Abstract
Purpose
It has recently been shown that chronic noninvasive ventilation (NIV) improves a number of outcomes including survival, in patients with stable hypercapnic COPD. However, the mechanisms responsible for these improved outcomes are still unknown. The aim of the present study was to identify parameters associated with: 1) an improved arterial partial pressure of carbon dioxide (PaCO2) and 2) survival, in a cohort of hypercapnic COPD patients treated with chronic NIV.
Patients and methods
Data from 240 COPD patients treated with chronic NIV were analyzed. Predictors for the change in PaCO2 and survival were investigated using multivariate linear and Cox regression models, respectively.
Results
A higher level of bicarbonate before NIV initiation, the use of higher inspiratory ventilator pressures, the presence of anxiety symptoms, and NIV initiated following an exacerbation compared to NIV initiated in stable disease were associated with a larger reduction in PaCO2. A higher body mass index, a higher FEV1, a lower bicarbonate before NIV initiation, and younger age and NIV initiation in stable condition were independently associated with better survival. The change in PaCO2 was not associated with survival, neither in a subgroup of patients with a PaCO2 >7.0 kPa before the initiation of NIV.
Conclusion
Patients with anxiety symptoms and a high bicarbonate level at NIV initiation are potentially good responders in terms of an improvement in hypercapnia. Also, higher inspiratory ventilator pressures are associated with a larger reduction in PaCO2. However, the improvement in hypercapnia does not seem to be associated with an improved survival and emphasizes the need to look beyond PaCO2 when considering NIV initiation.
Keywords: carbon dioxide, home mechanical ventilation, exacerbation, bicarbonate, chronic obstructive pulmonary disease, respiratory insufficiency
Introduction
COPD is a leading cause of morbidity and mortality worldwide and constitutes a major healthcare burden.1,2 In the advanced stages of the disease, patients frequently develop chronic hypercapnic respiratory failure (CHRF). Noninvasive ventilation (NIV) is the standard treatment for patients with CHRF due to restrictive lung disease. In patients with CHRF due to COPD, chronic NIV has led to clinically relevant improvements in dyspnea, gas exchange, lung function, and health-related quality of life (HRQL), when sufficient inspiratory pressures and backup respiratory rates were applied.3–5 This so called high-intensity NIV is aimed at a substantial reduction in arterial partial pressure of carbon dioxide (PaCO2).5 A randomized controlled trial (RCT) by Köhnlein et al, using this NIV strategy, was the first to demonstrate a survival benefit in stable hypercapnic COPD patients after 1 year of NIV.6 As a result, chronic NIV is nowadays a standard treatment for COPD patients with CHRF and a major indication for home mechanical ventilation (HMV) in Europe.7
Nevertheless, it remains unknown how chronic NIV actually leads to these improved outcomes. While it has been hypothesized that a substantial reduction in hypercapnia is responsible for the merits of NIV and improved survival of patients on chronic NIV, a survival benefit has also been shown without a change in daytime hypercapnia and not all studies showing a reduction in hypercapnia could demonstrate improvements in survival.5,8,9 Therefore, chronic NIV might affect outcomes through several different mechanisms. With a growing prevalence of COPD, it is expected that the number of patients requiring NIV will increase. As it is clear that not all patients respond favorably to NIV, it needs to be determined which subgroup benefits most from chronic NIV, before this costly therapy can be implemented on a broader scale.
The aim of the present study was to identify parameters associated with a favorable response to chronic NIV in terms of: 1) change in PaCO2 after 1 year of NIV and 2) survival, in a cohort of hypercapnic COPD patients treated with chronic NIV.
Patients and methods
This is a prospective observational cohort study of a population of COPD patients treated with chronic NIV by the HMV center of the University Medical Center Groningen from 2005 to 2015. Data were collected in two RCTs; the RECOVER and the RESCUE trial. The RECOVER trial (ClinicalTrial NCT00135538) aimed to investigate the addition of chronic NIV to pulmonary rehabilitation in patients with stable CHRF (daytime PaCO2 >6.0 kPa). In the RESCUE trial (trial registration number NTR1100), patients with acute respiratory failure (ARF) continued NIV at home, if they had persistent hypercapnia after treatment with ventilator support. Both trials excluded patients with severe cardiac or neuromuscular disease and patients with an apnea–hypopnea index >15 per hour. Medical Ethical Committee approval (Medisch Ethische Toetsingscommissie of the University Medical Center Groningen) and written informed consent were obtained for the two trials.
As part of regular clinical practice in our center, COPD patients are initiated on chronic NIV in addition to pulmonary rehabilitation and are followed prospectively. Patients have an indication for chronic NIV once they have COPD GOLD stage III or IV combined with CHRF (PaCO2 >6.0 kPa) in a stable clinical condition. Data from these patients were also analyzed for this study.
Measurements
The following parameters were recorded before NIV initiation (baseline) and during follow-up after 3 and 12 months: demographics, comorbidities, body mass index (BMI), blood gasses, lung function, medication, and ventilator settings. Lung function was measured on patients’ own bronchodilators, and arterial blood gasses were drawn with the regular amount of oxygen applied. In patients initiated outside the trials, 3- and 12-month follow-up outcomes were collected between 2–3 months and 9–14 months, respectively, after NIV initiation. In both trials, the severe respiratory insufficiency (SRI) questionnaire was administered to assess HRQL. The SRI is a validated and highly specific tool for assessing HRQL in patients with CHRF.10 The validated Hospital Anxiety and Depression Scale (HADS) was administered in both studies for screening of symptoms of anxiety and depression (HADS score ≥8 point per domain).11 The HADS was not administered in patients initiated on NIV in regular care. These patients were assessed by a qualified psychologist, screening for symptoms of anxiety and depression (as part of our pulmonary rehabilitation program).
In both trials, polygraphies were conducted in patients with a BMI >30 kg/m2, who snored or had complaints of disrupted sleep, excessive daytime sleepiness, or morning headache. In patients initiated as part of regular care, polygraphies were conducted only in patients with a clinical suspicion. In addition, medical records were investigated for the diagnosis obstructive sleep apnea (OSA) or treatment with continuous positive airway pressure. Furthermore, the diagnosis of diabetes mellitus, hypertension, coronary artery disease, or congestive heart failure were identified in the medical records of all patients.
