Abstract
Hepatocellular carcinoma (HCC) represents ~90% of primary liver cancers and constitutes a major global health problem. Since a decade ago, the management of advanced disease that cannot be locally treated has mainly been based on multi-targeted antiangiogenic therapies. Some have demonstrated improvement in overall survival over best supportive care in first- and second-line treatment. This study focused on the efficacy of antiangiogenics in patients with advanced HCC and particularly the rising role of ramucirumab in patients with elevated alpha-fetoprotein at diagnosis.
Keywords: hepatocellular carcinoma, antiangiogenic drugs, ramucirumab
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related deaths with the incidence on the rise worldwilde.1 The incidence/mortality ratio is approximately 1:1, indicating that most of the patients who develop HCC die because of it.2 Five-year survival rates have improved modestly to ~15%–20%, an improvement that is believed to be associated, on the one hand, with improved surveillance in identifiable high-risk patients (ie, those with hepatitis B and C viruses) and on the other hand, with surgical intervention (resection or transplant) for patients with early stage disease.3 The majority of HCCs occur in patients with chronic liver disease from viral hepatitis, alcohol abuse, and/or nonalcoholic steatohepatitis. HCC is currently the leading cause of death among patients with cirrhosis.4 Indeed, once cirrhosis has developed, HCC will occur at a rate of 2%–7% per year.5
To standardize the approach to diagnosis and treatment, consensus guidelines have been published by several organizations, including the National Comprehensive Cancer Network (NCCN), American Association for the Study of Liver Disease (AASLD), and European Association for the Study of the Liver (EASL).6–8 Similar to most other cancers, HCC is more effectively treated when it is diagnosed at an early stage leading to recommended regular surveillance and early diagnosis screening in patients known to be at high risk, including patients with cirrhosis from any cause and carriers of hepatitis B. Several biomarkers for the detection of early HCC are promising9 but alpha-fetoprotein (AFP) is the only one that remains of possible interest for clinical use. Elevated AFP of more than 20 ng/mL associated with abnormal imaging currently remains the most discriminant tool for the diagnosis of HCC. The 2012 NCCN guidelines recommend screening high-risk patients with serum AFP and liver ultrasound (US) every 6–12 months, whereas the new surveillance guidelines recommend liver US every 6 months.10 A rising AFP level associated with a liver nodule measuring larger than 1 cm should raise suspicion for HCC and warrants evaluation with cross-sectional imaging.
Multiple therapeutic options are currently available for the treatment of HCC but liver function, tumor extension, and performance status need to be taken into account. Several algorithms have been used to attempt to stratify patients into subgroups and help to determine specific therapies. The Cancer of the Liver Italian Program system includes tumor morphology (uninodular, multinodular, or extensive), Child-Pugh score, AFP, and the presence or absence of portal vein thrombosis.11 The Barcelona Clinic Liver Cancer (BCLC) system12 includes the Child-Pugh score, clinical performance status, and tumor stage (solitary, multinodular, vascular invasion, or extrahepatic spread) and categorizes patients into: 1 – early HCC (BCLC stage A1–A4), including well compensated (Child-Pugh score A) liver reserve with an excellent performance status and limited tumor burden; 2 – intermediate HCC (BCLC stage B) including moderate liver reserve (Child-Pugh score A and B), excellent performance status, and multinodular tumors; and 3 – advanced HCC (BCLC stage C) including moderate liver reserve (Child-Pugh score A and B), vascular invasion or extrahepatic spread, and a vulnerable performance status (Eastern Cooperative Oncology Group 1–2). The BCLC staging system has been repeatedly modified and recently validated13 as recommended algorithm for prognostic prediction and treatment allocation. In its last version, it was recommended that AFP serum level could be taken into account in future versions of the BCLC system. Our review focused on systemic therapies for advanced disease and, in particular, documented the recent challenging role of ramucirumab, a monoclonal antibody specifically targeting VEGFR-2.
