Abstract
This study aimed to assess the role of the National Comprehensive Cancer Network (NCCN) risk classification in predicting biochemical recurrence (BCR) after radical prostatectomy (RP) in Chinese prostate cancer patients. We included a consecutive cohort of 385 patients with prostate cancer who underwent RP at Fudan University Shanghai Cancer Center (Shanghai, China) from March 2011 to December 2014. Gleason grade groups were applied at analysis according to the 2014 International Society of Urological Pathology Consensus. Risk groups were stratified according to the NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer version 1, 2017. All 385 patients were divided into BCR and non-BCR groups. The clinicopathological characteristics were compared using an independent sample t-test, Chi-squared test, and Fisher's exact test. BCR-free survival was compared using the log-rank test and multivariable Cox proportional hazard analysis. During median follow-up of 48 months (range: 1–78 months), 31 (8.05%) patients experienced BCR. The BCR group had higher prostate-specific antigen level at diagnosis (46.54 ± 39.58 ng ml-1 vs 21.02 ± 21.06 ng ml−1, P = 0.001), more advanced pT stage (P = 0.002), and higher pN1 rate (P < 0.001). NCCN risk classification was a significant predictor of BCR (P = 0.0006) and BCR-free survival (P = 0.003) after RP. As NCCN risk level increased, there was a significant decreasing trend in BCR-free survival rate (Ptrend = 0.0002). This study confirmed and validated that NCCN risk classification was a significant predictor of BCR and BCR-free survival after RP.
Keywords: biochemical recurrence, prostate cancer, radical prostatectomy, National Comprehensive Cancer Network risk classification
INTRODUCTION
Prostate cancer is the most common malignant tumor among men in the United Sates, and it is estimated that there will be 164 690 new cases and 29 430 deaths in 2018.1 Although both incidence and mortality rate of prostate cancer are lower in China, there has been a dramatic increase over the last few decades. In 2015, it was estimated that there would be 60 300 new cases and 26 600 deaths from prostate cancer in China.2 The National Comprehensive Cancer Network (NCCN) guidelines suggested a new prostate cancer risk classification system depending on clinicopathological characteristics. Since 2010, two new groups have been added to the traditional three-group classification: very low-risk and very high-risk groups.3 NCCN risk classification is mainly designed for deciding on treatment strategy for prostate cancer. It has also been validated for prediction of outcomes after radical prostatectomy (RP) or radiation therapy. However, most of those validations were not performed in Asian populations.4,5,6
RP is one of the curative treatments for localized prostate cancer. For high-risk prostate cancer, RP is a reasonable first step in patients without tumor fixation to the pelvic wall and urethral sphincter invasion, combined with adjuvant or salvage androgen deprivation therapy (ADT) or radiation therapy.7 After RP, prostate-specific antigen (PSA) is supposed to decrease to an undetectable level. If PSA is consecutively higher than 0.2 ng ml−1 twice and keeps increasing, it is defined as biochemical recurrence (BCR).8 BCR is an early indication of clinical progression, which is estimated to precede the appearance of clinical metastasis by 8 years after RP.9 Secondary treatment is usually offered to patients once BCR occurs. The aim of this study was to assess the role of NCCN risk classification in predicting BCR after RP in Chinese prostate cancer patients.
PATIENTS AND METHODS
Patient and data collection
We performed a retrospective study after obtaining approval from the Institutional Review Board of Fudan University Shanghai Cancer Center. We included a consecutive cohort of 891 patients with prostate cancer who underwent RP at Fudan University Shanghai Cancer Center (Shanghai, China) from March 2011 to December 2014. Each patient was informed about the aims of this study, and informed consent for participation was obtained in accordance with the institutional guidance at Fudan University Shanghai Cancer Center. We excluded patients who received neoadjuvant ADT or radiation therapy prior to RP and patients with distant metastasis (M1). We also excluded from our analysis patients who were lost to follow-up. Our analysis eventually included 385 patients with prostate cancer. All these patients had complete clinical and follow-up records, including age, height, weight, PSA level at diagnosis, Gleason score (grade groups 1–5 were applied at analysis according to the 2014 International Society of Urological Pathology Consensus10), date of RP, surgical technique, pathological stage and Gleason score, surgical margins and lymph node stage, and BCR information. Pathological data were reviewed and modified according to the American Joint Committee on Cancer eighth edition Cancer Staging Manual.11 NCCN risk groups were divided according to the NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer Version 1, 2017, and depending on clinical Gleason score and tumor stage. The risk groups defined by the NCCN guidelines were as follows: low risk: T1–T2a, Gleason score ≤6, and PSA <10 ng ml−1; intermediate risk: T2b–T2c or Gleason score 7 or PSA 10–20 ng ml−1; high risk: T3a or Gleason score 8–10 or PSA >20 ng ml−1; very high risk for locally advanced prostate cancer: T3b–T4 or primary Gleason pattern 5 or >5 cores with Gleason score 8–10; and metastatic risk: N1 or M1 with any T stage.12 However, in this study, pathological Gleason score and pathological stage were used instead, which were considered to be more accurate. BCR after RP was defined as two consecutive rises in PSA >0.2 ng ml−1, which was also applied in our study. Patients who were classified into the very high-risk group or whose pathological results showed pN1 were closely monitored and followed up for postoperative PSA level. Adjuvant ADT or radiation therapy was offered to these patients. Patients who chose to receive adjuvant ADT or radiation therapy shortly after surgery had already been excluded from the analysis. Adjuvant ADT or radiation therapy was not applied if PSA level decreased dramatically and reached <0.2 ng ml−1.
