Sir,
A 75-year-old female presented to our emergency department with cough, intermittent low-grade fever, joint pain, and progressive shortness of breath for the last 4 months that had worsened over the last 2 weeks. There was no history of rashes, myalgia, or dysphagia. On clinical examination, the patient was febrile with Spo2 of 92% at room air and bilateral lower zone fine crepitations. There was hyperkeratosis in fingers of both hands, which could not be attributed to her occupational history of rubber tapping, raising suspicion for a “mechanic's hand” due to an underlying collagen vascular disease. Her initial laboratory workup was within normal limits except for mildly raised C-reactive protein and D-dimer. Frontal chest radiograph revealed symmetrical volume loss and consolidation in bilateral lower zones, with blurring of the hemidiaphragm [Figure 1]. A computed tomography pulmonary angiogram (CTPA) was performed that did not show pulmonary embolism. However, the CTPA showed symmetrical extensive peribronchial consolidative opacities with bronchiectasis in the lower lobes associated with striking volume loss in the lower lungs [Figure 2]. There was no honeycombing. This is considered as a mixed organizing pneumonia-nonspecific interstitial pneumonia (OP-NSIP) pattern. She was admitted in the hospital and initially treated with oxygen therapy and antibiotics without much improvement. Meanwhile, serological examination revealed elevated antinuclear antibody level while antineutrophil cytoplasmic autoantibodies (p-[ANCA] and c-ANCA), dsDNA, and lupus anticoagulant were negative. Based on the clinical suspicion and CT features, an antisynthetase antibody panel was ordered that was ultimately positive for the presence of anti-Jo-1 antibodies. These results confirmed the diagnosis of antisynthetase syndrome (AS). The patient was treated with immunosuppressive therapy and steroids that resulted in significant improvement in her dyspnea on the short-term follow-up. A repeat chest radiograph and high-resolution CT (HRCT) after 2 months of treatment revealed significant improvement in the bilateral lower lobe peribronchial consolidation and scattered areas of ground glass attenuation and reticulation [Figure 1 and 2]. Mild improvement in overall lung aeration was also evident. However, the traction bronchiectasis and scarring within bilateral lower lobes and inferior lingula persisted [Figure 3]. Decision to continue the steroid therapy was made, which resulted in complete clinical remission.
Figure 1.
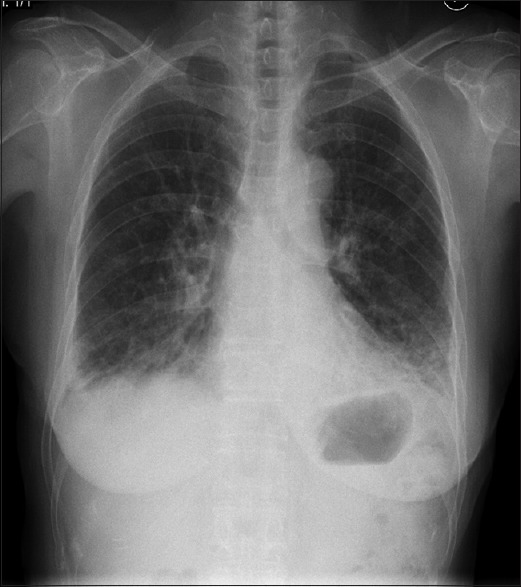
Initial chest radiograph shows bibasal consolidations with volume loss in bilateral lower lungs without cardiomegaly
Figure 2.
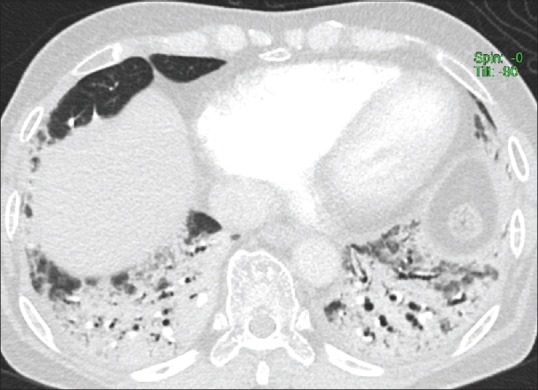
Initial computed tomography pulmonary angiogram image from basal region shows extensive symmetrical bibasilar peribronchial consolidations with bronchiectasis
Figure 3.