Noninvasive ventilation
All patients were initiated on NIV by the same registered nurse according to a standardized high-intensity protocol. NIV was supplied through a pressure cycled ventilator, applying both an inspiratory and expiratory positive airway pressure, with a backup respiratory rate (BiPAP Synchrony® and A30®, Respironics, Murrysville, PA, USA). The effectiveness of NIV during the night was observed by monitoring the transcutaneous PCO2 and O2 saturation (TOSCA® 500, Linde Medical Sensors AG, Basel, Switzerland). Supplementary oxygen was applied through the ventilator in regular amount. Monitoring of efficacy was done ambulatory using the TOSCA device.
Statistical analyses
Results are presented as mean±SD or medians and inter-quartile range (IQR). Normality of the distribution was tested by visual inspection of normal probability plots and histograms and checked with the Kolmogorov–Smirnov test. The independent samples t-test and chi-squared test, or nonparametric equivalent, were used to compare baseline characteristics of patients initiated in stable condition and patients initiated after an exacerbation. Variables with more than 40% of missing values were excluded from the analysis.
To assess prognostic value of baseline variables on the change in PaCO2, univariate regression analyses were performed with percentage of change in PaCO2 after 12 months as the dependent variable and baseline measurements as independent variables, ie, predictors. As the dependent variable is a change score, baseline PaCO2 was only excluded from this analysis. All variables that were associated with the change in PaCO2 in the univariate analysis (ie, P-value <0.1), and age, gender and diuretic use were entered in the final multivariate linear regression model.
The prognostic value of each baseline variable on mortality was assessed in the intention-to-treat population by a univariate Cox regression from initiation of NIV until 1 January 2016. The proportional hazards assumption was assessed by visual inspection of log–log plots. All variables that were associated with survival in a univariate analysis (P-value <0.1) and age, gender, and diuretic use were entered in the final multivariate Cox regression. Due to the strong association between baseline PaCO2 and bicarbonate levels, the variable with the lowest P-value was included.
All statistical analyses were performed using IBM SPSS Statistics V22 (Armonk, NY, USA). A P-value <0.05 was considered statistically significant.
Results
Study population
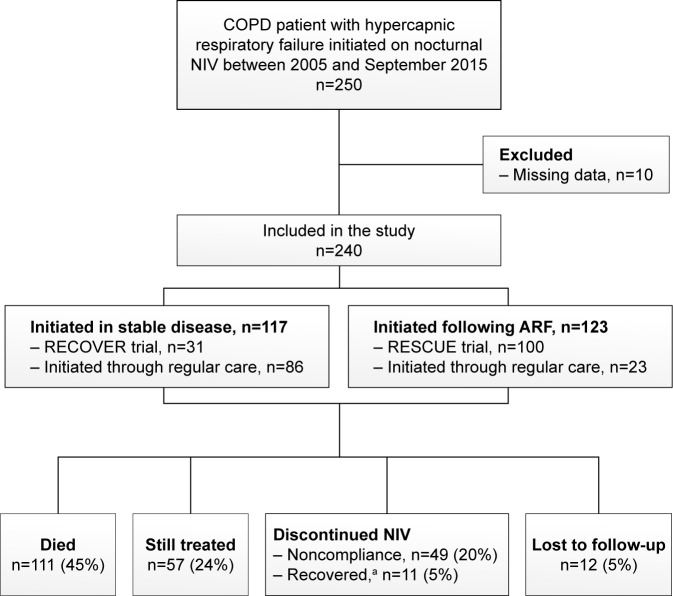
As depicted in Figure 1, 250 patients started with chronic NIV. Ten patients were excluded from the analyses because insufficient data were available. Table 1 shows the baseline characteristics for the whole group as well as separately for the COPD patients initiated in stable condition and for the COPD patients initiated after an episode of ARF. Anxiety and depression symptoms were reported in 39% and 42% of the patients, respectively. Diabetes was present in 19% of the patients, hypertension in 34%, coronary artery disease in 14%, and congestive heart failure in 13% of the patients.
Figure 1.
Flowchart of the study.
Note: aDiscontinued NIV due to lung transplantation, lung volume reduction therapy, or recovery of respiratory failure.
Abbreviations: NIV, noninvasive ventilation; ARF, acute respiratory failure.
Table 1.
