Abstract
The autonomic nervous system contributes to prostate cancer proliferation and metastasis. However, the exact molecular mechanism remains unclear. In this study, muscarinic acetylcholine receptor M1 (CHRM1) expression was measured via immunohistochemical analysis in human prostate cancer tissue array slides. PC-3, LNCaP, and A549 cells were treated with pirenzepine or carbachol, and the cell migration and invasion abilities were evaluated. Western blotting and quantitative real-time PCR were performed to measure GLI family zinc finger 1 (GLI1), patched 1 (PTCH1), and sonic hedgehog (SHH) expression levels. High expression of CHRM1 was found in early-stage human prostate cancer tissues. In addition, the selective CHRM1 antagonist pirenzepine inhibited PC-3, LNCaP, and A549 cell migration and invasion, but the agonist carbachol promoted the migration and invasion of these three cell lines. Muscarinic signaling can be relayed by hedgehog signaling. These data show that CHRM1 is involved in the regulation of prostate cancer migration and invasion through the hedgehog signaling pathway.
Keywords: hedgehog signaling, muscarinic acetylcholine receptor M1 (CHRM1), metastasis, prostate cancer
INTRODUCTION
Recent evidence has demonstrated the complementary role of two branches of the autonomic nervous system in prostate cancer initiation and progression.1 On the one hand, sympathetic nervous system plays an important role in the early stages of tumorigenesis through β2- and β3-adrenergic receptors; on the other hand, parasympathetic nervous system plays predominant role in cancer metastasis through muscarinic acetylcholine receptor M1 (CHRM1). These findings indicate a crucial role for muscarinic signaling in prostate cancer dissemination, but the molecular mechanism underlying the involvement of CHRM1 in prostate cancer invasion and metastasis remains unclear.
Muscarinic receptors are G protein-coupled receptors (GPCRs) composed of seven transmembrane domains located in the parasympathetic nerve subsynaptic membrane.2 With the aid of a molecular cloning technique, five muscarinic receptors (CHRM1-CHRM5) have been described.3 These receptors are expressed in the nervous system and the target organs of parasympathetic neurons. After binding with the endogenous neurotransmitter acetylcholine (Ach), muscarinic receptors activate guanosine triphosphate (GTP)-binding regulatory proteins (G proteins). While odd-numbered muscarinic receptors couple to Gq-type proteins, even-numbered muscarinic receptors couple to Gi/Go-type proteins. Activated G proteins initiate a number of intracellular signals: Gq-type proteins activate phospholipase C-β to catalyze phosphatidylinositol (PI) turnover and inward-rectified potassium channels, and Gi/Go-type proteins inhibit adenylate cyclase activity. Thus, muscarinic receptors regulate many fundamental physiological functions, such as slowing of the heart, vasodilation of vessels, and secretion of glands.4,5,6,7,8 In human prostate tissue, all five muscarinic receptors are expressed in the glandular epithelium. Muscarinic acetylcholine receptor M1, also called M1 muscarinic receptor, is the predominant receptor.9,10 These muscarinic receptors, together with parasympathetic neurons, have an established role in normal prostate growth and low-level glandular secretion. Consequently, muscarinic receptors have been suggested to play an important role in tumor progression. Early studies revealed that muscarinic receptors promote the growth of cancers, such as colorectal, lung, and prostate cancers. In colorectal cancer, the pro-proliferative action of the muscarinic acetylcholine receptor M3 (CHRM3) has been demonstrated to depend on the transactivation of epidermal growth factor receptor (EGFR) and post-EGFR signaling.11,12 Another study revealed that muscarinic receptors block apoptosis through protein kinase B (AKT)- and nuclear factor (NF)-kappa B-mediated mechanisms.13 The growth of human lung cancer cells involves intracellular calcium, mitogen-activated protein kinase (MAPK), and AKT phosphorylation.14 With regard to prostate cancer, PC-3, LNCaP, and DU145 cells proliferate in response to carbachol. Carbachol stimulates the mitogen-activated kinase (ERK) in PC-3 and DU145, but not in LNCaP.15,16 Recent studies have demonstrated that parasympathetic nerves and muscarinic receptors play a critical role in tumor metastasis, including prostate cancer metastasis.1,17,18 Denervation, pharmacological inhibition, or genetic disruption of the corresponding receptors significantly blocks the spread of tumors, leading to improved survival of the recipient animal. Nevertheless, the downstream molecular mechanisms underlying CHRM1 signaling in prostate cancer metastasis have not yet been explored. Thus, the function and underlying mechanisms of the CHRM1 in prostate cancer, especially in prostate cancer metastasis, remain to be elucidated.
Hedgehog (Hh) signaling primarily involves sonic hedgehog (SHH), patched 1 (PTCH1), smoothened (SMO), and GLI family zinc finger 1 (GLI1).19 Once the SHH ligand binds to the membrane anchoring receptor PTCH1, SMO is relieved from PTCH1-mediated repression, which leads to dissociation of the suppressor of fused (SUFU)-GLI1 complex and translocation of the GLI1 transcription factor. Hh signaling plays a central role in embryonic development, histological differentiation, and organ formation.20 Moreover, Hh signaling pathway activation contributes to tumor aggressiveness by inducing cell proliferation, stimulating angiogenesis, and inhibiting cell apoptosis.21,22,23,24 In many prostate cancer tissues, SHH, PTCH1, GLI1, GLI2, and GLI3 are upregulated compared with their levels in matched normal tissues.21 Pathway activity is closely related to prostate cancer development and invasiveness.25,26 Recent studies have reported that SHH promotes perineural invasion (PNI) in pancreatic cancer.27 Other studies have indicated that Hh signaling cross talks with other signaling pathways, such as transforming growth factor beta (TGF-β) and Notch signaling,28 suggesting that there is a possible connection between the CHRM1 and Hh signaling. A previous study demonstrated that CHRM1 promotes prostate cancer metastasis.1 However, it remains unclear whether the CHRM1 and Hh signaling are correlated in prostate cancer invasion.
The aim of this study was to investigate the molecular mechanisms that promote prostate cancer, including changes in the hedgehog signaling pathway and the migration and invasion abilities of prostate cancer cells after treatment with a CHRM1 antagonist or agonist.
