Abstract
Hair is a defining mammalian feature that serves as a hallmark of human communication. Given the critical significance of hair in social, religious, and political contexts, it is important to understand factors that play a role in hair loss disorders. The hair follicle is an immune privileged site, and mounting evidence suggests that the collapse of immune privilege contributes to the pathogenesis of autoimmune hair loss disorders, including alopecia areata and lichen planopilaris. This review comprehensively appraises the current literature to shed light on mechanisms for immune privilege collapse, and examines the role of neurogenic stress in triggering this process.
Keywords: Autoimmune alopecia, Hair follicle immune privilege, Alopecia areata, Lichen planopilaris, Stress
Introduction
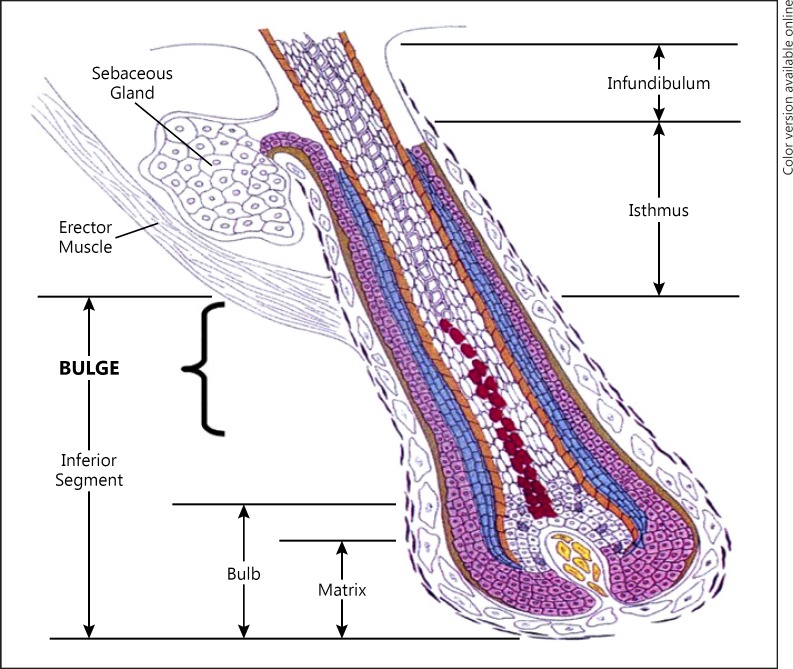
A discussion of the mechanisms underlying autoimmune hair loss requires understanding the hair follicle (HF) and its cycle. The proximal HF continually cycles through alternating phases of growth (anagen), apoptosis-mediated regression (catagen), rest (telogen), and shedding (exogen) [1, 2]. Within the isthmus at the insertion of the arrector pili lies the bulge region as depicted in Figure 1. It is thought that HF immune privilege (HFIP) aims to protect the bulge, which houses the epithelial HF stem cells (HFSC) that are essential to follicle regeneration [3, 4, 5, 6].
Fig. 1.
Hair follicle anatomy. Adapted from Color Atlas of Differential Diagnosis of Hair Loss by David Whiting, courtesy of Cranfield Publishing.
Alopecia is classified as cicatricial (scarring), which results in permanent hair loss, or noncicatricial, which preserves regenerative potential. This may be accounted for by varying locations of inflammatory processes. HFSC renewal is thought to be maintained in the upper portion of the follicle. Inflammatory processes associated with noncicatricial alopecias spare this fragile upper portion, permitting the possibility of hair regrowth.
This review will focus on two forms of hair loss: lichen planopilaris (LPP) and alopecia areata (AA). These disorders represent examples of cicatricial and noncicatricial hair loss, respectively. In LPP, activated T lymphocytes obliterate HFs, ultimately begetting irreversible hair loss. Clinical findings include follicular hyperkeratosis, perifollicular erythema and perifollicular scaling [3, 6, 7, 8, 9]. Women appear to be more frequently affected than men, and it is primarily observed in postmenopausal females [10, 11, 12]. AA, a noncicatricial autoimmune alopecia, involves T-cell-mediated disruption of the HF cycle. Peritubular inflammation induces premature termination of the growth phase [13, 14]. Approximately 2% of the global population suffers from AA [15]. It can occur at any age, is frequently associated with other autoimmune conditions, and affects both sexes similarly [16]. Both LPP and AA have the potential to lead to extensive hair loss with significant psychosocial impact.
While the exact pathogenesis of LPP and AA remains unknown, evidence increasingly implicates HFIP collapse as a possible initial step [17]. This begs the question if an inciting event elicits collapse. One proposed factor is emotional stress. In soon to be published data, over 65% of AA patients in our clinic identify emotional stress as a trigger for initial AA episode or flare.
Hair Follicle Immune Privilege
Over 140 years ago, it was observed that introducing tissue grafts to specific anatomical compartments enables prolonged survival, suggesting that certain sites are exempt from standard immune surveillance [18]. Prominent examples of immune privileged sites include the ocular anterior chamber, fetomaternal placental unit, and parts of the gonads. Immune privilege is a dynamic process maintained by several mechanisms that collude to limit recognition of foreign antigens, deviate immune responses to favor tolerance, and suppress immune-mediated inflammation [18, 19, 20, 21, 22].
One of the earliest observations of HFIP was reported when black murine ear epidermis was transplanted onto genetically incompatible white guinea pigs. Donor melanocytes were recognized as foreign and rejected, as evidenced by transplanted black skin rapidly losing pigment. However, black hair shafts grew through the white epidermis, indicating that some donor melanocytes could evade immune rejection [23, 24, 25].
Upholders of Immune Privilege
Several mechanisms that are believed to uphold HFIP are shown in Figure 2a, and are discussed in more detail below.
Fig. 2.
Immune privilege. a Summary of proposed mechanisms involved in the maintenance and collapse of hair follicle immune privilege (HFIP). b Proposed connections between neurogenic stress and HFIP collapse.
Impeding immune cell trafficking is one described mechanism of HFIP. The HF lacks lymphatic drainage [26]. The connective tissue sheath may serve a similar purpose, as it generates proteoglycans during anagen in rats, which are thought to guard against immune cell infiltration [27].
