Abstract
A radial artery pseudoaneurysm represents a rare, potentially catastrophic complication of arterial cannulation that has been reported after cardiac catheterization. Treatment options are limited to chemical, mechanical, and combined approaches to obliterate the radial artery pseudoaneurysm and tract. Manual compression protocols using the TR Band have been variableand anecdotal, without objective measurements of adequate compression, making this technique prone to failure (1). In this report, we present an efficient, safe, and noninvasive management for treatment of radial artery pseudoaneurysms that is cost-effective and efficient and ensures correction without occlusion of the radial artery.
Key words: Cardiac catheterisation, radial artery pseudoaneurysm, ultrasound
INTRODUCTION
The use of radial access for catheterization and cardiac intervention is becoming more popular, due to its lack of complications. Radial artery pseudoaneurysm (RAp) is an extremely rare complication, and hence, many of its clinical features are unknown, and treatment is not systematic.[1] In the few reported cases of RAp, surgical repair was the most commonly used treatment, although recently there have been reports of successful nonsurgical treatment in patients.[2] We report a case of 70-year-old female who had large radial pseudoaneurysm which was managed conservatively with the ultrasound-guided compression.
CASE PRESENTATION
A 70-year-old female patient presented to our hospital with the complaints of chest pain for 1 week duration suggestive of unstable angina. She is a known type 2 diabetic patient on irregular treatment. She was admitted. Coronary angiogram was done through the right radial artery. Radial artery puncture was done by Jelco needle with a single puncture and 5F sheath was secured in place. Patient's coronary angiogram showed double vessel disease. (significant left anterior descending and obtuse marginal) which was planned for double vessel angioplasty. The patient was loaded with dual antiplatelet therapy (aspirin 300 mg and clopidogrel 300 mg) with heparin of 5000U.
Hemostasis was achieved completely after the procedure by using TR band with 11 cc of air for compression. The TR Band was deflated as per protocol over the next 2 h and discontinued. There was forearm ecchymosis and hematoma noted on removal of the TR Band, without oozing or tenderness to palpation. Next day, patient complained of right wrist swelling with the extension of swelling with ecchymosis [Figures 1 and 2] in the dorsum of the right hand despite ice-cold compression and limb elevation [Video 1].
Figure 1.
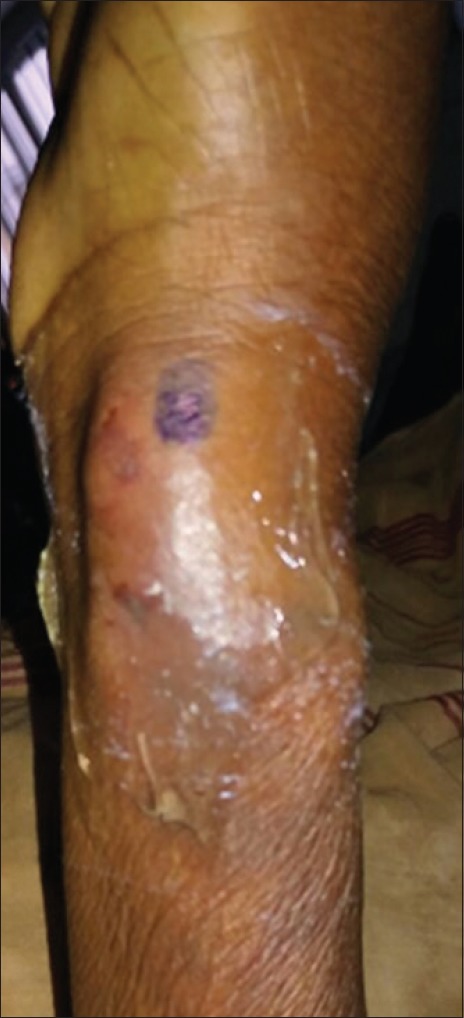
Large haematoma with ecchymosis
Figure 2.
