Abstract
Brunsting-Perry type pemphigoid (BPP) is a rare subepidermal blistering disease and a cause of secondary cicatricial alopecia. It was originally described by Brunsting and Perry in 1957 as a rare variant of cicatricial pemphigoid, characterized by bullous lesions limited to the head, neck, scalp, and upper trunk with mild or no mucosal involvement. We report 2 cases of BPP cicatricial alopecia with histopathology of subepidermal blister formation, different clinical presentation, and different salt-split test results. One patient had features of bullous pemphigoid (BP) with important oral mucosal involvement (not yet reported in the literature), and the second patient had typical features of epidermolysis bullosa acquisita (EBA). The secondary cicatricial alopecia may be due to different antigens associated with either BP or EBA. The phenomenon of epitope spreading could explain the association between 2 distinctive bullous diseases in the same patient, justifying the divergent findings of the immunofluorescence. The specific target antigen of BPP is yet to be defined.
Keywords: Pemphigoid, Brunsting-Perry type pemphigoid, Alopecia
Established Facts
Brunsting-Perry type pemphigoid (BPP) is a rare cause of secondary cicatricial alopecia.
BPP is a rare subepidermal blistering disease and was originally described by Brunsting and Perry in 1957 as a rare variant of cicatricial pemphigoid, characterized by bullous lesions limited to the head, neck, scalp, and upper trunk with mild or no mucosal involvement.
BPP has also been described, in a few reports, as a possible clinical variant of epidermolysis bullosa acquisita (EBA); however, the pathomechanisms and autoantigens in this condition remain unknown.
Novel Insights
We report 2 cases of BPP cicatricial alopecia with histopathology of subepidermal blister formation, different clinical presentation, and different salt-split test results. One patient had features of bullous pemphigoid (BP) with important oral mucosal involvement (not yet reported in the literature), and the second patient had typical features of EBA.
The secondary cicatricial alopecia of BPP may be associated with clinical manifestations of either BP or EBA.
The phenomenon of epitope spreading could explain the association of cicatricial alopecia of BPP with either BP or EBA, justifying the divergent findings in the salt-split immunofluorescence test.
Introduction
Brunsting-Perry type pemphigoid (BPP) was originally described by Brunsting and Perry in 1957 as a rare variant of cicatricial pemphigoid, characterized by bullous lesions limited to the head, neck, scalp, and upper trunk with mild or no mucosal involvement. Blisters on the scalp lead to secondary cicatricial alopecia [1, 2]. Autoantibodies to BP180, type VII collagen, and laminin-332 have already been detected; however, the pathomechanisms and autoantigens in this condition remain unknown [2, 3, 4].
We report 2 cases of BPP with cicatricial alopecia, with a histopathology of subepidermal blister formation, different clinical presentation, and different salt-split test results. One patient had important oral mucosal involvement, and the second patient had typical clinical features of epidermolysis bullosa acquisita (EBA).
Case Reports
Case 1
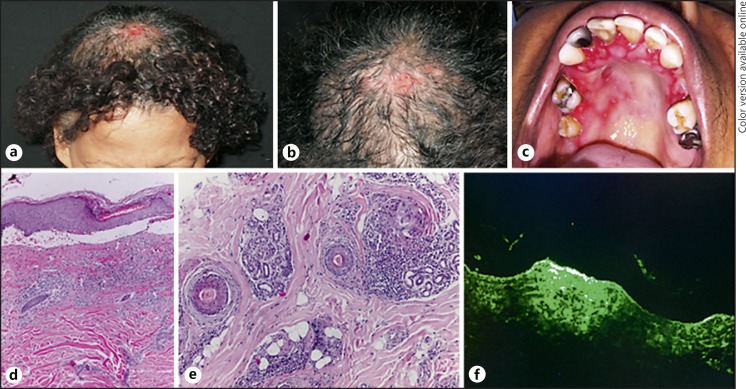
A 62-year-old black female presented with a 3-year history of bullous lesions in the oral mucosa associated with scalp blisters and cicatricial alopecia (Fig. 1a, b, c). Histological examination of the scalp biopsy showed subepidermal bullous formation (Fig. 1d) associated with scarring alopecia (Fig. 1e). The direct immunofluorescence with salt-split analyses demonstrated linear positivity with IgG and C3 antibodies in the epidermal portion of the blister (Fig. 1f). Biopsy of the oral mucosa revealed subepithelial blister with eosinophils, and immunofluorescence, performed through cytopathological examination, showed immunoreaction for IgG and C3. She was diagnosed as having BPP with exuberant oral mucous membrane involvement. She was treated with oral dapsone, oral corticosteroids, and topical corticosteroids with good improvement.
