Abstract
Introduction
Excess weight is associated with subclinical myocardial damage, as reflected by high sensitivity cardiac troponin-T (hs-cTnT) concentrations, which portends high heart failure risk. However, the association between weight history and myocardial damage is unknown.
Methods
We evaluated 9,062 ARIC Visit 4 (1996–99) participants with body-mass index (BMI) ≥ 18.5 kg/m2 and no prior cardiovascular disease. We cross-tabulated Visit 4 (“current”) BMI categories of normal weight, overweight and obese with those at Visit 1 (1987–89) and with BMI categories calculated from self-reported weight at age 25. Duration of obesity was calculated in years. A cumulative weight measure of “excess BMI years” was also calculated (product of mean BMI [centered at 25 kg/m2] over all ARIC time points x follow-up duration). We used logistic regression to estimate associations of weight history metrics with increased hs-cTnT (≥14 ng/L) at Visit 4.
Results
Overall, 623 individuals (7%) had increased hs-cTnT at Visit 4. Within each current BMI category, prior excess weight was associated with increased hs-cTnT, with the strongest associations for those with past and current obesity (OR 3.85 [2.51–5.90] for obesity at age 25 and Visit 4). Each 10-years longer obesity duration was associated with increased hs-cTnT (OR 1.26; 1.17–1.35). Each 100 higher excess BMI years was also progressively associated with increased hs-cTnT (OR 1.21; 1.14–1.27).
Conclusion
Prior obesity and greater cumulative weight from young adulthood increase the likelihood of myocardial damage, indicating chronic toxic effects of adiposity on the myocardium and the need for weight maintenance strategies targeting the entire lifespan.
Keywords: Obesity, Troponin, Heart Failure
Introduction
Obesity is a well-established risk factor for several types of cardiovascular disease (CVD), but the pathways underlying these associations are incompletely understood(1;2). This is particularly true for the relationship between obesity and heart failure (HF), where several large epidemiologic studies demonstrate a risk association independent of traditional mediators of the relationship between obesity and CVD, such as hypertension, diabetes and dyslipidemia(3–6). A growing body of laboratory data suggest direct toxic effects of obesity on the myocardium that predispose to the development of heart failure(7;8). Similarly, clinical studies demonstrate a strong association between obesity and high sensitivity cardiac troponin-T (hs-cTcT), a biomarker of subclinical myocardial damage, (9;10), and that the combination of obesity and increased hs-cTnT is linked to a markedly increased risk for future HF(10).
While most studies evaluating the relationship between excess weight and cardiovascular disease risk utilize anthropometric measures from one time point, a growing body of data demonstrates that weight history may significantly influence the likelihood of developing cardiovascular events. In past studies, both a longer duration of obesity and higher measures of cumulative weight were linked to increased risk for incident HF(11;12). While the mechanisms for this association are not yet known, imaging studies indicate that a history of excess weight is linked to several abnormalities of myocardial structure and function(13;14). However, it is presently unknown how weight history impacts the likelihood of subclinical myocardial damage, as indexed by hs-cTnT. Understanding the relationship between weight history and hs-cTnT will both elucidate the timing of myocardial damage related to obesity and inform recommendations for weight maintenance to promote optimal cardiovascular prevention. Additionally, given the strong association of minute increases in hs-cTnT among those without clinical CVD with future cardiovascular events and mortality(15;16), the association between past weight patterns and subclinical myocardial damage is of considerable clinical significance.
Therefore, in the present analysis, we examined the relationship between various weight history metrics and increased hs-cTnT in the predominately bi-racial, community-based Atherosclerosis Risk in Communities (ARIC) study. With repeated measures of weight across the adult life span, detailed risk factor characterization, and continuous cardiovascular event ascertainment allowing for the exclusion of individuals with clinical CVD, the ARIC study is well-suited to evaluate the link between weight history and subclinical myocardial damage.
Materials and Methods
The ARIC study was originally designed to investigate the etiology of CVD and initially recruited 15,792 participants from four U.S. population centers: Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; and suburbs of Minneapolis, Minnesota. ARIC participants aged 45–64 years were recruited in 1987–89 (Visit 1) and subsequently examined at visits in 1990–92 (Visit 2), 1993–95 (Visit 3), 1996–98 (Visit 4) and, most recently, from 2011–13 (Visit 5). In addition to undergoing detailed history and examinations, including anthropometry, at each study visit, participants have been followed continuously for cardiovascular events since baseline at Visit 1. Previous papers described the design of ARIC in detail(17). The institutional review boards associated with each ARIC site approved the study protocol and all ARIC participants provided informed consent.
