Abstract
Background:
Whether the indications and diagnostic yield of endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA) vary according to the socioeconomic status of the patient, remains unknown. Herein, we evaluate this aspect in participants who underwent EBUS-TBNA.
Materials and Methods:
This is a retrospective analysis of all participants who underwent EBUS-TBNA for the evaluation of intrathoracic lymphadenopathy. We evaluated the indications and outcome of EBUS-TBNA in participants with and without economic disadvantage (issuance of a below poverty line card by the government).
Results:
Of the EUBUS procedures performed on 1582 participants (mean [standard deviation] age, 46.1 [15.7] years; 593 [37.5%] women) performed during the study, 61 (3.9%) were done in the economically disadvantaged (ED) group. Individuals in the ED group were younger (median age, 40 vs. 46 years; P = 0.002) and more likely to have tuberculosis (42.6% vs. 26.2%, P = 0.005) or malignancy (39.3% vs. 26.9%, P = 0.032) as a presumptive diagnosis. The overall diagnostic yield of EBUS was 63% and was significantly lower in the ED group (49.2% vs. 63.5%, P = 0.023). Previously used EBUS-TBNA needles were more commonly employed in the ED participants (62.7% vs. 20.1%, P < 0.001). On multivariate logistic regression analysis, younger age, larger size, and number of nodes sampled, and the use of new (versus reused) needles were independent predictors of higher diagnostic yield. There was no difference in the complication rate between the two groups.
Conclusion:
The diagnostic yield of EBUS was significantly lower in the ED participants, which is due to the differences in the clinical and procedural characteristics.
KEY WORDS: Ebus, endobronchial ultrasonography, lung cancer, sarcoidosis, transbronchial needle aspiration, tuberculosis
INTRODUCTION
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is the investigation of choice for the evaluation of intrathoracic lymphadenopathy as it is minimally invasive and allows sampling of most intrathoracic lymph node stations under “direct” endosonographic vision.[1] The procedure is now widely practiced in India with more than 130 EBUS installations across the country.
Unfortunately, more than one-fifth of the Indian population lives below the poverty line.[2] Based on the family income, the government assigns a below poverty line (BPL) status to these individuals and issues them a BPL card. Patients with a BPL status are entitled to concessions at various public-sector hospitals for most diagnostic procedures and treatments. The socioeconomic profile of patients may have a bearing on the spectrum of diseases encountered. For example, patients with sarcoidosis are more likely to belong to an affluent socioeconomic status than those with tuberculosis.[3]
Whether the indications and diagnostic yield of EBUS-TBNA are also different in varying socioeconomic strata, remains unknown. Herein, we compare the indications and outcome of EBUS-TBNA in participants with intrathoracic lymphadenopathy, based on their economic status.
MATERIALS AND METHODS
This was a retrospective analysis of all participants who underwent EBUS-TBNA between July 2011 and October 2017 in the bronchoscopy suite of this institute. The study protocol was approved by the Institute Ethics Committee. The requirement for informed consent was waived due to the retrospective nature of the study and the use of anonymized patient data. We routinely obtain a procedural consent from all the participants undergoing the EBUS procedure. A part of the data has been previously published.[4,5,6,7,8]
Study subjects
All participants who underwent EBUS-TBNA for the evaluation of intrathoracic lymphadenopathy during the study were included in the analysis. We classified the participants into two groups, economically disadvantaged (ED) or others. ED participants were defined as those who carried a BPL status. For these participants, the fee for all diagnostic procedures is waived off as a policy in public sector hospitals. All subjects (including those in the ED group) who could afford a new needle underwent EBUS-TBNA with a new needle. Those who could not purchase a new needle were given the option to undergo the procedure with a needle that had been previously used, albeit after an elaborate sterilization process, as previously described.[7] They were also counseled regarding the risk of acquiring infections or needle malfunction while employing the used needle. The EBUS needles were reused only once. Moreover, needles that were used in participants with serological evidence of infection with hepatitis B virus, hepatitis C virus, or human immunodeficiency virus or in those with clinical suspicion of tuberculosis (or final diagnosis of tuberculosis) were not reused.
Procedure
EBUS-TBNA was performed as an outpatient procedure under local anesthesia and conscious sedation, as previously described.[6,9] The convex probe EBUS scope (BF-UC 180F; Olympus Medical Systems, Japan) was used for the procedure. Sedation depth was assessed using the Ramsay sedation scale. The intensity of the subject's cough and the amount of airway secretions during the procedure were scored by the operator using visual analog scale on a horizontal line, 100 mm in length, immediately after the procedure. All participants were observed for complications (fever, chills, excessive cough, chest pain, bradycardia, hypotension, sustained hypoxemia, bleeding, and need for escalation of care) for at least 2 h after the procedure.