Systemic therapies for advanced HCC
HCC and chemotherapies
Advanced HCC is recognized to be chemo-resistant to most common chemotherapies which, as single agents, have shown modest anti-tumoral responses.14 Systemic doxorubicin is the most commonly evaluated agent in clinical trials with response rates of ~20%.15 The phase III CALGB trial 80802 study failed to show the benefit of adding doxorubicin to sorafenib. The median overall survival (OS) with sorafenib monotherapy was 10.5 months vs 9 months with doxorubicin plus sorafenib.16 Two other regimens, the PIAF (cisplatin/interferon 2b/doxorubicin/fluorouracil) and FOLFOX regimens have also shown negative results with no improvement in OS.17,18
Use of chemotherapy is a category 2B recommendation as per NCCN guidelines although they are not commonly used in daily practice. Moreover, with the new data regarding tyrosine kinase inhibitors (TKI) and immunotherapies, there is no room anymore for systemic chemotherapy in HCC in 2018.
HCC and immunotherapies
Multiple checkpoint inhibitors have gained US Food and Drug Administration (FDA) approval for many different types of malignancies,19 and several clinical trials for advanced HCC patients are currently ongoing. Nivolumab, an immune checkpoint inhibitor, was studied in a phase I/II multi-cohort trial (Check Mate 040).20 A total of 262 eligible patients were enrolled, with HCC and Child-Pugh score A score cirrhosis, who were either sorafenib-naïve or had progression on sorafenib. The objective response rate was 15%–20%, irrespective of line of therapy and occurred within 3 months in 69% of responders. This study also showed 18-month OS rates of 57% in sorafenib-naïve and 44% in sorafenib-experienced patients. Nivolumab is currently being evaluated in Check Mate 459 phase III trial (NCT02576509) in comparison to sorafenib as a first-line treatment in patients with advanced HCC.
Pembrolizumab is another checkpoint inhibitor. It was studied in the single arm, open-label phase II KEYNOTE-224 study,21 that included a total of 105 patients with objective response rate of 16.3% (95% CI 9.8%–24.9%) and one complete response. Median progression-free survival (PFS) was 4.8 months and median OS was not reached. The 6-month PFS and OS rates were 43.1% and 77.9%, respectively. The phase III KEYNOTE-240 trial recently assessed the efficacy of pembrolizumab for pretreated patients with HCC (NCT02702401). Data from this study may soon be available.
One of the major striking points of the use of immunotherapies in HCC is duration of response, with some being very long-lasting. Many other clinical trials using immunotherapies alone or combined with other drugs are currently ongoing for advanced HCC, with promising results, especially with the use of immunotherapies combined with TKI.
HCC and antiangiogenics
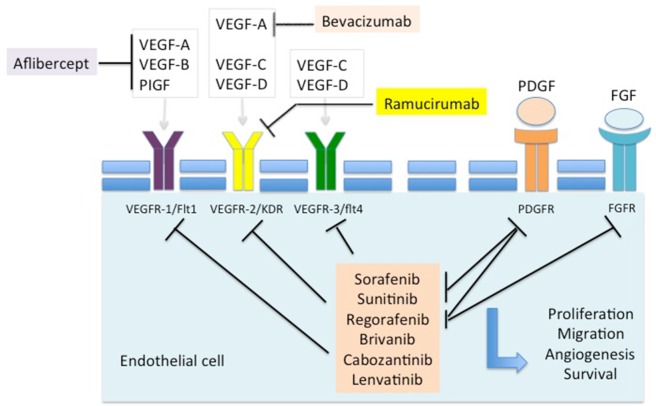
Since 2007, molecular targeted therapies have changed the landscape of advanced HCC management. As the disease is characterized by vascular recruitment as well as invasion and high microvessel density predicting early recurrence after potentially curative surgery,22 the VEGF signaling pathway is broadly involved in HCC angiogenesis and lymphangiogenesis and seems to play a crucial role in disease pathogenesis.23,24 The use of antiangiogenic molecules, and in particular TKI, is now well established and so far seems to be the most effective compounds to treat advanced HCC (Figure 1).
Figure 1.
Targets of inhibition in approved antiangiogenic agents.