MATERIALS AND METHODS
Cell culture and drugs
The human androgen-independent prostate cancer cell line PC-3 was obtained from BeNa Culture Collection (BNCC, Beijing, China). The androgen-dependent prostate cancer cell line LNCaP and the non-small cell lung cancer cell line A549 (this tumor cell line was randomly selected to compare CHRM1 expression between prostate and other derived cancers) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The human normal epithelial prostate cell line RWPE-1 was obtained from China Center for Type Culture Collection (CCTCC, Wuhan, China). PC-3 and LNCaP cells were grown in Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Cellmax, Lanzhou, China) and 1% antibiotic mixture (penicillin/streptomycin; Solarbio, Beijing, China). A549 cells were maintained in Roswell Park Memorial Institute (RPMI 1640; Thermo Fisher Scientific) medium supplemented with 10% FBS and 1% antibiotic mixture. RWPE-1 cells were grown in keratinocyte serum-free medium (K-SFM; Gibco, Carlsbad, CA, USA) supplemented with 10% FBS and 1% antibiotic mixture. These four cell lines were cultured at 37°C in a 5% CO2 and 95% humidified atmosphere. The nonselective muscarinic receptor agonist carbamoylcholine chloride (carbachol; T0105, Targetmol, Shanghai, China), CHRM1-specific antagonist pirenzepine (T1542, Targetmol), and Hh pathway antagonist GANT61 (HY-13901, MCE, Monmouth Junction, NJ, USA) were dissolved in dimethyl sulfoxide (DMSO; Sigma, Milwaukee, WI, USA) and stored at 4°C. The solutions were diluted to the final concentration with culture medium, and treatment with vehicle (DMSO, Sigma) was used for control cells.
Cell proliferation assay
PC-3 cells were seeded in 96-well plates and were left untreated (control) or treated with pirenzepine or carbachol the next day at different concentrations according to prior studies.29 Cell proliferation was assessed with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma) assays after incubation with drugs for 24 h. At the indicated time point, 10 μl of MTT solution was added to each well, and the cells were incubated at 37°C for an additional 4 h. Formazan was dissolved in DMSO, and absorbance was measured at 490 nm using a spectrophotometer (BioTeK, Vinooski, VT, USA). Each concentration was tested in five replicates. The results are expressed as the mean ± standard error of the mean (s.e.m.).
Cell migration and invasion assays
In the migration assays, 20 000–100 000 cells in 100 μl of serum-free medium were seeded on the top of a Transwell chamber (8.0 μm pore size, Corning, Vineland, NJ, USA), and 600 μl of medium containing 20% FBS was added in the bottom of 24-well plates to induce cell migration. In the invasion assays, an additional 60 μl of Matrigel matrix (356234, Corning) was added on the top of the Transwell chamber before seeding cells as described.30 Cells were left untreated (control) or treated with carbachol (2 μg ml−1) or pirenzepine (110 μg ml−1) for 24 h. The culture inserts were fixed with 4% paraformaldehyde and then stained with crystal violet (Beyotime, Shanghai, China) for 15 min. Cells that stayed on the top of the membrane were lightly scraped away with a cotton swab. Migrated cells were counted under a high-power microscope (OLYMPUS, Tokyo, Japan) in five random fields.
Western blotting analysis
PC-3 cells were seeded in 6-well or 12-well plates and left untreated (control) or treated with carbachol (2 μg ml−1) or pirenzepine (110 μg ml−1) for 2, 4, 6, 12, and 24 h. Cells were lysed in NP 40 buffer (Solarbio, Beijing, China) containing protease inhibitors. Total protein was measured using the Bicinchoninic acid (BCA) method (Beyotime). Primary antibodies against SHH (1:2000, Abcam, Cambridge, MA, USA), PTCH1 (1:1000, Abcam), GLI1 (1:1000, CST, Beverly, MA, USA), and CHRM1 (1:1000, Abcam) as well as horseradish peroxidase (HRP)-conjugated secondary antibodies (ZSbio, Beijing, China) were applied. Equal protein sample loading was monitored using an anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (1:5000, Ray Antibody, Beijing, China). The probed proteins were visualized with an enhanced chemiluminescence (ECL) blotting detection kit (BIO-RAD, Chengdu, China).
Real-time PCR assays
PC-3 cells were seeded in 6-well plates and left untreated (control) or treated with different concentrations of pirenzepine at 37°C for 24 h. Total RNA was extracted with Trizol reagent (TaKaRa, Kusatsu, Japan) according to the standard protocol. cDNA was synthesized using a Primescript™ RT reagent kit (TaKaRa). Conventional and quantitative real-time PCR assays were performed as described.10,31 The oligonucleotide primers are listed in Table 1. The conditions for RT-PCR amplification were as follows: 95°C for 30 s followed by 39 cycles of 95°C for 5 s, annealing temperature for 30 s, and a final cycle at 65°C for 5 s and 95°C for 15 s. Each sample was run in triplicate. The data were analyzed with the comparative ΔΔCT method, with actin beta (ATCB) gene expression as an endogenous reference.
Table 1.
Primer sequences used for real-time PCR

Immunohistochemical staining
The human prostate cancer tissue array slides (PR633, Alenabio, Xi’an, China) included 60 spots characterized as follows: 34 cases of early-stage prostate cancer, 26 cases of late-stage prostate cancer, 50 cases of non-metastatic prostate cancer, and 10 cases of metastatic prostate cancer. Early-stage prostate cancer included stage I and stage II patients, and late-stage prostate cancers included stage III and stage IV patients. The median age of the patients was 69.5 years old. Case data included pathology diagnosis, Gleason score, stage, and tumor node matastasis (TNM) status. The cultured PC-3, LNCaP, and A549 cells are described above. The three cell lines were not treated with any drugs before staining. A primary antibody against CHRM1 (1:500) and a HRP-conjugated secondary antibody (PV-9000, Zsbio) were applied. The immunohistochemical staining was visualized with 3,3’-diaminobenzidine (DAB; Zsbio). Images of five random fields were captured using a microscope under the same exposure conditions and analyzed with Image-pro Plus 6.0 software (Media Cybernetics, Silver Spring, MD, USA).