This analysis included participants who attended ARIC Visit 4 and who had available data on body mass index (BMI) from each of the prior ARIC visits. Of 11,656 participants at Visit 4, we excluded: 1,572 participants with a history of HF or CHD at, or prior to, Visit 4 (self-reported HF or CHD at Visit 1; or HF-related hospitalization or death, adjudicated non-fatal myocardial infarction or coronary revascularization event, or silent myocardial infarction by ECG criteria at or prior to Visit 4); 214 participants missing data on prevalent HF or CHD; 417 participants missing data on BMI at one of the study visits; 222 participants missing data on hs-cTnT at Visit 4; 138 participants with BMI values less than 18.5 kg/m2 at one or more of the study visits (due to the confounding associated with underweight); and 31 not of black or white race, for a final study population of 9,062 individuals.
The primary exposure was BMI, based on measured weight and height at each visit and calculated in kg/m2. BMI was categorized as normal weight (18.5 to <25 kg/m2), overweight (25 to < 30 kg/m2) and obese (≥ 30 kg/m2). BMI measured at Visit 4, the time point of hs-cTnT measurement, was defined as “current BMI”, while BMI measures from the past ARIC study visits were defined as “past BMIs”. We additionally calculated BMI from self-reported weight at age 25, a variable that was collected at ARIC Visit 1. We used these data to evaluate the associations of weight history from ARIC Visits 1 through 4 (a 9-year span in middle age) and from age 25 through Visit 4 (from young adulthood to late middle age) with the likelihood of subclinical myocardial damage at Visit 4.
Information regarding demographics, health behaviors and cardiovascular risk factors was obtained at ARIC Visit 4. Smoking status was categorized as current, former, and never smoker. Alcohol intake was self-reported and calculated in grams per week. Diabetes was defined as meeting one of more of the following criteria: a prior diagnosis of diabetes, the use of hypoglycemic medications, a fasting blood glucose ≥ 126 mg/dl or a non-fasting blood glucose ≥ 200 mg/dl. Systolic BP was measured twice at the study visit using standardized techniques, and the mean of the measurements was used for analysis. Total cholesterol, HDL-cholesterol (HDL-C) and triglycerides were measured using enzymatic assays. LDL-C was subsequently calculated using the Friedewald equation [TC – HDL-C – (triglycerides/5)] for those with triglycerides less than 400 mg/dL. Estimated glomerular filtration rate (eGFR) was calculated using the MDRD equation. NT-proBNP was measured using the Elecsys proBNP II immunoassay (Roche Diagnostics).
hs-cTnT was measured in 2010 from thawed Visit 4 plasma samples. Between Visit 4 and 2010, the samples were stored at -80 degrees C. hs-cTnT measurements were performed using the Elecsys Troponin T high-sensitivity assay (Roche Diagnostics) on an automated Cobas e411 analyzer. The between assay coefficients of variation for control materials with mean hs-cTnT concentrations of 2,378 ng/L and 29 ng/L were 2.6% and 6.9%, respectively. The primary outcome in the present study was increased hs-cTnT, defined as ≥14 ng/L, a cutpoint that has been used in several prior analyses relating hs-cTnT to cardiovascular endpoints and mortality(15;16).
Statistical Analysis
We performed univariate comparisons of demographic and clinical variables across BMI categories at Visit 4, using chi-square tests for categorical variables and ANOVA for continuous variables.
Logistic regression was used to assess the associations of different weight history patterns with increased hs-cTnT concentrations at Visit 4. We used two regression models: Model 1 was adjusted for the potential confounders of age, sex, race, smoking status and alcohol intake. Model 2 was adjusted for the Model 1 variables plus additional variables potentially on the causal pathway between obesity and myocardial damage, including diabetes, systolic blood pressure, anti-hypertensive medication use, LDL-C, HDL-C, triglycerides, eGFR, and NT-proBNP.