Expression of major histocompatibility complex classes (MHC-Ia and II) is markedly reduced in the proximal follicular epithelium during anagen [28, 29, 30]. MHC-Ia is expressed on all nucleated cells, unless an IP- protected area, and functions to present antigens to cytotoxic CD8+ T cells [6]. Two related molecules, β2-microglobulin and TAP (transporter associated with antigen processing), are crucial for stabilization of MHC-Ia [31]. Absence of MHC-Ia and β2-microglobulin, and reduced expression of TAP-1 have been documented in murine HFs [32, 33]. In human proximal HFs, decreased MHC-Ia expression is observed at protein and transcriptional levels [2, 3, 28, 29, 30], and anagen bulb melanocytes lack MHC-Ia [28, 34]. Downregulation of the MHC-Ia pathway minimizes opportunities for misinterpretation of autoantigens, thus preventing possibly destructive immune responses. However, cells lacking MHC-Ia are susceptible to destruction by natural killer (NK) cells [35]. MHC class II, expressed predominantly on antigen presenting cells (APCs), interacts with helper CD4+ T cells to engage phagocytes and trigger activation of antibody-producing B cells. Studies reveal total absence of MHC-II expression from the hair bulb and reduced expression in the proximal HF, which results in functional impairment of APCs [28, 36]. It is important to note that downregulation of MHC Ia and II was largely observed during anagen [2, 28]. Inflammatory assault against the bulge during anagen could thwart HF growth, and compromise regenerative capacity. In contrast, MHC-Ib expression may uphold HFIP [37].
Immunomodulation through local production of immunosuppressants also contributes to HFIP. Transforming growth factor-β (TGFβ), a powerful immunosuppressive growth factor, has been demonstrated to impede APC activity and T-cell activation [38, 39, 40]. TGFβ1 expression is highest in the outer root sheath during late anagen[41]. Therefore, its major contribution to HFIP may be insulating autoantigens associated with anagen and/or melanogenesis from CD8+ T cell-mediated destruction [23, 42, 43]. TGFβ2 is expressed in the bulge region, where it is thought to help preserve melanocyte stem cell quiescence [44, 45]. Proopiomelanocortin- derived hormones, adrenocorticotropic hormone and α-melanocyte stimulating hormone (α-MSH) act as powerful immunosuppressants [23, 46, 47, 48]. Interestingly, proopiomelanocortin gene transcription and translation exhibit an HF cycle-dependent pattern, rising during anagen in C57BL/6 mice and contributing to HFIP [49, 50, 51]. Adrenocorticotropic hormone is detected in the outer root sheath of anagen follicles, while α-MSH is found in both the outer root sheath and hair matrix [46, 47, 52].
α-MSH is generated within the follicle and likely acts as an immunomodulator via its effects on APCs that express melanocortin receptor [47, 51, 53, 54, 55]. α-MSH suppresses NFκB activation and upregulates cytokine synthesis inhibitor IL-10 [54, 55]. Insulin-like growth factor-1, a local immunomodulator, downregulates ectopic MHC-I expression, as do α-MSH and TGFβ1 [42]. Macrophage migration inhibitory factor (MIF) expression is increased in the proximal follicular epithelium compared to the distal follicle [6]. With a location near the vital eHFSC population, MIF is thought to contribute to HFIP via its immunomodulating properties and suppression of NK attack [35, 56, 57].
Another HFIP mechanism is “no danger” signaling via the type-1 transmembrane glycoprotein CD200, which is prominently expressed in the bulge region [3, 6, 58]. Interaction of CD200 and its receptor, CD200R significantly diminishes APC activity and secretion of proinflammatory cytokines by activated T cells [59, 60]. CD200-CD200R interaction is thought to promote tolerance and prevent autoimmunity within the HF [61].
The immune cell milieu, or lack thereof, protects against HFIP collapse. In human HFs, CD4+ and CD8+ T cells are localized primarily to the distal epithelium and connective tissue sheath [2]. No intraepithelial T cells have been appreciated in the hair bulb [28]. Regulatory T cells are postulated to preserve IP regions by promoting tolerance, although this has yet to be observed in the HF [42]. As mentioned, NK cells target MHC-I-negative cells (such as melanocytes), raising a question about how the proximal follicular epithelium is spared from NK attack. Firstly, NK cells within the follicle are scarce and exclusive to the distal HF [28]. Previously noted IP mechanisms (expression of nonclassical MHC molecules, production of local immunomodulators) may also contribute to NK inhibition [6, 35, 56, 62]. Additionally, killer cell Ig-like receptors, which are essential to preventing NK cell-mediated destruction of MHC-I cells, are demonstrated at high levels in the HF [35]. By a combination of these mechanisms, the MHC-I-negative HF epithelium is exempt from NK cell destruction.
Collapsers of Immune Privilege
Collapse of mechanisms that maintain HFIP renders the HF susceptible to inflammatory assault. When subjected to immune attack, the hair growth cycle may adjust to enable follicle rehabilitation. Hair shaft proliferation might even persist, albeit inadequately, throughout the inflammatory response. Alternatively, collapse of immune privilege may engender HF destruction, which is one proposed mechanism for the pathogenesis of certain alopecias.
Collapse of immune privilege can be characterized by upregulation of MHC-I and -II expression. Growing evidence implicates the proinflammatory cytokine interferon-γ (IFNγ) in triggering HFIP collapse and ensuing immune-mediated eHFSC damage by enhancing expression of MHC I and II [6, 30, 63]. In AA, it has been proposed that greater expression of MHC-I may facilitate autoimmune attack by CD8+ T cells [64]. Compared to other cytokines believed to induce MHC-I (i.e., IL-1β, TNF-α), IFNγ has been shown to act as a robust upregulator of MHC-I in vivo in anagen hair bulbs from murine back skin [30, 33]. Furthermore, Ito and colleagues treated human scalp HFs with IFNγ; minimal doses were sufficient to trigger ectopic MHC class 1 expression, and higher doses led to premature induction of catagen in vitro [3].
Not only does IFNγ appear to compromise HFIP, it also directly threatens the eHFSC population [23, 30, 33, 35, 43, 65]. Keratin 15, β1-integrin and CD200, all reliable eHFSC markers, are markedly reduced following IFNγ stimulation [2, 58, 63, 66, 67]. Furthermore, LPP bulge cells have diminished expression of keratin 15, β1-integrin and CD200, inciting IFNγ as a possible mechanism for LPP permanent hair loss [2, 66]. LPP bulge epithelium also demonstrates reduced expression of the immunosuppressant TGFβ [2]. Furthermore, deletion of CD200 in mice stimulates enormous perifollicular inflammation and scarring hair loss [60, 61].
Mast cells induce cytotoxic CD8+ T cell proliferation [68]. Higher levels of degranulated mast cells and immature mast cell progenitors (cKit+ cells) are found in the LPP perifollicular infiltrate compared with controls [2]. Double staining reveals greater physical connection between mast cells and cytotoxic CD8+ T cells, suggesting that mast cell degranulation incites the immune response that ushers in HFIP collapse.