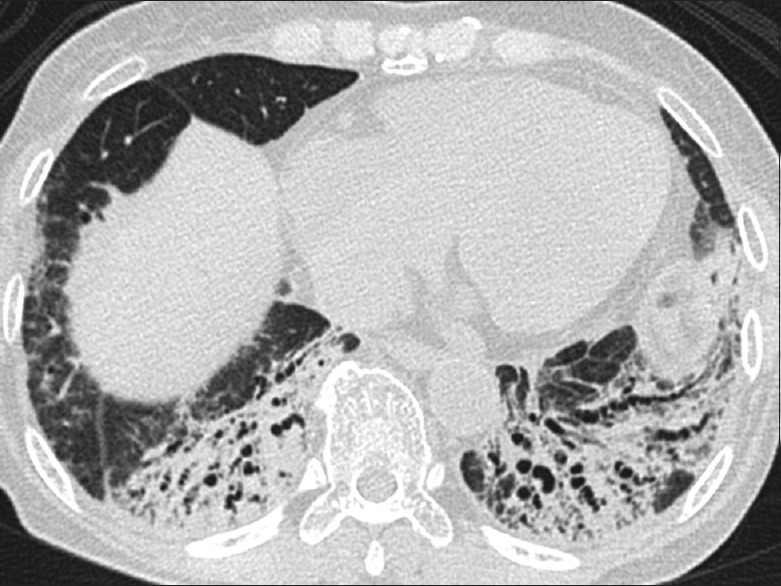
Follow-up high-resolution computed tomography image from basal region demonstrates improvement in consolidations and aeration but persistence of traction bronchiectasis
Patients with myositis can be subclassified according to the various myositis-related antibodies, including anti-aminoacyl-tRNA synthetase (anti-ARS), anti-MDA5 antibody, and anti-transcriptional intermediary factor 1 antibody. The subcategory patients have different clinical profile and imaging features. AS is a specific subset of inflammatory myositis (polymyositis/dermatomyositis) patients, those have a clinical syndrome characterized by the presence of anti-ARS antibodies, interstitial lung disease (ILD), and some of the following clinical features: fever, arthralgias, Raynaud's phenomenon, and exanthema on the hands (mechanic's hands).[1] Anti-ARS antibodies are directed against a family of cytoplasmic enzymes (anti-ARS) that catalyze the formation of the aminoacyl-tRNA complex from an amino acid and its cognate tRNA and play a vital role in protein synthesis. Antisynthetase antibodies so far described include anti-Jo-1 (anti-histidyl), anti-PL-7 (anti-threonyl), anti-PL-12 (anti-alanyl), anti-OJ (anti-isoleucyl), anti-EJ (anti-glycyl), anti-KS (anti-asparaginyl), anti-KS (anti-asparaginyl), anti-ZO (anti-phenylalanyl) snit-YRS (anti-tyrosyl).[1,2] The most commonly detected anti-ARS antibody is anti-Jo-1.
Diagnosis is considered in patients with an antisynthetase antibody plus two major criteria or one major criterion and two minor criteria.[1] Major criteria include (a) ILD (not explained by environmental, occupational, medication exposure, and not related to any other base disease) and (b) polymyositis or dermatomyositis. Minor criteria include (a) arthritis, (b) Raynaud's phenomenon, and (c) fever. In a 2-year retrospective study by Maturu et al., the authors found that at the time of diagnosis of AS, all nine patients had radiologic evidence of ILD, whereas inflammatory myositis and arthritis were present in seven and five patients, respectively.[3] In all the nine patients diagnosed with anti-Jo-1-related AS, ILD was present in all patients. Our patient had ILD and joint pain. Thickened skin of tips and margins of fingers termed “mechanic's hand” was also present in the patient.
AS is a subset of myopathy, but myositis may be absent or delayed after lung involvement in more than one-third of the patients. ILD is found in 70%–90% of patients with AS and is a major cause of morbidity and mortality.[4] Patients belonged to wide age range from 18 to 79 years with female preponderance.[5] The clinical presentation of lung involvement includes persistent cough, chest pain, diminished exercise tolerance, and dyspnea at rest, and even acute respiratory failure can occur.[1] The pulmonary function tests reveal typical restrictive pattern and the total lung capacity varies according to the severity of the disease. Chest radiograph usually reveals lower lung predominant interstitial pattern. Given that pulmonary involvement is the most common cause of morbidity in AS, HRCT plays a pivotal role in diagnosis and monitoring treatment response. On HRCT, three common patterns are seen in patients with AS: a nonspecific (NSIP) pattern, OP pattern, or mixed NSIP-OP pattern.[2,5] Rarely, AS presents with usual interstitial pattern. Commonly, the ground-glass opacities and consolidation are basal predominant and peribronchovascular in distribution. Classically, on follow-up imaging, the consolidations decrease or resolve in most of the cases with residual fibrosis.[2] The ground-glass opacities and traction bronchiectasis may improve or remain unchanged.
Steroids and immunosuppressive therapy are the mainstay of treatment for AS and are often required for several months or years. Physical therapy and rehabilitation should start early to prevent muscle deconditioning and to improve weakness. This case highlights the typical imaging and clinical features of AS that should prompt the physician to raise suspicion for AS and ask for laboratory workup for it.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that her name and initials will not be published and due efforts will be made to conceal her identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Solomon J, Swigris JJ, Brown KK. Myositis-related interstitial lung disease and antisynthetase syndrome. J Bras Pneumol. 2011;37:100–9. doi: 10.1590/s1806-37132011000100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Debray MP, Borie R, Revel MP, Naccache JM, Khalil A, Toper C, et al. Interstitial lung disease in anti-synthetase syndrome: Initial and follow-up CT findings. Eur J Radiol. 2015;84:516–23. doi: 10.1016/j.ejrad.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Maturu VN, Lakshman A, Bal A, Dhir V, Sharma A, Garg M, et al. Antisynthetase syndrome: An under-recognized cause of interstitial lung disease. Lung India. 2016;33:20–6. doi: 10.4103/0970-2113.173055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katzap E, Barilla-Labarca ML, Marder G. Antisynthetase syndrome. Curr Rheumatol Rep. 2011;13:175. doi: 10.1007/s11926-011-0176-8. [DOI] [PubMed] [Google Scholar]
- 5.Waseda Y, Johkoh T, Egashira R, Sumikawa H, Saeki K, Watanabe S, et al. Antisynthetase syndrome: Pulmonary computed tomography findings of adult patients with antibodies to aminoacyl-tRNA synthetases. Eur J Radiol. 2016;85:1421–6. doi: 10.1016/j.ejrad.2016.05.012. [DOI] [PubMed] [Google Scholar]