Effective first-line targeted therapies
Sorafenib (Bayer AG, Leverkusen, Germany), an oral multi-TKI, has been approved by the FDA and the European Medicines Agency (EMA) for the treatment of HCC patients with well-preserved liver function (Child-Pugh score A), advanced tumors (BCLC–C) or earlier stage tumors progressing upon or unsuitable for loco-regional therapies. Its use as a standard first-line systemic therapy is based on phase III data in Western countries and in Asia-Pacific region, showing a survival benefit of almost 3 months with an OS of 10.7 months compared to 7.9 months in the treated group and nontreated group, respectively (HR 0.69; 95% CI 0.55–0.87; P=0.00058).25 Over 30% of the patients in the phase III SHARP study had been previously treated with liver-directed therapies (chemoembolization) prior to entry. Although the objective response rate (according to modified WHO criteria) was ~2%, most patients obtained disease control (disease control rates up to 70% in several phase III trials). Of note, neither the use of sorafenib in the adjuvant setting after resection,26 nor combined with embolization27 is convincing, with reasonable toxicity profiles but a slight improvement in efficacy.28 Sorafenib, like other angiogenesis inhibitors, has known therapeutic class side effects and the discontinuation due to adverse events (AEs) might reach up to 15% of patients.29 The most common drug-related AEs include skin-related toxicities such as rash and pruritus, hypertension, proteinuria, diarrhea, and an increased risk for thromboembolism and bleeding events.29 While initial responses can be observed with sorafenib, over time a loss of efficacy is apparent that may be due to “resistance” via escape/compensatory mechanisms.
On the other hand, the recent multicenter phase III trial of lenvatinib, an oral multi-TKI of VEGF receptors 1–3, FGF receptors 1–4, PDGF receptor α, RET, and KIT showed positive data in the first-line setting. A total of 954 eligible patients were randomly assigned to lenvatinib (12 or 8 mg/day, depending on bodyweight) (n=478) or sorafenib 800 mg/day (n=476). Median survival time for lenvatinib of 13.6 months (95% CI 12.1–14.9) was noninferior to sorafenib (12.3 months [10.4–13.9]; HR 0.92, 95% CI 0.79–1.06).30 As a result, lenvatinib is also recommended in first-line therapy for HCC patients with well-preserved liver function (Child-Pugh score A), good performance status, and without main portal vein invasion.
Several other antiangiogenic therapies have been tested in the advanced HCC setting. Among them, the most studied multi-targeted TKI, sunitinib (Pfizer, Inc., New York, NY, USA), was compared to sorafenib in patients with advanced HCC. The phase III SUN 1170 trial was discontinued early due to increased serious sunitinib-related AEs and futility, with improbability to achieve noninferiority.31 Finally, both the dual inhibitor of VEGF and FGF receptors brivanib32 and the anti-VEGF and PDGF compound linifanib33 have also failed to improve outcome over sorafenib.
Effective second-line targeted therapies
In the second-line setting, regorafenib was approved in 2017 by the FDA and EMA due to the demonstration of an improved OS in the RESORCE study, with an HR of 0.63 (95% CI 0.50–0.79; P<0.0001) over placebo in second line after failure of sorafenib in patients who can tolerate it. The median survival was 10.6 months for regorafenib vs 7.8 months for placebo.34 Another agent with antiangiogenic property, cabozantinib, a multikinase inhibitor that targets c-MET, VEGFRs, AXL, RET, KIT, and FLT3, showed, in a phase II trial, clinical activity including objective tumor responses, disease stabilization, and reductions in AFP in patients with HCC in both treatment-naïve and sorafenib-treated patients.35 The phase III trial, CELESTIAL, compared cabozantinib at a dose of 60 mg/day vs placebo in second or third line of advanced HCC.36 The study was not only positive on primary endpoint with an improved OS of 2.2 months in the experimental arm (10.2 vs 8.0 months; HR=0,76; P=0.0005) but also on secondary objectives with improved PFS (5.2 vs 1.9 months; P<0.001) and response rate (4% vs 0% [P=0.009]). In the cohort receiving sorafenib in first line then cabozantinib in second line, median OS improved from 7.2 months in the placebo arm up to 11.3 months with manageable toxicities.