In recent years, NK cells have been studied for their potential role in HFIP collapse, particularly in AA. In healthy HFs, there is almost no expression of MHC class I chain-related A gene (MICA), a stress-induced ligand that activates NKG2D recognition receptors on NK and CD8+ T cells [30, 35, 69, 70, 71, 72, 73, 74, 75, 76]. NKG2D has been implicated in other autoimmune diseases, including rheumatoid arthritis and type I diabetes [35, 77, 78, 79]. Lesional AA HFs demonstrate extensive MICA immunoreactivity, and are observed to be surrounded by NKG2D+ NK and CD8+T cells [35]. Abnormally increased MICA expression may facilitate HF attack via activated NKG2D+ cells, leading to compromise of the anagen phase and hair loss. HF damage in AA may also arise from impeded suppression of NK cells [2, 35]. Compared to controls, AA patients have a significantly greater percentage of NK cells that lack the inhibitory receptor KIR-2D2/2D3 [35]. Additionally, there is markedly decreased MIF immunoreactivity, which, as noted above, helps prevent NK attack [55].
Could Stress Play a Role?
Psychological stress has garnered increased attention as a possible contributor to autoimmune pathogenesis [80]. Stress is known to impact the immune system. Several studies have observed that up to 80% of patients endorse a major psychological stressor preceding the onset of an autoimmune disease [81, 82, 83]. How stress relates to pathogenesis remains controversial, because while stress affects the immune system, autoimmune diseases in turn can also trigger stress [84, 85].
Psychological stress has been recognized as a trigger for AA [38, 86, 87, 88, 89, 90, 91]. The association of stress and LPP is not as clear, although the oral variant of lichen planus has been linked with stress [92]. Oral lichen planus often occurs 1-2 weeks following an intense psychological stressor, and erosive disease has been shown to be associated with stress [93, 94, 95].
To better characterize the role of stress in hair loss, it is important to understand how skin responds to stress. The skin utilizes a system analogous to the hypothalamic-pituitary-adrenal (HPA) axis to confront a diversity of insults [96]. During periods of physical and psychological stress, the HPA axis is activated to secrete corticotropin-releasing hormone (CRH). CRH has been found in murine HFs, and the CRH gene is transcribed by the human hair bulb [96, 97, 98, 99, 100].
Mast Cell Degranulation and CRH
As mentioned, mast cell degranulation can lead to collapse of anagen HFIP. In human HFs, CRH has been shown to trigger production of mature mast cells from local precursors and promote degranulation, suggesting that the local HF neuroendocrine axis and the immune system are intertwined [101]. Acute stress can stimulate greater CRH expression in skin. During stressful situations that lead to CRH release, mast cells are vital to the regulation of neurogenic inflammation [101, 102, 103, 104, 105, 106, 107, 108]. Researchers showed that sonic stress in mice triggers mast cell degranulation [109]. In mice lacking mast cells, sonic stress fails to induce the neurogenic inflammation and HF apoptosis that are observed in murine skin following a distressing sonic stimulus [101, 105]. In rats, acute stress has also been shown to increase the CRH skin [110, 111]. Therefore, both peripheral CRH and acute stress can trigger mast cell degranulation in the skin.
One possible mechanism for CRH influence on mast cells is stem cell factor, which is known to trigger mast cell differentiation, facilitate mast cell cytokine release, and impede mast cell apoptosis. In human HFs, CRH enhances stem cell factor transcription and translation, providing a possible link for CRH-induced mast cell degranulation [101, 102, 112].
Neurotrophins
Nerve growth factor (NGF), a broadly studied neurotrophin that has been noted in murine skin, plays a major role in stress responses [113, 114, 115, 116]. Increased NGF release during stress is thought to facilitate catagen induction by activating the p75NTR receptor expressed on the outer root sheath [117, 118, 119, 120]. Contrarily, NGF fails to elicit premature catagen when p75NTR is antagonized [117]. P75NTR directly triggers apoptosis of HF keratinocytes, as well as downregulates keratinocyte growth factor effects [121, 122]. Similar to CRH, NGF is known to stimulate mast cell degranulation, which may facilitate the neurogenic inflammation undermining HFIP [121, 123].
Substance P
Substance P (SP) is downstream of NGF. It is a neuropeptide that has been implicated in the stress response and is thought to be enhanced by NGF. Firstly, SP yields upregulation of MHC-I and β2-microglobulin, and stimulates ectopic MHC-I expression in the anagen HF [124]. Secondly, SP can activate perifollicular mast cells, leading to damaging intercutaneous neurogenic inflammation [124, 125, 126]. Thirdly, SP can stimulate growth factor cascades which favor catagen by selectively upregulating NGF and p75NTR [124]. Additionally, SP may stimulate murine mast cells to release TNFα, which has been demonstrated to prevent hair growth and induce keratinocyte apoptosis [124, 127, 128, 129, 130]. Interestingly, administration of systemic SP recapitulated sonic stress-induced apoptosis of murine HFs, whereas co-injecting a selective SP receptor antagonist prevented it.
Connecting Stress and Autoimmune Hair Loss
Taken together, these findings elucidate a plausible mechanism to connect stressful phenomenon with subsequent hair loss, summarized in Figure 2b. Psychological stress triggers the HF equivalent of the HPA axis, resulting in increased CRH secretion, which stimulates mast cell production and degranulation. The resulting neurogenic inflammation collapses HFIP and induces premature destruction of the follicle. To date, whether NGF and/or SP exist downstream of CRH is not well defined [131]. However, NGF can also act to trigger mast cell degranulation, either directly or possibly via SP.
Conclusions
Several mechanisms exist to protect the delicate HFSC population, collectively establishing HFIP to ensure the regenerative capacity of HFs. Collapse of HFIP is thought to contribute to the pathogenesis of autoimmune hair loss disorders. While both LPP and AA constitute examples of autoimmune hair loss, certain mechanisms may be more important for triggering HFIP collapse in one disease than the other.
Despite the complex etiology of autoimmune disease and the missing links in the relationship between psychological stress and hair loss, we conclude that psychological stress plays a key role in autoimmune hair loss. The skin features a local neuroendocrine axis enabling it to respond to stress. Increased activity of this HPA axis equivalent can promote neurogenic inflammation that facilitates HFIP loss.
Disclosure Statement
The authors have no conflicts of interest to disclose.
Funding Sources
This article has no funding source.
