Abstract
Peroxynitrite is a highly reactive nitrogen species and a potent inducer of apoptosis and necrosis in somatic cells. Peroxynitrite-induced nitrosative stress has emerged as a major cause of impaired sperm function; however, its ability to trigger cell death has not been described in human spermatozoa. The objective here was to characterize biochemical and morphological features of cell death induced by peroxynitrite-mediated nitrosative stress in human spermatozoa. For this, spermatozoa were incubated with and without (untreated control) 3-morpholinosydnonimine (SIN-1), in order to generate peroxynitrite. Sperm viability, mitochondrial permeability transition (MPT), externalization of phosphatidylserine, DNA oxidation and fragmentation, caspase activation, tyrosine nitration, and sperm ultrastructure were analyzed. The results showed that at 24 h of incubation with SIN-1, the sperm viability was significantly reduced compared to untreated control (P < 0.001). Furthermore, the MPT was induced (P < 0.01) and increment in DNA oxidation (P < 0.01), DNA fragmentation (P < 0.01), tyrosine nitration (P < 0.0001) and ultrastructural damage were observed when compared to untreated control. Caspase activation was not evidenced, and although phosphatidylserine externalization increased compared to untreated control (P < 0.001), this process was observed in <10% of the cells and the gradual loss of viability was not characterized by an important increase in this parameter. In conclusion, peroxynitrite-mediated nitrosative stress induces the regulated variant of cell death known as MPT-driven necrosis in human spermatozoa. This study provides a new insight into the pathophysiology of nitrosative stress in human spermatozoa and opens up a new focus for developing specific therapeutic strategies to better preserve sperm viability or to avoid cell death.
Keywords: necrosis, nitrosative stress, oxidative stress, peroxynitrite, sperm cell death
INTRODUCTION
It is currently recognized that eukaryotic cells react to potentially dangerous perturbations of the intracellular or extracellular microenvironment by activating mechanisms that attempt to restore homeostasis; however, if these adaptive responses are not successful, they may actively engage in programmed cell death.1 The mechanism by which a cell dies depends on various exogenous factors as well as the cell's ability to handle the stress to which it is exposed.2 Thus, a cell can die by different mechanisms including apoptosis, regulated necrosis, and autophagy.2 Programmed cell death manifests with various characteristics, including a series of complex pathways that lead to the elimination of the cells in a regulated manner [for extensive reviews see 1,3,4].
The process of cell death in spermatozoa is also of great biological importance because almost all the millions of spermatozoa that are permanently produced are destined to die in the testes or in the female genital tract, with the exceptions being those that fertilize an oocyte.5 Moreover, it is very striking that in a normal ejaculate from men with verified fertility, more than 40% of spermatozoa are dead.6
Given that spermatozoa are transcriptionally silent cells with a unique nuclear constitution and a highly specialized architecture, they cannot undergo a programmed death in the conventional sense as has been described for somatic cells.7 Thus, the mechanisms that underlie cell death in human spermatozoa remain incompletely understood, and although this subject has been studied, the information available is mainly centered on a type of cell death with partially apoptotic features.7,8,9,10 It is currently accepted that spermatozoa exposed to oxidative stress default to an intrinsic apoptotic pathway after the start of a lipid peroxidation cascade.11
However, in addition to oxidative stress, nitrosative stress caused by the excessive levels of reactive nitrogen species (RNS) also leads to impaired sperm function.12,13,14,15,16,17 Nitrosative stress in sperm cells can be induced by the RNS produced by the spermatozoa themselves15,18 and by other cells in the male reproductive tract.12 Overproduction of RNS in the male reproductive system is observed in lifestyles and pathophysiological conditions including diabetes mellitus,19 idiopathic asthenozoospermia,14,20 varicocele,21 and smoking.22 Moreover, the sperm cells can be exposed to nitrosative stress during their transit through the female genital tract, where RNS are also produced.23,24 The RNS include the peroxynitrite anion, a powerful oxidant whose toxicity is mediated by both peroxynitrite directly and peroxynitrite-derived radicals.25 High levels of peroxynitrite have been associated with impaired sperm function in infertile men.14,15,26 Furthermore, in vitro studies have demonstrated that peroxynitrite induces lipid peroxidation of the plasma membrane,27 impairs motility and mitochondrial membrane potential,13 decreases the ATP production affecting both glycolysis and oxidative phosphorylation,16 and causes thiol oxidation in human spermatozoa.17 In somatic cells, peroxynitrite is a potent inducer of apoptosis and necrosis;28 however, neither the ability of peroxynitrite to induce cell death nor the morphological and biochemical correlates of peroxynitrite-mediated cytotoxicity in human spermatozoa have been described to date. Thus, the aim of this study was to characterize the biochemical and morphological features of cell death induced in vitro by peroxynitrite-mediated nitrosative stress in human spermatozoa.
MATERIALS AND METHODS
Donors of semen samples and ethical approval
Semen samples from healthy donors who were students at the University of La Frontera, Temuco, Chile, and the National University of Cuyo, Mendoza, Argentina, were used. The study was approved by the Scientific Ethics Committee at the University of La Frontera. Furthermore, normozoospermic semen samples from male members of couples consulting for infertility at the Department of Obstetrics and Gynecology at the University of Cologne, Cologne, Germany, were used and the use of these samples for research was approved by the Ethics Committee of the Faculty of Medicine at the University of Cologne. These semen samples were obtained from 29 men. All participants in this study signed written informed consent.
Semen preparation
Semen samples were obtained by masturbation, after at least 3 days of sexual abstinence, collected in sterile containers, and delivered to the laboratory in <60 min. Standard semen analysis was performed according to the World Health Organization (WHO) guidelines,6 and only normal semen samples according to the WHO criteria were used.
The swim-up technique with modified human tubular fluid (HTF) medium (Irvine Scientific, Santa Ana, CA, USA) was used to select the motile fraction from the semen samples.
In vitro generation of peroxynitrite-mediated nitrosative stress
In vivo peroxynitrite is usually produced at lower rates, but for prolonged time periods. Thus, approaches to properly mimic the pathophysiological generation of peroxynitrite include infusion of peroxynitrite to biological systems, or the simultaneous generation of its precursors nitric oxide (NO) and superoxide anion (O2−), either using compounds such as 3-morpholinosydnonimine (SIN-1) or independent NO and O2− generation systems.25 According to this, the compound SIN-1 (Enzo Life Science Inc., Farmingdale, NY, USA) was used in this study to generate nitrosative stress in human spermatozoa. This compound in solution and in the presence of molecular oxygen releases NO in a process associated with the generation of O2−, both of them react together and form peroxynitrite.29 A previous report of our work group demonstrated the proper generation of peroxynitrite in suspensions of human spermatozoa using SIN-1.13 SIN-1 solution was freshly prepared at 100 mmol l−1 as previously described.