Of note, some phase II clinical trials using bevacizumab, a monoclonal antibody against VEGF, demonstrated interesting results in terms of response rates, but severe toxicities including digestive hemorrhages.37,38 No phase III studies have been carried out, but the impressive results of synergistic clinical activity associated with checkpoint inhibitor atezolizumab in a phase I/II trial has revived this option.39 An expansion of this HCC cohort and evaluation of atezolizumab+bevacizumab in a phase III study are underway (clinical trial information: NCT02715531).
Potential role of ramucirumab in HCC
Ramucirumab
Ramucirumab (IMC-1121B) is a fully human IgG1 monoclonal antibody that selectively targets VEGFR-2. This agent was discovered from an antibody phage display library constructed from the pooled B-lymphocytes of nonimmunized healthy human donors.40 The pharmacokinetics of ramucirumab was studied in a phase I trial that enrolled 37 subjects. Patients diagnosed with advanced malignancies were treated with doses ranging from 2 to 16 mg/kg infused weekly.41 All patients demonstrated trough levels >20 µg/mL, which was the target value associated with anticancer activity. In the phase II trial with advanced HCC patients, treatment with ramucirumab 8 mg/kg every 2 weeks demonstrated an increase in serum VEGF and PlGF and a transient decrease in soluble VEGFR-2 levels,42 leading to the dose of 8 mg/kg every 2 weeks as the recommended dose for future trials. In 2015, the FDA and EMA first approved ramucirumab in patients with advanced gastric cancer based on two randomized, double-blind, positive placebo-controlled phase III trials, the REGARD and RAINBOW trials. In the REGARD trial,43 patients with disease progression after first-line therapy, including a fluoropyrimidine and platinum agent, were treated either with ramucirumab or placebo. The median OS was 5.2 months in the experimental arm vs 3.8 months (HR 0.776, 95% CI 0.603–0.998; P=0.047). The RAINBOW trial44 compared, as a second line, the combination of paclitaxel plus ramucirumab vs paclitaxel alone and demonstrated a significant improvement in survival, with a median OS of 9.6 vs 7.4 months (HR 0.807, 95% CI 0.678–0.962; P=0.017).
Ramucirumab and HCC
In recent years, ramucirumab was also used for treating advanced HCC. A phase II study of 42 patients with advanced HCC and well-preserved liver function (75% Child-Pugh score A status) showed that first-line ramucirumab monotherapy gave a disease control rate of 50% and median PFS of 4.3 months.45 This positive study prompted the initiation of the phase III REACH trial,46 which compared ramucirumab/supportive care vs placebo/supportive care in patients with advanced HCC who had received first-line sorafenib. A total of 644 patients with HCC were enrolled and initially stratified according to Child-Pugh score (A or B). However, based on the independent data monitoring committee evaluation, the protocol was amended to exclude patients with Child- Pugh score B disease due to severe toxicities. The final intent-to-treat (ITT) population comprised only patients with Child-Pugh score A disease (n=565). They received either ramucirumab 8 mg/kg or placebo intravenously every 2 weeks. The study was negative in terms of primary endpoint. The experimental arm did not demonstrate a significant OS improvement over best supportive care; however, improvements in PFS and response rate were observed. A post hoc analysis of East Asians (n=252) and non-East Asians (n=313) in the ITT population was