References
- 1.Sperling LC. Hair anatomy for the clinician. J Am Acad Dermatol. 1991;25:1–17. doi: 10.1016/0190-9622(91)70167-z. [DOI] [PubMed] [Google Scholar]
- 2.Harries MJ. The immunopathobiology of lichen planopilaris; PhD thesis. Manchester, University of Manchester. 2011 [Google Scholar]
- 3.Harries MJ, Paus R. The pathogenesis of primary cicatricial alopecias. Am J Pathol. 2010;177:2152–2162. doi: 10.2353/ajpath.2010.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 5.Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009;19:R132–R142. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Meyer KC, Klatte JE, Dinh HV, Harries MJ, Reithmayer K, Meyer W, Sinclair R, Paus R. Evidence that the bulge region is a site of relative immune privilege in human hair follicles. Br J Dermatol. 2008;159:1077–1085. doi: 10.1111/j.1365-2133.2008.08818.x. [DOI] [PubMed] [Google Scholar]
- 7.Harries MJ, Trueb RM, Tosti A, Messenger AG, Chaudhry I, Whiting DA, Sinclair R, Griffiths CE, Paus R. How not to get scar(r)ed: pointers to the correct diagnosis in patients with suspected primary cicatricial alopecia. Br J Dermatol. 2009;160:482–501. doi: 10.1111/j.1365-2133.2008.09008.x. [DOI] [PubMed] [Google Scholar]
- 8.Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1–37. doi: 10.1016/j.jaad.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Kang H, Alzolibani AA, Otberg N, Shapiro J. Lichen planopilaris. Dermatol Ther. 2008;21:249–256. doi: 10.1111/j.1529-8019.2008.00206.x. [DOI] [PubMed] [Google Scholar]
- 10.Soares VC, Mulinari-Brenner F, Souza TE. Lichen planopilaris epidemiology: a retrospective study of 80 cases. An Bras Dermatol. 2015;90:666–670. doi: 10.1590/abd1806-4841.20153923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meinhard J, Stroux A, Lünnemann L, Vogt A, Blume-Peytavi U. Lichen planopilaris: epidemiology and prevalence of subtypes - a retrospective analysis in 104 patients. J Dtsch Dermatol Ges. 2014;12:229-235–229-236. doi: 10.1111/ddg.12264. [DOI] [PubMed] [Google Scholar]
- 12.Handa S, Sahoo B. Childhood lichen planus: a study of 87 cases. Int J Dermatol. 2002;41:423–427. doi: 10.1046/j.1365-4362.2002.01522.x. [DOI] [PubMed] [Google Scholar]
- 13.Gilhar A, Paus R, Kalish RS. Lymphocytes, neuropeptides, and genes involved in alopecia areata. J Clin Invest. 2007;117:2019–2027. doi: 10.1172/JCI31942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messenger AG, Slater DN, Bleehen SS. Alopecia areata: alterations in the hair growth cycle and correlation with the follicular pathology. Br J Dermatol. 1986;114:337–347. doi: 10.1111/j.1365-2133.1986.tb02825.x. [DOI] [PubMed] [Google Scholar]
- 15.Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397–403. doi: 10.2147/CCID.S53985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ., 3rd Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628–633. doi: 10.4065/70.7.628. [DOI] [PubMed] [Google Scholar]
- 17.Harries MJ, Meyer KC, Chaudhry IH, Griffiths CE, Paus R. Does collapse of immune privilege in the hair-follicle bulge play a role in the pathogenesis of primary cicatricial alopecia? Clin Exp Dermal. 2010;35:637–644. doi: 10.1111/j.1365-2230.2009.03692.x. [DOI] [PubMed] [Google Scholar]
- 18.Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immuneprivilege. Nat Immunol. 2006;7:354–359. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- 19.Hong S, Van Kaer L. Immune privilege: keeping an eye on natural killer T cells. J Exp Med. 1999;190:1197–1200. doi: 10.1084/jem.190.9.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Streilein JW, Ksander BR, Taylor AW. Immune deviation in relation to ocular immune privilege. J Immunol. 1997;158:3557–3560. [PubMed] [Google Scholar]
- 21.Streilein JW, Takeuchi M, Taylor AW. Immune privilege, T-cell tolerance, and tissue-restricted autoimmunity. Hum Immunol. 1997;52:138–143. doi: 10.1016/S0198-8859(96)00288-1. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson TA, Griffith TS. A vision of cell death: insights into immune privilege. Immunol Rev. 1997;156:167–184. doi: 10.1111/j.1600-065x.1997.tb00967.x. [DOI] [PubMed] [Google Scholar]
- 23.Paus R, Ito N, Takigawa M, Ito T. The hair follicle and immune privilege. J Investig Dermatol Symp Proc. 2003;8:188–194. doi: 10.1046/j.1087-0024.2003.00807.x. [DOI] [PubMed] [Google Scholar]
- 24.Billingham RE, Transplantation immunity evoked by skin allografts and expressed in intact skin . Immunology of the Skin. Adv Biol Skin. In: Montagna W, Billingham RE, editors. vol XI. New York: Appleton-Century-Crofts; 1971. pp. pp 183–198. [PubMed] [Google Scholar]
- 25.Billingham RE, Silvers WK. A biologist's reflections on dermatology. J Invest Dermatol. 1971;57:227–240. doi: 10.1111/1523-1747.ep12261543. [DOI] [PubMed] [Google Scholar]
- 26.Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 27.Westgate GE, Craggs RI, Gibson WT. Immune privilege in hair growth. J Invest Dermatol. 1991;97:417–420. doi: 10.1111/1523-1747.ep12481002. [DOI] [PubMed] [Google Scholar]
- 28.Christoph T, Müller-Röver S, Audring H, Tobin DJ, Hermes B, Cotsarelis G, Rückert R, Paus R. The human hair follicle immune system: cellular composition and immune privilege. Br J Dermatol. 2000;142:862–873. doi: 10.1046/j.1365-2133.2000.03464.x. [DOI] [PubMed] [Google Scholar]
- 29.Bröcker EB, Echternacht-Happle K, Hamm H, Happle R. Abnormal expression of class I and class II major histocompatibility antigens in alopecia areata: modulation by topical immunotherapy. J Invest Dermatol. 1987;88:564–568. doi: 10.1111/1523-1747.ep12470166. [DOI] [PubMed] [Google Scholar]