Fig. 1.
a, b Area of cicatricial alopecia in the parietal region with exulcerations. c Erosions on the hard palate and desquamative gingivitis. Histopathology of the scalp: subepidermal vesicle with influx of lymphocytes and eosinophils in the papillary dermis (d); concentric fibroplasia in the terminal follicles with lichenoid infiltrate with lymphocytes, plasma cells, and rare eosinophils (e). f Direct immunofluorescence with salt-split test of the scalp positive for IgG antibody on the epidermal side of the blister.
Case 2
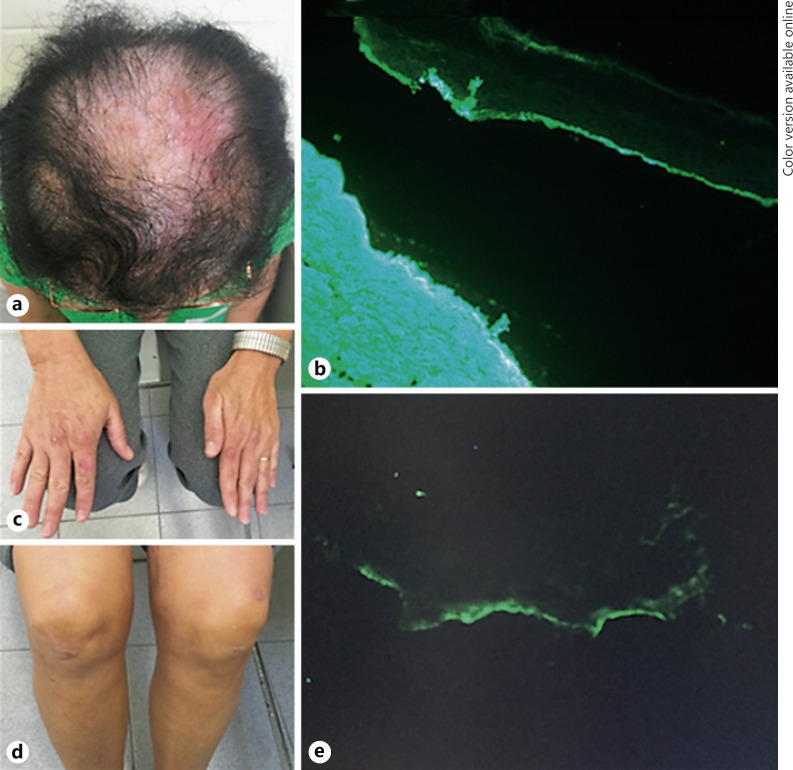
A 45-year-old white female with a 12-year history of blisters on the face, ears, scalp, hands, and knees related to minor trauma presented an extensive area of cicatricial alopecia on the parietal scalp (Fig. 2a) and atrophic scars and milia on the hands and knees (Fig. 2c, d). Scalp and face biopsy showed subepidermal bullous formation with lymphocytes, neutrophils, and eosinophils. Direct immunofluorescence with salt-split test of the scalp skin showed immunopositivity for IgA, IgG, and fibrinogen in the epidermal portion of the blister (Fig. 2b). Salt-split test of the knee skin showed linear immunopositivity for IgG in the dermal portion of the blister (Fig. 2e). She was treated with oral colchicine 0.5 mg/day and topical corticosteroids on the scalp with some improvement.
Fig. 2.
a Extensive area of cicatricial alopecia with erythema and erosions. b Direct immunofluorescence with salt-split test of the scalp positive for IgG antibody on the epidermal side of the blister. c, d Cicatricial lesions with milia formation on the dorsum of the hands and on the knees. e Direct immunofluorescence with salt-split test of the knee skin positive for IgG antibody on the dermal side of the blister.
Discussion
We report 2 cases of BPP with secondary cicatricial alopecia. The first case showed extensive mucosal involvement and a positive salt-split test of the scalp biopsy in the epidermal portion, as observed in cases of bullous pemphigoid (BP), suggesting the involvement of the antigens BP180 or BP230. This case resembles the first description of the disease, but with extensive mucosal lesions.
In the second case, the patient presented typical lesions of EBA and different salt-split test skin patterns: scalp biopsy showed a positive test on the epidermal side, suggesting PB180 and PB230 as antigens; and there was a positive knee skin biopsy on the dermal side, as observed in EBA, suggesting collagen type VII or laminin-332 as antigens. Immunoblotting and ELISA complementary exams were not performed.