performed in 2016 and did not show any differences between the two groups neither in terms of OS nor toxicity.47 A secondary prespecified subgroup analysis according to Child-Pugh score48 showed a potential OS benefit for patients with a Child-Pugh score of 5 (HR 0.80, 95% CI 0.63–1.02, P=0.06), but no apparent benefit for patients with Child-Pugh scores of 6 or 7 and 8 (HR 0.96, 95% CI 0.71–1.28, P=0.76 and HR 1.00, 95% CI 0.62–1.60, P>0.99, respectively). The PFS HRs were 0.59 (95% CI 0.47–0.74, P<0.001) for patients with a Child-Pugh score of 5, 0.78 (95% CI 0.58–1.04, P=0.09) for patients with a Child-Pugh score of 6, and 0.74 (95% CI 0.46–1.19, P=0.22) for patients with Child-Pugh scores of 7 and 8. In another prespecified analysis, patients with Child-Pugh scores of 5 and 6 and baseline serum AFP of 400 ng/mL or more (n=290), derived a clear OS benefit (HR 0.61 and 0.64, respectively; P<0.05); however, the PFS was favorable only for patients with a Child-Pugh score of 5 (HR 0.66, 95% CI 0.47–0.93, P=0.02). In patients with baseline AFP levels less than 400 ng/mL (n=344), no apparent OS improvement was observed in any Child-Pugh subgroup. Due to these aforementioned observations, ramucirumab was then investigated in a designed randomized 2:1, double-blind, placebo-controlled phase III study, REACH-2, which enrolled 292 Child-Pugh score A score, performance status 0–1 patients with elevated AFP levels at baseline (≥400 ng/mL) with OS as primary endpoint (Figure 2). Earlier in 2018, a Lilly Press release announced that the study met the OS endpoint,49 the first results were presented at an ASCO Meeting in 2018 in Chicago, USA, IL. The study was positive in its primary objective as well as in its secondary objectives (disease control rate, PFS). The median OS in the ramucirumab arm was 8.5 months vs 7.3 months in the placebo arm (HR 0.710, 95% CI 0.531–0.949, P=0.01). The survival rate at 12 and 18 months were 36.8% and 24.5% in the ramucirumab arm vs 30.3% and 11.3% in the placebo arm. The median PFS was also improved in the ramucirumab arm (2.8 vs 1.6 months, P<0.001), however, even though the response rate was better in the experimental arm, it was still very low (4.6% vs 1.1%).50 Similar to what was observed in the first REACH study, significant improvement of disease control rate was observed in the majority of patients (59.9% vs 38.9%), however, a significant improvement of objective response rate with ramucirumab treatment was only observed in a subgroup of patients with Child-Pugh scores of 5.
Figure 2.
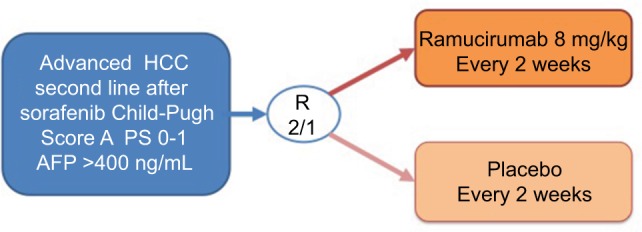
The REACH-2 trial design.
Abbreviations: AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma; PS, performance status.
For both REACH and REACH-2 trials, the most frequent AEs in the ramucirumab arm included hypertension (12.2%, grade 3), decreased appetite, nausea, asthenia (7%, grade 3), headache, and peripheral edema. However, the tolerance profile of ramucirumab seemed to be globally better than TKI profile.34 The incidence of any-grade liver injury and/or failure AEs including clinical and laboratory events was higher in the ramucirumab arm (81.6%) compared with the placebo arm (55.3%). The number of patients who experienced hepatic encephalopathy in the treatment arm was low (n=4). These results are consistent with the observed survival benefit in the ITT population with baseline AFP levels either 400 ng/mL or more or less than 400 ng/mL reported previously.46 So far, except for this new “biomarker AFP” used in advanced