Statistical analyses
The data in this study are expressed as the means ± s.e.m. All statistical analyses were performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Statistical significance for three or more groups was assessed by ANOVA and Mann–Whitney U test (2-tailed). Two groups were compared with Student's t-test. Significance was set at P < 0.05 (*P < 0.05, **P < 0.01, and ***P < 0.001).
RESULTS
CHRM1 expression is upregulated in the early stages of prostate cancer
To confirm CHRM1 expression in human prostate cancers, we conducted immunohistochemical staining of prostate cancer tissue array slides. We observed frequent CHRM1 expression in the prostate cancer tissues (60/60) with different degrees of staining intensities. CHRM1 was mainly expressed in epithelial cells surrounding the prostate gland (Figure 1a–1d). We analyzed the relationship between CHRM1 expression and clinicopathological features in the prostate cancer samples and found that CHRM1 was remarkably overexpressed in early-stage (stage I and II) prostate cancer compared with its expression level in late-stage (stage III and IV) prostate cancer (P = 0.008; Figure 1e), whereas there were no significant differences in CHRM1 expression between nonmetastatic and metastatic prostate cancers (Figure 1f). These results suggest that CHRM1 upregulation might occur in the early stages of prostate cancer.
Figure 1.
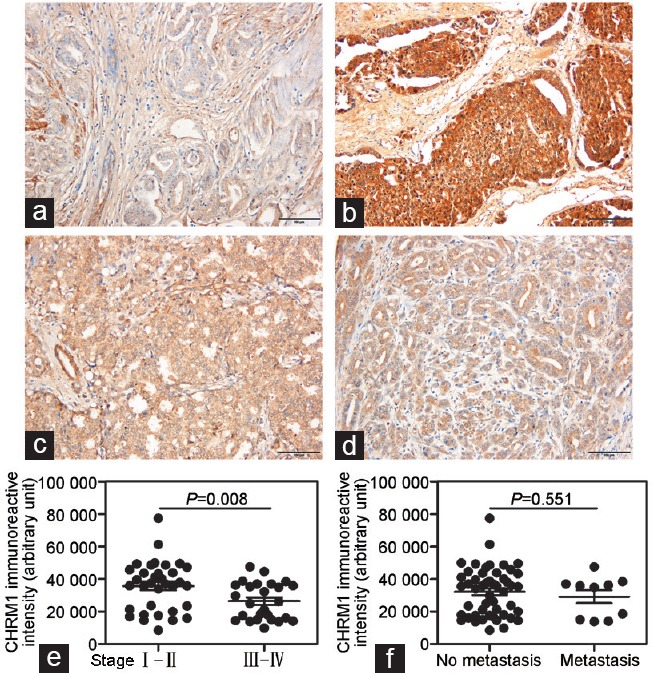
CHRM1 expression is upregulated in the early stages of prostate cancer. Representative images of immunohistochemical staining of CHRM1 in human prostate cancer tissue arrays: (a) stage I patient, (b) stage II patient, (c) stage III patient, and (d) stage IV patient tissue samples are shown. Note that CHRM1 is widely expressed in human prostate cancer tissues and mainly localized in glandular epithelium. Scale bars = 100 μ m. (e) Quantification of CHRM1 staining intensities in early-stage (I–II, n = 34) and late-stage (III–IV, n = 26) prostate adenocarcinomas. (f) Quantification of CHRM1 staining intensities in nonmetastatic (n = 50) and metastatic (n = 10) prostate adenocarcinomas. CHRM1: muscarinic acetylcholine receptor M1.
CHRM1 expression in prostate cancer cells
We compared the expression of CHRM1 in the normal epithelial prostate cell line RWPE-1 and two prostate cancer cell lines (PC-3 and LNCaP) via western blotting analysis. The protein expression level of CHRM1 was much higher in the two prostate cancer cell lines than that in RWPE-1 cell line (Figure 2a). Furthermore, we made an analysis of the protein expression level of CHRM1 in the two prostate cancer cell lines (PC-3 and LNCaP) and in a non-small cell lung cancer cell line A549. The results showed that the expression of CHRM1 in PC-3 and LNCaP cells was approximately 65% more than that in A549 cells at protein level (Figure 2b). Cell immunohistochemical staining showed that CHRM1 was localized in cell membranes and cytoplasm (Figure 2c–2e). These results suggest that substantial CHRM1 is expressed in PC-3 and LNCaP prostate cancer cell lines.
Figure 2.
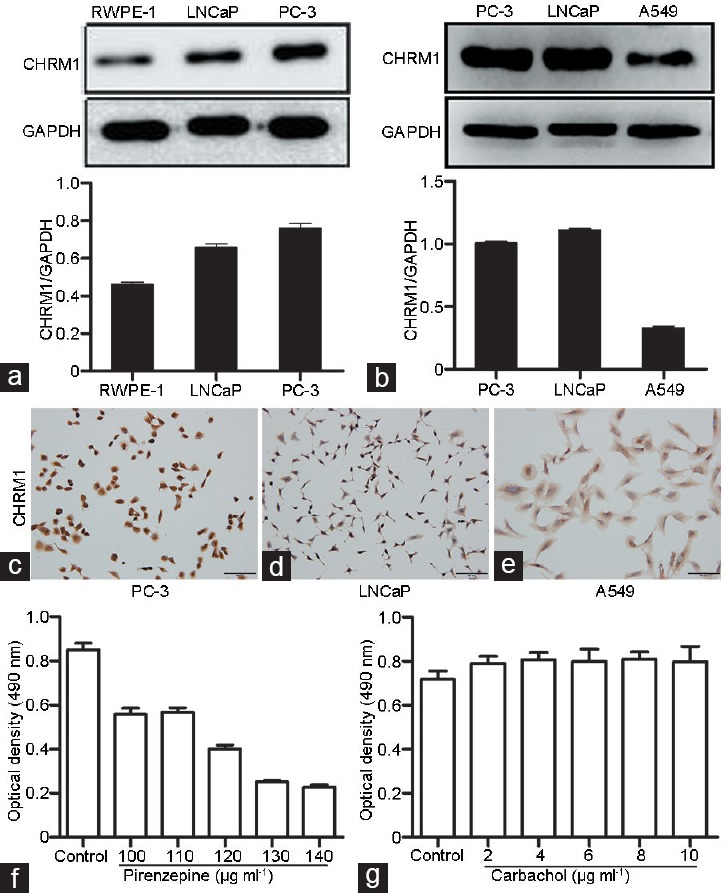
CHRM1 expression in prostate cancer cells. (a) Western blotting images of CHRM1 in RWPE-1, LNCaP, and PC-3 cells. (b) Western blotting images of CHRM1 in PC-3, LNCaP, and A549 cells. Immunohistochemical staining of CHRM1 in (c) PC-3, (d) LNCaP, and (e) A549 cell lines. Scale bars = 100 μm. (f) Pirenzepine at 100–140 μg ml-1 inhibited PC-3 cell proliferation in a concentration-dependent manner. (g) Carbachol promoted PC-3 cell proliferation at 2–10 μg ml-1 even though these changes did not reach statistical significance. Each concentration was tested in five replicates. The results are shown as the mean ± s.e.m. CHRM1: muscarinic acetylcholine receptor M1; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; s.e.m.: standard error of the mean.
