Abstract
Once-daily tadalafil administration has been well established; however, studies about tadalafil once-daily treatment in the Chinese population are lacking. In this phase 4, postmarketing study, we ascertained the long-term safety and effectiveness of tadalafil 2.5 mg and 5.0 mg once daily in Chinese men with erectile dysfunction (n = 635). The primary endpoint of the study was safety at 12 months as assessed by the proportion of patients experiencing at least one treatment-emergent adverse event (serious or nonserious). The secondary endpoints included safety and effectiveness, measured by the International Index of Erectile Function-Erectile Function (IIEF-EF) domain scores. Similar adverse events to the known safety profile of tadalafil, such as nasopharyngitis, upper respiratory tract infection, headache, and dizziness, were detected. No new cardiovascular safety concerns were observed. After 3 months of treatment, significant increases in IIEF-EF domain scores were detected for both 2.5-mg (least squares [LS] mean change: 6.3; 95% confidence interval [CI]: 5.4–7.1; P < 0.001) and 5.0-mg (LS mean change: 7.4; 95% CI: 6.8–7.9; P < 0.001) tadalafil doses, and significance was maintained up to 12 months. In addition, approximately 40% of patients regained normal erectile function (IIEF-EF ≥26) following 1 year of tadalafil once-daily treatment. The findings in this study provide evidence for the extended effectiveness and tolerability of tadalafil, demonstrating no new safety concerns, in a Chinese population and make once-daily tadalafil administration a viable option for improving sexual performance and satisfaction in Chinese men with erectile dysfunction.
Keywords: erectile dysfunction, long-term safety, once-daily dosing, tadalafil
INTRODUCTION
Tadalafil is approved for erectile dysfunction (ED) in more than 90 countries, including the United States, European Union, Australia, and China.1,2,3,4 Previously, clinical development of tadalafil focused on on-demand oral administration at 10-mg or 20-mg doses.5,6,7,8 While phosphodiesterase type-5 (PDE5) inhibitors are effective on-demand, they require time after administration to achieve full efficacy, which may reduce spontaneity in sexual activity.9,10 Pharmacokinetic analyses demonstrate that the 17.5-h half-life of tadalafil allows for once-daily dosing, with stable serum concentrations attained within 5 days.11 Under steady-state conditions, tadalafil exposure is 1.6-fold more than that after a single dose, which may increase the rate of obtaining an erection upon sexual stimulation.11,12 Tadalafil once-daily dosing has been extensively tested in multiple clinical trials, with results suggesting that once-daily administration boosts the patient's ability for spontaneity while still maintaining confidence in and effectiveness of treatment.12,13,14,15,16 Extended safety and tolerability of tadalafil after long-term use has also been reported in an open-label study with 5.0-mg once-daily dosing.17 The most frequent treatment-emergent adverse events (TEAEs) were back pain, influenza, headache, and dyspepsia, while clinical laboratory measures as well as electrocardiograms did not indicate any meaningful abnormalities after 1- and 2-year tadalafil once-daily dosing.17 However, the long-term safety and effectiveness of tadalafil once-daily administration in Chinese men with ED has not been studied. In this study, we report the effectiveness and safety results of 2.5-mg and 5.0-mg once-daily dosing after 1–3 months (period 1) and 5.0-mg once-daily dosing after 12 months (period 2) of tadalafil treatment in Chinese men.
PATIENTS AND METHODS
Study design
This study was a phase 4, prospective, multicenter, randomized, open-label study to determine the effectiveness and long-term safety of tadalafil given once daily to Chinese men with ED (NCT02224846). Patients were enrolled in the study between October 2014 and May 2015, provided that they met the following inclusion criteria. Briefly, the population included Chinese men ≥22 years old and <70 years old, with a 3-month history of ED and a monogamous sexual relationship with a female partner. Exclusion criteria included sexual disorders, such as premature ejaculation or ED caused by untreated endocrine disease (e.g., hypothyroidism, hypopituitarism, or hypogonadism); clinically meaningful hepatobiliary disease or renal insufficiency; history of cardiac conditions; recent central nervous system injury; uncontrolled diabetes; or current use of nitrates, chemotherapy, androgens, antiandrogens, estrogens, anabolic steroids, or other approved or traditional Chinese medicine as ED treatment. All patients provided written informed consent, and the study was conducted in accordance with the Declaration of Helsinki. An ethical review board at each site approved the study protocol. The study was conducted at 24 centers in China. The last patient visit was in July 2017.