Lymph node stations were categorized according to the classification proposed by the International Association for the Study of Lung Cancer.[10] All the lymph node stations were systematically examined using the echobronchoscope, and endosonographic lymph nodes characteristics were recorded.[4]
Transbronchial needle aspiration
TBNA was performed using either 21 or 22G EBUS-TBNA needle (Vizishot, NA-201SX-4021/4022, Olympus Medical Systems, Japan). According to the number and size of the lymph nodes on computed tomography of the thorax, one to four lymph node stations were sampled per patient. Two to three aspirations were performed from each lymph node station with 10–20 revolutions during every pass. Aspirated material was used to prepare slides and cell block and was subsequently subjected to cytological examination. Additional investigations such as the Xpert MTB/RIF assay, Ziehl-Neelsen staining for acid-fast bacilli, mycobacterial culture, and fungal smear and culture were performed, as clinically indicated. Rapid on-site cytological examination (ROSE) was not available.
Outcomes
The primary outcome measure was the diagnostic yield of EBUS-TBNA in the two groups (ED vs. others). The TBNA sample was considered adequate if there was preponderance of lymphocytes on cytological examination. Lymphadenopathy was considered reactive if the aspirate was adequate but did not yield a specific diagnosis. EBUS-TBNA was deemed as diagnostic if the cytological examination resulted in a definite pathological diagnosis (tuberculosis, lymphoma, malignancy, sarcoidosis, or others) rather than a diagnosis of reactive lymphadenopathy. If the aspirate did not yield a specific diagnosis or did not meet the criterion for adequacy, it was labeled as inadequate. We defined the diagnostic yield of EBUS-TBNA as the proportion of participants with a diagnostic EBUS divided by the total number of participants. We also recorded the complications due to the EBUS-TBNA procedure.
Statistical analysis
Statistical analyses were performed utilizing the Statistical Package for Social Sciences software (IBM SPSS Statistics, version 22; IBM Corporation, Armonk, NY, USA). Descriptive data are presented as number and percentage or mean (standard deviation) or median (interquartile range [IQR]). Continuous variables were compared using the Student's t-test or the Mann–Whitney U-test, while categorical variables were compared with the Chi-square test or the Fisher's exact test, as applicable. A multivariate logistic regression analysis was performed to assess the factors that predicted a successful diagnostic yield of EBUS-TBNA. The results of the logistic regression are presented as adjusted odds ratio with 95% confidence intervals. P < 0.05 was considered statistically significant.
RESULTS
During the study period, 1699 EBUS procedures were performed. A total of 1582 participants were finally included in the study; 117 were excluded either because TBNA was not performed owing to various reasons or due to the lack of diagnostic details. Among the included participants, 61 (3.9%) belonged to the ED group. The median (IQR) age of the participants (593 [37.5%] women) was 46 (33–60) years [Table 1]. The baseline characteristics were significantly different between the two groups. The ED participants were significantly younger (median age, 40 vs. 46 years, P = 0.002). A higher proportion of participants in the ED group had a presumptive clinical diagnosis of tuberculosis (43.6% vs. 26.2%, P = 0.005) or malignancy (39.3% vs. 26.9%, P = 0.032). On the other hand, the presumptive clinical diagnosis of sarcoidosis was higher (44.7% vs. 18%, P < 0.001) in participants who were not ED. There was no significant difference in tuberculin reactivity between the two groups.
Table 1.