Baseline characteristics of the total group of COPD patients (n=240) and separately of patients initiated in stable condition (n=117) and of patients initiated after an episode of ARF (n=123)
| Total group (n=240) | Stable COPD (n=117) | After ARFb (n=123) | P-value | |
|---|---|---|---|---|
| Age (years) | 62.5 (8.9) | 61.8 (9.1) | 63.2 (8.6) | 0.235 |
| Gender (M), n (%) | 104 (43) | 50 (43) | 54 (44) | 0.855 |
| Smoker, n (%) | 55 (23) | 24 (21) | 31 (25) | 0.369 |
| Pack yearsa | 39 (23–50) | 40 (27–45) | 38 (21–56) | 0.850 |
| Living alone, n (%) | 50 (23) | 19 (16) | 31 (25) | 0.087 |
| Exacerbations (n)a,c | 2 (0–3) | 1 (0–2) | 2 (1–4) | 0.001 |
| BMI (kg/m2) | 25.6 (5.9) | 26.5 (6.0) | 24.8 (5.6) | 0.033 |
| PaO2 (kPa) | 7.9 (1.4) | 8.1 (1.4) | 7.8 (1.5) | 0.269 |
| PaCO2 (kPa)a | 7.4 (6.8–8.4) | 7.2 (6.6–7.9) | 7.8 (7.1–9.0) | <0.001 |
| HCO3− (mmol/L) | 33.0 (1.5) | 31.7 (3.9) | 34.0 (4.7) | <0.001 |
| FVC (L) | 2.1 (0.7) | 2.2 (0.6) | 2.1 (0.7) | 0.188 |
| FEV1 (L)a | 0.63 (0.49–0.81) | 0.64 (0.49–0.79) | 0.62 (0.49–0.83) | 0.741 |
| FEV1/FVC (%) | 33 (9) | 31 (8) | 34 (10) | 0.051 |
| TLC (L) | 7.3 (1.5) | 7.3 (1.5) | 7.2 (1.6) | 0.918 |
| RV (L) | 4.8 (1.3) | 4.9 (1.3) | 4.7 (1.6) | 0.696 |
| RV/TLC (%)a | 69 (60–72) | 69 (62–72) | 66 (54–73) | 0.475 |
| SRI, total score | 49.9 (15.0) | 54.6 (14.3) | 47.9 (14.9) | 0.020 |
| HADS, total score | 15.3 (8.7) | 13.9 (7.1) | 16.0 (9.2) | 0.214 |
| LTOT, n (%) | 170 (72) | 80 (68) | 90 (73) | 0.352 |
| Medication, n (%) | ||||
| Inhaled corticosteroid | 210 (88) | 107 (92) | 103 (84) | 0.096 |
| Oral corticosteroid | 142 (59) | 59 (50) | 83 (68) | 0.006 |
| Beta agonist | 233 (98) | 113 (97) | 120 (98) | 0.379 |
| Anticholinergics | 227 (95) | 111 (95) | 116 (94) | 0.941 |
| Theophylline | 74 (31) | 35 (30) | 39 (32) | 0.732 |
| Diuretics | 105 (44) | 55 (47) | 50 (41) | 0.321 |
Notes: Last column shows P-value for comparison between patients initiated in stable condition and patients initiated after acute respiratory failure ceased but remaining hypercapnic. Data are presented as mean (SD) or
median (IQR);
NIV initiated after acute respiratory failure resolved and patients remained hypercapnic;
Number of exacerbations in 12 months prior to NIV initiation. The values in bold are considered statistically significant (P<0.05). HADS, a higher score indicates more symptoms of anxiety and depression.
Abbreviations: ARF, acute respiratory failure; HADS, Hospital Anxiety and Depression Scale; HCO3−, arterial bicarbonate; LTOT, long-term oxygen therapy; NIV, noninvasive ventilation; PaCO2, arterial carbon dioxide pressure; PaO2, arterial oxygen pressure; RV, residual volume; SRI, severe respiratory insufficiency questionnaire; TLC, total lung capacity.
Follow-up
During follow-up, 111 patients died at a median time of 14 (IQR 8–31) months after NIV initiation. All-cause mortality after 1 year was 23%. Fifty-seven patients were still treated at the time of this evaluation (median duration on NIV: 44 [IQR 23–70] months) and 49 patients dropped out because of nontolerance/noncompliance (after a median duration of NIV of 2 [IQR 1–7] months). These patients did not differ from compliant patients in baseline characteristics and comorbidities. In 11 patients, NIV was no longer needed due to lung transplantation (n=8), lung volume reduction therapy (n=1), or recovery of respiratory failure (n=2). Finally, long-term follow-up data of 12 patients could not be retrieved.
Noninvasive ventilation
After 3 months, mean inspiratory positive airway pressure was 21.9±3.9 (IQR 20–24), expiratory positive airway pressure was 5.6±1.6 (IQR 4–6) cm H2O, and backup respiratory rate was 15.9±2.8 breaths per minute. Compliance to the ventilator after 3 months was 6.6±2.0 hours per night and 80% of the patients used NIV for more than 5 hours per night. Compliance among patients who died was not different from patients who were alive at the time of the analysis (P=0.302), and compliance did not differ between patients feeling anxious (P=0.520) or depressed (P=0.220) and patients without these symptoms.
Predictors of change in PaCO2
According to the univariate regression, age, timing of NIV initiation, the SRI total score, and baseline bicarbonate levels were associated with the change in PaCO2 after 12 months (Table S1). In the multivariate regression model, a higher baseline bicarbonate level and inspiratory pressure, initiation after an exacerbation instead of initiation in stable disease, and the presence of anxiety symptoms were related to a larger decrease in PaCO2 after 12 months (Table 2).
Table 2.
Prognostic value of baseline parameters for the percentage of change in PaCO2 after 12 months for the total group of COPD patients according to the multivariate regression model
| ΔPaCO2a | |||
|---|---|---|---|
| B | 95% CI of B | P-value | |
| Constant | 38.84 | 8.59 to 69.10 | 0.013 |
| HCO3− (mmol/L) | −1.29 | −1.89 to −0.70 | <0.001 |
| NIV started after ARF | −5.54 | −10.73 to −0.36 | 0.036 |
| Anxiety | −7.48 | −13.64 to −1.32 | 0.018 |
| IPAP (cmH2O)b | −0.78 | −1.49 to −0.07 | 0.032 |
| Age (years) | 0.26 | −0.04 to 0.57 | 0.088 |
| Gender (F) | −0.16 | −5.11 to 4.79 | 0.949 |
| FEV1 (% predicted) | −0.19 | −0.46 to 0.86 | 0.177 |
| Depression | −0.71 | −5.14 to 6.56 | 0.810 |
| Diuretics | 3.46 | −1.44 to 8.37 | 0.164 |
| Congestive heart failure | −1.32 | −7.95 to 5.31 | 0.693 |
| R2 | 0.435 | ||
| n | 84 | ||
Notes:
Percentage of change in PaCO2 after 12 months, a negative value indicates a decrease in PaCO2;
IPAP after 12 months. The values in bold are considered statistically significant (P<0.05).
Abbreviations: ARF, acute respiratory failure; HCO3−, arterial bicarbonate; IPAP, inspiratory positive airway pressure; NIV, noninvasive ventilation; PaCO2, arterial carbon dioxide pressure.