The study was conducted in three phases: the first phase was a 0.5- to 1-month run-in period for baseline sexual function assessment; the second phase (period 1) was a 3-month treatment period with 2.5 mg or 5.0 mg of oral tadalafil once daily to determine effectiveness and safety; and the third phase (period 2) was a 21-month extension period of the 5.0-mg oral tadalafil once-daily treatment to assess long-term safety. Upon completion of the run-in phase, patients were randomized (1:2) to the tadalafil 2.5-mg treatment group (n = 211) or 5.0-mg treatment group (n = 424) at the baseline visit. Study treatments were not blinded since no comparisons between the two doses were conducted. At the end of period 1, patients initially in the 2.5-mg treatment group were switched to the 5.0-mg tadalafil once-daily dose and those initially in the 5.0-mg treatment group remained on the same dose for the 21-month extension period (period 2). Figure 1 displays the study design.
Figure 1.
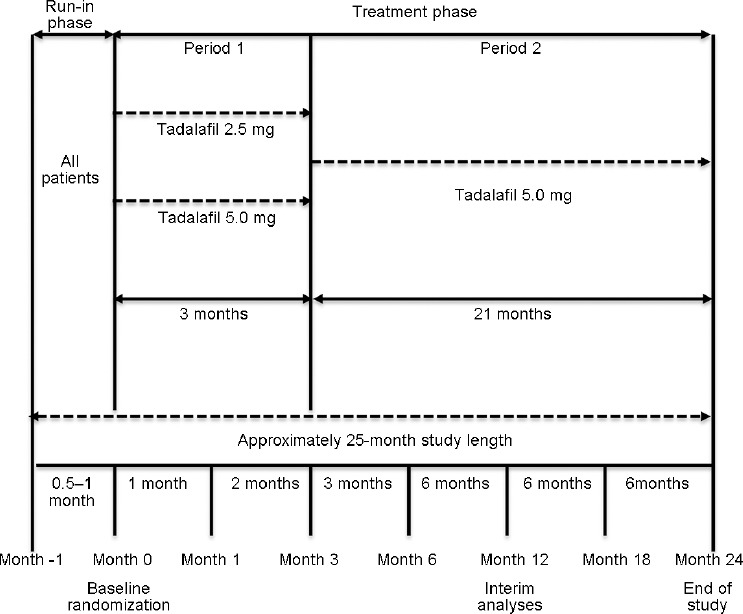
Study design flowchart.
A preplanned interim analysis was conducted after all patients completed 1 year of tadalafil treatment, and those results are presented here. A final analysis will be conducted after all patients complete the 2-year treatment.
The primary objective of the trial was safety of tadalafil once-daily treatment at 12 months. The secondary objectives included 1-month and 3-month effectiveness of 2.5-mg and 5.0-mg tadalafil once-daily dosing, 12-month effectiveness of 5.0-mg tadalafil once-daily dosing, and 3-month safety of 2.5-mg and 5.0-mg tadalafil once-daily dosing.
Safety analyses
The safety population included all enrolled patients who received at least one dose of tadalafil, and patients without follow-up data were excluded. Safety was analyzed via the proportion of patients at 3 and 12 months who experienced at least one TEAE (serious or nonserious). A TEAE was defined as an event that first occurred or worsened after baseline. Treatment-related adverse events were defined as events the investigator indicated to be related to treatment. Patient vital signs (blood pressure and heart rate) and laboratory assessments (hematology and clinical chemistry) were also reported.
Effectiveness outcome measures
The effectiveness population included all enrolled patients who received at least one dose of tadalafil and had both baseline and postbaseline data. The effectiveness of tadalafil once daily was measured by a change from baseline in the International Index of Erectile Function-Erectile Function (IIEF-EF) domain questionnaire18 (sum of questions Q1–5 and Q15) at months 1, 3, and 12; the proportion of patients who achieved normal erectile function (IIEF-EF ≥26) at months 1, 3, and 12; and the proportion of patients who responded “yes” to the Sexual Encounter Profile (SEP) diary Q2 (SEP2) and Q3 (SEP3)14 at months 1 and 3 upon a minimum of four sexual intercourse attempts.