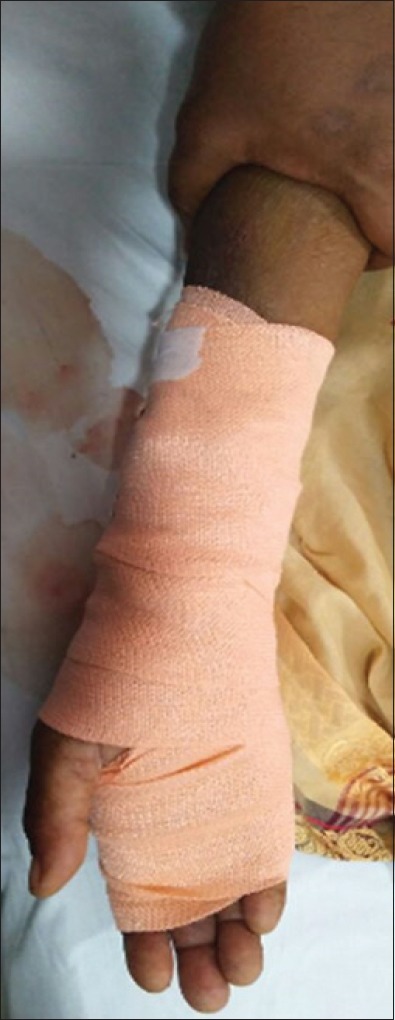
Coservative bandage application after manual compression
An ultrasound of the right wrist demonstrated a pseudoaneurysm (PSA) of the radial artery with a communicating channel between the aneurysm and the radial artery [Figures 3 and 4]. Ultrasound-guided compression was given for 45 min over the communicating neck between the radial artery and PSA for 3 days. After 3 days, the swelling and ecchymosis completely disappeared. Serial subsequent ultrasounds confirmed successful treatment of the radial PSA with preserved radial artery flow.
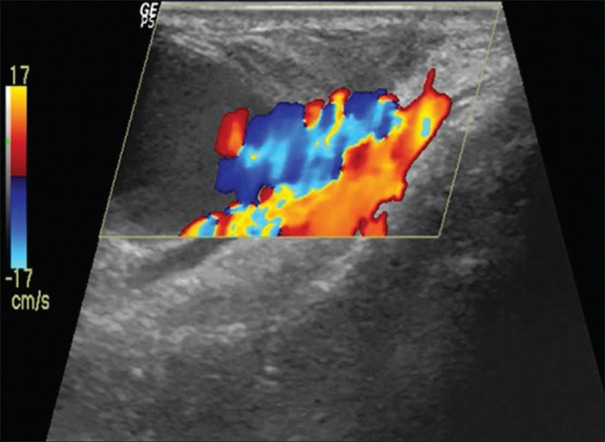
Figure 3.
Channel with the aneurysm by the colour flow turbulance
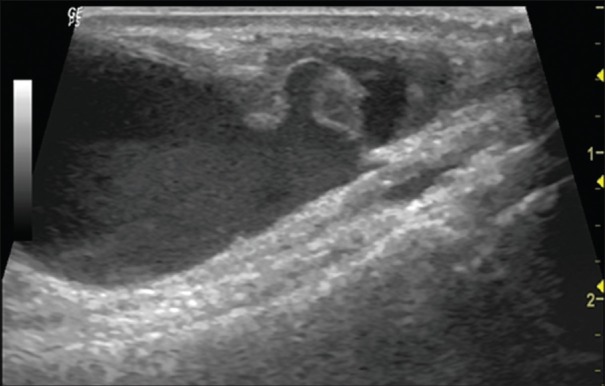
Figure 4.
Ultrasound showing aneurysmal sac
DISCUSSION
PSA is described as a tear through all the layers of the artery with resultant hemorrhage and hematoma formation contained by the surrounding tissue creating a false sac. PSA with TR access is rare, with <0.1% incidence reported in a large case series.[3] There are several factors that predispose TR access to the development of PSA, including multiple puncture attempts, ongoing systemic anticoagulation, inadequate hemostasis or postprocedure compression, vascular site infection, and the use of larger sheaths.[4]
RAp is a very rare complication but potentially harmful one due to the risk of external bleeding because of the rupture of its fragile outer wall.[5] The appearance of an internal bruise on forearm after the initial compression is an important sign that alert us about the possibility of a subsequent RAp development, especially if the patient was anticoagulated and if big sheaths were used during the procedure. A nonsurgical approach is an effective strategy, starting initially with mechanical compression and if this fails, use a thrombin injection.[5] However unlike femoral PSAs, we should avoid the direct compression of the RAp to avoid the RAp rupture. We recommend performing a radial artery compression but proximal to the RAp.[6]
Treatment of radial artery PSA is based on the anatomic characteristics of the PSA. A small PSA can be treated with compression to occlude flow into the PSA, while a large PSA require surgical intervention.[7] Other treatment strategies include the use of an external compression device or thrombin injection when the PSA has a narrow neck. The recommendations for preventing RAp:
Prevention is better – For anticoagulated patients, we recommend firm and more prolonged compression of the puncture point, as well as a thorough exploration of the puncture site and forearm after compression. If a hematoma appears, the patient should be informed of the signs of radial artery RAp and it should be reported immediately, to allow an early diagnosis[7]
-
If the patient does develop radial artery RAp, we recommend conservative treatment initially (without anticoagulating the patient, if possible) using compression with a pneumatic device TR bandage applied against the radial artery proximally to the RAp and not directly against the RAp (as is done currently, imitating the treatment of femoral RAp). By this way, potential iatrogenic rupture of the RAp is avoided. This proximal compression should be fully occlusive. Due to the possibility that blood may enter the RAp through the palmar arch via the ulnar artery, the absence of flow in the RAp should be verified by vascular ultrasound, or if not possible, by palpation by the absence of pulsatility. We then recommend using a finger to apply direct but mild compression against the RAp to expel the blood from the cavity into the radial artery and collapse it.