Predictors of survival
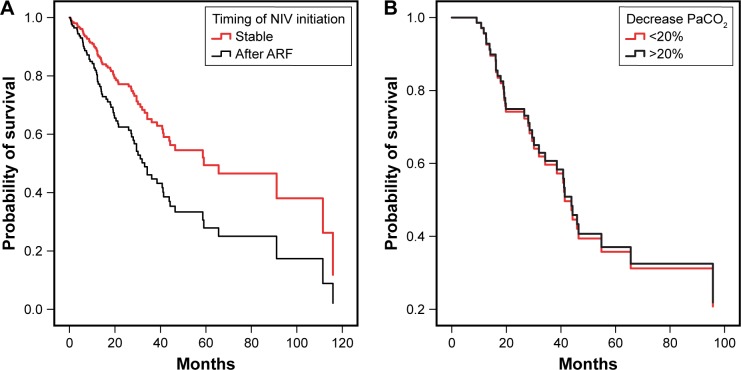
According to univariate regression, age, BMI, FEV1, PaCO2, bicarbonate levels, OSA, and timing of NIV initiation were significant determinants of survival (Table S2). Of note, the absolute and percentage of change in PaCO2 after 12 months were not associated with survival. Therefore, we conducted a subgroup analysis on patients with severe hypercapnia (baseline PaCO2 >7.0 kPa, (median 8.0 [7.3–9.0] kPa, n=163)). Also in this subgroup, the change in PaCO2 after 12 months was not associated with survival.
The multivariate Cox regression revealed that a higher age and baseline bicarbonate levels, and initiation after an exacerbation remained independently associated with an impaired prognosis, while patients with a higher BMI and FEV1 seemed to have a better prognosis (Table 3). Figure 2 illustrates the prognostic value of the timing of NIV initiation (Figure 2A) and of the percentage of change in PaCO2 after 12 months (Figure 2B) on survival.
Table 3.
Prognostic value for baseline parameters on survival according to the multivariate Cox regression analysis
| Parameter estimate | Standard error | HR | 95% CI | P-value | |
|---|---|---|---|---|---|
| Age (years) | 0.05 | 0.02 | 1.05 | 1.01 to 1.08 | 0.008 |
| BMI (kg/m2) | −0.07 | 0.03 | 0.94 | 0.89 to 0.99 | 0.011 |
| NIV started after ARF | 0.59 | 0.26 | 1.81 | 1.06 to 3.08 | 0.029 |
| HCO3− | 0.06 | 0.03 | 1.06 | 1.00 to 1.12 | 0.049 |
| FEV1 (% predicted) | −0.04 | 0.02 | 0.96 | 0.93 to 0.99 | 0.007 |
| Diuretics | 0.46 | 0.25 | 1.59 | 0.97 to 2.61 | 0.069 |
| Gender | 0.09 | 0.25 | 1.09 | 0.66 to 1.79 | 0.737 |
| OSA | −0.27 | 0.55 | 0.76 | 0.26 to 2.23 | 0.620 |
Notes: Model coefficients: -2 log likelihood 606.568; χ2=38.938; P<0.001; n=166. The values in bold are considered statistically significant (P<0.05).
Abbreviations: ARF, acute respiratory failure; HCO3−, arterial bicarbonate; NIV, noninvasive ventilation; OSA, obstructive sleep apnea.
Figure 2.
Survival curves of patients initiated on NIV. (A) Patients initiated on NIV in stable condition and after a COPD exacerbation, corrected for the remaining predictors. (B) Survival curves referring to the percentage of change in PaCO2 after 12 months for a subgroup of patient (n=69) with baseline PaCO2 >7.0 kPa.
Abbreviations: ARF, acute respiratory failure; NIV, noninvasive ventilation; PaCO2, arterial carbon dioxide pressure.
Discussion
The present study suggests that COPD patients with anxiety symptoms and high bicarbonate levels at initiation of NIV benefit most of chronic NIV in terms of improvement in PaCO2. Also, we confirmed that with higher inspiratory ventilator pressures a more pronounced improvement in gas exchange can be achieved. However, improvements in PaCO2 do not seem to influence survival. This suggests that NIV might improve survival in a subgroup of patients irrespective of the ability to improve PaCO2 and emphasizes the need to look beyond PaCO2 when treating COPD patients with chronic NIV.
Chronic NIV as a treatment for CHRF in COPD patients has long been controversial. In 2014, Köhnlein et al published the results of an important landmark study, as it showed an impressive survival benefit when chronic NIV was applied in COPD patients with severe CHRF.6 With this study, evidence for the use of NIV received an enormous boost. However, it is still unclear exactly how and why chronic NIV improves survival.
The focus of high-intensity NIV is currently on achieving a maximal reduction in PaCO2.5 Previous studies have shown conflicting results regarding the influence of chronic hypercapnia on prognosis.12,13 If hypercapnia is associated with bad prognosis, it would be more logical that by decreasing daytime PaCO2, survival would be positively influenced. In the present study, we did not find this, even not in a separate analysis of those patients with severe hypercapnia before NIV initiation.
The present study did show that the level of arterial bicarbonate at initiation is a strong predictor for both the change in PaCO2 and prognosis, independent from confounders as diuretic use, which is known to affect arterial bicarbonate. This is interesting as in clinical practice, NIV is both initiated and monitored based on PaCO2. Budweiser et al demonstrated that arterial base excess, in addition to BMI and hyperinflation, was an independent predictor for prognosis in COPD patients treated with NIV.14 They suggested that daytime base excess might better represent nocturnal hypoventilation than daytime PaCO2 as the increased level of base excess is a renal compensatory mechanism for chronically elevated levels of PaCO2.14 As bicarbonate is the predominant base contributing to base excess, our study supports this finding. We suggest that this marker of CHRF might be a therapeutic target for NIV and that NIV should be initiated and monitored based on bicarbonate. In addition, this finding suggests that patients with high bicarbonate levels will potentially be better responders to NIV.
The present study also shows that a higher BMI is associated with a better survival, independent from confounders like the presence of OSA. This is not a new finding as a cohort study by Borel et al showed that NIV is associated with better prognosis only in a subgroup of obese patients (BMI >30 kg/m2),15 a finding supported by Budweiser et al and Yang et al.13,14 We hypothesize that in the obese patients, hypercapnia is probably not solemnly a marker of severe lung disease. Sleep hypoventilation due to obesity itself can contribute to the hypercapnia in these patients. This mechanism is potentially less harmful to the patient and may explain the better prognosis of these obese patients.