- 30.Ito T, Ito N, Bettermann A, Tokura Y, Takigawa M, Paus R. Collapse and restoration of MHC class-I-dependent immune privilege: exploiting the human hair follicle as a model. Am J Pathol. 2004;164:623–634. doi: 10.1016/S0002-9440(10)63151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prasanna SJ, Nandi D. The MHC-encoded class I molecule, H-2Kk, demonstrates distinct requirements of assembly factors for cell surface expression: roles of TAP, Tapasin and beta2-microglobulin. Mol Immunol. 2004;41:1029–1045. doi: 10.1016/j.molimm.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Paus R, Eichmüller S, Hofmann U, Czarnetzki BM, Robinson P. Expression of classical and non-classical MHC class I antigens in murine hair follicles. Br J Dermatol. 1994;131:177–183. doi: 10.1111/j.1365-2133.1994.tb08488.x. [DOI] [PubMed] [Google Scholar]
- 33.Rückert R, Hofmann U, van der Veen C, Bulfone-Paus S, Paus R. MHC class I expression in murine skin: developmentally controlled and strikingly restricted intraepithelial expression during hair follicle morphogenesis and cycling, and response to cytokine treatment in vivo. J Invest Dermatol. 1998;111:25–30. doi: 10.1046/j.1523-1747.1998.00228.x. [DOI] [PubMed] [Google Scholar]
- 34.Moseley RP, Brown JI, Auld J, Mumtaz H, Rainey AJ, Kirkham N, Gelsthorpe K, Masters R, Smith ME. An immunohistochemical study of MHC class I expression on human Langerhans cells and melanocytes. J Pathol. 1997;181:419–425. doi: 10.1002/(SICI)1096-9896(199704)181:4<419::AID-PATH796>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 35.Ito T, Ito N, Saatoff M, Hashizume H, Fukamizu H, Nickoloff BJ, Takigawa M, Paus R. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. J Invest Dermal. 2008;128:1196–1206. doi: 10.1038/sj.jid.5701183. [DOI] [PubMed] [Google Scholar]
- 36.Bröcker EB, Echternacht-Happle K, Hamm H, Happle R. Abnormal expression of class I and class II major histocompatibility antigens in alopecia areata: modulation by topical immunotherapy. J Invest Dermatol. 1987;88:564–568. doi: 10.1111/1523-1747.ep12470166. [DOI] [PubMed] [Google Scholar]
- 37.Braud VM, Allan DS, O'Callaghan CA, Söderström K, D'Andrea A, Ogg GS, Lazetic S, Young NT, Bell JI, Phillips JH, Lanier LL, McMichael AJ. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 38.Ito T. Hair follicle is a target of stress hormone and autoimmune reactions. J Dermatol Sci. 2010;60:67–73. doi: 10.1016/j.jdermsci.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Cerwenka A, Swain SL. TGF-beta1: immunosuppressant and viability factor for T lymphocytes. Microbes Infect. 1999;1:1291–1296. doi: 10.1016/s1286-4579(99)00255-5. [DOI] [PubMed] [Google Scholar]
- 40.Wahl SM, Wen J, Moutsopoulos N. TGF-beta: a mobile purveyor of immune privilege. Immunol Rev. 2006;213:213–227. doi: 10.1111/j.1600-065X.2006.00437.x. [DOI] [PubMed] [Google Scholar]
- 41.Foitzik K, Lindner G, Mueller-Roever S, Maurer M, Botchkareva N, Botchkarev V, Handjiski B, Metz M, Hibino T, Soma T, Dotto GP, Paus R. Control of murine hair follicle regression (catagen) by TGF-beta1 in vivo. FASEB J. 2000;14:752–760. doi: 10.1096/fasebj.14.5.752. [DOI] [PubMed] [Google Scholar]
- 42.Paus R, Nickoloff BJ, Ito T. A “hairy” privilege. Trends Immunol. 2005;26:32–40. doi: 10.1016/j.it.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 43.Gilhar A, Kalish RS. Alopecia areata: a tissue specific autoimmune disease of the hair follicle. Autoimmun Rev. 2006;5:64–69. doi: 10.1016/j.autrev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Nishimura EK, Suzuki M, Igras V, Du J, Lonning S, Miyachi Y, Roes J, Beermann F, Fisher DE. Key roles for transforming growth factor beta in melanocyte stem cell maintenance. Cell Stem Cell. 2010;6:130–140. doi: 10.1016/j.stem.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanimura S, Tadokoro Y, Inomata K, Binh NT, Nishie W, Yamazaki S, Nakauchi H, Tanaka Y, McMillan JR, Sawamura D, Yancey K, Shimizu H, Nishimura EK. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 2011;8:177–187. doi: 10.1016/j.stem.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 46.Slominski A, Wortsman J, Mazurkiewicz JE, Matsuoka L, Dietrich J, Lawrence K, Gorbani A, Paus R. Detection of proopiomelanocortin-derived antigens in normal and pathologic human skin. J Lab Clin Med. 1993;122:658–666. [PubMed] [Google Scholar]
- 47.Slominski A, Botchkareva NV, Botchkarev VA, Chakraborty A, Luger T, Uenalan M, Paus R. Hair cycle-dependent production of ACTH in mouse skin. Biochim Biophys Acta. 1998;1448:147–152. doi: 10.1016/s0167-4889(98)00124-4. [DOI] [PubMed] [Google Scholar]
- 48.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 49.Slominski A, Paus R, Mazurkiewicz J. Proopiomelanocortin expression in the skin during induced hair growth in mice. Experientia. 1992;48:50–54. doi: 10.1007/BF01923606. [DOI] [PubMed] [Google Scholar]
- 50.Slominski A, Ermak G, Hwang J, Mazurkiewicz J, Corliss D, Eastman A. The expression of proopiomelanocortin (POMC) and of corticotropin releasing hormone receptor (CRH-R) genes in mouse skin. Biochim Biophys Acta. 1996;1289:247–251. doi: 10.1016/0304-4165(95)00159-x. [DOI] [PubMed] [Google Scholar]
- 51.Slominski A, Botchkareva NV, Botchkarev VA, Chakraborty A, Luger T, Uenalan M, Paus R. ACTH production in C57BL/6 mouse skin. Ann NY Acad Sci. 1999;885:448–450. doi: 10.1111/j.1749-6632.1999.tb08709.x. [DOI] [PubMed] [Google Scholar]
- 52.Paus R, Botchkarev VA, Botchkareva NV, Mecklenburg L, Luger T, Slominski A. The skin POMC system (SPS). Leads and lessons from the hair follicle. Ann NY Acad Sci. 1999;885:350–363. doi: 10.1111/j.1749-6632.1999.tb08690.x. [DOI] [PubMed] [Google Scholar]
- 53.Slominski A, Heasley D, Mazurkiewicz JE, Ermak G, Baker J, Carlson JA. Expression of proopiomelanocortin (POMC)-derived melanocyte-stimulating hormone (MSH) and adrenocorticotropic hormone (ACTH) peptides in skin of basal cell carcinoma patients. Hum Pathol. 1999;30:208–215. doi: 10.1016/s0046-8177(99)90278-2. [DOI] [PubMed] [Google Scholar]