HCC, no other consistent predictive cancer biomarkers are currently being used to guide patient selection for systemic antiangiogenic treatments. Surprisingly, in the BRISK-FL trial comparing brivanib, a dual inhibitor of FGF and VEGF receptors, to placebo, in second-line postsorafenib, the effi-cacy of brivanib was borderline significant for patients with AFP levels less than 200 ng/mL! As described previously, mounting evidence suggests that in a particular subclass of patients with HCC who have elevated AFP levels and poor prognosis, intratumoral conditions may exist that enhance sensitivity to VEGFR-2 inhibition. A recent study51 reported that TP53 mutations are strongly correlated to the novel macrotrabecular-massive subtype of HCC (MTM-HCC). This subtype, representing 10% of all HCC, is characterized by a predominant macrotrabecular growth pattern, more frequent in hepatitis virus B infected patients, with high AFP serum levels and histological features of aggressive ness (satellite nodules, macrovascular and microvascular invasion). TP53 mutations were mostly identified in poorly differentiated tumors with frequent vascular invasion and activation of cell proliferation, angiogenesis, and epithelial-to-mesenchymal transition. Fang et al demonstrated that silencing the expression of ANGPT2 growth factor, known to promote neoangiogenesis and endothelial sprouting in cooperation with VEGFA,52 by neoplastic cells was able to disrupt the formation of the MTM-HCC pattern and reduce both intrahepatic and pulmonary metastases.53 Higher ANGPT2 mRNA levels in MTM-HCC and a trend toward an increase in VEGFA expression may open novel therapeutic strategies for this highly aggressive HCC subtype, as inhibitors of both ANGPT2 and VEGFA signaling have shown great in vivo anti-tumor efficacy if used in combination.54
Conclusion
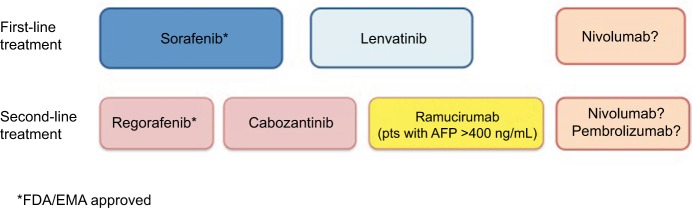
In mid-2018, sorafenib remains the gold standard first-line therapy for advanced HCC; recently, lenvatinib has been shown to be noninferior to sorafenib in first-line therapy. In patients who tolerated and progressed under sorafenib, regorafenib is the second-line therapy of choice. In 2018, two other drugs, a multi-TKI, cabozantinib, and a monoclonal antibody, ramucirumab (only in patients with high baseline AFP serum levels), were demonstrated as efficient in second-line therapy (Figure 3).
Figure 3.
The hepatocellular carcinoma management landscape in 2018.
Abbreviations: AFP, alpha-fetoprotein; EMA, European Medicines Agency; FDA, US Food and Drug Administration; pts, patients.
Currently, immunotherapy seems very promising in advanced HCC treatment, with an improved tumor response rate and durability of response in first-line (nivolumab) therapy and for patients who had failed on sorafenib (nivolumab and pembrolizumab). However, nowadays, systemic targeted therapies in the treatment of advanced HCC remain the most effective compounds even though most of them have multi-target antiangiogenic functions. This is the first time that a one-target antiangiogenic agent (ramucirumab – VEGFR-2) demonstrated positive results in a phase III trial underlining the potential antitumor effect of these new classes of angiogenesis inhibitors in HCC patients; moreover, this drug is not efficient for all patients but works mainly in patients with high AFP levels. In the coming years, we may have many trials testing these different agents alone or in combination (TKI+immunotherapies), providing many new treatment options for patients with HCC.