Experimental protocol of exposure to peroxynitrite
Aliquots of sperm suspension in HTF medium were exposed to 0.8 mmol l−1 of SIN-1 for 4 h and for 24 h at 37°C. The generation of peroxynitrite in human sperm suspensions by this SIN-1 concentration was previously demonstrated by our work group.13 Furthermore, the induction of nitrosative stress by SIN-1 which led to impairment of sperm parameters was previously reported.13,16,17 Thus, the SIN-1 concentration selected for this study was based on these previous reports. The incubation times were selected in order to obtain an early and a late incubation times. For each experimental condition, aliquots of untreated sperm were included as controls. After incubation, the spermatozoa, including controls, were washed twice with HTF by centrifugation (Biofuge Fresco, Heraeus, Hayward, CA, USA) at 500 g for 5 min and resuspended with HTF medium for subsequent analysis.
Analysis of sperm viability
The sperm viability was evaluated by incorporating propidium iodide (PI; Sigma-Aldrich Inc., St. Louis, MO, USA). For this, 1 ml of sperm aliquots (1 × 106 spermatozoa per ml) previously exposed to SIN-1 and controls was incubated with 1 μmol l−1 of PI, incubated for 5 min at room temperature, washed once with Dulbecco's phosphate-buffered saline (DPBS; IrvineScientific), and resuspended with 300 μl of DPBS for flow cytometry analysis. The sperm viability was determined as the percentage of PI-negative cells.
Analysis of mitochondrial permeability transition
The mitochondrial permeability transition (MPT) was evaluated by the MitoProbe™ Transition Pore Assay kit (Molecular Probes, Invitrogen, Eugene, OR, USA), which has been previously used to assess the mitochondrial permeability transition pore (mPTP) opening.30 This method uses calcein-acetoxymethyl (calcein-AM) staining, which passively diffuses into cells and accumulates in the cytosolic compartments, including the mitochondria. Cytosolic calcein fluorescence is quenched by the addition of cobalt chloride (CoCl2) while mitochondrial calcein fluorescence is maintained, since the CoCl2 is not able to pass through intact inner mitochondrial membrane. The mPTP opening alters the permeability of the mitochondria, allowing either the release of calcein or the entry of CoCl2 and the complete quenching of calcein fluorescence. Thus, MPT is evidenced by a drastic decrease in calcein fluorescence.30
For the experiments, a previously described procedure was followed.31 Briefly, 1 ml of sperm aliquot (1 × 106 spermatozoa per ml) exposed to SIN-1 and its respective untreated controls was incubated with 0.01 μmol l−1 of calcein-AM and 0.4 μmol l−1 of CoCl2. Three technique controls were also included by incubating sperm aliquots with (i) calcein-AM; (ii) calcein-AM and CoCl2; and (iii) calcein-AM, CoCl2, and 0.5 μmol l−1 ionomycin of which the latter induces the mPTP opening. The cells were incubated at 37°C for 15 min in darkness, washed once, and resuspended in 300 μl of DPBS for flow cytometry analysis. The results were expressed as mean fluorescence intensity (MFI) of calcein.
Analysis of phosphatidylserine (PtdSer) externalization
The PtdSer externalization was evaluated using the Annexin V-Alexa Fluor® 488 conjugate (Thermo Fisher Scientific Inc., Waltham, MA, USA). For experiments, 1 ml of sperm aliquots (1 × 106 spermatozoa per ml) previously exposed to SIN-1 and controls was washed twice and resuspended with 100 μl of binding buffer (MACS ART Binding Buffer; Miltenyi Biotec, Cologne, Germany). Then, 5 μl of Annexin V-Alexa Fluor® 488 conjugate was added and the cells were incubated in the dark for 15 min at room temperature. After the incubation period, 400 μl of binding buffer and 1 μmol l−1 of PI were added and the fluorescence intensity was analyzed by flow cytometry. The externalization of PtdSer was considered as the percentage of Annexin V positive and PI negative cells.
Analysis of caspase activation
Caspase activation was evaluated using the FAM-FLICA® in vitro Caspase-3/7 detection kit (ImmunoChemistry Technologies, Bloomington, MN, USA). The fluorescent-labeled inhibitors of caspase (FLICA) probes covalently link to the active caspase, being retained within the cell, allowing fluorescence detection by flow cytometry.
For the experiments, 1 ml of sperm aliquots (1 × 106 spermatozoa per ml) previously exposed to SIN-1 and controls was washed twice, resuspended with 300 μl of DPBS, and incubated with 10 μl of 30 × FLICA solution for 60 min at 37°C. After that, the cells were washed twice with 1 × apoptosis wash buffer and 1 μmol l−1 of PI was added. Fluorescence intensity was analyzed by flow cytometry and the results were expressed as the MFI of FLICA.
Analysis of DNA oxidation
The DNA oxidation was evaluated by the OxyDNA Assay kit (Merck KGaA, Darmstadt, Germany), which is based on direct binding of a fluorescein isothiocyanate (FITC)-conjugated probe to the 8-oxoguanine, a product of DNA oxidation induced by free radicals.
For the experiments, 1 ml of sperm aliquot (4 × 106 spermatozoa per ml) previously exposed to SIN-1 and its untreated controls was fixed in 2% paraformaldehyde for 15 min at 4°C and permeabilized with 0.1% Triton X-100 for 10 min at room temperature. Subsequently, sperm were incubated with FITC conjugate for 1 h at room temperature in the dark. Finally, the cells were washed twice and resuspended in DPBS. Fluorescence intensity was analyzed by flow cytometry and the results were expressed as the percentage of FITC-positive cells.
Analysis of DNA fragmentation
The In Situ Cell Death Detection kit (Roche, Mannheim, Germany), which is based on the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) technique, was used to analyze the sperm DNA fragmentation. For the experiments, 1 ml of sperm aliquot (4 × 106 spermatozoa per ml) previously exposed to SIN-1 and its untreated controls was incubated with 2 mmol l−1 dithiothreitol for 45 min at room temperature in order to relax the chromatin and allow the enzyme better access to the DNA.32 Subsequently, the cells were washed twice with PBS, fixed in 2% paraformaldehyde for 15 min at 4°C, and permeabilized with 0.1% Triton X-100 for 10 min at room temperature. Then, the sperm were incubated with the TUNEL reaction solution and incubated for 1 h at 37°C. Finally, the cells were washed twice, resuspended in DPBS, and analyzed by flow cytometry. The results were expressed as the percentage of FITC-positive cells.
Analysis of tyrosine nitration
The analysis of the tyrosine nitration was performed by immunofluorescence using an antibody against 3-nitrotyrosine (3-NT). For the experiments, sperm aliquots (1 × 106 spermatozoa per ml) previously exposed to SIN-1 and their untreated control were attached to coverslips, fixed in 2% paraformaldehyde for 10 min at room temperature, and permeabilized with 0.1% Triton X-100 for 10 min at room temperature. After that, the cells were covered for 30 min with blocking solution (PBS supplemented with 0.5% bovine serum albumin [BSA]). The spermatozoa were incubated with an anti-3-NT antibody (sc-55256; Santa Cruz Biotechnology, Inc., Dallas, TX, USA.) diluted 1:100 in blocking solution overnight at 4°C. Then, the spermatozoa were washed 3 times for 5 min with PBS and incubated for 30 min at room temperature with an anti-rabbit IgG biotin conjugate (Sigma-Aldrich Inc.) diluted 1:800 with blocking solution. Once the incubation time ended, the sperm were washed 3 times with PBS and incubated for 15 min at room temperature with ExtrAvidin®-FITC (Sigma-Aldrich Inc.) diluted 1:600 with blocking solution. The absence of nonspecific staining was assessed by processing a control without primary antibody. Subsequently, the cells were washed 3 times with PBS, 1 μmol l−1 of PI was added, incubated for 5 min at room temperature, and washed twice with PBS. Finally, the coverslips were mounted on slides using Fluoroshield™ Mounting Medium (Sigma-Aldrich Inc.) for subsequent analysis. Images of sperm cells were acquired with a confocal microscope (FV10-ASW; Olympus, New York, NY, USA). The relative fluorescence intensity (RFI) in the principal piece and in the entire flagellum (middle piece plus principal piece) of the sperm was analyzed using ImageJ software (Image J 1.32j, National Institutes of Health, Bethesda, MD, USA).