- 54.Luger TA, Scholzen T, Brzoska T, Becher E, Slominski A, Paus R. Cutaneous immunomodulation and coordination of skin stress responses by alpha-melanocyte-stimulating hormone. Ann NY Acad Sci. 1998;840:381–394. doi: 10.1111/j.1749-6632.1998.tb09577.x. [DOI] [PubMed] [Google Scholar]
- 55.Luger TA, Scholzen TE, Brzoska T, Böhm M. New insights into the functions of alpha-MSH and related peptides in the immune system. Ann NY Acad Sci. 2003;994:133–140. doi: 10.1111/j.1749-6632.2003.tb03172.x. [DOI] [PubMed] [Google Scholar]
- 56.Apte RS, Sinha D, Mayhew E, Wistow GJ, Niederkorn JY. Cutting edge: role of macrophage migration inhibitory factor in inhibiting NK cell activity and preserving immune privilege. J Immunol. 1998;160:5693–5696. [PubMed] [Google Scholar]
- 57.Arcuri F, Cintorino M, Carducci A, Papa S, Riparbelli MG, Mangioni S, Di Blasio AM, Tosi P, Viganò P. Human decidual natural killer cells as a source and target of macrophage migration inhibitory factor. Reproduction. 2006;131:175–182. doi: 10.1530/rep.1.00857. [DOI] [PubMed] [Google Scholar]
- 58.Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB, Brady JN, Udey MC, Vogel JC. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. 2006;116:249–260. doi: 10.1172/JCI26043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barclay AN, Wright GJ, Brooke G, Brown MH. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 2002;23:285–290. doi: 10.1016/s1471-4906(02)02223-8. [DOI] [PubMed] [Google Scholar]
- 60.Rosenblum MD, Olasz EB, Yancey KB, Woodliff JE, Lazarova Z, Gerber KA, Truitt RL. Expression of CD200 on epithelial cells of the murine hair follicle: a role in tissue-specific immune tolerance? J Invest Dermatol. 2004;123:880–887. doi: 10.1111/j.0022-202X.2004.23461.x. [DOI] [PubMed] [Google Scholar]
- 61.Rosenblum MD, Yancey KB, Olasz EB, Truitt RL. CD200, a “no danger” signal for hair follicles. J Dermatol Sci. 2006;41:165–174. doi: 10.1016/j.jdermsci.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 62.Apte RS, Niederkorn JY. Isolation and characterization of a unique natural killer cell inhibitory factor present in the anterior chamber of the eye. J Immunol. 1996;156:2667–2673. [PubMed] [Google Scholar]
- 63.Harries MJ, Meyer K, Chaudhry I, E Kloepper J, Poblet E, Griffiths CE, Paus R. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle's epithelial stem cell niche. J Pathol. 2013;231:236–247. doi: 10.1002/path.4233. [DOI] [PubMed] [Google Scholar]
- 64.Paus R, Slominski A, Czarnetzki BM. Is alopecia areata an autoimmune-response against melanogenesis-related proteins, exposed by abnormal MHC class I expression in the anagen hair bulb? Yale J Biol Med. 1993;66:541–554. [PMC free article] [PubMed] [Google Scholar]
- 65.Freyschmidt-Paul P, McElwee KJ, Hoffmann R, et al. Interferon-gamma-deficient mice are resistant to the development of alopecia areata. Br J Dermatol. 2006;155:515–521. doi: 10.1111/j.1365-2133.2006.07377.x. [DOI] [PubMed] [Google Scholar]
- 66.Cotsarelis G. Gene expression profiling gets to the root of human hair follicle stem cells. J Clin Invest. 2006;116:19–22. doi: 10.1172/JCI27490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tiede S, Koop N, Kloepper JE, Fässler R, Paus R. Nonviral in situ green fluorescent protein labeling and culture of primary, adult human hair follicle epithelial progenitor cells. Stem Cells. 2009;27:2793–2803. doi: 10.1002/stem.213. [DOI] [PubMed] [Google Scholar]
- 68.Stelekati E, Bahri R, D'Orlando O, Orinska Z, Mittrücker HW, Langenhaun R, Glatzel M, Bollinger A, Paus R, Bulfone-Paus S. Mast cell-mediated antigen presentation regulates CD8+ T cell effector functions. Immunity. 2009;31:665–676. doi: 10.1016/j.immuni.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 69.Groh V, Bahram S, Bauer S, Herman A, Beauchamp M, Spies T. Cell stress-regulated human major histocompatibility complex class I gene expressed in gastrointestinal epithelium. Proc Natl Acad Sci USA. 1996;93:12445–12450. doi: 10.1073/pnas.93.22.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamamoto K, Fujiyama Y, Andoh A, Bamba T, Okabe H. Oxidative stress increases MICA and MICB gene expression in the human colon carcinoma cell line (CaCo-2) Biochim Biophys Acta. 2001;1526:10–12. doi: 10.1016/s0304-4165(01)00099-x. [DOI] [PubMed] [Google Scholar]
- 71.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 72.Carosella ED, Rouas-Freiss N, Paul P, Dausset J. HLA-G: a tolerance molecule from the major histocompatibility complex. Immunol Today. 1999;20:60–62. doi: 10.1016/s0167-5699(98)01387-5. [DOI] [PubMed] [Google Scholar]
- 73.Wu J, Song Y, Bakker AB, et al. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285:730–732. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 74.Menier C, Riteau B, Carosella ED, Rouas-Freiss N. MICA triggering signal for NK cell tumor lysis is counteracted by HLA-G1-mediated inhibitory signal. Int J Cancer. 2002;100:63–70. doi: 10.1002/ijc.10460. [DOI] [PubMed] [Google Scholar]
- 75.Middleton D, Curran M, Maxwell L. Natural killer cells and their receptors. Transpl Immunol. 2002;10:147–164. doi: 10.1016/s0966-3274(02)00062-x. [DOI] [PubMed] [Google Scholar]
- 76.Pende D, Rivera P, Marcenaro S, Chang CC, Biassoni R, Conte R, Kubin M, Cosman D, Ferrone S, Moretta L, Moretta A. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res. 2002;62:6178–6186. [PubMed] [Google Scholar]
- 77.Groh V, Bruhl A, El-Gabalawy H, Nelson JL, Spies T. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc Natl Acad Sci USA. 2003;100:9452–9457. doi: 10.1073/pnas.1632807100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Caillat-Zucman S. How NKG2D ligands trigger autoimmunity? Hum Immunol. 2006;67:204–207. doi: 10.1016/j.humimm.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 79.Jie HB, Sarvetnick N. The role of NK cells and NK cell receptors in autoimmune disease. Autoimmunity. 2004;37:147–153. doi: 10.1080/0891693042000196174. [DOI] [PubMed] [Google Scholar]
