Abstract
Inflammatory myofibroblastic tumor of the airway is a very uncommon benign primary neoplasm in pediatric age group with increased local recurrence rate and potential metastatic spread. We describe a case of a 6-year boy who was brought to the pediatric emergency with severe respiratory distress, dry cough, and stridor. Contrast-enhanced computed tomography and magnetic resonance imaging (MRI) of the neck showed a polypoidal mass lesion in the right anterolateral trachea causing significant airway narrowing. Bronchoscopic findings correlated with the imaging. The lesion was confirmed at surgery and was completely removed by surgical excision. Histopathology revealed an inflammatory myofibroblastic tumor. MRI findings of this entity in a child have not been reported before.
KEY WORDS: Inflammatory myofibroblastic tumor, inflammatory pseudotumor, tracheal tumor
INTRODUCTION
Inflammatory myofibroblastic tumor (IMT), also known as inflammatory pseudotumor, is a prototype of a group of rare pseudoneoplastic lesions commonly seen in the lung. However, alike entities have been reported in various extrapulmonary locations as well.[1,2] The natural course of this pathological entity is not very well recognized. Although IMT is a commonly encountered lesion in pediatric age group, not many reports of this lesion in trachea have been published so far, where it is seen in approximately 0.04%–0.07% cases of all respiratory tract neoplasms.[3] Here, we report computed tomography (CT) scan and magnetic resonance imaging (MRI) findings in a 6-year boy with inflammatory myofibroblastic tumor of the trachea, treated with surgical resection of IMT. With increasing role of MRI as a radiation-free modality in evaluation of pediatric chest and airways, it is desirable for the radiologists to be aware of the MRI findings of this rare entity.
CASE REPORT
A 6-year-male child was brought to the pediatric emergency with complaints of dry cough, wheezing, stridor, and severe respiratory distress. He had similar such episodes in the past as well with complaints of dry cough and dyspnea for the last 6 months and underwent treatment for presumed bronchial asthma. Clinical diagnosis of endotracheal tuberculosis or extratracheal airway compression was made. Chest X-ray at admission was unremarkable.
Further diagnostic work-up included bronchoscopy and CT scan. Subsequently, MRI was also performed. The diagnostic rigid bronchoscopy showed a smooth-surfaced, vascular, lobulated, polypoidal mass lesion visible through the vocal cords in proximal trachea, causing significant airway narrowing. Bronchoscopy probe could not be negotiated beyond the mass lesion. A diagnosis of polyp/hemangioma was made on bronchoscopy. These findings were reasserted by Contrast enhanced CT (CECT) study of chest [Figure 1]. CT revealed a well-defined, homogeneous, mildly enhancing, lobulated, polypoidal, intraluminal mass lesion, ~10 mm below the vocal cords on the right lateral wall of trachea, causing significant narrowing of the proximal trachea. There was seen mild adjacent tracheal wall thickening. No intralesional calcification or necrosis was noted. No significant cervical lymphadenopathy was seen.
Figure 1.
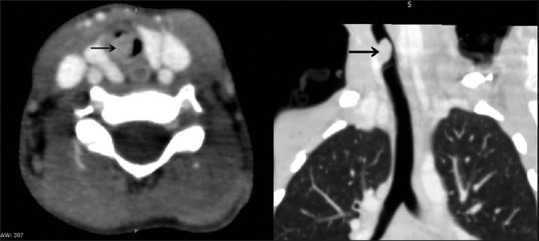
Contrast-enhanced computed tomography of the chest reveals a well-defined endoluminal tracheal mass (black arrow) arising from the right lateral wall and causing airway narrowing
Subsequently, MRI of airways was performed on a 3T MRI (Ingenia, Phillips) [Figure 2]. A well-defined, ovoid mass lesion, measuring 7.5 × 6.4 × 10 mm, which appeared homogeneously hypointense on T1-weighted images and hyperintense on T2-weighted images, was seen arising from right lateral wall of the proximal trachea. Multiplanar images in the sagittal and coronal axis demonstrated its exact extent and the distal airways were normal. This hyperintense soft tissue lesion was causing significant airway narrowing. No evidence of extratracheal extension of the lesion was noted.