Analysis of sperm ultrastructure
The analysis of sperm ultrastructure was performed by transmission electron microscopy (TEM) using a Zeiss EM 900 transmission electron microscope (Zeiss, Oberkochen, Germany). For the experiments, sperm aliquots (2 × 106 spermatozoa per ml) from three different donors previously exposed to SIN-1 and controls were fixed for 2 h at 0°C–4°C with fixative solution consisting of 4% paraformaldehyde (w/v), 4% glutaraldehyde (w/v), and 20% picric acid (v/v) saturated in PBS. Fixed sperm were washed twice with PBS for 10 min at 600 g. Then, the pellet containing the spermatozoa was postfixed by adding 30 ml of 1% osmium (VIII) oxide (OsO4; w/v) and incubated overnight at 4°C. Osmified samples were dehydrated in ethanol-acetone up to absolute acetone and embedded in Epon 812 epoxy resin (Ted Pella Inc., Redding, CA, USA). Ultrathin sections were obtained by Ultracut R microtome (Leica Biosystems, Vienna, Austria) and stained with classical uranyl acetate and lead citrate TEM stain. The ultrathin sections were examined with a TEM at 80 kV. The results were presented as percentage of alterations on sperm ultrastructure. Representative images of each condition were also obtained.
Analysis by flow cytometry
The analyses of viability, MPT, PtdSer externalization, DNA oxidation, and DNA fragmentation were made using a flow cytometer FACSCalibur (Becton, Dickinson and Company, BD Biosciences, San Jose, CA, USA). Samples were acquired and analyzed with the software Cell Quest Pro (Becton, Dickinson and Company). The fluorescence analysis of caspase activation and the analysis of the single sample over time for PtdSer externalization were performed in a FACSCanto II flow cytometer, and data were analyzed with the software FACSDiva™ version 6.1.3 (Becton, Dickinson and Company). The fluorescence from FITC, calcein, and FLICA were detected using the band pass filter of 530/30 nm, and the fluorescence from PI was detected with a band pass filter of 585/42 nm. All analyses by flow cytometry were done on logarithmic scales and data from 10 000 sperm events were analyzed in each experiment.
Statistical analyses
The treatment of spermatozoa with SIN-1 and its controls was carried out in duplicate, and independent experiments were performed at least 3 times on different days with different semen samples. Results were expressed as mean ± standard deviation (s.d.). Statistical evaluation was performed with the Prism 6 software (GraphPad, La Jolla, CA, USA), applying D’Agostino-Pearson K2 test to assess Gaussian distribution, and numeric results were transformed to a logarithmic scale when did not pass the normality test. One-way analysis of variance (ANOVA) was used to analyze the MPT whereas two-way ANOVA was used to analyze sperm viability, PtdSer externalization, caspase activation, and DNA oxidation and fragmentation. For analysis of tyrosine nitration, Students’ t-test was applied, and for TEM analysis, Mann–Whitney comparison test was performed. P < 0.05 was considered statistically significant.
RESULTS
Sperm viability
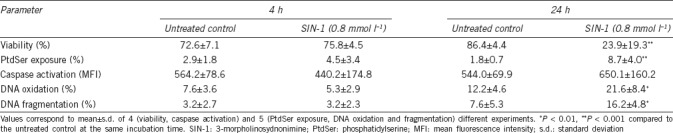
First, experimental conditions in which peroxynitrite causes cell death of human spermatozoa were established. The current recommendations suggest considering as dead cells those that either exhibit irreversible plasma membrane permeabilization or have undergone complete fragmentation.33 Thus, sperm viability, measured as the integrity of plasma membrane after exposure to SIN-1 for 4 h and for 24 h, was analyzed. No significant differences between the untreated control and spermatozoa exposed to peroxynitrite for 4 h were observed (P > 0.05), whereas a significant decrease (P < 0.001) in sperm viability was observed at 24 h of incubation with 0.8 mmol l−1 of SIN-1 (Table 1). Thus, these experimental conditions were used to analyze the biochemical markers and morphological alterations associated with cell death induced by peroxynitrite in human spermatozoa.
Table 1.
Analysis of biochemical markers of cell death on human spermatozoa at 4 h and 24 h of incubation with 0.8 mmol l−1 of 3-morpholinosydnonimine
MPT
The MPT in human spermatozoa exposed to 0.8 mmol l−1 of SIN-1 for 4 h and 24 h was analyzed by a technique that uses three controls, which are spermatozoa incubated with: (i) calcein (Cal), (ii) calcein and cobalt chloride (Cal-Co), and (iii) calcein, cobalt chloride, and ionomycin (Cal-Co-Io). The results in Figure 1 show that in the Cal group, the fluorescence was the highest, consistent with the calcein distribution in all cellular compartments. In Cal-Co spermatozoa, the fluorescence of calcein decreased, due to calcein was limited to mitochondria, where cobalt chloride cannot pass indicating the intactness of the inner mitochondrial membrane. In the control group with Cal-Co-Io, the calcein fluorescence was negligible, indicating that MPT process had occurred allowing access of cobalt chloride to the mitochondrial matrix. In the experimental group treated with SIN-1 (Cal-Co-SIN) for 4 h, the MFI of calcein did not differ significantly compared with the Cal-Co spermatozoa (P > 0.05), indicating that at this incubation time, the MPT did not occur (Figure 1a). However, in spermatozoa exposed for 24 h to SIN-1, the MFI of calcein was significantly decreased compared to Cal-Co spermatozoa (P < 0.01), indicating that the MPT process had occurred (Figure 1b).
Figure 1.