Statistical analyses
Tadalafil exposure (in days) was calculated as the difference between the first day and the last day of treatment in each period. Treatment was self-administered. To establish treatment compliance, the number of tablets dispensed, the date of tadalafil dispensed, and the number of tablets returned were recorded at each patient visit.
The overall incidence and proportion of patients experiencing a TEAE (95% confidence interval [CI]) or discontinuation due to adverse events during the 1st year of tadalafil once-daily treatment were calculated for all patients combined and for each treatment group.
A mixed-model with repeated measures (MMRM) was used for calculation of the adjusted mean changes in IIEF-EF domain scores from baseline to each follow-up visit, and the least squares (LS) mean changes and 95% CI are presented. For MMRM, the fixed effects of visit, pooled investigator, baseline score, and the interaction of visit and baseline score were fitted. The percentage of patients with IIEF-EF domain scores <26 and ≥26 were summarized. The SEP questions were tabulated into a baseline score and an endpoint score, based on the percentages of “yes” responses to a particular question requesting the number of sexual encounters during the run-in period and in the postbaseline period, respectively. No comparisons between treatment groups were performed for the effectiveness population. All tests of treatment effects were conducted with a two-sided alpha = 0.05. P values for significance of change from baseline to the follow-up visit were determined for each treatment group.
RESULTS
Patient disposition
A total of 689 patients entered into the study, and the study had 54 (7.8%) screen failures: 21 (38.9%) due to not meeting entry criteria, 29 (53.7%) due to withdrawal by patients, and 4 (7.4%) due to loss to follow-up (Figure 2). The remaining 635 patients were randomized (1:2) to the 2.5-mg treatment group (n = 211) or the 5.0-mg treatment group (n = 424). By month 3, 9.0% of patients in the 2.5-mg treatment group and 8.5% of patients in the 5.0-mg treatment group had discontinued. The most common reason for discontinuation within period 1 was patient withdrawal (n = 26). Other reasons included protocol violation (n = 12), loss to follow-up (n = 11), adverse event (n = 3), and sponsor decision (n = 3). A total of 530 (83.5%) patients completed period 2, with 116 (18.3%) patients discontinuing up to the interim analysis. The most common reason for discontinuation within period 2 was patient withdrawal (n = 68). Other reasons included loss to follow-up (n = 20), protocol violation (n = 15), adverse event (n = 7), sponsor decision (n = 3), physician decision (n = 2), and death (n = 1).
Figure 2.
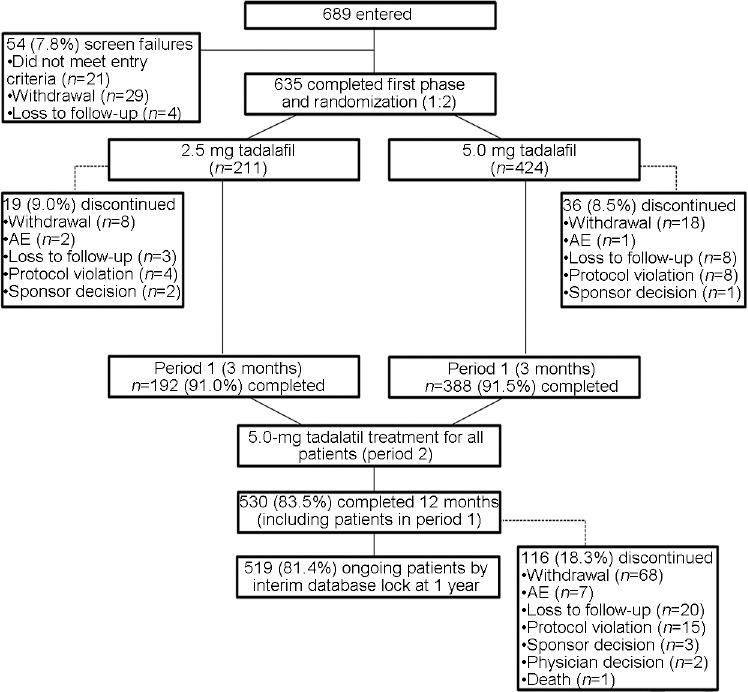
Flow diagram of patient disposition. AE: adverse event.