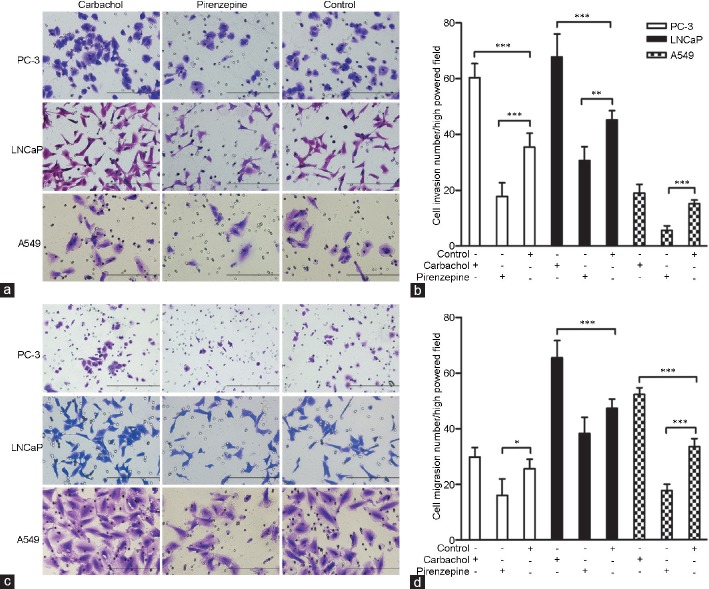
CHRM1 activation promotes prostate cancer cell migration and invasion
To determine an optimal drug concentration for use in this study, experiments on PC-3 cells were conducted with CHRM1-specific antagonist pirenzepine and nonselective muscarinic receptor agonist carbachol. Pirenzepine inhibited the PC-3 cell proliferation at 100-140 μg ml−1 in a concentration-dependent manner (Figure 2f). In contrast, carbachol promoted PC-3 cell proliferation at concentration of 2–10 μg ml−1 even though these changes did not reach statistical significance (Figure 2g). Thus, we used 110 μg ml−1 pirenzepine and 2 μg ml−1 carbachol in subsequent studies. To investigate the role of CHRM1 in regulating cell migration and invasion, cell migration and invasion assays were performed. Carbachol (2 μg ml−1) dramatically stimulated the invasion of PC-3 and LNCaP cell lines, while pirenzepine (110 μg ml−1) markedly inhibited the invasion of all three cell lines (P = 0.006; Figure 3a and 3b). Similarly, carbachol promoted the migration of LNCaP and A549 cell lines, and pirenzepine significantly inhibited the migration of PC-3 and A549 cell lines (P = 0.014; Figure 3c and 3d). Thus, these data suggest that CHRM1 activation promotes prostate cancer cell migration and invasion and has a similar effect on lung cancer cells expressing CHRM1.
Figure 3.
CHRM1 activation promotes prostate cancer cell migration and invasion. (a) Representative images of the membrane infiltration of three cancer cell lines (PC-3, LNCaP, and A549 cells) after carbachol (a muscarinic receptor agonist) or pirenzepine (a CHRM1-specific antagonist) administration. Scale bars = 200 μm. (b) Quantification of infiltrated cells in the cell invasion assays. (c) Images of migration of the three tumor cell lines through the Matrigel-coated filter upon carbachol or pirenzepine administration. (d) Quantification of migrated cell numbers in the cell migration assays. Note that activation of CHRM1 significantly promoted the migration and invasion of the three cell lines. Cells were counted in five random fields per section. Error bars indicate standard error. *P < 0.05; **P < 0.01; ***P < 0.001. CHRM1: muscarinic acetylcholine receptor M1.
CHRM1 stimulation promotes hedgehog signaling activation
Western blotting was applied to detect protein expression levels of GLI1, PTCH1, and SHH. GLI1 and PTCH1 protein levels were increased in a time-dependent manner after exposure to carbachol (Figure 4a and 4b). In addition, the expression of GLI1 and PTCH1 was prevented by pirenzepine, a CHRM1-specific antagonist (Figure 4c and 4d), while the expression of SHH was scarcely affected by carbachol or pirenzepine (Figure 4a–4d). Furthermore, we measured the mRNA abundance of GLI1, PTCH1, and SHH in PC-3 cells treated with multiple concentrations of pirenzepine. GLI1 mRNA expression was clearly inhibited by pirenzepine at all the applied concentrations (P = 0.037; Figure 4e). Although PTCH1 mRNA level was slightly increased after treatment with pirenzepine in PC-3 cells, it did not reach statistical significance (Figure 4f). In addition, the SHH mRNA expression level was scarcely affected by pirenzepine (Figure 4g). Together, these data support the concept that CHRM1 stimulation promotes Hh signaling activation mainly by regulating GLI1 and PTCH1.
Figure 4.