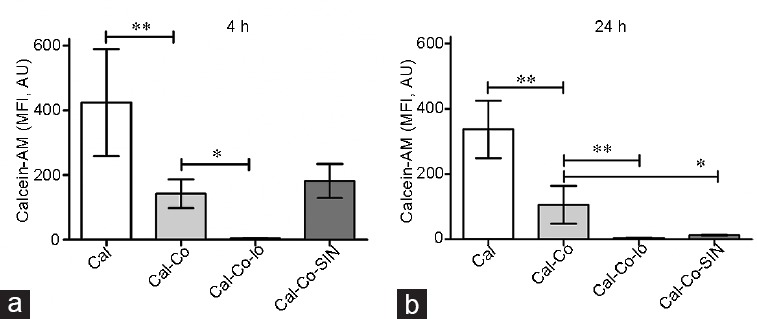
Analysis of mitochondrial permeability transition by the calcein-CoCl2 method in human spermatozoa exposed to peroxynitrite. Sperm cells were incubated with 0.8 mmol l−1 of SIN-1 (a) for 4 h and (b) for 24 h and the calcein-CoCl2 method was performed (Cal-Co-SIN). Three technique controls were also included by incubating sperm with calcein-AM (Cal), calcein-AM and CoCl2 (Cal-Co) and calcein-AM, CoCl2 and ionomycin (Cal-Co-Io). The results are presented as mean ± standard deviation of five different experiments. *P < 0.01, **P < 0.001. MFI: mean fluorescence intensity; AM: acetoxymethyl; AU: arbitrary units; SIN-1: 3-morpholinosydnonimine.
PtdSer externalization
The percentage of living cells displaying translocation of PtdSer to the outer leaflet of the plasma membrane (Annexin V-positive and PI-negative cells), after 4 h of incubation with peroxynitrite, was not statistically significant compared to the untreated control (P > 0.05); and although was significantly increased after 24 h of incubation (P < 0.001), the percentage of cells with this characteristic was <10% (Table 1). Considering that at 24 h of incubation, the sperm viability was near to 20% and in order to test whether externalization of PtdSer to the outer leaflet of plasma membrane occurs before 24 h of incubation, when the integrity of plasma membrane was still preserved, a single semen sample was tracked from 4 h to 20 h of incubation with 0.8 mmol l−1 of SIN-1. Figure 2 shows that from 12 h of incubation with peroxynitrite, the sperm cells gradually lost their viability, but no important increase in the percentage of PtdSer externalization (Annexin V-positive and PI-negative cells, lower-right quadrant in each dot plot) was observed (Figure 2), indicating that peroxynitrite induces sperm cell death but did not trigger a process of PtdSer translocation.
Figure 2.
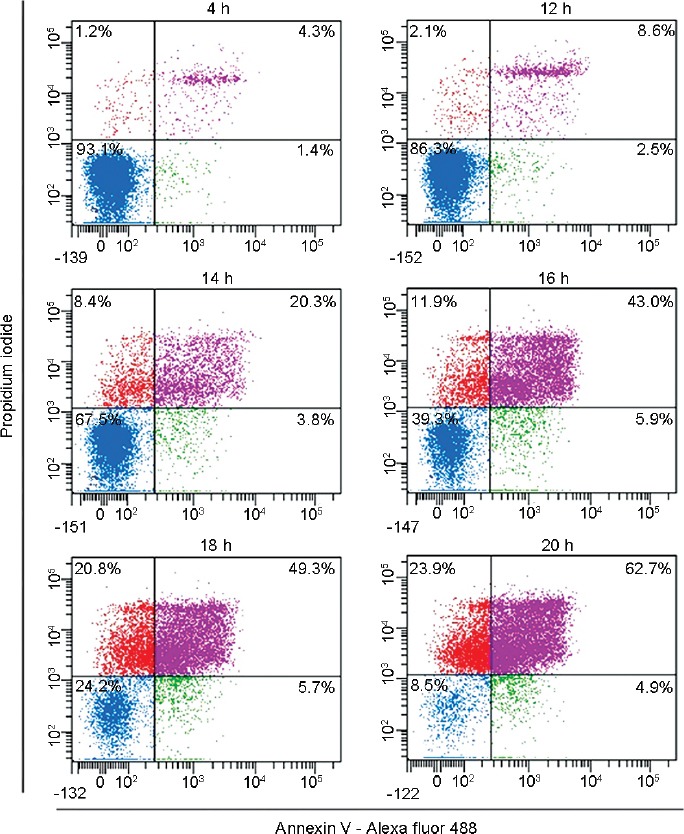
Externalization of phosphatidylserine through the time in spermatozoa exposed to peroxynitrite. Images correspond to representative dot plots from flow cytometry analysis of a single sperm sample. The spermatozoa were incubated with 0.8 mmol l−1 of SIN-1 and phosphatidylserine exposure was analyzed through the time. SIN-1: 3-morpholinosydnonimine.
Caspase activation
The results of caspase activation analyzed in spermatozoa incubated with SIN-1 showed that at 4 h or 24 h, the MFI of FLICA did not differ significantly compared to the untreated control (P > 0.05, Table 1), indicating that under the experimental conditions tested, peroxynitrite did not induce caspase activation in human spermatozoa.
DNA oxidation and fragmentation
DNA integrity was analyzed in terms of DNA oxidation and fragmentation in spermatozoa exposed to SIN-1 for 4 h and 24 h. The results showed that at 4 h of incubation, the percentage of sperm displaying oxidized DNA was similar to the untreated control spermatozoa (P > 0.05). However, after 24 h of incubation, the DNA oxidation was increased in spermatozoa exposed to SIN-1 compared to the untreated control (P < 0.01, Table 1). In the same way, DNA fragmentation increased in spermatozoa exposed to SIN-1 only after 24 h of incubation compared to the untreated control (P < 0.01, Table 1).
Tyrosine nitration
Tyrosine nitration analyzed in untreated human spermatozoa demonstrated positivity for this posttranslational protein modification mainly in the acrosomal region and middle piece (Figure 3a–3c). However, in spermatozoa treated with 0.8 mmol l−1 of SIN-1, the signal increased, and a strongly positive signal for 3-NT was observed in the acrosome region and flagellum, which was already evident after 4 h of incubation with SIN-1 (Figure 3d–3f). The sperm incubated without the first antibody showed only a weak signal in the acrosome and middle piece region (Figure 3g–3i). When the relative fluorescence intensity (RFI) was analyzed in the sperm, a significant difference in spermatozoa treated with SIN-1 compared to the untreated control was observed both in the principal piece (11.1 ± 2.3 vs 8.7 ± 1.7, respectively; P < 0.0001) and in the entire flagellum (middle piece plus principal piece; 11.8 ± 2.3 vs 9.6 ± 1.9, respectively; P < 0.0001). These results indicate that peroxynitrite caused an increase in tyrosine nitration in human spermatozoa which was evident after 4 h of incubation.
Figure 3.