Patient demographics
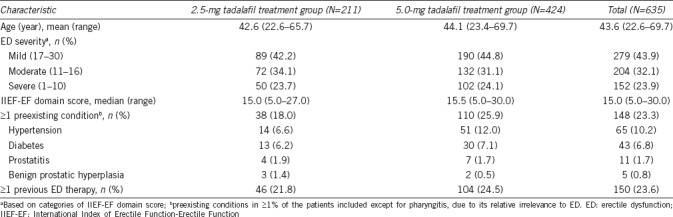
Baseline characteristics of patients, with preexisting conditions that occurred in at least 1% of the patients in any treatment group, are displayed in Table 1. Patients had the mean age of 43.6 (range: 22.6–69.7) years, and 23.6% of the patients had previously been treated with an ED therapy, including tadalafil, sildenafil, and vardenafil. The severity of ED was mostly mild to moderate, with a median IIEF-EF domain score of 15.0 (range: 5.0–30.0). Overall, clinical characteristics and domain scores were well balanced between the two treatment groups.
Table 1.
Baseline characteristics
Tadalafil once daily exposure and compliance
The mean ± standard deviation of total treatment exposure time during period 1 and period 2 was 89.5 ± 11.7 days and 271.9 ± 18.6 days, respectively. By month 12, total exposure time was 332.5 ± 89.4 days. Total treatment compliance (taking 70%–100% of prescribed tablets) at month 12 was 86.0%.
Safety of tadalafil once daily
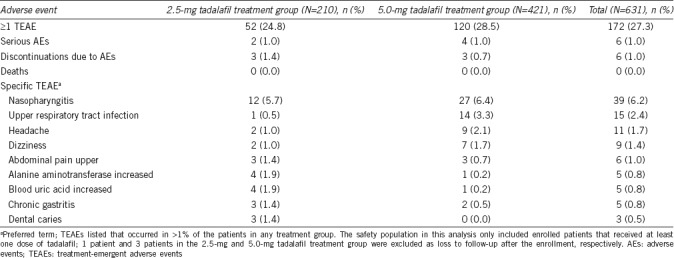
At the end of the 12-month treatment period, no deaths were reported. Serious adverse events (SAEs) included unstable angina, coronary artery disease, duodenal ulcer hemorrhage, osteoporosis, spouse pregnancy, ureterolithiasis, and sleep apnea syndrome, none of which were considered related to study drug. The study reported TEAEs in 172 (27.3%) of the patients who were mostly mild (Table 2). The most frequent TEAEs (occurring in >1% of patients in any treatment group) at 3 months included nasopharyngitis, upper respiratory tract infection, headache, dizziness, alanine aminotransferase increased, and blood uric acid increased; in addition, at 12 months, chronic gastritis, abdominal pain upper, and dental caries were also reported (Table 2). A total of 6 (1.0%) patients were discontinued from the study due to AEs of eye swelling, duodenal ulcer hemorrhage, chest discomfort, back pain, coronary artery disease, and hemoptysis (1 [0.2%] patient for each AE).
Table 2.
Treatment-emergent adverse events during first 12-month treatment
At month 3, several statistically significant changes from baseline were measured for hematological and chemistry laboratory values, such as decreases in erythrocytes, hematocrit, hemoglobin, leukocytes, lymphocytes, and monocytes; decreases in albumin, alanine aminotransferase, aspartate aminotransferase/serum glutamic oxaloacetic transaminase, cholesterol, calcium, glucose, potassium, and sodium; and increases in creatinine and uric acid. However, all of the median changes in the laboratory values were within the normal range and were not considered clinically meaningful.
A median change from baseline in systolic blood pressure was detected at month 3 in the 5.0-mg treatment group (−1.0 mmHg; P = 0.003). At month 12, slight increases from baseline in heart rate were observed in the 2.5-mg to 5.0-mg (median change: 2.0 bpm; P = 0.014) and 5.0-mg (median change: 2.0 bpm; P = 0.012) treatment groups.
Overall, safety results at 1 year suggest similar AEs to the known safety profile of tadalafil. No new cardiovascular concerns were observed.