Statistical analysis
All 385 patients were divided into the BCR or non-BCR group. The clinicopathological characteristics were compared with an independent sample t-test, Chi-squared test, and Fisher's exact test. The BCR-free survival was compared using the log-rank test. Multivariable Cox proportional hazard analysis was conducted to confirm significance of BCR after RP. P < 0.05 was considered to be statistically significant. Another multivariable model was also conducted, which replaced PSA level at diagnosis, pT stage, and pN stage by NCCN risk groups. The statistical analysis was done by SPSS version 21.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism software 7 (GraphPad Software Inc., La Jolla, CA, USA).
RESULTS
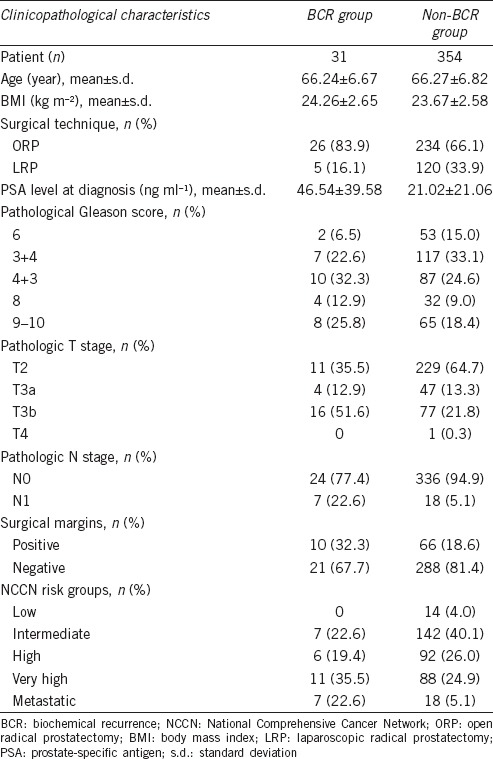
The clinicopathological characteristics of the 385 patients are listed in Table 1. During median follow-up of 48 (range: 1–78) months, 31 (8.05%) patients experienced BCR. Among the 385 patients, 125 received laparoscopic radical prostatectomy (LRP) and 260 received open radical prostatectomy (ORP). All LRP procedures were performed via an extraperitoneal approach. The BCR group had higher PSA level at diagnosis (46.54 ± 39.58 ng ml−1 vs 21.02 ± 21.06 ng ml−1, P = 0.001), more advanced pT stage (P = 0.002), and higher pN1 rate (P < 0.001). The BCR group also had more very high and metastatic NCCN risk groups than the non-BCR group had (P = 0.001). The BCR group tended to be more likely to undergo ORP than LRP (P = 0.043).
Table 1.
Patient clinicopathological characteristics
Survival analysis is shown in Figure 1, and 3-year BCR-free survival rates are shown in Table 2. Patients with PSA >20 ng ml−1 at diagnosis had significantly worse BCR-free survival compared with patients with PSA level <20 ng ml−1 (Figure 1a, P = 0.0004). Among the 385 patients included in the analysis, there were 201 patients with recorded PSA level at day 7 after RP. We also found that the higher short-term PSA level after RP was a significant predictor of BCR (Supplementary Table 1 (212.5KB, tif) ).
Figure 1.