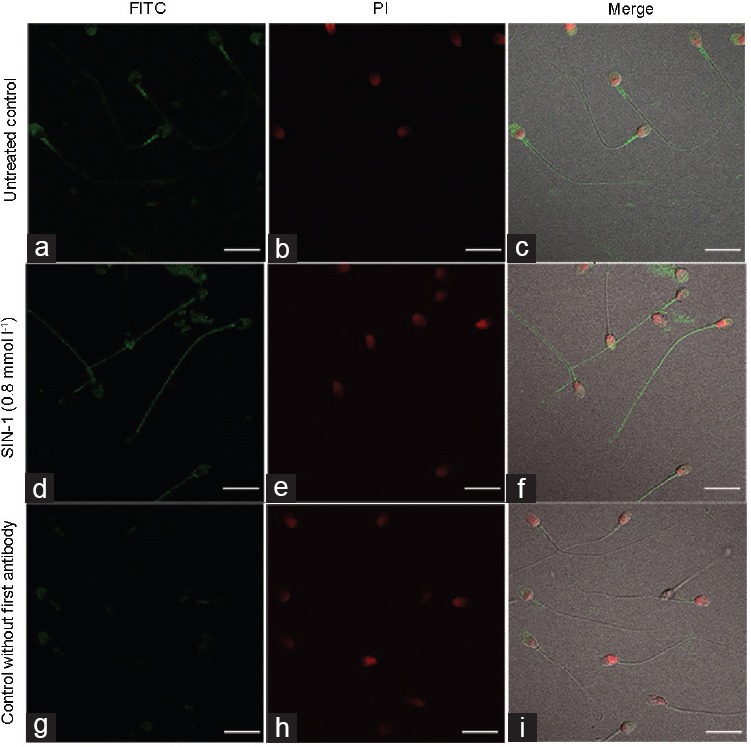
Analysis of tyrosine nitration in human spermatozoa exposed to peroxynitrite. Immunofluorescence representative images of (a–c) untreated control, (d–f) sperm cells exposed to 0.8 mmol l−1 of SIN-1 for 4 h at 37°C, and (g–i) a control without first antibody. Left column (FITC) indicates positivity for 3-nitrotyrosine. PI stain and merge are also shown. Scale bars = 10 μm. SIN-1: 3-morpholinosydnonimine; FITC: fluorescein isothiocyanate; PI: propidium iodide.
Ultrastructure
The ultrastructure of spermatozoa was analyzed under the same experimental conditions. Figure 4 shows representative images obtained by transmission electron microscopy analysis from untreated spermatozoa and from spermatozoa exposed to SIN-1. It is noted that the untreated control at 4 h and 24 h of incubation showed a conserved ultrastructure in the acrosomal region, head and middle piece (Figure 4a–4d). The majority of sperm showed nuclei with an appropriate condensation (asterisk in Figure 4c) and normal mitochondria (arrows in Figure 4d), corresponding to selected spermatozoa from normozoospermic donors.
Figure 4.
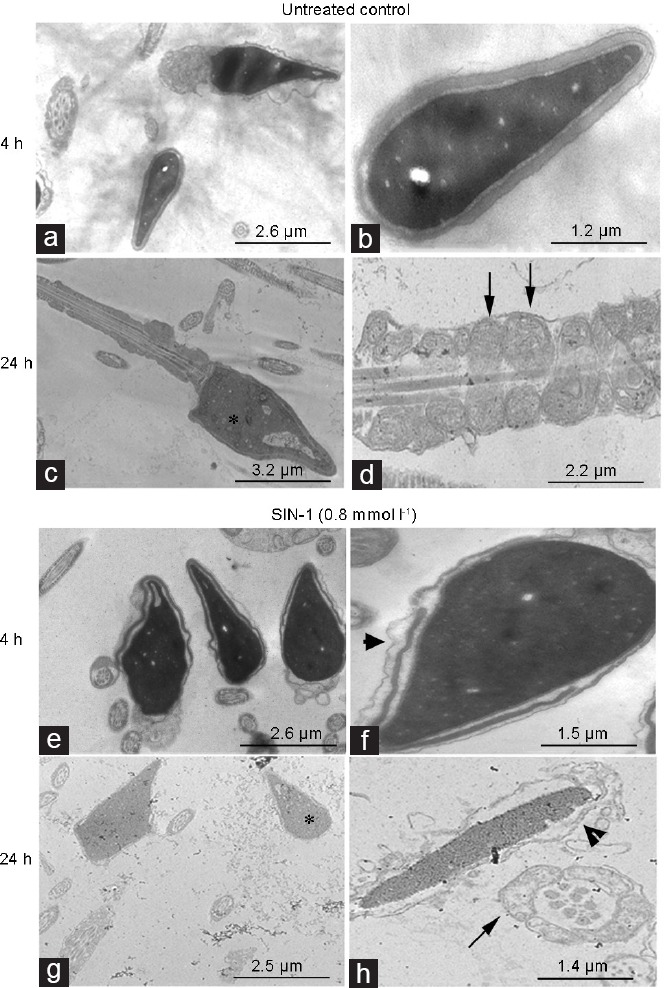
Analysis of ultrastructure of human spermatozoa exposed to peroxynitrite. Transmission-electron micrographs of human sperm incubated for 4 h and 24 h without (untreated control; a–d) and with 0.8 mmol l−1 of SIN-1 (e–h). The asterisk indicates normal nuclei in c but indicates degranulated nuclei in g. Arrows indicate preserved mitochondria in d, and impaired mitochondria in h. Arrow heads in f and h indicate acrosome membrane undulation. Magnification is ×7000 in a, c, e and g; ×20 000 in d; ×30 000 in b, f and h. SIN-1: 3-morpholinosydnonimine.
However, compared to the untreated control, in the spermatozoa treated with SIN-1, changes attributable to nitrosative stress were observed in the ultrastructure already at 4 h (Figure 4e and 4f). These changes included swelling in the plasma membrane, evident in 70% of sperm cells (P < 0.05); acrosome undulation and detaching of the acrosomal vesicles from the nuclear membrane (arrow head in Figure 4f) were observed in 26% of spermatozoa (P < 0.05). Some of sperm mitochondria (19%) displayed mitochondrial swelling (P > 0.05). The sperm tails show normal ultrastructural features. Moreover, these alterations were accentuated in the cells exposed to SIN-1 for 24 h (Figure 4g and 4h). The nuclei were found to be less electron-dense and displayed a granulated appearance, evident in 20% of sperm head (P < 0.05; asterisk in Figure 4g). Ninety percent of spermatozoa with broken plasma membrane (P < 0.05) and 80% of sperm with reacted or absent acrosome (P < 0.05) were observed (arrow head in Figure 4h). The cells showing mitochondrial swelling, characterized by electron-lucid areas (arrow in Figure 4h), increased to 60% (P < 0.05).
DISCUSSION
Peroxynitrite is a highly reactive nitrogen species which causes nitrosative stress and is a potent inducer of apoptosis and necrosis in somatic cells.25,34 Our study demonstrated for the first time that cell death induced by peroxynitrite in human spermatozoa is characterized by induction of the MPT process accompanied by DNA oxidation and fragmentation, tyrosine nitration, and damage to the ultrastructure; however, it is not associated with PtdSer externalization or caspase activation. These results suggest that nitrosative stress induces the regulated variant of cell death known as MPT-driven necrosis. MPT is characterized by the assembly and prolonged opening of the mitochondrial permeability transition pore which, in somatic cells, is modulated by calcium, reactive oxygen species (ROS), and RNS.35 MPT causes a rapid increase in the permeability of the inner mitochondrial membrane, leading to dissipation of the mitochondrial membrane potential (ΔΨm), uncoupling of the respiratory chain, and influx of water and ions, which drives the osmotic swelling of the mitochondrial matrix, causing a mechanical breakdown of the outer mitochondrial membrane.4 Widespread MPT can cause cell death via regulated necrosis or apoptosis. MPT-driven necrosis is induced when the dissipation of the ΔΨm leads to arrest in ATP synthesis. On the contrary, MPT-driven apoptosis is carried out by mitochondrial intermembrane proteins released into the cytoplasm as consequence of MPT and requires the activation of the proteolytic enzyme caspases in an ATP-dependent manner. Thus, MPT may induce apoptosis or regulated necrosis depending on the intracellular availability of ATP (reviewed by Bonora et al.36). We have already reported that peroxynitrite decreases the ATP production in sperm cells;16 thus, we propose that the ATP depletion along with the MPT leads to the regulated variant of cell death known in somatic cells as MPT-driven necrosis.