Effectiveness of tadalafil once daily
Significant improvement in erectile function, as measured by IIEF-EF, was observed as early as 1 month for 2.5-mg (LS mean change: 4.7; 95% CI: 3.9–5.5; P < 0.001), 5.0-mg (LS mean change: 6.1; 95% CI: 5.6–6.7; P < 0.001), and total tadalafil (LS mean change: 5.7; 95% CI: 5.2–6.1; P < 0.001) once-daily dosing. The same significance was present for individual once-daily doses (LS mean change for 2.5-mg: 6.3; 95% CI: 5.4–7.1; P < 0.001; LS mean change for 5.0-mg: 7.4; 95% CI: 6.8–7.9; P < 0.001) and total tadalafil dose (LS mean change: 7.0; 95% CI: 6.5–7.4; P < 0.001) at 3 months and was maintained to 12 months (LS mean change for 2.5-mg to 5.0-mg dose: 8.0; 95% CI: 7.1–8.8; P < 0.001; for 5.0-mg dose: 7.9; 95% CI: 7.4–8.5; P < 0.001; for total tadalafil dose: 7.9; 95% CI: 7.5–8.4; P < 0.001) (Figure 3a). By month 1, 45 (22.2%) patients in the 2.5-mg treatment group and 127 (30.7%) patients in the 5.0-mg treatment group regained normal erectile function (IIEF-EF ≥26) (total of 172 [27.9%] patients); by month 3, 61 (31.6%) patients in the 2.5-mg treatment group and 147 (37.6%) patients in the 5.0-mg treatment group (total of 208 [35.6%] patients) regained normal erectile function; and by month 12, the numbers of patients in the 2.5–5.0 mg and 5.0 mg dose groups that returned to normal erectile function were 78 (44.1%) and 157 (43.5%), respectively (total of 235 [43.7%] patients) (Figure 3b).
Figure 3.
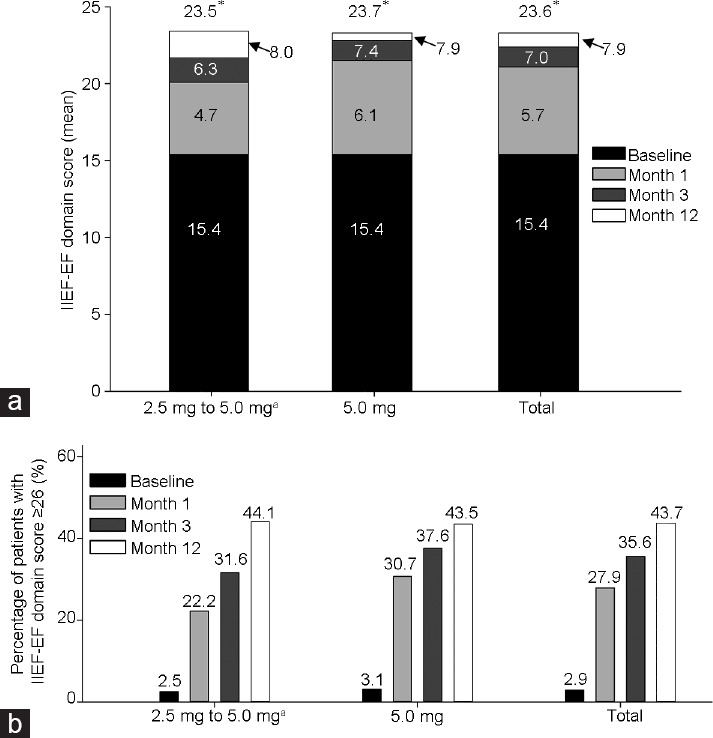
IIEF-EF domain score LS mean change from baseline and achievement of normal erectile function. (a) Mean IIEF-EF domain score at baseline, month 1, month 3, and month 12. LS mean change from baseline indicated within bar. *P < 0.001. (b) Percentage of patients with IIEF-EF domain score ≥26 at baseline, month 1, month 3, and month 12. aPatients given the 2.5-mg dose were switched to 5.0-mg dose starting at 3 months (end of period 1). IIEF-EF: International Index of Erectile Function-Erectile Function; LS: least squares.