By using this simple digital compression, the swelling disappears completely. Once the RAp has collapsed, it is recommended to apply a semi-compressive bandage directly over it. After proximal occlusive compression of the RAp for 3–4 h, we then recommend a semiocclusive compression of the RAp and proximally for an additional 24 h by using a creep bandage. Due to the risk of external breakage, we recommend hospitalization for the following 24 h[7]
If the above method is not successful, we recommend treatment with ultrasound-guided injection of thrombin (1 mL, 500 IU)
Surgery should be considered for cases if the conservative management strategy has not been effective.
A nonsurgical approach is an effective strategy, starting initially with mechanical compression and if it does not work use a thrombin injection. However unlike femoral PSAs, we should avoid direct compression of the RAp prevent rupture.[7]
CONCLUSION
RAp is a rare complication (0.1%) but potentially harmful one due to the risk of rupture. The appearance of an internal bruise on forearm after the initial compression is an important sign that should alert us about the possibility of a subsequent RAp development.
A nonsurgical approach is an effective strategy, starting initially with mechanical compression and if this fails, to use a thrombin injection. Nonoperative management of uncomplicated PA of the radial artery is a safe and valuable treatment strategy that may be useful. Compression bandage provides a simple, noninvasive, and cost-effective procedure which may be first-line treatment for radial PA.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Video Available on: www.heartviews.org
REFERENCES
- 1.Matthew P Cauchi, DO, Paul M Robb, MD, Robert P Zemple, MD, Timothy C Ball., MD, PhD [Last accessed on 2017 Jul 01];J Ultrasound Med. 2014 33:1505–9. Available from: www.aium.org . [Google Scholar]
- 2.Rodrı´guez-Olivares R, Garcı´a-Touchard A, Ferna´ndez-Dı´az JA, Oteo JF, Zorita B, Goicolea J. Abordaje transulnar con arteria radial homolateral ocluida: descripcio´n de la vascularizacio´n del antebrazo y seguimiento a largo plazo. Rev Esp Cardiol. 2014;67:854–5. [Google Scholar]
- 3.Cozzi DA, Morini F, Casati A, Pacilli M, Salvini V, Cozzi F. Radial artery pseudoaneurysm successfully treated by compression bandage. Arch Dis Child. 2003;88:165–6. doi: 10.1136/adc.88.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerr CD, Duffey TP. Traumatic false aneurysm of the radial artery. J Trauma. 1988;28:1603–4. doi: 10.1097/00005373-198811000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Collins N, Wainstein R, Ward M, Bhagwandeen R, Dzavik V. Pseudoaneurysm after transradial cardiac catheterization: case series and review of the literature. Catheter Cardiovasc Interv. 2012;80:283–7. doi: 10.1002/ccd.23216. doi: 10.1002/ccd.23216. [DOI] [PubMed] [Google Scholar]
- 6.Kiemeneji F, Laarman GJ, Odekerken D. A randomized comparison of percutaneous transluminal coronary angioplasty by the radial, brachial, and femoral approaches: the access study. J Am Coll Cardiol. 1997;29:1269–75. doi: 10.1016/s0735-1097(97)00064-8. [DOI] [PubMed] [Google Scholar]
- 7.Radial Artery Pseudoaneurysm Following Cardiac Catheterization: Scientific letters. Rev Esp Cardiol. 2015;68(4):343–54. doi: 10.1016/j.rec.2014.11.013. Available from: http://dx.doi.org/10.1016/j.rec.2014.11.013 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.