We assessed how changes across the BMI categories of normal weight, overweight and obese from Visit 1 to Visit 4 were associated with the likelihood of increased hs-cTnT at Visit 4. We compared the likelihood of increased hs-cTnT between those individuals with stable normal weight or overweight from Visit 1 to Visit 4 and those who transitioned from normal weight to overweight or obesity, or from overweight to obesity (those whose BMI category increased). Similarly, we compared those individuals with stable overweight or obesity from Visit 1 to Visit 4 to those who transitioned from overweight to normal weight, or from obesity to overweight or normal weight (those whose BMI category decreased). We performed identical analyses using the time points of age 25 and Visit 4. We assessed the improvements in model quality associated with separately adding BMI category at Visit 1 and BMI category at age 25 to regression models including BMI category at Visit 4 using likelihood ratio tests.
We created cross-tabulations of BMI categories at Visits 1 and 4, with those individuals with normal weight at both time-points serving as the reference, and estimated each subgroup’s likelihood of increased hs-cTnT. We performed the same analysis using cross-tabulations of BMI categories at age 25 and Visit 4. We similarly cross-tabulated tertiles of waist circumference at Visits 1 and 4 as an additional measure of adiposity, and estimated each subgroup’s odds of increased hs-cTnT (with those in the lowest waist circumference tertile at both time points serving as the reference). We tested for interactions between the weight change patterns above and race, sex and age (≥ or < 65 years at Visit 4) on the outcome of increased hs-cTnT.
To further evaluate the effects of chronic excess weight on the likelihood of myocardial damage, we assessed the associations of duration of obesity and cumulative weight, expressed in “excess BMI years” with increased hs-cTnT. Duration of obesity was calculated by coding obesity at each time point from age 25 to Visit 4 as 0 or 1 (for absent or present, respectively), and then multiplying the product of the obesity variable at two consecutive time points by the duration in years between those time points and summing each of the products. For those with obesity at Visit 1 but not at age 25, we assumed obesity onset starting at age 40. We then evaluated the association between each 10 years longer duration of obesity and the odds of increased hs-cTnT. This was performed for the entire population, and in subgroups stratified by race, sex, age (>= or < 65 years), hypertension status, diabetes status and presence of chronic kidney disease (CKD; eGFR < 60 ml/min/1.73 m2). Excess BMI years was calculated by centering the BMI variable at 25 kg/m2 (the upper limit of normal weight), averaging BMI across the time points and multiplying the average of centered BMI by the duration in years of observation (from age 25 to age at Visit 4). Using this calculation, an average BMI of 30 kg/m2 (5 units above 25 kg/m2) for 20 years would equate to 100 excess BMI years. Analyses were performed assessing the odds of myocardial damage associated with each 100 units higher excess BMI years. A restricted cubic spline model (using Harrell’s method) was also constructed to assess the continuous association between excess BMI years and increased hs-cTnT.
In sensitivity analyses, we evaluated the associations of duration of obesity and excess BMI years with increased hs-cTnT using only BMI calculated from measurements from ARIC Visits 1 through 4. In additional sensitivity analyses, we evaluated the associations of weight and height separately with increased hs-cTnT, to assess how these two components of the BMI calculation were associated with myocardial damage.
All analyses were performed using Stata 13.1 (StataCorp). All P values presented are 2-sided.
Results
The mean age of the study population at Visit 4 was 63 years, with 58% women and 21% African American. Those individuals in higher BMI categories were slightly younger, more likely to be African American, and less likely to be current smokers (Table 1). Individuals with obesity also had higher mean systolic blood pressures, a higher prevalence of anti-hypertensive medication use and diabetes, generally more abnormal lipid values and lower NT-proBNP concentrations.
Table 1.
