRNA levels correlate with vascular endothelial growth factor mRNA levels, tumor angiogenesis and prognosis in non-small cell lung cancer patients. Int J Cancer. 2005;115(4):545–555. doi: 10.1002/ijc.20898. [DOI] [PubMed] [Google Scholar]
- 22.Vermeulen PB, Gasparini G, Fox SB, et al. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer. 2002;38(12):1564–1579. doi: 10.1016/s0959-8049(02)00094-1. [DOI] [PubMed] [Google Scholar]
- 23.Li SH, Tian H, Yue WM, et al. Metastasis-associated protein 1 nuclear expression is closely associated with tumor progression and angiogenesis in patients with esophageal squamous cell cancer. World J Surg. 2012;36(3):623–631. doi: 10.1007/s00268-011-1421-z. [DOI] [PubMed] [Google Scholar]
- 24.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 25.Okawa T, Naomoto Y, Nobuhisa T, et al. Heparanase is involved in angiogenesis in esophageal cancer through induction of cyclooxygenase-2. Clin Cancer Res. 2005;11(22):7995–8005. doi: 10.1158/1078-0432.CCR-05-1103. [DOI] [PubMed] [Google Scholar]
- 26.Cui XB, Zhang SM, Xu YX, et al. PFN2, a novel marker of unfavorable prognosis, is a potential therapeutic target involved in esophageal squamous cell carcinoma. J Transl Med. 2016;14(1):137. doi: 10.1186/s12967-016-0884-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He Z, Yi J, Liu X, et al. MiR-143-3p functions as a tumor suppressor by regulating cell proliferation, invasion and epithelial-mesenchymal transition by targeting QKI-5 in esophageal squamous cell carcinoma. Mol Cancer. 2016;15(1):51. doi: 10.1186/s12943-016-0533-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Zhou X, Wen W, Zhu J, et al. A six-microRNA signature in plasma was identified as a potential biomarker in diagnosis of esophageal squamous cell carcinoma. Oncotarget. 2017;8(21):34468–34480. doi: 10.18632/oncotarget.16519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dote H, Toyooka S, Tsukuda K, et al. Aberrant promoter methylation in human DAB2 interactive protein (hDAB2IP) gene in breast cancer. Clin Cancer Res. 2004;10(6):2082–2089. doi: 10.1158/1078-0432.ccr-03-0236. [DOI] [PubMed] [Google Scholar]
- 30.Dote H, Toyooka S, Tsukuda K, et al. Aberrant promoter methylation in human DAB2 interactive protein (hDAB2IP) gene in gastrointestinal tumour. Br J Cancer. 2005;92(6):1117–1125. doi: 10.1038/sj.bjc.6602458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yano M, Toyooka S, Tsukuda K, et al. Aberrant promoter methylation of human DAB2 interactive protein (hDAB2IP) gene in lung cancers. Int J Cancer. 2005;113(1):59–66. doi: 10.1002/ijc.20531. [DOI] [PubMed] [Google Scholar]
- 32.Zhang R, He X, Liu W, Lu M, Hsieh JT, Min W. AIP1 mediates TNF-alpha-induced ASK1 activation by facilitating dissociation of ASK1 from its inhibitor 14-3-3. J Clin Invest. 2003;111(12):1933–1943. doi: 10.1172/JCI17790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, He Y, Dai S, et al. AIP1 functions as an endogenous inhibitor of VEGFR2-mediated signaling and inflammatory angiogenesis in mice. J Clin Invest. 2008;118(12):3904–3916. doi: 10.1172/JCI36168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peinado H, Rafii S, Lyden D. Inflammation joins the “niche”. Cancer Cell. 2008;14(5):347–349. doi: 10.1016/j.ccr.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franses JW, Drosu NC, Gibson WJ, Chitalia VC, Edelman ER. Dysfunctional endothelial cells directly stimulate cancer inflammation and metastasis. Int J Cancer. 2013;133(6):1334–1344. doi: 10.1002/ijc.28146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Y, He J, Wang Y, et al. miR-889 promotes proliferation of esophageal squamous cell carcinomas through DAB2IP. FEBS Lett. 2015;589(10):1127–1135. doi: 10.1016/j.febslet.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 38.Li B, Xu WW, Han L, et al. MicroRNA-377 suppresses initiation and progression of esophageal cancer by inhibiting CD133 and VEGF. Oncogene. 2017;36(28):3986–4000. doi: 10.1038/onc.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu J, Wu G, Li Q, et al. Overexpression of Suprabasin is Associated with Proliferation and Tumorigenicity of Esophageal Squamous Cell Carcinoma. Sci Rep. 2016;6:21549. doi: 10.1038/srep21549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu L, Li X, Ye L, et al. Vascular endothelial growth inhibitor 174 is a negative regulator of aggressiveness and microvascular density in human clear cell renal cell carcinoma. Anticancer Res. 2014;34(2):715–722. [PubMed] [Google Scholar]
- 41.Ammendola M, Sacco R, Marech I, et al. Microvascular density and endothelial area correlate with Ki-67 proliferative index in surgically-treated pancreatic ductal adenocarcinoma patients. Oncol Lett. 2015;10(2):967–971. doi: 10.3892/ol.2015.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang K, Huang Q, Qiu F, Sui M. Non-viral Delivery Systems for the Application in p53 Cancer Gene Therapy. Curr Med Chem. 2015;22(35):4118–4136. doi: 10.2174/0929867322666151001121601. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Li N, Li X, et al. Low expression of DAB2IP contributes to malignant development and poor prognosis in hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27(6):1117–1125. doi: 10.1111/j.1440-1746.2011.07049.x. [DOI] [PubMed] [Google Scholar]
- 44.Smits M, van Rijn S, Hulleman E, et al. EZH2-regulated DAB2IP is a medulloblastoma tumor suppressor and a positive marker for survival. Clin Cancer Res. 2012;18(15):4048–4058. doi: 10.1158/1078-0432.CCR-12-0399. [DOI] [PubMed] [Google Scholar]