- 80.Shoenfeld Y, Isenberg DA. The mosaic of autoimmunity. Immunol Today. 1989;10:123–126. doi: 10.1016/0167-5699(89)90245-4. [DOI] [PubMed] [Google Scholar]
- 81.Brickman CM, Shoenfeld Y. The mosaic of autoimmunity. Scand J Clin Lab Invest Suppl. 2001;235:3–15. [PubMed] [Google Scholar]
- 82.Herrmann M, Schölmerich J, Straub RH. Stress and rheumatic diseases. Rheum Dis Clin North Am. 2000;26:737–763. doi: 10.1016/s0889-857x(05)70167-8. [DOI] [PubMed] [Google Scholar]
- 83.Stojanovich L. Stress and autoimmunity. Autoimmun Rev. 2010;9:A271–A276. doi: 10.1016/j.autrev.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 84.Shepshelovich D, Shoenfeld Y. Prediction and prevention of autoimmune diseases: additional aspects of the mosaic of autoimmunity. Lupus. 2006;15:183–190. doi: 10.1191/0961203306lu2274rr. [DOI] [PubMed] [Google Scholar]
- 85.Huerta PT, Kowal C, DeGiorgio LA, Volpe BT, Diamond B. Immunity and behavior: antibodies alter emotion. Proc Natl Acad Sci USA. 2006;103:678–83. doi: 10.1073/pnas.0510055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brauner GJ, Goodheart HP. Dermatologic care behind bars. J Am Acad Dermatol. 1988;18:1066–1073. doi: 10.1016/s0190-9622(88)70107-3. [DOI] [PubMed] [Google Scholar]
- 87.Muller SA, Winkelmann RK. Alopecia areata: an evaluation of 736 patients. Arch Dermatol. 1963;88:290–297. doi: 10.1001/archderm.1963.01590210048007. [DOI] [PubMed] [Google Scholar]
- 88.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 89.García-Hernández MJ, Ruiz-Doblado S, Rodriguez-Pichardo A, Camacho F. Alopecia areata, stress and psychiatric disorders: a review. J Dermatol. 1999;26:625–632. doi: 10.1111/j.1346-8138.1999.tb02063.x. [DOI] [PubMed] [Google Scholar]
- 90.Inui S, Nakajima T, Itami S. Two cases of alopecia areata responsive to fexofenadine. J Dermatol. 2007;34:852–854. doi: 10.1111/j.1346-8138.2007.00401.x. [DOI] [PubMed] [Google Scholar]
- 91.Kakourou T, Karachristou K, Chrousos G. A case series of alopecia areata in children: impact of personal and family history of stress and autoimmunity. J Eur Acad Dermatol Venereol. 2007;21:356–359. doi: 10.1111/j.1468-3083.2006.01931.x. [DOI] [PubMed] [Google Scholar]
- 92.Hampf BG, Malmström MJ, Aalberg VA, Hannula JA, Vikkula J. Psychiatric disturbance in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol. 1987;63:429–432. doi: 10.1016/0030-4220(87)90254-4. [DOI] [PubMed] [Google Scholar]
- 93.Kaplan B, Barnes L. Oral lichen planus and squamous carcinoma. Case report and update of the literature. Arch Otolaryngol. 1985;111:543–547. doi: 10.1001/archotol.1985.00800100091015. [DOI] [PubMed] [Google Scholar]
- 94.Lowental U, Pisanti S. Oral lichen planus according to the modern medical model. J Oral Med. 1984;39:224–226. [PubMed] [Google Scholar]
- 95.Boyd AS, Neldner KH. Lichen planus. J Am Acad Dermatol. 1991;25:593–619. doi: 10.1016/0190-9622(91)70241-s. [DOI] [PubMed] [Google Scholar]
- 96.Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005;19:1332–1334. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- 97.Arck PC, Handjiski B, Peters EM, Peter AS, Hagen E, Fischer A, Klapp BF, Paus R. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am J Pathol. 2003;162:803–814. doi: 10.1016/S0002-9440(10)63877-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roloff B, Fechner K, Slominski A, Furkert J, Botchkarev VA, Bulfone-Paus S, Zipper J, Krause E, Paus R. Hair cycle-dependent expression of corticotropin-releasing factor (CRF) and CRF receptors in murine skin. FASEB J. 1998;12:287–297. doi: 10.1096/fasebj.12.3.287. [DOI] [PubMed] [Google Scholar]
- 99.Slominski A, Pisarchik A, Tobin DJ, Mazurkiewicz JE, Wortsman J. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology. 2004;145:941–950. doi: 10.1210/en.2003-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ito N, Ito T, Betterman A, Paus R. The human hair bulb is a source and target of CRH. J Invest Dermal. 2004;122:235–237. doi: 10.1046/j.1523-1747.2003.22145.x. [DOI] [PubMed] [Google Scholar]
- 101.Ito N, Sugawara K, Bodó E, Takigawa M, van Beek N, Ito T, Paus R. Corticotropin-releasing hormone stimulates the in-situ generation of mast cells from precursors in the human hair follicle mesenchyme. J Invest Dermatol. 2010;130:995–1004. doi: 10.1038/jid.2009.387. [DOI] [PubMed] [Google Scholar]
- 102.Maurer M, Echtenacher B, Hültner L, Kollias G, Männel DN, Langley KE, Galli SJ. The c-kit ligand, stem cell factor, can enhance innate immunity through effects on mast cells. J Exp Med. 1998;188:2343–2348. doi: 10.1084/jem.188.12.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weber A, Knop J, Maurer M. Pattern analysis of human cutaneous mast cell populations by total body surface mapping. Br J Dermatol. 2003;148:224–228. doi: 10.1046/j.1365-2133.2003.05090.x. [DOI] [PubMed] [Google Scholar]
- 104.Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 105.Arck PC, Handjiski B, Kuhlmei A, Peters EM, Knackstedt M, Peter A, Hunt SP, Klapp BF, Paus R. Mast cell deficient and neurokinin-1 receptor knockout mice are protected from stress-induced hair growth inhibition. J Mol Med (Berl) 2005;83:386–396. doi: 10.1007/s00109-004-0627-z. [DOI] [PubMed] [Google Scholar]
- 106.Maurer M, Metz M. The status quo and quo vadis of mast cells. Exp Dermatol. 2005;14:923–929. doi: 10.1111/j.1600-0625.2005.00369.x. [DOI] [PubMed] [Google Scholar]
- 107.Weller K, Foitzik K, Paus R, Syska W, Maurer M. Mast cells are required for normal healing of skin wounds in mice. FASEB J. 2006;20:2366–2368. doi: 10.1096/fj.06-5837fje. [DOI] [PubMed] [Google Scholar]