Consistent with this cell death modality, the results of this study show that caspase activation did not occur even after prolonged incubation with peroxynitrite, when cell death had been induced. Furthermore, the sperm cell death induced by peroxynitrite did not trigger PtdSer translocation, which is considered a hallmark of apoptosis.37 In healthy cells, phospholipids are maintained asymmetrically in the plasma membrane with PtdSer confined to the cytoplasmic leaflet of the plasma membrane by activity of ATP-dependent aminophospholipid translocases or flippases, which specifically translocates PtdSer and phosphatidylethanolamine from the outer to the inner leaflet of the lipid bilayer.38 The distribution of phospholipids is also regulated by phospholipid scramblases, which nonspecifically translocate or scramble phospholipids between the lipid bilayers in both directions, allowing PtdSer exposure on the outer leaflet of the plasma membrane.39 When cells undergo apoptosis, flippases are inactivated and scramblases are activated, both by caspase-mediated cleavage, leading to irreversible PtdSer exposure.40 In this study, peroxynitrite-induced cell death was not associated with PtdSer externalization, coinciding with the nonactivation of caspase and ATP unavailability. These results allow to discard the cell death modality displaying features of MPT-driven apoptosis.
An important characteristic of cell death observed in this study after peroxynitrite exposure was the decrease of DNA integrity evidenced as increased percentage of sperm displaying DNA oxidation and DNA fragmentation, which was near to 20% and 15%, respectively. These levels could seem low; however, it is important to consider that men with more than 30% of spermatozoa with DNA damaged are infertile.41 In somatic cells, peroxynitrite has detrimental effects on DNA integrity, leading to important DNA modifications including the formation of 8-nitroguanine and 8-oxoguanine as well as the induction of DNA strand breakage and sugar-phosphate backbone damage.28,42,43 DNA damage induced by peroxynitrite triggers the activation of the DNA repair enzyme poly(ADP-ribose) polymerase-1 (PARP-1), of which over-activation consumes nicotinamide adenine dinucleotide (NAD) leading to ATP depletion, culminating in cell dysfunction and death44 coinciding with what was observed in this study. There are no previous reports regarding the effect of peroxynitrite on sperm DNA, although a positive correlation between RNS and DNA fragmentation has been reported.26,45 Sperm DNA damage has an important clinical impact since it is not only associated with reduced male fertility but also associated with birth defects and several forms of morbidity in the offspring (reviewed by Gharagozloo and Aitken46).
The exposure of spermatozoa to peroxynitrite also resulted in an increase in tyrosine nitration, which is a posttranslational protein modification due to the addition of a nitro group adjacent to the hydroxyl group on the aromatic ring of tyrosine residues.47 Tyrosine nitration has been associated with at least 50 human diseases affecting protein structure and function, being considered an essential feature of peroxynitrite-mediated cytotoxicity.34 In our experimental settings, the basal level of tyrosine nitration was significantly increased in spermatozoa after 4 h of peroxynitrite exposure. This increase was mainly found in sperm flagella, suggesting the involvement of structural proteins and metabolic enzymes, which can explain, at least in part, the decrease in sperm motility also reported for us under the same conditions.13 In agreement with our results, it was previously reported that the exposure to peroxynitrite caused wider tyrosine nitration in the flagellum,48 and the exposure to 1,1-diethyl-2-hydroxy-2-nitroso-hydrazine sodium (diethylamine NONOate), a nitric oxide donor, increased the levels of tyrosine nitration, which were also located at the head and tail regions being associated to impaired sperm capacitation.49 In this regard, it is noteworthy that in asthenozoospermic men, an increase in tyrosine nitration has been associated with impairment of sperm function, and this may be attributed to negative effects on structure, intracellular compartmentalization, catalytic activity, or rate of degradation and protein turnover.15 Moreover, an increase in tyrosine nitration, inducible nitric oxide synthase (iNOS) expression, and citrulline formation is observed in infertile men, suggesting that increased NOS activity and excess of tyrosine nitration are involved in the pathogenesis of idiopathic asthenozoospermia causing male infertility.14
Finally, when the ultrastructure in spermatozoa exposed to peroxynitrite was analyzed, an impairment of ultramorphological characteristics was clearly appreciated, especially after prolonged incubation. This is the first report about the deleterious effect of peroxynitrite or nitrosative stress on sperm ultrastructure. The results presented here show that plasma membrane, mitochondria, and nuclei were affected. The impairment of plasma membrane integrity is consistent with the results of sperm viability while the morphological alterations of mitochondria are consistent with the MPT process, which is characterized in somatic cells by swelling of the mitochondria, release of mitochondrial content, and cell death.4
The scheme shown in Figure 5 summarizes these and previous results of our work group regarding the negative effect of peroxynitrite on human sperm.13,16,17 Based on the above, we propose that peroxynitrite-mediated nitrosative stress accounts for impairment of sperm function and loss of viability as follows: peroxynitrite directly causes protein modifications including thiol oxidation17 and tyrosine nitration, both of which may account for the decrease in ATP production by glycolysis and by oxidative phosphorylation,16 likely due to modifications in metabolic enzymes. The decrease in ATP together with the excess of tyrosine nitration and thiol group oxidation in proteins responsible for the movement on sperm flagella is the likely cause of the impairment of sperm motility. Particularly, at the middle piece, the posttranslational modifications of electron transport chain proteins can induce a decrease in mitochondrial membrane potential contributing to the decrease on ATP production by oxidative phosphorylation.34 The oxidation of critical thiol groups within a protein component of the mitochondrial permeability transition pore probably mediates the MPT process.34 As a consequence of the MPT process, the increase in ROS production can induce DNA oxidation and fragmentation,31 although it may also be a direct effect of peroxynitrite as has been reported in somatic cells.50 Finally, the ATP depletion along with the MPT leads to the regulated variant of cell death known in somatic cells as MPT-driven necrosis, which happens without caspase activation or PtdSer externalization. This cell death process is accompanied by ultrastructural damage in plasma membrane, nuclei, and mitochondria as showed in this study in human spermatozoa.
Figure 5.
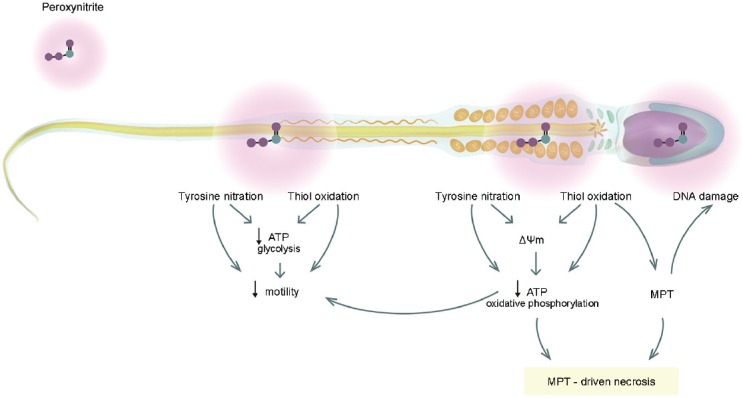
Diagram of the observed effects after peroxynitrite-mediated nitrosative stress in human spermatozoa and its possible interrelationships. ΔΨm: mitochondrial membrane potential; ATP: adenosine triphosphate; MPT: mitochondrial permeability transition.