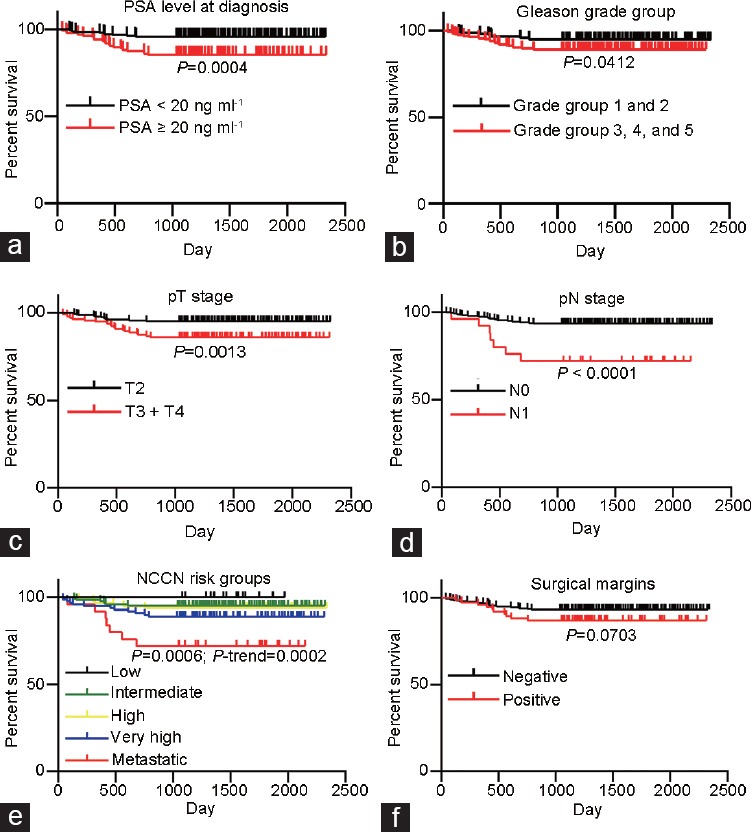
BCR-free survival among all patients. (a) BCR-free survival by prostate-specific antigen level at diagnosis; (b) BCR-free survival by pathological Gleason grade group; (c) BCR-free survival by pathological T stage; (d) BCR-free survival by pathological N stage; (e) BCR-free survival by NCCN risk group; (f) BCR-free survival by surgical margins. NCCN: National Comprehensive Cancer Network; BCR: Biochemical recurrence.
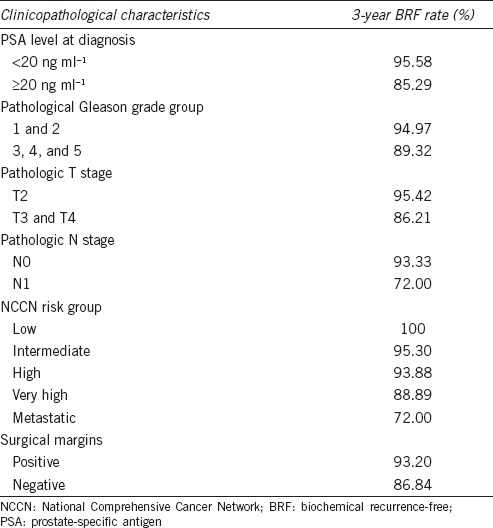
Table 2.
Three years biochemical recurrence-free survival rate
Multivariate analysis of biochemical recurrence-free survival
When stratified by Gleason grade group, patients with Grade 1 (Gleason score 3 + 3) and Grade 2 (Gleason score 3 + 4) had significantly better BCR-free survival than patients with Grade 3 (Gleason score 4 + 3), Grade 4 (Gleason score 4 + 4, 3 + 5, or 5 + 3), and Grade 5 (Gleason score 4 + 5, 5 + 4, or 5 + 5) (Figure 1b, P = 0.0412). Pathological T stage and N stage were also analyzed. Patients with organ-confined prostate cancer (pT2) had better BCR-free survival than patients with pT3 and pT4 prostate cancer (Figure 1c, P = 0.0013). Patients with regional lymph node metastasis (pN1) also had worse BCR-free survival than patients with pN0 (Figure 1d, P < 0.0001). We applied the NCCN classification system to pool these clinicopathological factors together and found that BCR-free survival significantly deteriorated as NCCN risk level increased (Figure 1e, P = 0.0006, Ptrend = 0.0002). Positive surgical margins also contributed to worse BCR-free survival (Figure 1f, P = 0.0703).