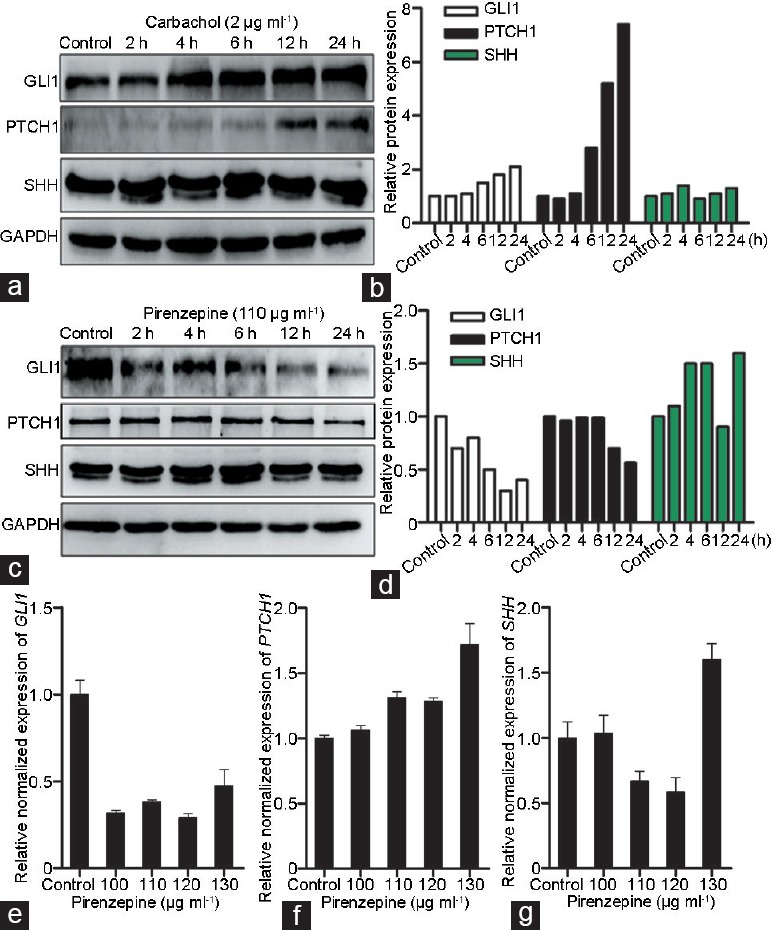
CHRM1 stimulation promotes hedgehog signaling activation. (a) Western blotting images of GLI1, PTCH1, and SHH in PC-3 cells treated with 2 μg ml−1 carbachol for the indicated time. (b) Quantification of GLI1, PTCH1, and SHH expression in PC-3 cells treated with carbachol. (c) Images of GLI1, PTCH1, and SHH immunoblotting in PC-3 cells treated with 110 μg ml-1 pirenzepine for the indicated time. (d) Quantification of GLI1, PTCH1, and SHH in PC-3 cells treated with pirenzepine. Note that the expression of GLI1 and PTCH1 was mediated by CHRM1 activation, but that the expression of SHH was not significantly affected by carbachol or pirenzepine. (e) Real-time PCR results showed that GLI1 mRNA expression was suppressed in PC-3 cells treated with different concentrations of pirenzepine. (f) Quantitative analysis of PTCH1 mRNA expression in PC-3 cells after treatment with pirenzepine. (g) Quantitative analysis of SHH mRNA expression in PC-3 cells treated with pirenzepine. Each sample was run in triplicate. The results are shown as the mean ± s.e.m. GLI1: GLI family zinc finger 1; PTCH1: patched 1; SHH: sonic hedgehog; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; s.e.m.: standard error of the mean.
Hedgehog signaling inhibition attenuates carbachol-induced prostate cancer cell migration
To further investigate whether carbachol affects prostate cancer cell migration through Hh signaling, we examined the effect of Hh signaling blockade on carbachol-induced cell migration. Compared with that in the control group, the migratory ability of PC-3 cells was inhibited after treatment with 3 μmol l-1 GANT61 (Figure 5a and 5b), which is a GLI inhibitor by suppressing the DNA-binding capacity of GLI.32 As expected, the migratory ability of PC-3 cells was stimulated by treatment with 10 μmol l-1 carbachol (Figure 5a and 5c). The inhibitory effect of GANT61 on muscarinic signaling is shown in Figure 5d. GANT61 counteracted the carbachol-stimulated migration of PC-3 cells (Figure 5c–5e). These results indicate that Hh signaling blockade attenuates carbachol-induced migration in PC-3 prostate cancer cells.
Figure 5.
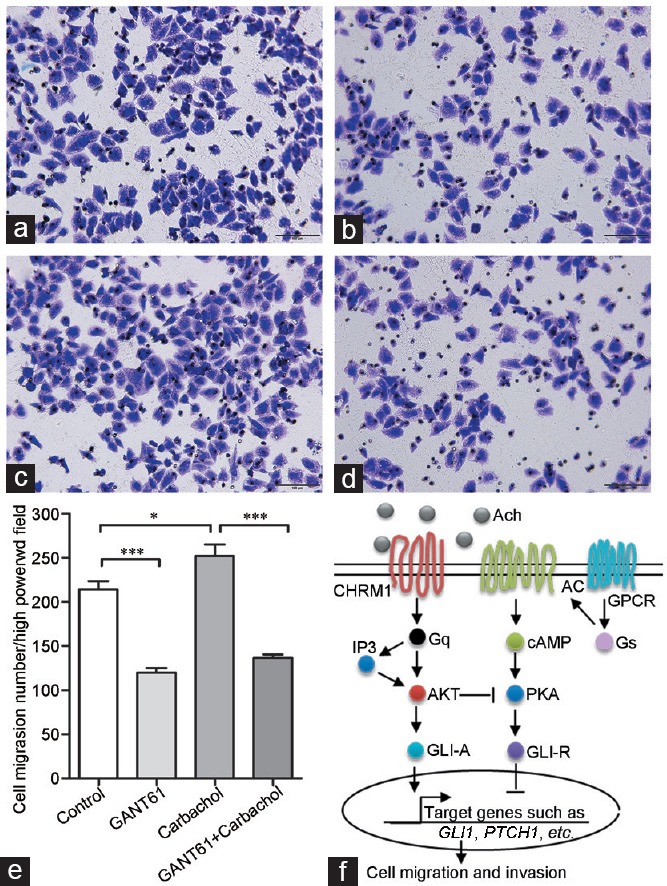
Hedgehog signaling inhibition attenuates carbachol-induced prostate cancer cell migration. Representative images of migrated PC-3 cells left (a) untreated or treated with (b) GANT61 at 3 μmol l−1, (c) carbachol at 10 μmol l−1, or (d) both carbachol and GANT61. Scale bars = 100 μm. (e) Quantification of migrated cells in the cell migration assays. Note that GANT61 (an inhibitor of hedgehog signaling) counteracted the carbachol-induced migration of PC-3 cells. Cells were counted in five random fields per section. Error bars indicate standard error. *P < 0.05; ***P < 0.001. (f) Proposed scheme for muscarinic receptor signaling transduction mechanisms in prostate cancer migration and invasion. Activation of CHRM1 through binding to Ach mediates PTCH1 and downstream GLI1 activation, resulting in gene transcription and ultimately promoting prostate cancer cell migration and invasion. Ach: acetylcholine; cAMP: cyclic adenosine monophosphate; PKA: protein kinase A; AKT: protein kinase B; GPCR: G protein-coupled receptors.