Figure 2.
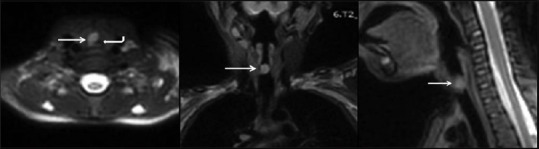
T2 weighted magnetic resonance imaging (Multiplanar images) shows circumscribed hyperintense endotracheal lesion (white arrows) causing luminal obliteration (curved white arrow). Findings correlated well with the computed tomography scan
The lesion was removed by complete surgical resection. Histopathologic examination of the lesion revealed stratified squamous epithelial lining and tumor in the subepithelium in the form of short fascicles. Individual tumor cells were spindle shaped, had moderate amount of cytoplasm, vesicular chromatin, and prominent nucleoli. Many of these cells showed intranuclear inclusions. Numerous scattered inflammatory cells consisting plasma cells, polymorphs, and lymphocytes were also noted. No significant mitoses or nuclear atypia was noted. The cells stained positively for cytoplasmic anaplastic lymphoma kinase and focally positive for smooth muscle antigen. Desmin was negative. Overall features were implicative of IMT [Figure 3].
Figure 3.
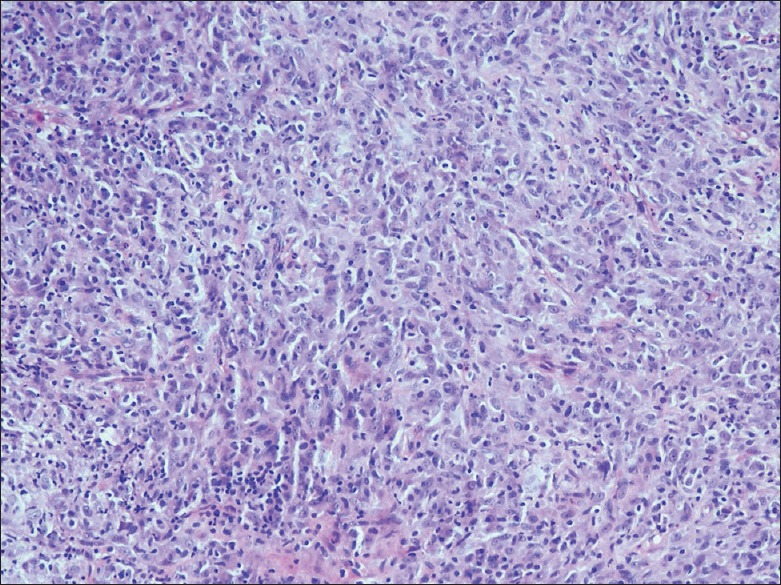
Photomicrograph of the fibroinflammatory polyp shows proliferating myofibroblasts, and inflammatory infiltrate of lymphomononuclear cells (H and E, ×20)
The postsurgical period was uneventful, and recovery was good. The child was discharged after 7 days of surgery. He is currently doing well and is under follow-up to look for tumor recurrence.
DISCUSSION
IMT is one of the very uncommon primary neoplasms of trachea. The World Health Organization (WHO) has graded it as an intermediate class of malignancy. It has been defined by the WHO as a lesion composed of a myofibroblastic spindle cell population along with an infiltrate of inflammatory cells likely lymphocytes, eosinophils, and plasma cells.[1]
The IMT has been referred to in literature with many synonyms due to the highly varied morphology as histiocytoma, inflammatory myofibrohistiocytic proliferation, plasma cell granuloma, fibrous histiocytoma, fibroxanthoma, mast cell tumor, plasma cell histiocytoma, xanthomatous pseudotumor, and postinflammatory pseudotumor.[4,5] It affects children most commonly, but any age group can be affected.[6] In pediatric age group, in approximately 50% of cases, tracheobronchial tree and lungs are involved.[7,8] As seen in our case stridor, dyspnea and wheezing are the classical symptoms, but hemoptysis, fever, and dysphagia have also been described.[6,9] It is for these symptoms that many of these patients are mistreated for asthma for a long time delaying the diagnosis as well as treatment of the cause.