- 108.Metz M, Maurer M. Mast cells-key effector cells in immune responses. Trends Immunol. 2007;28:234–241. doi: 10.1016/j.it.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 109.Arck PC, Handjiski B, Hagen E, Joachim R, Klapp BF, Paus R. Indications for a “brain-hair follicle axis (BHA)”: inhibition of keratinocyte proliferation and up-regulation of keratinocyte apoptosis in telogen hair follicles by stress and substance P. FASEB J. 2001;15:2536–2538. doi: 10.1096/fj.00-0699fje. [DOI] [PubMed] [Google Scholar]
- 110.Theoharides TC, Singh LK, Boucher W, et al. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinology. 1998;139:403–413. doi: 10.1210/endo.139.1.5660. [DOI] [PubMed] [Google Scholar]
- 111.Theoharides TC, Donelan JM, Papadopoulou N, Cao J, Kempuraj D, Conti P. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563–568. doi: 10.1016/j.tips.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 112.Maurer M, Theoharides T, Granstein RD, et al. What is the physiological function of mast cells? Exp Dermatol. 2003;12:886–910. doi: 10.1111/j.0906-6705.2003.0109a.x. [DOI] [PubMed] [Google Scholar]
- 113.Aloe L, Alleva E, Böhm A, Levi-Montalcini R. Aggressive behavior induces release of nerve growth factor from mouse salivary gland into the bloodstream. Proc Natl Acad Sci USA. 1986;83:6184–6187. doi: 10.1073/pnas.83.16.6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aloe L, Alleva E, De Simone R. Changes of NGF level in mouse hypothalamus following intermale aggressive behaviour: biological and immunohistochemical evidence. Behav Brain Res. 1990;39:53–61. doi: 10.1016/0166-4328(90)90120-4. [DOI] [PubMed] [Google Scholar]
- 115.Tron VA, Coughlin MD, Jang DE, Stanisz J, Sauder DN. Expression and modulation of nerve growth factor in murine keratinocytes (PAM 212) J Clin Invest. 1990;85:1085–1089. doi: 10.1172/JCI114539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paus R, Luftl M, Czarnetzki BM. Nerve growth factor modulates keratinocyte proliferation in murine skin organ culture. Br J Dermatol. 1994;130:174–180. doi: 10.1111/j.1365-2133.1994.tb02896.x. [DOI] [PubMed] [Google Scholar]
- 117.Botchkarev VA, Welker P, Albers KM, et al. A new role for neurotrophin-3:involvement in the regulation of hair follicle regression (catagen) Am J Pathol. 1998;153:785–799. doi: 10.1016/S0002-9440(10)65621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Casaccia-Bonnefil P, Carter BD, Dobrowsky RT, Chao MV. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;383:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- 119.Frade JM, Rodriguez Tebar A, Barde YA. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature. 1996;383:166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- 120.Yaar M, Zhai S, Pilch PF, et al. Binding of β-amyloid to the p75 neurotrophin receptor induces apoptosis: a possible mechanism for Alzheimer's disease. J Clin Invest. 1997;100:2333–2340. doi: 10.1172/JCI119772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Peters EM, Handjiski B, Kuhlmei A, Hagen E, Bielas H, Braun A, Klapp BF, Paus R, Arck PC. Neurogenic inflammation in stress-induced termination of murine hair growth is promoted by nerve growth factor. Am J Pathol. 2004;165:259–271. doi: 10.1016/S0002-9440(10)63294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Botchkareva NV, Botchkarev VA, Chen LH, Lindner G, Paus R. A role for p75 neurotrophin receptor in the control of hair follicle morphogenesis. Dev Biol. 1999;216:135–153. doi: 10.1006/dbio.1999.9464. [DOI] [PubMed] [Google Scholar]
- 123.Marshall JS, Gomi K, Blennerhassett MG, Bienenstock J. Nerve growth factor modifies the expression of inflammatory cytokines by mast cells via a prostanoid-dependent mechanism. J Immunol. 1999;162:4271–4276. [PubMed] [Google Scholar]
- 124.Peters EM, Liotiri S, Bodó E, Hagen E, Bíró T, Arck PC, Paus R. Probing the effects of stress mediators on the human hair follicle: substance P holds central position. Am J Pathol. 2007;171:1872–1886. doi: 10.2353/ajpath.2007.061206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Singh LK, Pang X, Alexacos N, Letourneau R, Theoharides TC. Acute immobilization stress triggers skin mast cell degranulation via corticotropin releasing hormone, neurotensin, and substance P: a link to neurogenic skin disorders. Brain Behav Immun. 1999;13:225–239. doi: 10.1006/brbi.1998.0541. [DOI] [PubMed] [Google Scholar]
- 126.Scholzen T, Armstrong CA, Bunnett NW, Luger TA, Olerud JE, Ansel JC. Neuropeptides in the skin: interactions between the neuroendocrine and the skin immune systems. Exp Dermatol. 1998;7:81–96. doi: 10.1111/j.1600-0625.1998.tb00307.x. [DOI] [PubMed] [Google Scholar]
- 127.Ansel JC, Brown JR, Payan DG, Brown MA. Substance P selectively activates TNF-alpha gene expression in murine mast cells. J Immunol. 1993;150:4478–4485. [PubMed] [Google Scholar]
- 128.Hoffmann R, Eicheler W, Huth A, Wenzel E, Happle R. Cytokines and growth factors influence hair growth in vitro. Possible implications for the pathogenesis and treatment of alopecia areata. Arch Dermatol Res. 1996;288:153–156. doi: 10.1007/BF02505825. [DOI] [PubMed] [Google Scholar]
- 129.Bläsing H, Hendrix S, Paus R. Pro-inflammatory cytokines upregulate the skin immunoreactivity for NGF, NT-3, NT-4 and their receptor, p75NTR in vivo: a preliminary report. Arch Dermatol Res. 2005;296:580–584. doi: 10.1007/s00403-005-0563-y. [DOI] [PubMed] [Google Scholar]
- 130.Tong X, Coulombe PA. Keratin 17 modulates hair follicle cycling in a TNF alpha-dependent fashion. Genes Dev. 2006;20:1353–1364. doi: 10.1101/gad.1387406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Arck PC, Slominski A, Theoharides TC, Peters EM, Paus R. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol. 2006;126:1697–1704. doi: 10.1038/sj.jid.5700104. [DOI] [PMC free article] [PubMed] [Google Scholar]