Characteristics of Study Population at Visit 4, Stratified by BMI Category
| Normal Weight N=2,352 |
Overweight N=3,748 |
Obese N=3,184 |
P value | |
|---|---|---|---|---|
| Mean Age, in years (SD) | 62.9 (5.8) | 62.8 (5.7) | 62.1 (5.5) | <0.001 |
| Female – % | 64.8 | 50.7 | 61.3 | <0.001 |
| African American – % | 12.5 | 19.2 | 28.7 | <0.001 |
| Current Smokers – % | 20.6 | 13.6 | 9.2 | <0.001 |
| Mean systolic blood pressure, in mmHg (SD) | 122.8 (19.3) | 126.7 (18.1) | 130.3 (17.9) | <0.001 |
| Use of anti-hypertensive medication – % | 19.5 | 29.9 | 44.1 | <0.001 |
| Diabetes – % | 5.9 | 12.5 | 23.2 | <0.001 |
| Mean LDL-C, in mg/dL (SD)* | 119.0 (33.5) | 124.5 (32.8) | 124.0 (33.8) | <0.001 |
| Mean HDL-C, in mg/dL (SD)* | 57.9 (18.0) | 49.3 (15.5) | 46.7 (14.5) | <0.001 |
| Median triglycerides, in mg/dL (Interquartile interval)* | 103 (75–143) | 123 (90–175) | 134 (99–187) | <0.001 |
| Median eGFR, in ml/min/1.73 m2 (Interquartile interval)) | 80.8 (70.8–93.0) | 81.0 (71.0–93.0) | 81.3 (70.3–94.4) | 0.11 |
| Median NT-proBNP, in pg/ml (Interquartile interval) | 80.6 (42.5–138.4) | 58.1 (29.4–111.3) | 55.9 (26.7–108.1) | <0.001 |
| Mean waist circumference, in cm (SD) | 87.3 (7.6) | 99.2 (6.9) | 115.3 (11.4) | <0.001 |
To convert mg/dL to mmol/L multiply by 0.2586 for cholesterol, by 0.01129 for triglycerides
Overall, 23% of individuals increased in BMI category from Visit 1 to Visit 4, while 72% remained in a stable BMI category, and 5% decreased in BMI category. At Visit 4, 7% of individuals (n=623) had increased hs-cTnT concentrations. Those individuals whose BMI category increased from Visit 1 to Visit 4 had an OR of 1.54 (95% CI: 1.20–1.97) for increased hs-cTnT, relative to those with stable normal weight or overweight. For those whose BMI category decreased from Visit 1 to Visit 4, there was no significant association with increased hs-cTnT (OR 1.12; 95% CI: 0.78–1.61) relative to those with stable overweight or obesity. From age 25 to Visit 4, 65% increased, 2% decreased and 33% remained in a stable BMI category. A BMI category increase from age 25 to Visit 4 was associated with an OR for increased hs-cTnT of 1.27 (95% CI: 1.02–1.59), whereas a BMI category decrease over the same time frame had no significant association with increased hs-cTnT (OR 1.06; 95% CI: 0.60–1.87). Overall, adding Visit 1 BMI category to a model including Visit 4 BMI category improved the model fit (likelihood ratio test P<0.01), as did adding BMI category at age 25 to Visit 4 BMI category (likelihood ratio test P<0.001).
In evaluating the multivariate associations of cross-tabulations of BMI at Visit 1 and Visit 4 with myocardial damage (Table 2), we generally found that within each current BMI category a history of excess weight was associated with a greater likelihood of increased hs-cTnT. Those with both current and past obesity had a tendency towards greater odds of increased hs-cTnT (OR 2.15; 95% CI: 1.57–2.92) than those with current obesity and past overweight at Visit 1 (OR 1.55; 95% CI: 1.07–2.22). Those with current overweight and past obesity also had a high likelihood of increased hs-cTnT (OR 2.05; 95% CI: 1.25–3.38). Interestingly, the odds of increased hs-cTnT (OR 1.57; 95% CI: 1.07–2.32) were significantly increased in those who transitioned from normal weight to overweight from Visit 1 to Visit 4, whereas no association was seen for those with persistent overweight over the same time period (OR 1.07; 95% CI: 0.79–1.45).
Table 2.