Patients initiated on NIV after an episode of ARF had worse survival rates compared to the patients initiated in stable condition, despite the fact that patients initiated following an acute exacerbation did respond well in terms of improvements in hypercapnia. Analysis revealed that these patients had a larger reduction in PaCO2 compared to stable patients, independent of bicarbonate levels. The RCT of Struik et al, from which trial patient data were used for the current analysis, revealed that initiating chronic NIV directly after an episode of ARF is not beneficial and that mortality of these patients remains high,9 despite the fact that PaCO2 was reduced more when NIV was applied compared to the standard arm in which spontaneous recovery was awaited. However, a major determinant for future exacerbations and death are a history of exacerbations and hospitalizations due to exacerbations of COPD, regardless of disease severity.16,17 As the patients initiated after an exacerbation indeed did suffer from more exacerbations compared to the patients initiated in stable disease, also after the start of the therapy, it was not surprising that the current evaluation identified that patients initiated after an exacerbation had a worse prognosis compared to the stable patients. Nevertheless, it is encouraging that recent evidence has shown that chronic NIV might prevent exacerbations in a subgroup of frail severe persistent hypercapnic patients, an effect that might eventually also affect survival.18,19
Some findings of our study were surprising. First, patients with anxious or depressive feelings did not have a worse outcome. Physicians are often reluctant toward initiating these patients on NIV as in daily practice, they are known to have difficulties adapting to NIV and show poor compliance. However, in this study, NIV was not terminated more often due to noncompliance in patients feeling anxious or depressed. Furthermore, compliance at 3 months did not differ between patients suffering of these symptoms, and those without anxious or depressed feelings. Although no data are available about the time and effort it took to adapt these patients to the ventilator, they eventually seem to adapt rather well. Notably, the present study revealed that patients with anxiety symptoms responded well to NIV as they demonstrated a larger reduction in PaCO2, independent from baseline bicarbonate levels (representing severity of hypercapnia). We speculate that anxious patients over time might adopt an unfavorable breathing pattern, with increased respiratory rates that can induce air trapping. If so, NIV has great potential in improving breathing patterns at night, perhaps explaining why these patients seem to respond well to NIV. Therefore, we believe that there is no reason to doubt the indication of NIV in anxious COPD patients with CHRF. Moreover, they might be a subgroup that could benefit most from NIV.
This study confirms that higher inspiratory pressures are necessary for improvements in hypercapnia and hereby strengthens the evidence for high-intensity NIV. Surprisingly, however, compliance was not associated with the change in PaCO2, a finding not in line with the general consensus. However, compliance of the patients in the present study was excellent. Approximately 80% of the patients used NIV for more than 5 hours per night, which is the suggested minimum to achieve a substantial improvement in hypercapnia and survival.15,20 In the Netherlands, the HMV centers accommodate a unique system with comprehensive care and monitoring at the patients’ home, frequent home visits, and a 24/7 service in case of problems. We acknowledge that this may enhance compliance. The general thought exists that adherence of COPD patients to the ventilator is poor. The present study indicates that this needs to be reconsidered, at least when sufficient care and monitoring is provided.
Finally, our study does have some limitations. First, we acknowledge the lack of a control group. In addition, missing data were a concern as we had to exclude potential significant parameters as hyperinflation and HRQL from the multivariate analysis.21–23 Also, the number of missing data might not be equal between patient initiated in stable condition compared to patients initiated following an exacerbation, as in the latter group more patients were RCT recruited. We also acknowledge that OSA was not assessed by a polygraphy in all patients and therefore possibly leading to an underestimation of the presence of OSA. These limitations might have biased our results. Finally, causes of death could not be retrieved for all patients, so we could not differentiate between COPD and other causes of death.
Conclusion
Patients with anxiety symptoms and patients with high bicarbonate levels at initiation of NIV might be potential responders to NIV in terms of an improvement in hypercapnia. This improvement in hypercapnia, however, seems not to be associated with an improved survival. This finding emphasizes the need to look beyond PaCO2 when considering NIV initiation, as other mechanisms and targets might be important when applying chronic NIV.
Supplementary materials
Table S1.
Prognostic value of baseline variables for the percentage of change in PaCO2 after 12 months for the whole group of COPD patients according to the univariate regression model
| ΔPaCO2a | |||||
|---|---|---|---|---|---|
| nb | R2 | B | 95% CI of B | P-value | |
| Age (years) | 105 | 0.042 | 0.314 | 0.022 to 0.606 | 0.035 |
| Gender (F) | 105 | 0.002 | −1.057 | −6.120 to 4.005 | 0.680 |
| BMI | 99 | 0.018 | 0.290 | −0.146 to 0.726 | 0.191 |
| Smoker | 105 | 0.002 | −1.340 | −7.086 to 4.406 | 0.645 |
| NIV started after ARF | 105 | 0.055 | −6.061 | −10.976 to 1.146 | 0.016 |
| PaO2 (kPa) | 102 | 0.018 | −1.127 | −2.776 to 0.523 | 0.178 |
| HCO3− (mmol/L) | 102 | 0.247 | −1.426 | −1.919 to −0.932 | <0.001 |
| FEV1 (% predicted) | 99 | 0.019 | 0.176 | −0.082 to 0.434 | 0.179 |
| FEV1/FVC (%) | 99 | 0.004 | −0.084 | −0.347 to 0.179 | 0.526 |
| RV/TLC (%) | 44 | 0.025 | −0.215 | −0.631 to 0.202 | 0.305 |
| Diuretics | 105 | 0.005 | 1.820 | −3.214 to 6.854 | 0.475 |
| SRI, total score | 68 | 0.110 | 0.288 | 0.087 to 0.490 | 0.006 |
| Comorbid disease | |||||
| Obstructive sleep apnea | 92 | 0.007 | 4.323 | −6.622 to 15.268 | 0.435 |
| Anxiety | 96 | 0.040 | −5.449 | −10.941 to 0.044 | 0.052 |
| Depression | 95 | 0.038 | −5.118 | −10.429 to 0.193 | 0.059 |
| Diabetes mellitus | 102 | 0.004 | −2.083 | −8.772 to 4.607 | 0.538 |
| Hypertension | 97 | 0.007 | 2.288 | −3.214 to 7.791 | 0.411 |
| Coronary artery disease | 105 | 0.015 | 3.787 | −2.114 to 9.688 | 0.206 |
| Congestive heart failure | 99 | 0.029 | −6.001 | −12,993 to 0.890 | 0.091 |
| NIV settingsc | |||||
| IPAP (cmH2O) | 103 | 0.035 | −0.638 | −1.297 to 0.021 | 0.058 |
| EPAP (cmH2O) | 103 | 0.003 | −0.421 | −1.849 to 1.006 | 0.560 |
| BURR (breaths per minute) | 103 | 0.004 | −0.292 | −1.209 to 0.625 | 0.529 |
| Compliance (hours)c | 78 | 0.014 | −0.701 | −2.048 to 0.647 | 0.304 |
Notes:
Percentage of change in PaCO2 after 12 months, a negative value indicates a decrease in PaCO2;
Number of patients included in the analysis;
NIV settings and compliance after 12 months. The values in bold are considered statistically significant (P<0.05).