CONCLUSIONS
Prolonged exposure of spermatozoa to peroxynitrite causes ultrastructural damage and biochemical markers of cell death compatible with MPT-driven necrosis. These markers include MPT induction, tyrosine nitration, and loss of DNA integrity without PtdSer externalization or caspase activation. This work provides a new insight into the pathophysiology and cytotoxicity induced by peroxynitrite in human spermatozoa. This study also supports the notion that sperm cells can develop cell death processes other than a truncated intrinsic apoptotic cascade and that different stress conditions can elicit different cell death modalities in spermatozoa. This knowledge opens new opportunities for studying the biology of sperm cells and could contribute to identify different cell death modalities displayed by sperm cells in men facing infertility or by sperm cells subjected to stressful conditions such as cryopreservation. This, in future, could make it possible to develop specific therapeutic strategies to better preserve sperm viability or to avoid sperm cell death.
AUTHOR CONTRIBUTIONS
PU contributed to the conception and design of the study, data acquisition, data analysis and interpretation, and drafting of the article. MEC performed the data acquisition, data analysis and interpretation, and critical review of the manuscript for important intellectual content. MWF participated in data analysis and interpretation and critical review of the manuscript for important intellectual content. FT, RB, EI, and VI contributed to the data analysis and interpretation and critical review of the manuscript for important intellectual content. RS participated in the study conception and design and critical review of the manuscript for important intellectual content. JVV contributed to the study conception and design, data analysis and interpretation, drafting of the article, and critical review of the article for important intellectual content. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This work was supported by the National Fund of Science and Technology (Grant number 1130888 to RS); the University of La Frontera (Grant number DI17-0035 to JVV); the CONICYT, Chile, PAI/Fund for Insertion in the Academy, Call 2016 (Grant number PAI79160030 to PU); and a scholarship from the German Academic Exchange Service, DAAD, Germany (to JVV).
REFERENCES
- 1.Galluzzi L, Bravo-San Pedro JM, Kepp O, Kroemer G. Regulated cell death and adaptive stress responses. Cell Mol Life Sci. 2016;73:2405–10. doi: 10.1007/s00018-016-2209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fulda S, Gorman AM, Hori O, Samali A. Cellular stress responses: cell survival and cell death. Int J Cell Biol. 2010;2010:214074. doi: 10.1155/2010/214074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–20. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green DR, Galluzzi L, Kroemer G. Cell biology. Metabolic control of cell death. Science. 2014;345:1250256. doi: 10.1126/science.1250256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aitken RJ, Gibb Z, Baker MA, Drevet J, Gharagozloo P. Causes and consequences of oxidative stress in spermatozoa. Reprod Fertil Dev. 2015;28:1–10. doi: 10.1071/RD15325. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. WHO laboratory manual for the examination and processing of human semen. Geneva: WHO Press; 2010. p. 271. [Google Scholar]
- 7.Aitken RJ, Baker MA, Nixon B. Are sperm capacitation and apoptosis the opposite ends of a continuum driven by oxidative stress? Asian J Androl. 2015;17:633–9. doi: 10.4103/1008-682X.153850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koppers AJ, Mitchell LA, Wang P, Lin M, Aitken RJ. Phosphoinositide 3-kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa. Biochem J. 2011;436:687–98. doi: 10.1042/BJ20110114. [DOI] [PubMed] [Google Scholar]
- 9.Aitken RJ, Whiting S, De Iuliis GN, McClymont S, Mitchell LA, et al. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J Biol Chem. 2012;287:33048–60. doi: 10.1074/jbc.M112.366690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aitken RJ, Gibb Z, Baker MA, Drevet J, Gharagozloo P. Causes and consequences of oxidative stress in spermatozoa. Reprod Fertil Dev. 2016;28:1–10. doi: 10.1071/RD15325. [DOI] [PubMed] [Google Scholar]
- 11.Aitken RJ, Jones KT, Robertson SA. Reactive oxygen species and sperm function – In sickness and in health. J Androl. 2012;33:1096–106. doi: 10.2164/jandrol.112.016535. [DOI] [PubMed] [Google Scholar]
- 12.Doshi SB, Khullar K, Sharma RK, Agarwal A. Role of reactive nitrogen species in male infertility. Reprod Biol Endocrinol. 2012;10:109. doi: 10.1186/1477-7827-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uribe P, Boguen R, Treulen F, Sanchez R, Villegas JV. Peroxynitrite-mediated nitrosative stress decreases motility and mitochondrial membrane potential in human spermatozoa. Mol Hum Reprod. 2015;21:237–43. doi: 10.1093/molehr/gau107. [DOI] [PubMed] [Google Scholar]
- 14.Salvolini E, Buldreghini E, Lucarini G, Vignini A, Di Primio R, et al. Nitric oxide synthase and tyrosine nitration in idiopathic asthenozoospermia: an immunohistochemical study. Fertil Steril. 2012;97:554–60. doi: 10.1016/j.fertnstert.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Vignini A, Nanetti L, Buldreghini E, Moroni C, Ricciardo-Lamonica G, et al. The production of peroxynitrite by human spermatozoa may affect sperm motility through the formation of protein nitrotyrosine. Fertil Steril. 2006;85:947–53. doi: 10.1016/j.fertnstert.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Uribe P, Treulen F, Boguen R, Sanchez R, Villegas JV. Nitrosative stress by peroxynitrite impairs ATP production in human spermatozoa. Andrologia. 2017;49:e12615. doi: 10.1111/and.12615. [DOI] [PubMed] [Google Scholar]
- 17.Cabrillana ME, Uribe P, Villegas JV, Alvarez J, Sanchez R, et al. Thiol oxidation by nitrosative stress: cellular localization in human spermatozoa. Syst Biol Reprod Med. 2016;62:325–34. doi: 10.1080/19396368.2016.1208782. [DOI] [PubMed] [Google Scholar]
- 18.Herrero MB, Perez Martinez S, Viggiano JM, Polak JM, de Gimeno MF. Localization by indirect immunofluorescence of nitric oxide synthase in mouse and human spermatozoa. Reprod Fertil Dev. 1996;8:931–4. doi: 10.1071/rd9960931. [DOI] [PubMed] [Google Scholar]
- 19.Amiri I, Karimi J, Piri H, Goodarzi MT, Tavilani H, et al. Association between nitric oxide and 8-hydroxydeoxyguanosine levels in semen of diabetic men. Syst Biol Reprod Med. 2011;57:292–5. doi: 10.3109/19396368.2011.621508. [DOI] [PubMed] [Google Scholar]
- 20.Balercia G, Moretti S, Vignini A, Magagnini M, Mantero F, et al. Role of nitric oxide concentrations on human sperm motility. J Androl. 2004;25:245–9. doi: 10.1002/j.1939-4640.2004.tb02784.x. [DOI] [PubMed] [Google Scholar]