DISCUSSION
The role of the autonomic nervous system in tumorigenesis and progression has received increasing attention in recent years. PNI, a process defined as cancer cell invasion along nerves, is a characteristic phenomenon in certain epithelial malignancies, including prostate and pancreatic carcinomas.33,34 This distinct pathological entity has been reported in approximately 75% of prostatectomy specimens and has been suggested to be correlated with poor prognosis.35,36 With regard to the parasympathetic branch of the nervous system, the integrated, functional loop comprises nerve bundles, acetylcholine, and corresponding receptors, and all of these have been associated with prostate cancer growth and progression. Our work focused on the effect of CHRM1 on disease aggressiveness and the downstream molecular mechanisms.
Previous studies have demonstrated the preponderance of type 1 muscarinic receptor among the five muscarinic receptor subtypes in healthy mouse prostate glands1 and in human benign prostatic hyperplasia (BPH) or prostate cancer tissues.9,10 Substantial CHRM1 expression was observed in human prostate adenoma and in the PC-3 and LNCaP prostate cancer cell lines, indicating a potential role of CHRM1 in prostate cancer progression. Surprisingly, the expression of CHRM1 was higher in early stages of human prostate adenoma than in late stages. This phenomenon may have been due to the low sample number (n = 60) in our study or to the greater number of early-stage cases (n = 34) than late-stage cases (n = 26). We analyzed the relationship between CHRM1 immune reactivity and Gleason score (Gleason 3 + 3, Gleason 3 + 4, Gleason 4 + 3, and Gleason 8-10). There was no significant difference in CHRM1 expression between different Gleason score groups (data not shown), similar to the results in nonmetastatic and metastatic groups. It has been reported that CHRM1 mRNA expression is associated with prostate size, but not associated with age, prostate-specific antigen level (PSA), and pathological diagnosis (BPH vs prostate cancer).10 Hence, our study may not take into account some potential-affecting factors. CHRM1 overexpression in early stages of human prostate cancer may partly explain why denervation should be performed in early stages of tumors and why later denervation does not prevent cancer development and metastasis.18 In light of the important role of type 1 muscarinic receptor in mouse prostate cancer metastasis, the role of type 1 muscarinic receptor in human prostate cancer deserves more investigation. Previous studies have showed that pirenzepine exerts an antiproliferative effect at dose of 100–125 μg ml−1 and carbachol promotes cell proliferation at 10 μmol l−1 in PC-3 cells.29 We observed similar effect induced by pirenzepine and carbachol in PC-3 cells, though the effect of carbachol did not reach statistical significance. In PC-3luc tumor-bearing mice, carbachol treatment enhances tumor cell invasion lymph nodes, and pirenzepine inhibits lymph node invasion.1 CHRM1 activation may also promote migration and invasion of prostate cancer cell lines. Activation of CHRM1 by carbachol promoted the migration and invasion of PC-3 and LNCaP prostate cancer cells and A549 lung cancer cells expressing CHRM1, while suppression of CHRM1 by pirenzepine inhibited the migration and invasion of PC-3, LNCaP, and A549 cells. Thus, our results strongly suggest a pivotal role for CHRM1 in regulating prostate cancer cell migration and invasion. However, the role of muscarinic receptors in cancer progression has been debated due to controversial research results. A recent study has suggested that the CHRM3 contributes to human colon cancer cell migration and invasion.37 In addition to neuronal cholinergic signaling, a more recent study has reported that autocrine acetylcholine and CHRM3 promote prostate cancer growth and castration-resistant prostate cancer (CRPC).38 Therefore, it remains unclear whether CHRM1, CHRM3, or the synergistic effect of CHRM1 and CHRM3 plays a major role in prostate cancer progression. Considering that CHRM1 and CHRM3 are both expressed in prostate tissues, single or co-knockout of CHRM1 and CHRM3 is likely a good approach to distinguish the different role of CHRM1 and CHRM3 in prostate cancer progression.
Moreover, previous studies have revealed that Hh signaling promotes the development and dissemination of multiple tumors, including prostate cancer,25,27 and that it also participates in the growth and maintenance of neurons in normal and tumor tissues.39,40,41 On the basis of the possible consistency between nerves and CHRM1, we investigated the relationship between Hh signaling and CHRM1 in PC-3 prostate cancer cells treated with carbachol or pirenzepine. Western blotting results showed that GLI1 and PTCH1 levels markedly increased in PC-3 cells after exposure to carbachol. In contrast, the expression of GLI1 and PTCH1 was prevented by pirenzepine, a CHRM1-specific antagonist. However, the expression of SHH was scarcely affected by carbachol or pirenzepine. RT-PCR analysis confirmed similar results, except for the expression of PTCH1, which was slightly increased in PC-3 cells treated with pirenzepine (P > 0.05). This result may be due to transcriptional regulation of Hh signaling, because PTCH1 is a negative target gene of Hh signaling.42 Moreover, the carbachol-induced migration of PC-3 cells was counteracted by GANT61, an antagonist of Hh signaling. Collectively, our data suggest that CHRM1 promote prostate cancer migration and invasion through Hh signaling-mediated activation of GLI1 and PTCH1 in PC-3 cells. However, it remains unclear how CHRM1 impacts Hh signaling. Studies have demonstrated that certain GPCRs regulate Hh signaling. For example, Gs-coupled Gpr161 is a negative regulator of Hh signaling by mediating cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling.43 Gpr161 increases cellular cAMP/PKA levels, increases PKA-phosphorylated GLI3, and ultimately downregulates Hh signaling. In contrast, Gi-coupled Gpr175 enhances Hh signaling by downregulating cAMP levels.44 Another report has suggested that phosphoinositide 3-kinase (PI3K) and AKT signaling are essential for Hh signaling.45 CHRM1 is a GPCR linked with Gq-type G proteins, which initiate PI metabolism and AKT phosphorylation. Thus, we hypothesized that CHRM1 affects Hh signaling (Figure 5f). Activation of Gq-coupled CHRM1 promotes AKT phosphorylation, thereby stabilizing the activation of GLI (GLI-A). AKT may also antagonize PKA-mediated repression of GLI (GLI-R),45 consequently activating Hh signaling. This hypothesis is important and should be further studied.