Abbreviations: ARF, acute respiratory failure; BURR, backup respiratory rate; EPAP, expiratory positive airway pressure; HCO3−, arterial bicarbonate; IPAP, inspiratory positive airway pressure; NIV, noninvasive ventilation; PaCO2, arterial carbon dioxide pressure; PaO2, arterial oxygen pressure; RV, residual volume; SRI, severe respiratory insufficiency questionnaire; TLC, total lung capacity.
Table S2.
Prognostic value of baseline variables and NIV settings on survival according to univariate Cox regression analysis
| na | HR | 95% CI | P-value | |
|---|---|---|---|---|
| Age (years) | 233 | 1.032 | 1.008 to 1.056 | 0.008 |
| BMI (kg/m2) | 222 | 0.938 | 0.905 to 0.973 | 0.001 |
| Pack years | 169 | 1.000 | 0.992 to 1.008 | 0.981 |
| Gender (female) | 233 | 0.733 | 0.504 to 1.068 | 0.106 |
| Living alone | 212 | 0.891 | 0.548 to 1.448 | 0.641 |
| NIV started after ARF | 233 | 2.106 | 1.420 to 3.124 | <0.001 |
| PaO2 (kPa) | 222 | 0.920 | 0.794 to 1.067 | 0.270 |
| PaCO2 (kPa) | 228 | 1.331 | 1.151 to 1.538 | <0.001 |
| ΔPaCO2 (%)b | 104 | 0.988 | 0.965 to 1.012 | 0.320 |
| ΔPaCO2 (kPa)c | 104 | 1.157 | 0.910 to 1.472 | 0.234 |
| HCO3− (mmol/L) | 221 | 1.115 | 1.067 to 1.165 | <0.001 |
| FEV1 (% predicted) | 217 | 0.968 | 0.947 to 0.990 | 0.005 |
| FEV1/FVC (%) | 215 | 0.993 | 0.970 to 1.016 | 0.526 |
| RV/TLC (%) | 110 | 1.036 | 0.988 to 1.076 | 0.066 |
| Diuretics | 233 | 1.011 | 0.695 to 1.471 | 0.955 |
| SRI, total score | 129 | 0.983 | 0.966 to 1.000 | 0.054 |
| Comorbid disease | ||||
| Obstructive sleep apnea | 192 | 0.423 | 0.185 to 0.968 | 0.042 |
| Anxiety | 209 | 1.347 | 0.900 to 2.016 | 0.148 |
| Depression | 207 | 0.849 | 0.567 to 1.273 | 0.428 |
| Diabetes mellitus | 225 | 1.369 | 0.859 to 2.182 | 0.186 |
| Hypertension | 221 | 0.864 | 0.574 to 1.301 | 0.484 |
| Coronary artery disease | 222 | 1.086 | 0.632 to 1.866 | 0.765 |
| Congestive heart failure | 223 | 1.206 | 0.714 to 2.037 | 0.483 |
| NIV settingsd | ||||
| IPAP (cmH2O) | 201 | 1.005 | 0.951 to 1.062 | 0.866 |
| EPAP (cmH2O) | 201 | 0.935 | 0.812 to 1.076 | 0.347 |
| BURR (breaths per minute) | 201 | 1.018 | 0.949 to 1.092 | 0.623 |
| Compliance (hours)d | 146 | 0.934 | 0.812 to 1.074 | 0.338 |
Notes:
Number of patients included in the analysis;
Percentage of change in PaCO2 between baseline and 12 months;
Absolute change in PaCO2 between baseline and 12 months;
NIV settings and compliance after 3 months. The values in bold are considered statistically significant (P<0.05).
Abbreviations: ARF, acute respiratory failure; BURR, backup respiratory rate; EPAP, expiratory positive airway pressure; HCO3−, arterial bicarbonate; IPAP, inspiratory positive airway pressure; NIV, noninvasive ventilation; PaCO2, arterial carbon dioxide pressure; PaO2, arterial oxygen pressure; RV, residual volume; SRI, severe respiratory insufficiency questionnaire; TLC, total lung capacity.
Acknowledgments
Both the RECOVER and the RESCUE trials, from which patient data were used for the current analysis, were funded by the Dutch Lung Foundation. The RESCUE trial was additionally funded by Philips/Respironics, Mediq TEFA, and Stichting Astma Bestrijding.
Footnotes
Disclosure
PJW reports grants and personal fees from Philips Respironics, RESMED, and VIVISOL, and grants from MediqTefa and Air Liquide, outside the submitted work. MD reports grants and personal fees from Philips Respironics, grants from RESMED Ltd, and Breas Medical, and personal fees from Vivisol BV, outside the submitted work. Funding sources had no involvement in the study design and in the collection, analysis, and interpretation of data. The other authors report no conflicts of interest in this work.