Some authors consider the existence of a special type of EBA, called the Brunsting-Perry pemphigoid-like presentation, which could explain the concomitant clinical features of BPP and EBA [5]. A case of EBA was described in the literature with clinical characteristics of BPP with reactivity for type VII collagen confirmed by immunoblotting and an IgG-positive salt-split test on the dermal side of the blister, therefore, suggesting BPP to be a variant of EBA [3]. Two cases of BPP were reported with a positive ELISA for laminin-332 (laminin-5) [4]. Also, there is a report of direct and indirect immunofluorescence IgG positivity in the basement membrane zone, but no known dermal or epidermal autoantigens were detected in the immunoblot analyzes, suggesting a rare variant of BPP with autoantibodies to an indeterminate antigen from the basal membrane zone [6]. Finally, a case was described with a discrepancy between the blistering level (intra-lamina lucida) and the location of antigen (lamina densa and sub-lamina densa areas); it can be concluded that BPP includes cases showing characteristics of EBA as well as BP [7].
Recent data from animal models of autoimmune diseases suggest that the targets of immune responses in autoimmunity do not remain fixed but can be extended to include other epitopes on the same protein or other proteins in the same tissue, a phenomenon termed “epitope spreading.” The “epitope spreading” phenomenon also applies to situations in which tissue damage from a primary inflammatory process causes the release and exposure of a previously “sequestered” antigen, leading to a secondary autoimmune response against the newly released antigen [8].
A patient with both BP and EBA was reported by Fairley et al. [9] (2004). This patient exhibited both subclass shifting and development of a second autoantibody system that correlated with a change in the clinical appearance of the disease. The analysis of the patient's autoantibodies provides strong evidence for the involvement of epitope spreading in the evolution of his autoimmune disease.
Conclusion
We conclude that, despite advances in immunopathological techniques, immunobullous diseases, such as BPP, remain a major challenge in dermatological practice. The secondary cicatricial alopecia may be due to different antigens associated with either BPP or EBA. The phenomenon of epitope spreading could explain the association between 2 distinctive bullous diseases in the same patient, justifying the divergent findings of the immunofluorescences, as occurred in the second case reported here. The specific target antigen of BPP is yet to be defined.
Statement of Ethics
The patients have provided informed consent for the publication of the case reports, and the study complies with human rights.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Brunsting LA, Perry HO. Benign pemphigoid? A report of seven cases with chronic, scarring, herpetiform plaques about the head and neck. Arch Dermatol. 1957;75:489–501. doi: 10.1001/archderm.1957.01550160015002. [DOI] [PubMed] [Google Scholar]
- 2.García-Martín P, Fraga J, Hashimoto T, García-Diez A. Brunsting-Perry-type cicatricial pemphigoid with IgG autoantibodies to LAD-1. Br J Dermatol. 2014;170:735–758. doi: 10.1111/bjd.12677. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka N, Dainichi T, Ohyama B, Yasumoto S, Oono T, Iwatsuki K, et al. A case of epidermolysis bullosa acquisita with clinical features of Brunsting-Perry pemphigoid showing an excellent response to colchicine. J Am Acad Dermatol. 2009;61:715–719. doi: 10.1016/j.jaad.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Jedlickova H, Niedermeier A, Zgažarová S, Hertl M. Brunsting-Perry pemphigoid of the scalp with antibodies against laminin 332. Dermatology. 2011;222:193–195. doi: 10.1159/000322842. [DOI] [PubMed] [Google Scholar]
- 5.Gupta R, Woodley DT, Chen M. Epidermolysis bullosa acquisita. Clin Dermatol. 2012;30:60–69. doi: 10.1016/j.clindermatol.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato-Shibuya M, Dainichi T, Egawa G, Honda T, Otsuka A, Ishii N. Case with Brunsting-Perry-like localized subepidermal blister formations and immunoglobulin G antibodies against unidentified basement membrane zone antigen. J Dermatol. 2016;43:426–428. doi: 10.1111/1346-8138.13084. [DOI] [PubMed] [Google Scholar]
- 7.Minato H, Ishii N, Fukuda S, Wakasa T, Wakasa K, Sogame R, et al. Heterogeneity of Brunsting-Perry type pemphigoid: a case showing blister formation at the lamina lucida, immune deposition beneath the lamina densa and autoantibodies against the 290-kD polypeptide along the lamina densa. Dermatology. 2011;38:887–892. doi: 10.1111/j.1346-8138.2010.01172.x. [DOI] [PubMed] [Google Scholar]
- 8.Chan LS1, Vanderlugt CJ, Hashimoto T, Nishikawa T, Zone JJ, Black MM, et al. Epitope spreading: lessons from autoimmune skin diseases. J Invest Dermatol. 1998;110:103–109. doi: 10.1046/j.1523-1747.1998.00107.x. [DOI] [PubMed] [Google Scholar]
- 9.Fairley JA, Woodley DT, Chen M, Giudice GJ, Lin MS. A patient with both bullous pemphigoid and epidermolysis bullosa acquisita: an example of intermolecular epitope spreading. J Am Acad Dermatol. 2004;51:118–122. doi: 10.1016/j.jaad.2003.12.033. [DOI] [PubMed] [Google Scholar]