Chest X-ray is usually noncontributory to any diagnosis. Cross-sectional imaging studies such as CT and MRI help in assessing the overall extent of the lesion as well as the degree of airways involvement. CT scan often reveals a well-defined, circumscribed, polypoidal endotracheal soft-tissue mass lesion. The lesion usually causes significant tracheal luminal obliteration. Calcification and necrosis are less commonly seen findings. Exophytic component is not associated. In the literature reviewed, we could not find any case with tracheal IMT in which MRI was done for the assessment of the lesion.
Children are at greater risk of getting affected by the deleterious consequences of radiation as compared to adults, because of higher sensitivity of their developing tissues to the radiation. In addition, children have more life years ahead of them as compared to adults, and hence, their body tissues get longer time to react to radiation-induced injuries. Therefore, it is of utmost importance to minimize the radiation exposure to the children, particularly when referring clinicians recommend CT scan as the preferred diagnostic imaging modality.[10] In current radiology practice, imaging of the airways is largely performed with conventional radiography, fluoroscopy, and CT. Each of these imaging modalities yields useful diagnostic information; however, all are performed with ionizing radiation, which is a major limitation in the care of pediatric patients. In a review by Liszewski et al., it has been inferred that high-quality MRI of the airways is an increasingly feasible alternative to conventional radiography, fluoroscopy, and CT in the evaluation of many diseases of the pediatric large airways.[11] Advances in technology have produced higher-resolution images (voxel size less 1 mm) that are less susceptible to motion artifact and allow image acquisition during free breathing, reducing the need for sedation.[12,13] These new sequences allow robust airway visualization up to the fourth bronchial generation. With these advances, radiologists should commence considering MRI as a preferred imaging modality over CT for assessing pediatric large airways diseases.
Histologically, tracheal IMTs often demonstrate a mixed, nonspecific inflammatory cell infiltrate, composing of histiocytes, lymphocytes, plasma cells and at times, neutrophils, and eosinophils as well. Spindle-cell component often predominates, as in our case. The admixture of cells with atypia and reactive cytological changes, spindle-cell population, and concomitant inflammatory infiltrate often makes the distinction of these lesions difficult from other benign and malignant entities.[14]
Even though the tumor is mostly benign, recurrence is common. Considering the higher recurrence rate, that ranges from 18% to 40%, locally invasive behavior of the lesion and its metastatic potential, complete margin free surgical resection of the lesion is the gold standard, whenever feasible.[15] In our case, the airway obstructive tracheal lesion was dealt with complete surgical resection and margins were free of tumor. This inflammatory pseudotumor or IMT carries an overall excellent long-term prognosis.