Tadalafil once-daily treatment led to a significant LS mean change per patient in the percentage of “yes” responses to the SEP questions. The following results were reported for SEP2 (the ability of the patient to insert penis into partner's vagina) LS mean change (95% CI): month 1, 24.5% (19.2%–29.8%), P < 0.001 for 2.5-mg treatment and 29.1% (26.4%–31.8%), P < 0.001 for 5.0-mg treatment; month 3, 30.6% (25.9%–35.4%), P < 0.001 for 2.5-mg treatment and 33.8% (31.3%–36.3%), P < 0.001 for 5.0-mg treatment. The following results were reported for SEP3 (an erection maintained long enough to have successful intercourse) LS mean change (95% CI): month 1, 27.3% (20.7%–33.9%), P < 0.001 for 2.5-mg treatment and 33.4% (29.0%–37.8%), P < 0.001 for 5.0-mg treatment; month 3, 41.1% (34.2%–48.1%), P < 0.001 for 2.5-mg treatment and 43.6% (39.2%–48.0%), P < 0.001 for 5.0-mg treatment (Figure 4).
Figure 4.
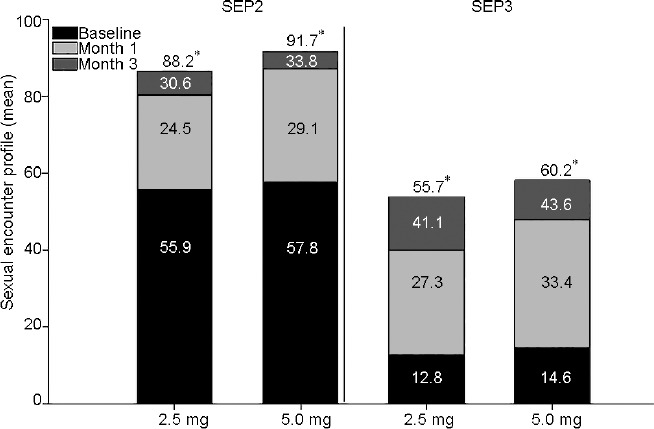
SEP question responses. Mean SEP at baseline, month 1, and month 3. LS mean change from baseline indicated within bar. *P < 0.001. SEP: sexual encounter profile; LS: least squares; SEP2: ability of patient to insert penis into partner's vagina; SEP3: erection maintained long enough to have successful intercourse.
DISCUSSION
The safety and effectiveness of long-term tadalafil once-daily administration were assessed in the Chinese population. The results reported in this study are from a 1-year interim analysis; the full 2-year analysis will be conducted once all patients have completed this period. At the time of this analysis, 530 (83.5%) of the 635 Chinese men who entered randomization completed 1 year of treatment and demonstrated a high treatment compliance rate of 86.0%.
Extended treatment with 5.0 mg tadalafil up to 1 year did not unveil any new safety concerns in Chinese patients with ED. Long-term tadalafil once-daily dosing resulted in mostly mild AEs (total TEAE incidence of 27.3%), only six discontinuations due to AEs, and no clinically meaningful laboratory or vital sign changes. No deaths were reported, and SAEs that occurred were mostly present in patients with preexisting conditions and therefore considered not related to study drug. The primary objective of this trial regarding long-term safety was met and the safety profile was consistent with the tolerability reported previously.17
Consistent with previous studies,12,13,14,15,16 tadalafil once daily improved erectile function. Based on the IIEF-EF domain score assessments, effectiveness was notable as early as 1 month after tadalafil once-daily dosing at 2.5 mg and 5.0 mg. After 1 month of tadalafil once-daily dosing, erectile function had returned to normal (IIEF-EF ≥26) for 27.9% of patients. This effectiveness was maintained at 3 months and continued up to 12 months, with 43.7% of patients having an IIEF-EF score ≥26. Responses for the SEP questions after once-daily treatment similarly supported long-term effectiveness and, importantly, patient fulfillment. Both treatment doses, 2.5 mg and 5.0 mg, led to increases in penetration (SEP2) and intercourse (SEP3). Largely, the effectiveness and improvements seen at month 1 were maintained to month 3 and then to month 12, after tadalafil once-daily dosing.
The long-term safety and effectiveness results of tadalafil once daily may afford several benefits to Chinese men. The rapid improvements in erectile function demonstrated after only 1 month of tadalafil once-daily dosing suggest the ability to quickly increase sexual performance. In addition, as demonstrated by the month 12 safety results, the extended tolerability of tadalafil once-daily dosing will enable greater administration compliance as well as a more spontaneous, natural sex life for Chinese men.