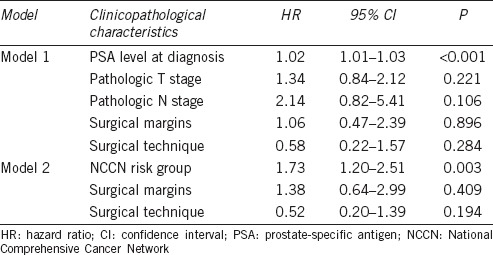
We then constructed two Cox proportional hazard models to confirm these potential risk factors of BCR-free survival (Table 3). Model 1 included PSA level at diagnosis, pathological T stage, pathological N stage, surgical margins, and surgical technique; Model 2 included NCCN risk group, surgical margins, and surgical technique. PSA level at diagnosis was a significant factor for BCR-free survival after other variables were adjusted in Model 1 (hazard ratio [HR]: 1.02, 95% confidence interval [CI]: 1.01–1.03, P < 0.001). In Model 2, NCCN risk group was also found to be a significant factor for BCR-free survival after other variables were adjusted (HR: 1.73, 95% CI: 1.20–2.51, P = 0.003).
Table 3.
Multivariable analysis of biochemical recurrence-free survival
DISCUSSION
In this study, we found that NCCN risk classification was a significant predictor of BCR after RP. As NCCN risk level increased, there was a significant decreasing trend in BCR-free survival rate. Therefore, NCCN risk classification was validated in Chinese prostate cancer patients and could be applied to predict BCR and BCR-free survival. NCCN risk classification was generated mainly based on American prostate cancer patients; thus, its validation among Chinese patients widens its application in prostate cancer management. However, the natural progression of prostate cancer might differ between American and Chinese patients. Some adjustment might need to be made to the original classification to predict BCR and BCR-free survival more accurately. Our study preliminarily indicated that NCCN risk classification could be applied to Chinese prostate cancer patients to predict BCR and BCR-free survival, which forms the basis for further study about modified risk classification among these patients.
BCR is a unique clinical phenomenon during prostate cancer treatment and progression. In general, patients experience BCR 3.5 years after RP.9 The 5-year BCR-free survival rate of prostate cancer patients is estimated as 80% and 68% for 10-year BCR-free survival rate.13 Patients who experience BCR have earlier clinical progression and worse overall survival. It is estimated that 27%–60% of patients with BCR experience clinical progression within 5 years.14 The median time from clinical progression to prostate cancer-specific mortality is estimated as 5 years. Therefore, accurate prediction of BCR would benefit prostate cancer patients after definitive therapy, like RP. More frequent and close follow-up, as well as more active adjuvant treatment, should be provided to those patients with higher BCR risk.
In this study, we found that PSA level at diagnosis, pathological Gleason grade group, pathological T stage, pathological N stage, and surgical margins were significantly associated with BCR-free survival. This was consistent with other studies.15,16 Preoperative PSA level as a factor predicting BCR was validated in most of the available studies. In this study, we also confirmed that PSA level at diagnosis, as well as short-term PSA after RP, was a significant predictor of BCR and BCR-free survival after adjusting other potential factors. We found that short-term PSA after RP, namely at 7 days after surgery, was a significant predictor of BCR-free survival. The reason why we focused on PSA level at that time was because a large number of patients came back to our hospital at that time. It usually took our pathologists 1 week to draw their final conclusions about the prostatectomy samples. In Shanghai, the practice of reviewing reports by mobile phone apps is not widespread. Hence, most patients choose to come to their urologists for follow-up immediately after the pathology results come out. Several other studies have examined the association between postoperative PSA level and BCR rate. Vesely et al.17 reported that patients with PSA >0.073 ng ml−1 at day 30 after surgery had significantly increased risk of BCR. Some studies used ultrasensitive PSA level after RP to identify BCR more accurately with a significant lead-time advantage.18,19 However, none of those studies focused on short-term PSA level after RP. Our study indicated that short-term PSA level after RP was also a significant predictor of BCR-free survival. We will continue with this study and further test the role of short-term PSA level in BCR-free survival as well as other RP outcomes.
In the second multivariable analysis model, we replaced PSA level at diagnosis, pathological T stage, and pathological N stage by NCCN risk group. We found that NCCN risk group was a significant predictor of BCR and BCR-free survival after adjusting for other potential factors. NCCN risk classification is mainly designed for treatment strategy for prostate cancer according to the NCCN guidelines. It stratifies patients into recurrence risk groups according to pretreatment clinicopathological characteristics, including clinical tumor stage, biopsy Gleason score, and PSA level. Patients within different risk groups are recommended different treatment modalities. In this analysis, we focused on whether NCCN risk classification could predict BCR after RP. Hence, we replaced clinical tumor stage and biopsy Gleason score in the original setting by pathological tumor stage and pathological Gleason score. Our study validated that NCCN risk classification could guide treatment strategy and predict BCR after definitive therapy like RP.