Footnotes
Author contributions
M Gilabert and JL Raoul contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015;61(1):191–199. doi: 10.1002/hep.27388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sangiovanni A, Prati GM, Fasani P, et al. The natural history of compensated cirrhosis due to hepatitis C virus: a 17-year cohort study of 214 patients. Hepatology. 2006;43(6):1303–1310. doi: 10.1002/hep.21176. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35(3):519–524. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018 doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Sherman M, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benson AB, D’Angelica MI, Abbott DE, et al. NCCN Guidelines Insights: Hepatobiliary Cancers, Version 1.2017. J Natl Compr Canc Netw. 2017;15(5):563–573. doi: 10.6004/jnccn.2017.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrero JA, Lok AS. Newer markers for hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S113–S119. doi: 10.1053/j.gastro.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Costentin CE, Layese R, Bourcier V, et al. Compliance with hepatocellular carcinoma surveillance guidelines associated with increased lead-time adjusted survival of patients with compensated viral cirrhosis: a multi-center cohort study. Gastroenterology. 2018;155(2):431–442. doi: 10.1053/j.gastro.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 11.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28(3):751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 13.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 14.Taieb J, Barbare JC, Rougier P. Medical treatments for hepatocellular carcinoma (HCC): what’s next? Ann Oncol. 2006;17(Suppl 10):x308–x314. doi: 10.1093/annonc/mdl279. [DOI] [PubMed] [Google Scholar]
- 15.Choi TK, Lee NW, Wong J. Chemotherapy for advanced hepatocellular carcinoma. Adriamycin versus quadruple chemotherapy. Cancer. 1984;53(3):401–405. doi: 10.1002/1097-0142(19840201)53:3<401::aid-cncr2820530306>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 16.Abou-Alfa GK, Niedzwieski D, Knox JJ, et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance) J Clin Oncol. 2016;34(4_suppl):192. [Google Scholar]
- 17.Yeo W, Mok TS, Zee B, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97(20):1532–1538. doi: 10.1093/jnci/dji315. [DOI] [PubMed] [Google Scholar]
- 18.Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31(28):3501–3508. doi: 10.1200/JCO.2012.44.5643. [DOI] [PubMed] [Google Scholar]
- 19.Liu B, Song Y, Liu D. Recent development in clinical applications of PD-1 and PD-L1 antibodies for cancer immunotherapy. J Hematol Oncol. 2017;10(1):174. doi: 10.1186/s13045-017-0541-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crocenzi TS, El-Khoueiry AB, Yau TC, et al. Nivolumab (nivo) in sorafenib (sor)-naive and -experienced pts with advanced hepatocellular carcinoma (HCC): CheckMate 040 study. J Clin Oncol. 2017;35(15_suppl):4013. [Google Scholar]
- 21.Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 22.Poon RT, Ng IO, Lau C, et al. Tumor microvessel density as a predictor of recurrence after resection of hepatocellular carcinoma: a prospective study. J Clin Oncol. 2002;20(7):1775–1785. doi: 10.1200/JCO.2002.07.089. [DOI] [PubMed] [Google Scholar]
- 23.Yao DF, Wu XH, Zhu Y, et al. Quantitative analysis of vascular endothelial growth factor, microvascular density and their clinicopathologic features in human hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2005;4(2):220–226. [PubMed] [Google Scholar]
- 24.Mäkinen T, Veikkola T, Mustjoki S, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. Embo J. 2001;20(17):4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Llovet JM, Ricci S, Mazzaferro V, SHARP Investigators Study Group Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 26.Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16(13):1344–1354. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 27.Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64(5):1090–1098. doi: 10.1016/j.jhep.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Meyer T, Fox R, Ma YT, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(8):565–575. doi: 10.1016/S2468-1253(17)30156-5. [DOI] [PubMed] [Google Scholar]
- 29.Roodhart JM, Langenberg MH, Witteveen E, Voest EE. The molecular basis of class side effects due to treatment with inhibitors of the VEGF/VEGFR pathway. Curr Clin Pharmacol. 2008;3(2):132–143. doi: 10.2174/157488408784293705. [DOI] [PubMed] [Google Scholar]