- 21.Mehraban D, Ansari M, Keyhan H, Sedighi Gilani M, Naderi G, et al. Comparison of nitric oxide concentration in seminal fluid between infertile patients with and without varicocele and normal fertile men. Urol J. 2005;2:106–10. [PubMed] [Google Scholar]
- 22.Ghaffari MA, Rostami M. Lipid peroxidation and nitric oxide levels in male smokers’ spermatozoa and their relation with sperm motility. J Reprod Infertil. 2012;13:81–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Machado-Oliveira G, Lefievre L, Ford C, Herrero MB, Barratt C, et al. Mobilisation of Ca2+ stores and flagellar regulation in human sperm by S-nitrosylation: a role for NO synthesised in the female reproductive tract. Development. 2008;135:3677–86. doi: 10.1242/dev.024521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu J, Ma S, Zou S, Li X, Cui P, et al. The regulation of nitric oxide synthase isoform expression in mouse and human fallopian tubes: potential insights for ectopic pregnancy. Int J Mol Sci. 2014;16:49–67. doi: 10.3390/ijms16010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–80. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 26.Khosravi F, Valojerdi MR, Amanlou M, Karimian L, Abolhassani F. Relationship of seminal reactive nitrogen and oxygen species and total antioxidant capacity with sperm DNA fragmentation in infertile couples with normal and abnormal sperm parameters. Andrologia. 2014;46:17–23. doi: 10.1111/and.12034. [DOI] [PubMed] [Google Scholar]
- 27.Oztezcan S, Turkoglu UM, Kervancioglu E, Kocak T, Kocak-Toker N, et al. In vitro effects of peroxynitrite on human spermatozoa. Andrologia. 1999;31:195–8. doi: 10.1046/j.1439-0272.1999.00279.x. [DOI] [PubMed] [Google Scholar]
- 28.Islam BU, Habib S, Ahmad P, Allarakha S, Moinuddin, et al. Pathophysiological role of peroxynitrite induced DNA damage in human diseases: a special focus on poly(ADP-ribose) polymerase (PARP) Indian J Clin Biochem. 2015;30:368–85. doi: 10.1007/s12291-014-0475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanco Garcia J, Aldinucci C, Maiorca SM, Palmi M, Valoti M, et al. Physiopathological effects of the NO donor 3-morpholinosydnonimine on rat cortical synaptosomes. Neurochem Res. 2009;34:931–41. doi: 10.1007/s11064-008-9854-y. [DOI] [PubMed] [Google Scholar]
- 30.Petronilli V, Miotto G, Canton M, Brini M, Colonna R, et al. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys J. 1999;76:725–34. doi: 10.1016/S0006-3495(99)77239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Treulen F, Uribe P, Boguen R, Villegas JV. Mitochondrial permeability transition increases reactive oxygen species production and induces DNA fragmentation in human spermatozoa. Hum Reprod. 2015;30:767–76. doi: 10.1093/humrep/dev015. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell LA, De Iuliis GN, Aitken RJ. The TUNEL assay consistently underestimates DNA damage in human spermatozoa and is influenced by DNA compaction and cell vitality: development of an improved methodology. Int J Androl. 2011;34:2–13. doi: 10.1111/j.1365-2605.2009.01042.x. [DOI] [PubMed] [Google Scholar]
- 33.Galluzzi L, Bravo-San Pedro JM, Vitale I, Aaronson SA, Abrams JM, et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 2015;22:58–73. doi: 10.1038/cdd.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaludercic N, Giorgio V. The dual function of reactive oxygen/nitrogen species in bioenergetics and cell death: the role of ATP synthase. Oxid Med Cell Longev. 2016;2016:3869610. doi: 10.1155/2016/3869610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonora M, Wieckowsk MR, Chinopoulos C, Kepp O, Kroemer G, et al. Molecular mechanisms of cell death: central implication of ATP synthase in mitochondrial permeability transition. Oncogene. 2015;34:1608. doi: 10.1038/onc.2014.462. [DOI] [PubMed] [Google Scholar]
- 37.Segawa K, Kurata S, Yanagihashi Y, Brummelkamp TR, Matsuda F, et al. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science. 2014;344:1164–8. doi: 10.1126/science.1252809. [DOI] [PubMed] [Google Scholar]
- 38.Segawa K, Nagata S. An apoptotic ‘eat me’ signal: phosphatidylserine exposure. Trends Cell Biol. 2015;25:639–50. doi: 10.1016/j.tcb.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Verhoven B, Schlegel RA, Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med. 1995;182:1597–601. doi: 10.1084/jem.182.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagata S, Suzuki J, Segawa K, Fujii T. Exposure of phosphatidylserine on the cell surface. Cell Death Differ. 2016;23:952–61. doi: 10.1038/cdd.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod. 1999;14:1039–49. doi: 10.1093/humrep/14.4.1039. [DOI] [PubMed] [Google Scholar]
- 42.Niles JC, Wishnok JS, Tannenbaum SR. Peroxynitrite-induced oxidation and nitration products of guanine and 8-oxoguanine: structures and mechanisms of product formation. Nitric Oxide. 2006;14:109–21. doi: 10.1016/j.niox.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Burney S, Caulfield JL, Niles JC, Wishnok JS, Tannenbaum SR. The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat Res. 1999;424:37–49. doi: 10.1016/s0027-5107(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 44.Pacher P, Szabo C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol. 2008;173:2–13. doi: 10.2353/ajpath.2008.080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amiri I, Sheikh N, Najafi R. Nitric oxide level in seminal plasma and its relation with sperm DNA damages. Iran Biomed J. 2007;11:259–64. [PubMed] [Google Scholar]
- 46.Gharagozloo P, Aitken RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod. 2011;26:1628–40. doi: 10.1093/humrep/der132. [DOI] [PubMed] [Google Scholar]
- 47.Gow AJ, Farkouh CR, Munson DA, Posencheg MA, Ischiropoulos H. Biological significance of nitric oxide-mediated protein modifications. Am J Physiol Lung Cell Mol Physiol. 2004;287:L262–8. doi: 10.1152/ajplung.00295.2003. [DOI] [PubMed] [Google Scholar]
- 48.Cassina A, Silveira P, Cantu L, Montes JM, Radi R, et al. Defective human sperm cells are associated with mitochondrial dysfunction and oxidant production. Biol Reprod. 2015;93:119. doi: 10.1095/biolreprod.115.130989. [DOI] [PubMed] [Google Scholar]
- 49.Morielli T, O’Flaherty C. Oxidative stress impairs function and increases redox protein modifications in human spermatozoa. Reproduction. 2015;149:113–23. doi: 10.1530/REP-14-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szabo C, Ohshima H. DNA damage induced by peroxynitrite: subsequent biological effects. Nitric Oxide. 1997;1:373–85. doi: 10.1006/niox.1997.0143. [DOI] [PubMed] [Google Scholar]