Benefits to Chinese men who previously did not see improvement with on-demand PDE5 inhibitors may also be possible. For example, in patients who received the maximum dose of PDE5 inhibitors on-demand (i.e., 100 mg sildenafil, 20 mg vardenafil, or 20 mg tadalafil) yet still had incomplete response (IIEF-EF <26), tadalafil once daily at both 2.5-mg and 5.0-mg dosing restored normal erectile function in almost 40% of those patients compared with placebo.19 This may be attributed to the pharmacokinetics of tadalafil once daily, reaching 1.6-fold higher steady state serum concentrations than on-demand dosing.20 Consequently, these findings along with the current report could potentially offer an option for improved effectiveness in Chinese men with ED who were once considered nonresponders. Tadalafil once-daily treatment may also provide benefit to the Chinese ED patients who failed in on-demand treatment, and this effectiveness deserves further evaluation.
This study presents some limitations. First, the study uses an open-labeled trial design with a focus on long-term safety results, instead of a randomized, controlled trial design. In addition, the current data are only interim analyses; the final analysis will be disclosed in the future.
CONCLUSION
The findings of this study in a Chinese population provide evidence for extended effectiveness and tolerability of tadalafil, demonstrating no new safety concerns, in this population and make once-daily tadalafil administration a viable option to improve sexual performance and satisfaction in Chinese men with ED.
AUTHOR CONTRIBUTIONS
HJ, LMZ, and YL participated in the study design. YL carried out the statistical analysis, interpretation of data, and revising the intellectual content. ZCZ and HCL played a critical role in coordinating study conduction and manuscript preparation. All other authors, including SY, JHL, ZHZ, YTD, and FBL, participated in the acquisition of data and revising the intellectual content. All authors read and approved the final version of the manuscript and agree with the order of presentation of the authors.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
The authors thank Meghan Greenwood, of inVentiv Health Clinical (Raleigh, NC, USA), for writing assistance, and Teri Tucker and Sarah Becker-Marrero, of inVentiv Health Clinical, for editing assistance. This work was sponsored by Eli Lilly and Company. Eli Lilly and Company paid no influence on the results and had no conflict of interests.
REFERENCES
- 1.Australian Government Therapeutic Goods Administration. AusPAR: Tadalafil. 2013. [Last accessed on 2017 Sep 29]. Available from: https://www.tga.gov.au/auspar/auspar-tadalafil .
- 2.China Food and Drug Administration. Database of approved Active Pharmaceutical Ingredients (APIs) and API manufacturers in China. Tadalafil. [Last accessed on 2018 Jun 21]. Available from: http://app2.sfda.gov.cn/datasearchp/index1.do?tableId=36&tableName=TABLE36&tableView=??3&Id=16333 .
- 3.U.S. Food & Drug Administration. Orange book: approved drug products with therapeutic equivalence evaluations. Product details for NDA 021368. [Last accessed on 2018 Jun 21]. Available from: https://www.accessdata.fda.gov/scripts/cder/ob/results_product.cfm?Appl_Type=N&Appl_No=021368 .
- 4.European Medicines Agency. Assessment Report: Cialis. 2013. [Last accessed on 2017 Sep 29]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000436/WC500137787.pdf .