There were several limitations to our study that need to be considered. First, the loss to follow-up rate was >50%. Our hospital is not only a tertiary care center but also the largest cancer center in Eastern China, and a large proportion of patients reside outside Shanghai. They would receive follow-up care at their local urological clinic. We are exploring other approaches for follow-up, such as surveys on mobile phone apps, remote on-line follow-up clinics, and automated calls. Second, the very high-risk group showed moderately worse BCR-free survival than the high-risk group showed, although the difference was not statistically significant (P = 0.210, data not shown). This might have been because of the high loss of follow-up data and the small sample size. We are still enrolling patients to our study cohort; therefore, we might be able to investigate further whether BCR-free survival differed between these two subgroups.
CONCLUSION
This study confirmed and validated that NCCN risk classification is a significant predictor of BCR and BCR-free survival after RP.
AUTHOR CONTRIBUTIONS
DWY designed this study and helped edit the manuscript. HX performed the statistical analysis and drafted the manuscript. BD and YZ participated in the statistical analysis. All authors have read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This study was sponsored by the National Natural Science Foundation of China (No. 81472377) and the Natural Science Foundation of Shanghai (No. 16ZR1406500). The authors also thank Wei-Yi Yang, Cui-Zhu Zhang, and Ying Shen for helping with follow-up of patients.
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Mohler J, Bahnson RR, Boston B, Busby JE, D’Amico A, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010;8:162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 4.Abdollah F, Sun M, Suardi N, Gallina A, Capitanio U, et al. National comprehensive cancer network practice guidelines 2011: need for more accurate recommendations for pelvic lymph node dissection in prostate cancer. J Urol. 2012;188:423–8. doi: 10.1016/j.juro.2012.03.129. [DOI] [PubMed] [Google Scholar]
- 5.Savdie R, Aning J, So AI, Black PC, Gleave ME, et al. Identifying intermediate-risk candidates for active surveillance of prostate cancer. Urol Oncol. 2017;35:605.e1–605.e8. doi: 10.1016/j.urolonc.2017.06.048. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen PL, Martin NE, Choeurng V, Palmer-Aronsten B, Kolisnik T, et al. Utilization of biopsy-based genomic classifier to predict distant metastasis after definitive radiation and short-course ADT for intermediate and high-risk prostate cancer. Prostate Cancer Prostatic Dis. 2017;20:186–92. doi: 10.1038/pcan.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yossepowitch O, Eggener SE, Serio AM, Carver BS, Bianco FJ, Jr, et al. Secondary therapy, metastatic progression, and cancer-specific mortality in men with clinically high-risk prostate cancer treated with radical prostatectomy. Eur Urol. 2008;53:950–9. doi: 10.1016/j.eururo.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moul JW. Prostate specific antigen only progression of prostate cancer. J Urol. 2000;163:1632–42. [PubMed] [Google Scholar]
- 9.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 10.Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–52. doi: 10.1097/PAS.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 11.Buyyounouski MK, Choyke PL, McKenney JK, Sartor O, Sandler HM, et al. Prostate cancer – major changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:245–53. doi: 10.3322/caac.21391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NCCN Clinical Practice Guidelines in Oncology Prostate Cancer Version 2. 2017. Feb 21, Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate_blocks.pdf .
- 13.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910–4. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 14.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–9. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 15.Hu XH, Cammann H, Meyer HA, Jung K, Lu HB, et al. Risk prediction models for biochemical recurrence after radical prostatectomy using prostate-specific antigen and Gleason score. Asian J Androl. 2014;16:897–901. doi: 10.4103/1008-682X.129940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herranz-Amo F, Molina-Escudero R, Ogaya-Pinies G, Ramirez-Martin D, Verdu-Tartajo F, et al. Prediction of biochemical recurrence after radical prostatectomy. New tool for selecting candidates for adjuvant radiation therapy. Actas Urol Esp. 2016;40:82–7. doi: 10.1016/j.acuro.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Vesely S, Jarolim L, Duskova K, Schmidt M, Dusek P, et al. The use of early postoperative prostate-specific antigen to stratify risk in patients with positive surgical margins after radical prostatectomy. BMC Urol. 2014;14:79. doi: 10.1186/1471-2490-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang JJ, Reiter RE, Steinberg ML, King CR. Ultrasensitive prostate specific antigen after prostatectomy reliably identifies patients requiring postoperative radiotherapy. J Urol. 2015;193:1532–8. doi: 10.1016/j.juro.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vesely S, Jarolim L, Schmidt M, Do Carmo Silva J, Duskova K, et al. Stratification model based on early postprostatectomy prostate-specific antigen kinetics may help to reduce the risk of overtreatment in candidates for adjuvant radiotherapy. Scand J Urol. 2017;51:114–9. doi: 10.1080/21681805.2017.1292545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multivariate analysis of biochemical recurrence-free survival