Baseline characteristics of the study participants
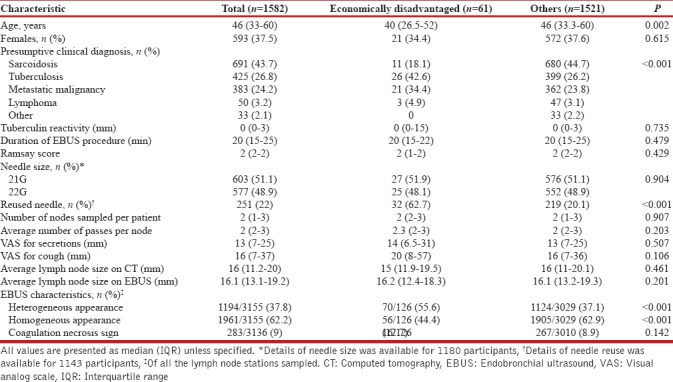
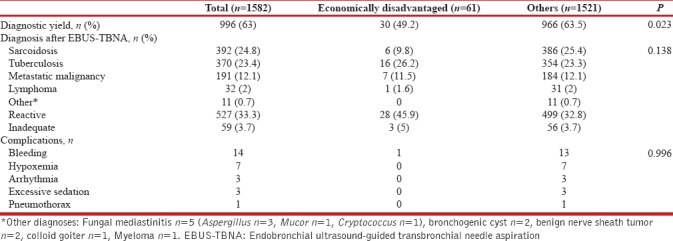
The depth of sedation, the duration of EBUS procedure, the severity of cough, and the airway secretions were not different between the two groups. The lymph nodes were more commonly sampled using a reused needle in ED group (62.7% vs. 20.1%, P < 0.001). On endosonography, the lymph nodes of patients in the ED group were more likely to be heterogeneous (55.6% vs. 37.1%, P < 0.001) as compared to others. The diagnostic yield was significantly lower in the ED group (49.2% vs. 63.5%, P = 0.023) [Table 2]. There was no difference in the overall spectrum of the cytological diagnoses obtained after EBUS-TBNA in between the two groups. However, the diagnosis of sarcoidosis was less frequent in the ED participants (9.8% vs. 25.4%; P = 0.006).
Table 2.
Diagnostic yield and complications of participants undergoing endobronchial ultrasound-guided transbronchial needle aspiration
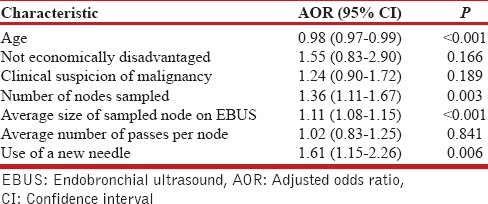
On multivariate logistic regression analysis, younger age, a higher number of nodes sampled, the larger size of the sampled nodes, and the use of new (vs. reused) EBUS needle were independent predictors of a definite diagnosis by EBUS-TBNA [Table 3].
Table 3.
Multivariate logistic regression analysis of factors affecting the diagnostic yield of endobronchial ultrasound-guided transbronchial needle aspiration
The complication rate was low (n = 28, 0.02%), with bleeding being the most frequent complication, followed by transient hypoxemia [Table 2]. There were no deaths related to the procedure. There was no difference in the complication rate between the two groups.
DISCUSSION
The results of this study indicate that the diagnostic yield of EBUS-TBNA was significantly lower in the ED participants. However, this was related to factors other than the socioeconomic status itself including the age, the number of nodes sampled, the average size of the sampled nodes on ultrasound, and the reuse of EBUS needles.
The overall diagnostic yield of EBUS-TBNA in the current study was 63% and is lower compared to previous studies.[11,12] The yield in the current study, however, is closer to the real-world scenario[13] and is similar to the yield reported in the multicentric AQuIRE registry.[14] The lower yield is likely due to a mix of patients with different etiologies (both benign and malignant) of undiagnosed mediastinal adenopathy in the current study, unlike many previous studies, where the predominant study population has been lung cancer.[11,12] In fact, the yield of EBUS-TBNA has been shown to be lower in benign disorders, especially sarcoidosis, than in patients with malignancy.[15,16] Technical differences in the EBUS-TBNA procedure may also have affected the diagnostic yield. We sampled 1–3 lymph nodes per patient and performed 2–3 needle passes per lymph node, employing 10–20 revolutions during each needle pass. Sampling more than one lymph node may increase the diagnostic yield in sarcoidosis; however, the results have been inconsistent.[17,18] Increasing the number of needle passes per lymph node (optimal 3–4) has been shown to improve the diagnostic yield in lung cancer and sarcoidosis.[17,18,19] The use of a greater number of revolutions (10 vs. 20) during each pass has not been shown to improve the diagnostic yield in sarcoidosis.[20]
In the current study, the diagnostic yield of EBUS-TBNA was significantly lower in the ED group compared to others, despite a similar sample adequacy rate in the two groups. The reason for this remains unclear, and we can only speculate. We did find an association between characteristics related to the patient or the procedure and outcome of EBUS-TBNA, as in previous studies.[7,14] Importantly, we also noted the possibility of a reduction in the diagnostic yield of EBUS-TBNA with the reuse of EBUS-TBNA needles. This finding has also been previously reported,[7] although in that study, the reuse of EBUS-TBNA needles was not a significant predictor of diagnostic yield on multivariate analysis.[7] In the current study, however, the reuse of EBUS needle did lower the diagnostic yield after adjusting for other covariates. In our practice, we have observed that traversing the airway with the EBUS needle becomes increasingly difficult after the needle has been used to perform about 15–20 passes. Therefore, the practice of reusing EBUS needles could have made the procedure technically more difficult, thereby reducing the diagnostic yield. In addition, there is a risk of transmission of infectious diseases if the EBUS needles are reused indiscriminately without performing the rigorous sterilization procedures and the necessary precautions described above. Hence, we suggest that the practice of reuse of EBUS needles should be avoided, wherever feasible.