- 5.Bai WJ, Li HJ, Dai YT, He XY, Huang YR, et al. An open-label, multicenter, randomized, crossover study comparing sildenafil citrate and tadalafil for treating erectile dysfunction in Chinese men naïve to phosphodiesterase 5 inhibitor therapy. Asian J Androl. 2015;17:61–7. doi: 10.4103/1008-682X.143244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Govier F, Potempa AJ, Kaufman J, Denne J, Kovalenko P, et al. A multicenter, randomized, double-blind, crossover study of patient preference for tadalafil 20mg or sildenafil citrate 50mg during initiation of treatment for erectile dysfunction. Clin Ther. 2003;25:2709–23. doi: 10.1016/s0149-2918(03)80328-4. [DOI] [PubMed] [Google Scholar]
- 7.Rosen RC, Padma-Nathan H, Shabsigh R, Saikali K, Watkins V, et al. Determining the earliest time within 30 minutes to erectogenic effect after tadalafil 10 and 20mg: a multicenter, randomized, double-blind, placebo-controlled, at-home study. J Sex Med. 2004;1:193–200. doi: 10.1111/j.1743-6109.2004.04028.x. [DOI] [PubMed] [Google Scholar]
- 8.Yip WC, Chiang HS, Mendoza JB, Tan HM, Li MK, et al. Efficacy and safety of on demand tadalafil in the treatment of East and Southeast Asian men with erectile dysfunction: a randomized double-blind, parallel, placebo-controlled clinical study. Asian J Androl. 2006;8:685–92. doi: 10.1111/j.1745-7262.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- 9.Costa P, Grivel T, Gehchan N. Tadalafil once daily in the management of erectile dysfunction: patient and partner perspectives. Patient Prefer Adherence. 2009;3:105–11. doi: 10.2147/ppa.s3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatzimouratidis K, Hatzichristou DG. A comparative review of the options for treatment of erectile dysfunction: which treatment for which patient? Drugs. 2005;65:1621–50. doi: 10.2165/00003495-200565120-00003. [DOI] [PubMed] [Google Scholar]
- 11.Forgue ST, Patterson BE, Bedding AW, Payne CD, Phillips DL, et al. Tadalafil pharmacokinetics in healthy subjects. Br J Clin Pharmacol. 2006;61:280–8. doi: 10.1111/j.1365-2125.2005.02553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubio-Aurioles E, Porst H, Kim ED, Montorsi F, Hackett G, et al. A randomized open-label trial with a crossover comparison of sexual self-confidence and other treatment outcomes following tadalafil once a day vs. tadalafil or sildenafil on-demand in men with erectile dysfunction. J Sex Med. 2012;9:1418–29. doi: 10.1111/j.1743-6109.2012.02667.x. [DOI] [PubMed] [Google Scholar]
- 13.Burns PR, Rosen RC, Dunn M, Baygani SK, Perelman MA. Treatment satisfaction of men and partners following switch from on-demand phosphodiesterase type 5 inhibitor therapy to tadalafil 5mg once daily. J Sex Med. 2015;12:720–7. doi: 10.1111/jsm.12818. [DOI] [PubMed] [Google Scholar]
- 14.Donatucci CF, Wong DG, Giuliano F, Glina S, Dowsett SA, et al. Efficacy and safety of tadalafil once daily: considerations for the practical application of a daily dosing option. Curr Med Res Opin. 2008;24:3383–92. doi: 10.1185/03007990802498440. [DOI] [PubMed] [Google Scholar]
- 15.Porst H, Giuliano F, Glina S, Ralph D, Casabé AR, et al. Evaluation of the efficacy and safety of once-a-day dosing of tadalafil 5mg and 10mg in the treatment of erectile dysfunction: results of a multicenter, randomized, double-blind, placebo-controlled trial. Eur Urol. 2006;50:351–9. doi: 10.1016/j.eururo.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 16.Porst H, Gacci M, Büttner H, Henneges C, Boess F. Tadalafil once daily in men with erectile dysfunction: an integrated analysis of data obtained from 1913 patients from six randomized, double-blind, placebo-controlled, clinical studies. Eur Urol. 2014;65:455–64. doi: 10.1016/j.eururo.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 17.Porst H, Rajfer J, Casabé A, Feldman R, Ralph D, et al. Long-term safety and efficacy of tadalafil 5mg dosed once daily in men with erectile dysfunction. J Sex Med. 2008;5:2160–9. doi: 10.1111/j.1743-6109.2008.00935.x. [DOI] [PubMed] [Google Scholar]
- 18.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 19.Kim ED, Seftel AD, Goldfischer ER, Ni X, Burns PR. A return to normal erectile function with tadalafil once daily after an incomplete response to as-needed PDE5 inhibitor therapy. J Sex Med. 2014;11:820–30. doi: 10.1111/jsm.12253. [DOI] [PubMed] [Google Scholar]
- 20.McMahon C. Efficacy and safety of daily tadalafil in men with erectile dysfunction previously unresponsive to on-demand tadalafil. J Sex Med. 2004;1:292–300. doi: 10.1111/j.1743-6109.04042.x. [DOI] [PubMed] [Google Scholar]