- 30.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 31.Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31(32):4067–4075. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 32.Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31(28):3517–3524. doi: 10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- 33.Cainap C, Qin S, Huang WT, et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33(2):172–179. doi: 10.1200/JCO.2013.54.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 35.Kelley RK, Verslype C, Cohn AL, et al. Cabozantinib in hepatocellular carcinoma: results of a phase 2 placebo-controlled randomized discontinuation study. Ann Oncol. 2017;28(3):528–534. doi: 10.1093/annonc/mdw651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siegel AB, Cohen EI, Ocean A, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26(18):2992–2998. doi: 10.1200/JCO.2007.15.9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas MB, Garrett-Mayer E, Anis M, et al. A randomized phase II open-label multi-institution study of the combination of bevacizumab and erlotinib compared to sorafenib in the first-line treatment of patients with advanced hepatocellular carcinoma. Oncology. 2018;94(6):329–339. doi: 10.1159/000485384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stein S, Pishvaian MJ, Lee PS, et al. Safety and clinical activity of 1L atezolizumab + bevacizumab in a phase Ib study in hepatocellular carcinoma (HCC). 2018 ASCO Annual Meeting; June 3, 2018; Chicago, IL. 2018. Abstract. Abstract 4074. [Google Scholar]
- 40.Lu D, Jimenez X, Zhang H, et al. Selection of high affinity human neutralizing antibodies to VEGFR2 from a large antibody phage display library for antiangiogenesis therapy. Int J Cancer. 2002;97(3):393–399. doi: 10.1002/ijc.1634. [DOI] [PubMed] [Google Scholar]
- 41.Spratlin JL, Cohen RB, Eadens M, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28(5):780–787. doi: 10.1200/JCO.2009.23.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu AX, Finn RS, Mulcahy M, et al. A phase II and biomarker study of ramucirumab, a human monoclonal antibody targeting the VEGF receptor-2, as first-line monotherapy in patients with advanced hepatocellular cancer. Clin Cancer Res. 2013;19(23):6614–6623. doi: 10.1158/1078-0432.CCR-13-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383(9911):31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 44.Wilke H, Muro K, van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 45.Zhu AX, Finn RS, Mulcahy MF. A phase II study of ramucirumab as first-line monotherapy in patients (pts) with advanced hepatocellular carcinoma (HCC) [abstract] J Clin Oncol. 2010;284083(15s) [Google Scholar]
- 46.Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859–870. doi: 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- 47.Park JO, Ryoo BY, Yen CJ, et al. Second-line ramucirumab therapy for advanced hepatocellular carcinoma (REACH): an East Asian and non-East Asian subgroup analysis. Oncotarget. 2016;7(46):75482–75491. doi: 10.18632/oncotarget.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu AX, Baron AD, Malfertheiner P, et al. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma: analysis of REACH trial results by Child-Pugh score. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.4115. [DOI] [PubMed] [Google Scholar]
- 49.Lilly . Lilly announces CYRAMZA® (ramucirumab) phase 3 REACH-2 study in second-line hepatocellular carcinoma patients met overall survival endpoint [press release] Indianapolis: Lilly; 2018. Apr 4, [Accessed September 13, 2018]. Available from: https://investor.lilly.com/news-releases/news-release-details/lilly-announces-cyramzar-ramucirumab-phase-3-reach-2-study. [Google Scholar]
- 50.Zhu AX, Kang Y-K, Yen CJ. REACH-2: A randomized, double-blind, placebo-controlled phase 3 study of ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated baseline alpha-fetoprotein (AFP) following first-line sorafenib. 2018 ASCO Annual Meeting; June 4, 2018; Arie Crown Theater. Abstract 4003. [Google Scholar]
- 51.Calderaro J, Couchy G, Imbeaud S, et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J Hepatol. 2017;67(4):727–738. doi: 10.1016/j.jhep.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 52.Gerald D, Chintharlapalli S, Augustin HG, Benjamin LE. Angiopoietin-2: an attractive target for improved antiangiogenic tumor therapy. Cancer Res. 2013;73(6):1649–1657. doi: 10.1158/0008-5472.CAN-12-4697. [DOI] [PubMed] [Google Scholar]
- 53.Fang JH, Zhou HC, Zhang C, et al. A novel vascular pattern promotes metastasis of hepatocellular carcinoma in an epithelial-mesenchymal transition-independent manner. Hepatology. 2015;62(2):452–465. doi: 10.1002/hep.27760. [DOI] [PubMed] [Google Scholar]
- 54.Rigamonti N, Kadioglu E, Keklikoglou I, et al. Role of angiopoietin-2 in adaptive tumor resistance to VEGF signaling blockade. Cell Rep. 2014;8(3):696–706. doi: 10.1016/j.celrep.2014.06.059. [DOI] [PubMed] [Google Scholar]