CONCLUSION
Even though IMT is an uncommon airway neoplasm, its accurate identification is mandatory to guide the management plan. A differential diagnosis of an obstructive tracheal mass lesion should be kept in mind in children who present with repeated episodes of respiratory distress and wheeze, which mimic asthma or a foreign body in respiratory tract. Awareness of imaging findings, especially MRI would be important for radiologists to arrive at the correct diagnosis, without exposing children to harmful radiation effects of CT.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Venizelos I, Papathomas T, Anagnostou E, Tsanakas J, Kirvassilis F, Kontzoglou G, et al. Pediatric inflammatory myofibroblastic tumor of the trachea: A case report and review of the literature. Pediatr Pulmonol. 2008;43:831–5. doi: 10.1002/ppul.20869. [DOI] [PubMed] [Google Scholar]
- 2.Browne M, Abramson LP, Chou PM, Acton R, Holinger LD, Reynolds M, et al. Inflammatory myofibroblastic tumor (inflammatory pseudotumor) of the neck infiltrating the trachea. J Pediatr Surg. 2004;39:e1–4. doi: 10.1016/j.jpedsurg.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 3.De Palma A, Loizzi D, Sollitto F, Loizzi M. Surgical treatment of a rare case of tracheal inflammatory pseudotumor in pediatric age. Interact Cardiovasc Thorac Surg. 2009;9:1035–7. doi: 10.1510/icvts.2009.216499. [DOI] [PubMed] [Google Scholar]
- 4.Lee HJ, Kim JS, Choi YS, Kim K, Shim YM, Han J, et al. Treatment of inflammatory myofibroblastic tumor of the chest: The extent of resection. Ann Thorac Surg. 2007;84:221–4. doi: 10.1016/j.athoracsur.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 5.Karnak I, Senocak ME, Ciftci AO, Cağlar M, Bingöl-Koloğlu M, Tanyel FC, et al. Inflammatory myofibroblastic tumor in children: Diagnosis and treatment. J Pediatr Surg. 2001;36:908–12. doi: 10.1053/jpsu.2001.23970. [DOI] [PubMed] [Google Scholar]
- 6.Boloursaz MR, Khalilzadeh S, Dezfoli AA, Kahkoee S, Karimi S, Abbaszadeh M, et al. Inflammatory myofibroblastic tumor of the trachea. Pediatr Surg Int. 2011;27:895–7. doi: 10.1007/s00383-011-2851-2. [DOI] [PubMed] [Google Scholar]
- 7.Vivero RJ, Dave SP, Roy S. Inflammatory pseudotumor of the trachea. Int J Pediatr Otorhinolaryngol. 2006;1:217–9. [Google Scholar]
- 8.Conforti S, Bonacina E, Ravini M, Torre M. A case of fibrous histiocytoma of the trachea in an infant treated by endobronchial ND: YAG laser. Lung Cancer. 2007;57:112–4. doi: 10.1016/j.lungcan.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Bumber Z, Jurlina M, Manojlović S, Jakić-Razumović J. Inflammatory pseudotumor of the trachea. J Pediatr Surg. 2001;36:631–4. doi: 10.1053/jpsu.2001.22306. [DOI] [PubMed] [Google Scholar]
- 10.Sodhi KS, Lee EY. What all physicians should know about the potential radiation risk that computed tomography poses for paediatric patients. Acta Paediatr. 2014;103:807–11. doi: 10.1111/apa.12644. [DOI] [PubMed] [Google Scholar]
- 11.Liszewski MC, Ciet P, Sodhi KS, Lee EY. Updates on MRI evaluation of pediatric large airways. AJR Am J Roentgenol. 2017;208:971–81. doi: 10.2214/AJR.16.17132. [DOI] [PubMed] [Google Scholar]
- 12.Sodhi KS, Sharma M, Saxena AK, Mathew JL, Singh M, Khandelwal N, et al. MRI in thoracic tuberculosis of children. Indian J Pediatr. 2017;84:670–6. doi: 10.1007/s12098-017-2392-3. [DOI] [PubMed] [Google Scholar]
- 13.Sodhi KS, Khandelwal N, Saxena AK, Singh M, Agarwal R, Bhatia A, et al. Rapid lung MRI in children with pulmonary infections: Time to change our diagnostic algorithms. J Magn Reson Imaging. 2016;43:1196–206. doi: 10.1002/jmri.25082. [DOI] [PubMed] [Google Scholar]
- 14.Hosler GA, Steinberg DM, Sheth S, Hamper UM, Erozan YS, Ali SZ, et al. Inflammatory pseudotumor: A diagnostic dilemma in cytopathology. Diagn Cytopathol. 2004;31:267–70. doi: 10.1002/dc.20113. [DOI] [PubMed] [Google Scholar]
- 15.Fabre D, Fadel E, Singhal S, de Montpreville V, Mussot S, Mercier O, et al. Complete resection of pulmonary inflammatory pseudotumors has excellent long-term prognosis. J Thorac Cardiovasc Surg. 2009;137:435–40. doi: 10.1016/j.jtcvs.2008.07.009. [DOI] [PubMed] [Google Scholar]