In the present study, the lymph nodes of the participants in the ED group were more likely to have a heterogeneous appearance (rather than a homogenous appearance) on endosonography. The discrepancy in the lymph node characteristic is probably due to the difference in the prevalence of sarcoidosis and tuberculosis among our study participants. On endosonographic evaluation, the nodes in sarcoidosis are more likely to have a homogeneous appearance while tuberculous lymph nodes are more likely to have a heterogeneous echotexture and coagulation necrosis.[4] Moreover, sarcoidosis is considered as a disease of the affluent while tuberculosis is considered as a disease of the underprivileged.[3] Although we found a lower prevalence of sarcoidosis in the ED participants (9.8% vs. 25.4%), we did not observe any difference in the prevalence of tuberculosis (26.2% vs. 23.3%). It is possible that India being a country with high tuberculosis burden, the disease may not be limited to the poor. It is also possible that participants who belonged to the ED group might have received empirical therapy for tuberculosis rather than being referred for evaluation with EBUS-TBNA, thus equalizing the prevalence of tuberculosis in the two groups. Conventional TBNA (cTBNA), wherein the lymph node aspiration, is performed utilizing anatomical landmarks in the airway is an alternative option for participants in the ED group, as the needle for cTBNA is cheaper compared to the EBUS needle. However, cTBNA has a lower diagnostic yield compared to EBUS-TBNA.[9] Moreover, cTBNA, being a “blind” technique has a lower yield in patients with smaller lymph nodes.
Finally, our study has certain limitations. This was a single-center retrospective study, with inherent limitations of the study design. The number of participants in the economically challenged group was small (3.9%) as we used the labeling of participants as ED based on the prevalent system at our institute and a detailed socioeconomic assessment was not made. We did not perform ROSE, which has now become a standard of care in most of the tertiary care centers performing EBUS. However, the use of ROSE has not been shown to improve the diagnostic yield in participants with mediastinal lymphadenopathy.[21] Finally, we do not have the follow-up data of the patients after treatment. The strength of the study is the large sample size and the availability of detailed EBUS-TBNA data including lymph node characteristics in the total study population.
CONCLUSION
The diagnostic yield of EBUS in participants undergoing the procedure for evaluation of intrathoracic lymphadenopathy is lower in the ED participants. This difference is due to variations in the clinical and procedural characteristics including the reuse of EBUS needles rather than the economic status of the patient.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Thangakunam B, Herth FJ. Endobronchial ultrasound: A new innovation in bronchoscopy. Lung India. 2009;26:17–21. doi: 10.4103/0970-2113.45199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Planning Commission of the Government of India. Press Note on Poverty Estimates, 2011-12. New Delhi: Press Bureau of India; 2013. pp. 1–9. [Google Scholar]
- 3.Gupta D, Vinay N, Agarwal R, Agarwal AN. Socio-demographic profile of patients with sarcoidosis vis-à -vis tuberculosis. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30:186–93. [PubMed] [Google Scholar]
- 4.Dhooria S, Agarwal R, Aggarwal AN, Bal A, Gupta N, Gupta D, et al. Differentiating tuberculosis from sarcoidosis by sonographic characteristics of lymph nodes on endobronchial ultrasonography: A study of 165 patients. J Thorac Cardiovasc Surg. 2014;148:662–7. doi: 10.1016/j.jtcvs.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 5.Dhooria S, Agarwal R, Aggarwal AN, Gupta N, Gupta D, Behera D, et al. Agreement of mediastinal lymph node size between computed tomography and endobronchial ultrasonography: A study of 617 patients. Ann Thorac Surg. 2015;99:1894–8. doi: 10.1016/j.athoracsur.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 6.Dhooria S, Sehgal IS, Gupta N, Aggarwal AN, Behera D, Agarwal R, et al. Diagnostic yield and complications of EBUS-TBNA performed under bronchoscopist-directed conscious sedation: Single center experience of 1004 subjects. J Bronchology Interv Pulmonol. 