References
- 1.Chapman KR, Mannino DM, Soriano JB, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J. 2006;27(1):188–207. doi: 10.1183/09031936.06.00024505. [DOI] [PubMed] [Google Scholar]
- 2.Mannino DM. Epidemiology and global impact of chronic obstructive pulmonary disease. Semin Respir Crit Care Med. 2005;26(2):204–210. doi: 10.1055/s-2005-869539. [DOI] [PubMed] [Google Scholar]
- 3.Dreher M, Storre JH, Schmoor C, Windisch W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax. 2010;65(4):303–308. doi: 10.1136/thx.2009.124263. [DOI] [PubMed] [Google Scholar]
- 4.Duiverman ML, Wempe JB, Bladder G, et al. Two-year home-based nocturnal noninvasive ventilation added to rehabilitation in chronic obstructive pulmonary disease patients: a randomized controlled trial. Respir Res. 2011;12:112–9921. doi: 10.1186/1465-9921-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Windisch W, Storre JH, Köhnlein T. Nocturnal non-invasive positive pressure ventilation for COPD. Expert Rev Respir Med. 2015;9(3):295–308. doi: 10.1586/17476348.2015.1035260. [DOI] [PubMed] [Google Scholar]
- 6.Köhnlein T, Windisch W, Köhler D, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014;2(9):698–705. doi: 10.1016/S2213-2600(14)70153-5. [DOI] [PubMed] [Google Scholar]
- 7.Crimi C, Noto A, Princi P, et al. Domiciliary non-invasive ventilation in COPD: an international survey of indications and practices. COPD. 2016;13(4):483–490. doi: 10.3109/15412555.2015.1108960. [DOI] [PubMed] [Google Scholar]
- 8.Mcevoy RD, Pierce RJ, Hillman D, et al. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax. 2009;64(7):561–566. doi: 10.1136/thx.2008.108274. [DOI] [PubMed] [Google Scholar]
- 9.Struik FM, Sprooten RT, Kerstjens HA, et al. Nocturnal non-invasive ventilation in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure: a randomised, controlled, parallel-group study. Thorax. 2014;69(9):826–834. doi: 10.1136/thoraxjnl-2014-205126. [DOI] [PubMed] [Google Scholar]
- 10.Struik FM, Kerstjens HA, Bladder G, et al. The Severe Respiratory Insufficiency Questionnaire scored best in the assessment of health-related quality of life in chronic obstructive pulmonary disease. J Clin Epidemiol. 2013;66(10):1166–1174. doi: 10.1016/j.jclinepi.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 12.Aida A, Miyamoto K, Nishimura M, Aiba M, Kira S, Kawakami Y. Prognostic value of hypercapnia in patients with chronic respiratory failure during long-term oxygen therapy. Am J Respir Crit Care Med. 1998;158(1):188–193. doi: 10.1164/ajrccm.158.1.9703092. [DOI] [PubMed] [Google Scholar]
- 13.Yang H, Xiang P, Zhang E, et al. Is hypercapnia associated with poor prognosis in chronic obstructive pulmonary disease? A long-term follow-up cohort study. BMJ Open. 2015;5(12):e008909. doi: 10.1136/bmjopen-2015-008909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Budweiser S, Jörres RA, Riedl T, et al. Predictors of survival in COPD patients with chronic hypercapnic respiratory failure receiving noninvasive home ventilation. Chest. 2007;131(6):1650–1658. doi: 10.1378/chest.06-2124. [DOI] [PubMed] [Google Scholar]
- 15.Borel JC, Pepin JL, Pison C, et al. Long-term adherence with non-invasive ventilation improves prognosis in obese COPD patients. Respirology. 2014;19(6):857–865. doi: 10.1111/resp.12327. [DOI] [PubMed] [Google Scholar]
- 16.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 17.Müllerova H, Maselli DJ, Locantore N, et al. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147(4):999–1007. doi: 10.1378/chest.14-0655. [DOI] [PubMed] [Google Scholar]
- 18.Murphy PB, Rehal S, Arbane G, et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: a randomized clinical trial. JAMA. 2017;317(21):2177–2186. doi: 10.1001/jama.2017.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ankjærgaard KL, Maibom SL, Wilcke JT. Long-term non-invasive ventilation reduces readmissions in COPD patients with two or more episodes of acute hypercapnic respiratory failure. Eur Clin Respir J. 2016;3:28303. doi: 10.3402/ecrj.v3.28303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Struik FM, Lacasse Y, Goldstein RS, Kerstjens HA, Wijkstra PJ. Nocturnal noninvasive positive pressure ventilation in stable COPD: a systematic review and individual patient data meta-analysis. Respir Med. 2014;108(2):329–337. doi: 10.1016/j.rmed.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Budweiser S, Harlacher M, Pfeifer M, Jörres RA. Co-morbidities and hyperinflation are independent risk factors of all-cause mortality in very severe COPD. COPD. 2014;11(4):388–400. doi: 10.3109/15412555.2013.836174. [DOI] [PubMed] [Google Scholar]
- 22.Casanova C, Cote C, de Torres JP, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(6):591–597. doi: 10.1164/rccm.200407-867OC. [DOI] [PubMed] [Google Scholar]
- 23.Oga T, Taniguchi H, Kita H, et al. Analysis of the relationship between health status and mortality in hypercapnic patients with noninvasive ventilation. Clin Respir J. 2017;11(6):772–780. doi: 10.1111/crj.12415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Prognostic value of baseline variables for the percentage of change in PaCO2 after 12 months for the whole group of COPD patients according to the univariate regression model