In summary, the present study results suggest that CHRM1 is involved in regulating prostate cancer migration and invasion through hedgehog signaling pathway activation. CHRM1-specific antagonist pirenzepine inhibit the dissemination of PC-3 and LNCaP prostate cancer cells and A549 lung cancer cells. Muscarinic receptor antagonists have a wide variety of clinical applications, such as for the treatment of gastric ulcer (GU), chronic obstructive pulmonary disease (COPD), and schizophrenia;5 clinical strategies such as CHRM1 antagonists and Hh signaling inhibitors may be potential therapeutic options for prostate cancer treatment.
AUTHOR CONTRIBUTIONS
CX put forward the concept of the study, designed the study, contributed to the statistical analysis, and revised the manuscript. QQY and LHX performed the experiments, collected and analyzed the data, and drafted the manuscript. MZ participated in the acquisition of data and discussion of results. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This work was supported by the Natural Science Foundation of Chongqing (CSTC, 2009BA5081).
REFERENCES
- 1.Magnon C, Hall SJ, Lin J, Xue X, Gerber L, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 2.Peralta EG, Ashkenazi A, Winslow JW, Smith DH, Ramachandran J, et al. Distinct primary structures, ligand-binding properties and tissue-specific expression of four human muscarinic acetylcholine receptors. EMBO J. 1987;6:3923–9. doi: 10.1002/j.1460-2075.1987.tb02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonner TI, Buckley NJ, Young AC, Brann MR. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987;237:527–32. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- 4.Hulme EC, Birdsall NJ, Buckley NJ. Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol. 1990;30:633–73. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- 5.Haga T. Molecular properties of muscarinic acetylcholine receptors. Proc Jpn Acad Ser B Phys Biol Sci. 2013;89:226–56. doi: 10.2183/pjab.89.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutkind JS, Novotny EA, Brann MR, Robbins KC. Muscarinic acetylcholine receptor subtypes as agonist-dependent oncogenes. Proc Natl Acad Sci U S A. 1991;88:4703–7. doi: 10.1073/pnas.88.11.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah N, Khurana S, Cheng K, Raufman JP. Muscarinic receptors and ligands in cancer. Am J Physiol Cell Physiol. 2009;296:C221–32. doi: 10.1152/ajpcell.00514.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spindel ER. Muscarinic receptor agonists and antagonists: effects on cancer. Handb Exp Pharmacol. 2012:451–68. doi: 10.1007/978-3-642-23274-9_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruggieri MR, Colton MD, Wang P, Wang J, Smyth RJ, et al. Human prostate muscarinic receptor subtypes. J Pharmacol Exp Ther. 1995;274:976–82. [PMC free article] [PubMed] [Google Scholar]
- 10.Witte LP, Teitsma CA, de La Rosette JJ, Michel MC. Muscarinic receptor subtype mRNA expression in the human prostate: association with age, pathological diagnosis, prostate size, or potentially interfering medications? Naunyn Schmiedebergs Arch Pharmacol. 2014;387:207–14. doi: 10.1007/s00210-013-0934-4. [DOI] [PubMed] [Google Scholar]
- 11.Cheng K, Zimniak P, Raufman JP. Transactivation of the epidermal growth factor receptor mediates cholinergic agonist-induced proliferation of H508 human colon cancer cells. Cancer Res. 2003;63:6744–50. [PubMed] [Google Scholar]
- 12.Frucht H, Jensen RT, Dexter D, Yang WL, Xiao Y. Human colon cancer cell proliferation mediated by the M3 muscarinic cholinergic receptor. Clin Cancer Res. 1999;5:2532–9. [PubMed] [Google Scholar]
- 13.Shant J, Cheng K, Marasa BS, Wang JY, Raufman JP. Akt-dependent NF-kappaB activation is required for bile acids to rescue colon cancer cells from stress-induced apoptosis. Exp Cell Res. 2009;315:432–50. doi: 10.1016/j.yexcr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song P, Sekhon HS, Lu A, Arredondo J, Sauer D, et al. M3 muscarinic receptor antagonists inhibit small cell lung carcinoma growth and mitogen-activated protein kinase phosphorylation induced by acetylcholine secretion. Cancer Res. 2007;67:3936–44. doi: 10.1158/0008-5472.CAN-06-2484. [DOI] [PubMed] [Google Scholar]
- 15.Rayford W, Noble MJ, Austenfeld MA, Weigel J, Mebust WK, et al. Muscarinic cholinergic receptors promote growth of human prostate cancer cells. Prostate. 1997;30:160–6. doi: 10.1002/(sici)1097-0045(19970215)30:3<160::aid-pros3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Luthin GR, Wang P, Zhou H, Dhanasekaran D, Ruggieri MR. Role of m1 receptor-G protein coupling in cell proliferation in the prostate. Life Sci. 1997;60:963–8. doi: 10.1016/s0024-3205(97)00035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao CM, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6:250ra115. doi: 10.1126/scitranslmed.3009569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saloman JL, Albers KM, Rhim AD, Davis BM. Can stopping nerves, stop cancer? Trends Neurosci. 2016;39:880–9. doi: 10.1016/j.tins.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin H, Jackson GA, Lu Y, Drenkhahn SK, Brownstein KJ, et al. Inhibition of Gli/hedgehog signaling in prostate cancer cells by “cancer bush” Sutherlandia frutescens extract. Cell Biol Int. 2016;40:131–42. doi: 10.1002/cbin.10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barakat MT, Humke EW, Scott MP. Learning from Jekyll to control hyde: hedgehog signaling in development and cancer. Trends Mol Med. 2010;16:337–48. doi: 10.1016/j.molmed.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez P, Hernandez AM, Stecca B, Kahler AJ, DeGueme AM, et al. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc Natl Acad Sci U S A. 2004;101:12561–6. doi: 10.1073/pnas.0404956101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathew E, Zhang Y, Holtz AM, Kane KT, Song JY, et al. Dosage-dependent regulation of pancreatic cancer growth and angiogenesis by hedgehog signaling. Cell Rep. 2014;9:484–94. doi: 10.1016/j.celrep.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Mauro C, Rosa R, D’Amato V, Ciciola P, Servetto A, et al. Hedgehog signalling pathway orchestrates angiogenesis in triple-negative breast cancers. Br J Cancer. 2017;116:1425–35. doi: 10.1038/bjc.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkinson SE, Furic L, Buchanan G, Larsson O, Pedersen J, et al. Hedgehog signaling is active in human prostate cancer stroma and regulates proliferation and differentiation of adjacent epithelium. Prostate. 2013;73:1810–23. doi: 10.1002/pros.22720. [DOI] [PubMed] [Google Scholar]
- 25.Datta S, Datta MW. Sonic hedgehog signaling in advanced prostate cancer. Cell Mol Life Sci. 2006;63:435–48. doi: 10.1007/s00018-005-5389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–12. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Wang Z, Ma Q, Xu Q, Liu H, et al. Sonic hedgehog paracrine signaling activates stromal cells to promote perineural invasion in pancreatic cancer. Clin Cancer Res. 2014;20:4326–38. doi: 10.1158/1078-0432.CCR-13-3426. [DOI] [PubMed] [Google Scholar]
- 28.Javelaud D, Pierrat MJ, Mauviel A. Crosstalk between TGF-beta and hedgehog signaling in cancer. FEBS Lett. 2012;586:2016–25. doi: 10.1016/j.febslet.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Mannan Baig A, Khan NA, Effendi V, Rana Z, Ahmad HR, et al. Differential receptor dependencies: expression and significance of muscarinic M1 receptors in the biology of prostate cancer. Anticancer Drugs. 2017;28:75–87. doi: 10.1097/CAD.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 30.Guo K, Ma Q, Li J, Wang Z, Shan T, et al. Interaction of the sympathetic nerve with pancreatic cancer cells promotes perineural invasion through the activation of STAT3 signaling. Mol Cancer Ther. 2013;12:264–73. doi: 10.1158/1535-7163.MCT-12-0809. [DOI] [PubMed] [Google Scholar]
- 31.Berman DM, Karhadkar SS, Hallahan AR, Pritchard JI, Eberhart CG, et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297:1559–61. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- 32.Tong W, Qiu L, Qi M, Liu J, Hu K, et al. GANT-61 and GDC-0449 induce apoptosis of prostate cancer stem cells through a GLI-dependent mechanism. J Cell Biochem. 2018;119:3641–52. doi: 10.1002/jcb.26572. [DOI] [PubMed] [Google Scholar]
- 33.Magnon C. Role of the autonomic nervous system in tumorigenesis and metastasis. Mol Cell Oncol. 2015;2:e975643. doi: 10.4161/23723556.2014.975643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11:695–707. doi: 10.1038/nrc3131. [DOI] [PubMed] [Google Scholar]
- 35.Maru N, Ohori M, Kattan MW, Scardino PT, Wheeler TM. Prognostic significance of the diameter of perineural invasion in radical prostatectomy specimens. Hum Pathol. 2001;32:828–33. doi: 10.1053/hupa.2001.26456. [DOI] [PubMed] [Google Scholar]
- 36.Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379–91. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 37.Belo A, Cheng K, Chahdi A, Shant J, Xie G, et al. Muscarinic receptor agonists stimulate human colon cancer cell migration and invasion. Am J Physiol Gastrointest Liver Physiol. 2011;300:G749–60. doi: 10.1152/ajpgi.00306.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang N, Yao M, Xu J, Quan Y, Zhang K, et al. Autocrine activation of CHRM3 promotes prostate cancer growth and castration resistance via CaM/CaMKK-mediated phosphorylation of Akt. Clin Cancer Res. 2015;21:4676–85. doi: 10.1158/1078-0432.CCR-14-3163. [DOI] [PubMed] [Google Scholar]
- 39.Feijoo CG, Onate MG, Milla LA, Palma VA. Sonic hedgehog (Shh)-Gli signaling controls neural progenitor cell division in the developing tectum in zebrafish. Eur J Neurosci. 2011;33:589–98. doi: 10.1111/j.1460-9568.2010.07560.x. [DOI] [PubMed] [Google Scholar]
- 40.Wu CL, Chen SD, Hwang CS, Yang DI. Sonic hedgehog mediates BDNF-induced neuroprotection against mitochondrial inhibitor 3-nitropropionic acid. Biochem Biophys Res Commun. 2009;385:112–7. doi: 10.1016/j.bbrc.2009.04.145. [DOI] [PubMed] [Google Scholar]
- 41.Peterson SC, Eberl M, Vagnozzi AN, Belkadi A, Veniaminova NA, et al. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell. 2015;16:400–12. doi: 10.1016/j.stem.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kasper M, Regl G, Frischauf AM, Aberger F. GLI transcription factors: mediators of oncogenic hedgehog signalling. Eur J Cancer. 2006;42:437–45. doi: 10.1016/j.ejca.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 43.Mukhopadhyay S, Wen X, Ratti N, Loktev A, Rangell L, et al. The ciliary G-protein-coupled receptor Gpr161 negatively regulates the sonic hedgehog pathway via cAMP signaling. Cell. 2013;152:210–23. doi: 10.1016/j.cell.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 44.Singh J, Wen X, Scales SJ. The orphan G protein-coupled receptor Gpr175 (Tpra40) enhances hedgehog signaling by modulating cAMP levels. J Biol Chem. 2015;290:29663–75. doi: 10.1074/jbc.M115.665810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riobo NA, Lu K, Ai X, Haines GM, Emerson CP., Jr Phosphoinositide 3-kinase and Akt are essential for sonic hedgehog signaling. Proc Natl Acad Sci U S A. 2006;103:4505–10. doi: 10.1073/pnas.0504337103. [DOI] [PMC free article] [PubMed] [Google Scholar]