2017;24:7–14. doi: 10.1097/LBR.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 7.Dhooria S, Sehgal IS, Gupta N, Ram B, Aggarwal AN, Behera D, et al. Yield of new versus reused endobronchial ultrasound-guided transbronchial needle aspiration needles: A retrospective analysis of 500 patients. Lung India. 2016;33:367–71. doi: 10.4103/0970-2113.184867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muthu V, Gupta N, Dhooria S, Sehgal IS, Bal A, Aggarwal AN, et al. A prospective, randomized, double-blind trial comparing the diagnostic yield of 21- and 22-gauge aspiration needles for performing endobronchial ultrasound-guided transbronchial needle aspiration in sarcoidosis. Chest. 2016;149:1111–3. doi: 10.1016/j.chest.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Gupta D, Dadhwal DS, Agarwal R, Gupta N, Bal A, Aggarwal AN, et al. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146:547–56. doi: 10.1378/chest.13-2339. [DOI] [PubMed] [Google Scholar]
- 10.Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P, et al. The IASLC lung cancer staging project: A proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568–77. doi: 10.1097/JTO.0b013e3181a0d82e. [DOI] [PubMed] [Google Scholar]
- 11.Casal RF, Lazarus DR, Kuhl K, Nogueras-González G, Perusich S, Green LK, et al. Randomized trial of endobronchial ultrasound-guided transbronchial needle aspiration under general anesthesia versus moderate sedation. Am J Respir Crit Care Med. 2015;191:796–803. doi: 10.1164/rccm.201409-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ernst A, Anantham D, Eberhardt R, Krasnik M, Herth FJ. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol. 2008;3:577–82. doi: 10.1097/JTO.0b013e3181753b5e. [DOI] [PubMed] [Google Scholar]
- 13.Lange TJ, Kunzendorf F, Pfeifer M, Arzt M, Schulz C. Endobronchial ultrasound-guided transbronchial needle aspiration in routine care – Plenty of benign results and follow-up tests. Int J Clin Pract. 2012;66:438–45. doi: 10.1111/j.1742-1241.2012.02907.x. [DOI] [PubMed] [Google Scholar]
- 14.Ost DE, Ernst A, Lei X, Feller-Kopman D, Eapen GA, Kovitz KL, et al. Diagnostic yield of endobronchial ultrasound-guided transbronchial needle aspiration: Results of the AQuIRE bronchoscopy registry. Chest. 2011;140:1557–66. doi: 10.1378/chest.10-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu P, Zhao YZ, Jiang LY, Zhang W, Xin Y, Han BH, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: A systematic review and meta-analysis. Eur J Cancer. 2009;45:1389–96. doi: 10.1016/j.ejca.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal R, Srinivasan A, Aggarwal AN, Gupta D. Efficacy and safety of convex probe EBUS-TBNA in sarcoidosis: A systematic review and meta-analysis. Respir Med. 2012;106:883–92. doi: 10.1016/j.rmed.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Sun J, Yang H, Teng J, Zhang J, Zhao H, Garfield DH, et al. Determining factors in diagnosing pulmonary sarcoidosis by endobronchial ultrasound-guided transbronchial needle aspiration. Ann Thorac Surg. 2015;99:441–5. doi: 10.1016/j.athoracsur.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 18.Oki M, Saka H, Ando M, Nakashima H, Shiraki A, Murakami Y, et al. How many passes are needed for endobronchial ultrasound-guided transbronchial needle aspiration for sarcoidosis. A Prospective multicenter study? Respiration. 2018;95:251–7. doi: 10.1159/000485661. [DOI] [PubMed] [Google Scholar]
- 19.Lee HS, Lee GK, Lee HS, Kim MS, Lee JM, Kim HY, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: How many aspirations per target lymph node station? Chest. 2008;134:368–74. doi: 10.1378/chest.07-2105. [DOI] [PubMed] [Google Scholar]
- 20.Dhooria S, Sehgal IS, Gupta N, Bal A, Prasad KT, Aggarwal AN, et al. A randomized trial evaluating the effect of 10 versus 20 revolutions inside the lymph node on the diagnostic yield of EBUS-TBNA in subjects with sarcoidosis. Respiration. 2018 doi: 10.1159/000490192. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Sehgal IS, Dhooria S, Aggarwal AN, Agarwal R. Impact of rapid on-site cytological evaluation (ROSE) on the diagnostic yield of transbronchial needle aspiration during mediastinal lymph node sampling: Systematic review and meta-analysis. Chest. 2018;153:929–38. doi: 10.1016/j.chest.2017.11.004. [DOI] [PubMed] [Google Scholar]