| ΔPaCO2a | |||||
|---|---|---|---|---|---|
| nb | R2 | B | 95% CI of B | P-value | |
| Age (years) | 105 | 0.042 | 0.314 | 0.022 to 0.606 | 0.035 |
| Gender (F) | 105 | 0.002 | −1.057 | −6.120 to 4.005 | 0.680 |
| BMI | 99 | 0.018 | 0.290 | −0.146 to 0.726 | 0.191 |
| Smoker | 105 | 0.002 | −1.340 | −7.086 to 4.406 | 0.645 |
| NIV started after ARF | 105 | 0.055 | −6.061 | −10.976 to 1.146 | 0.016 |
| PaO2 (kPa) | 102 | 0.018 | −1.127 | −2.776 to 0.523 | 0.178 |
| HCO3− (mmol/L) | 102 | 0.247 | −1.426 | −1.919 to −0.932 | <0.001 |
| FEV1 (% predicted) | 99 | 0.019 | 0.176 | −0.082 to 0.434 | 0.179 |
| FEV1/FVC (%) | 99 | 0.004 | −0.084 | −0.347 to 0.179 | 0.526 |
| RV/TLC (%) | 44 | 0.025 | −0.215 | −0.631 to 0.202 | 0.305 |
| Diuretics | 105 | 0.005 | 1.820 | −3.214 to 6.854 | 0.475 |
| SRI, total score | 68 | 0.110 | 0.288 | 0.087 to 0.490 | 0.006 |
| Comorbid disease | |||||
| Obstructive sleep apnea | 92 | 0.007 | 4.323 | −6.622 to 15.268 | 0.435 |
| Anxiety | 96 | 0.040 | −5.449 | −10.941 to 0.044 | 0.052 |
| Depression | 95 | 0.038 | −5.118 | −10.429 to 0.193 | 0.059 |
| Diabetes mellitus | 102 | 0.004 | −2.083 | −8.772 to 4.607 | 0.538 |
| Hypertension | 97 | 0.007 | 2.288 | −3.214 to 7.791 | 0.411 |
| Coronary artery disease | 105 | 0.015 | 3.787 | −2.114 to 9.688 | 0.206 |
| Congestive heart failure | 99 | 0.029 | −6.001 | −12,993 to 0.890 | 0.091 |
| NIV settingsc | |||||
| IPAP (cmH2O) | 103 | 0.035 | −0.638 | −1.297 to 0.021 | 0.058 |
| EPAP (cmH2O) | 103 | 0.003 | −0.421 | −1.849 to 1.006 | 0.560 |
| BURR (breaths per minute) | 103 | 0.004 | −0.292 | −1.209 to 0.625 | 0.529 |
| Compliance (hours)c | 78 | 0.014 | −0.701 | −2.048 to 0.647 | 0.304 |
Notes:
Percentage of change in PaCO2 after 12 months, a negative value indicates a decrease in PaCO2;
Number of patients included in the analysis;
NIV settings and compliance after 12 months. The values in bold are considered statistically significant (P<0.05).
Abbreviations: ARF, acute respiratory failure; BURR, backup respiratory rate; EPAP, expiratory positive airway pressure; HCO3−, arterial bicarbonate; IPAP, inspiratory positive airway pressure; NIV, noninvasive ventilation; PaCO2, arterial carbon dioxide pressure; PaO2, arterial oxygen pressure; RV, residual volume; SRI, severe respiratory insufficiency questionnaire; TLC, total lung capacity.
Table S2.
Prognostic value of baseline variables and NIV settings on survival according to univariate Cox regression analysis
| na | HR | 95% CI | P-value | |
|---|---|---|---|---|
| Age (years) | 233 | 1.032 | 1.008 to 1.056 | 0.008 |
| BMI (kg/m2) | 222 | 0.938 | 0.905 to 0.973 | 0.001 |
| Pack years | 169 | 1.000 | 0.992 to 1.008 | 0.981 |
| Gender (female) | 233 | 0.733 | 0.504 to 1.068 | 0.106 |
| Living alone | 212 | 0.891 | 0.548 to 1.448 | 0.641 |
| NIV started after ARF | 233 | 2.106 | 1.420 to 3.124 | <0.001 |
| PaO2 (kPa) | 222 | 0.920 | 0.794 to 1.067 | 0.270 |
| PaCO2 (kPa) | 228 | 1.331 | 1.151 to 1.538 | <0.001 |
| ΔPaCO2 (%)b | 104 | 0.988 | 0.965 to 1.012 | 0.320 |
| ΔPaCO2 (kPa)c | 104 | 1.157 | 0.910 to 1.472 | 0.234 |
| HCO3− (mmol/L) | 221 | 1.115 | 1.067 to 1.165 | <0.001 |
| FEV1 (% predicted) | 217 | 0.968 | 0.947 to 0.990 | 0.005 |
| FEV1/FVC (%) | 215 | 0.993 | 0.970 to 1.016 | 0.526 |
| RV/TLC (%) | 110 | 1.036 | 0.988 to 1.076 | 0.066 |
| Diuretics | 233 | 1.011 | 0.695 to 1.471 | 0.955 |
| SRI, total score | 129 | 0.983 | 0.966 to 1.000 | 0.054 |
| Comorbid disease | ||||
| Obstructive sleep apnea | 192 | 0.423 | 0.185 to 0.968 | 0.042 |
| Anxiety | 209 | 1.347 | 0.900 to 2.016 | 0.148 |
| Depression | 207 | 0.849 | 0.567 to 1.273 | 0.428 |
| Diabetes mellitus | 225 | 1.369 | 0.859 to 2.182 | 0.186 |
| Hypertension | 221 | 0.864 | 0.574 to 1.301 | 0.484 |
| Coronary artery disease | 222 | 1.086 | 0.632 to 1.866 | 0.765 |
| Congestive heart failure | 223 | 1.206 | 0.714 to 2.037 | 0.483 |
| NIV settingsd | ||||
| IPAP (cmH2O) | 201 | 1.005 | 0.951 to 1.062 | 0.866 |
| EPAP (cmH2O) | 201 | 0.935 | 0.812 to 1.076 | 0.347 |
| BURR (breaths per minute) | 201 | 1.018 | 0.949 to 1.092 | 0.623 |
| Compliance (hours)d | 146 | 0.934 | 0.812 to 1.074 | 0.338 |
Notes:
Number of patients included in the analysis;
Percentage of change in PaCO2 between baseline and 12 months;
Absolute change in PaCO2 between baseline and 12 months;
NIV settings and compliance after 3 months. The values in bold are considered statistically significant (P<0.05).
Abbreviations: ARF, acute respiratory failure; BURR, backup respiratory rate; EPAP, expiratory positive airway pressure; HCO3−, arterial bicarbonate; IPAP, inspiratory positive airway pressure; NIV, noninvasive ventilation; PaCO2, arterial carbon dioxide pressure; PaO2, arterial oxygen pressure; RV, residual volume; SRI, severe respiratory insufficiency questionnaire; TLC, total lung capacity.