Odds Ratios (95% CIs) for Associations of Cross-Tabulations of BMI Categories at Visit 4 and at an Earlier Time Point (Visit 1 or Age 25) With Increased hs-cTnT
| BMI Categories at Visit 1 and Visit 4 (9 years apart) | BMI Categories at age 25 (self-reported) and Visit 4 | ||||||
|---|---|---|---|---|---|---|---|
| Normal Weight at Visit 1 | Overweight at Visit 1 | Obesity at Visit 1 | Normal Weight at age 25 | Overweight at age 25 | Obesity at age 25 | ||
| Normal Weight at Visit 4 | n=83/2,086 [4.0%] Ref = 1 |
n=18/215 [8.4%] 1.31 (0.73–2.34) |
n=6 n/a |
Normal Weight at Visit 4 | n=78/1,916 [4.1%] Ref = 1 |
n=16/105 [15.2%] 2.34 (1.23–4.44) |
n=6 n/a |
| Over-weight at Visit 4 | n=51/1,047[4.9%] 1.57 (1.07–2.32) |
n=152/2,400 [6.3%] 1.07 (0.79–1.45) |
n=35/207=[16.9%] 2.05 (1.25–3.38) |
Over-weight at Visit 4 | n=156/2,808 [5.6%] 1.23 (0.90–1.68) |
n=63/601 [10.5%] 1.51 (1.03–2.21) |
n=8/62 [12.9%] 1.69 (0.69–4.13) |
| Obesity at Visit 4 | n=47 n/a |
n=77/1,021 [7.5%] 1.55 (1.07–2.22) |
n=205/2,033 [10.1%] 2.15 (1.57–2.92) |
Obesity at Visit 4 | n=120/1,815 [6.6%] 1.71 (1.22–2.39) |
n=101/899 [11.2%] 2.04 (1.44–2.90) |
n=57/288 [19.8] 3.85 (2.51–5.90) |
n = number with increased hs-cTnT within subgroup [proportion with increased hs-cTnT]
n/a = associations cannot be estimated due to the high imprecision resulting from the small sample size (< 50 individuals) in this group
Adjusted for age, sex, race, cigarette smoking, alcohol use, LDL-C, HDL-C, triglycerides, systolic blood pressure, anti-hypertensive medication use, diabetes, NT-proBNP and estimated GFR
In the multivariate cross-tabulations of BMI at age 25 and Visit 4 (Table 2), we similarly found that within each current weight category, a higher past weight category in young adulthood was associated with an increased likelihood of increased hs-cTnT. For example, among those individuals with current obesity, a progressive increase in the odds of increased hs-cTnT was seen for those with past normal weight (OR 1.71; 95% CI: 1.22–2.39), past overweight (OR 2.04; 95% CI: 1.44–2.90) and past obesity (OR 3.85; 95% CI: 2.51–5.90) at age 25. Overall, when considering cross-tabulations of BMI at Visit 1 and Visit 4 or at age 25 and Visit 4, those individuals with both past and current obesity had the greatest likelihood of increased hs-cTnT.
We found similar patterns across subgroups defined by race, gender, and age, with no significant interactions between these demographic variables and changes in weight categories on the outcome of increased hs-cTnT. Similar patterns were also seen when we evaluated cross-tertiles of waist circumference at Visits 1 and 4 (Table 3), with those in the highest waist circumference tertile at both time points having the greatest likelihood of increased hs-cTnT (OR 2.67; 95% CI: 1.91–3.72).
Table 3.
Hazard Ratios (95% CIs) for Associations of Cross-Tabulations of Waist Circumference Tertiles at Visit 1 and Visit 4 With Increased hs-cTnT
| 1st Waist Circumference Tertile at Visit 1 | 2nd Waist Circumference Tertile at Visit 1 | 3rd Waist Circumference Tertile at Visit 1 | |
|---|---|---|---|
| 1st Waist Circumference Tertile at Visit 4 | n=57/2,212 [2.6%] Ref = 1 |
n=55/614 [9.0%] 1.92 (1.26–2.94) |
n=28 n/a |
| 2nd Waist Circumference Tertile at Visit 4 | n=20/633 [3.2%] 1.36 (0.77–2.40) |
n=118/1,807 [6.5] 1.53 (1.07–2.18) |
n=53/555 [9.6%] 1.66 (1.08–2.55) |
| 3rd Waist Circumference Tertile at Visit 4 | n=2/98 [2.0%] 2.04 (0.47–8.75) |
n=38/670 [5.7%] 1.91 (1.21–3.04) |
n=278/2,441 [11.4%] 2.67 (1.91–3.73) |
n = number with increased hs-cTnT within subgroup [proportion with increased hs-cTnT]
n/a = associations cannot be estimated due to the high imprecision resulting from the small sample size (< 50 individuals) in this group
Adjusted for age, sex, race, cigarette smoking, alcohol use, LDL-C, HDL-C, triglycerides, systolic blood pressure, anti-hypertensive medication use, diabetes, NT-proBNP and estimated GFR
The duration of obesity within the study population ranged from 0 to 50.1 years. On average, each 10 years longer duration of obesity was associated with an OR of 1.26 (95% CI: 1.17–1.35) for increased hs-cTnT. Significant associations between duration of obesity and increased hs-cTnT were seen in all demographic subgroups and among those participants with and without hypertension, diabetes and CKD at Visit 4 (Figure 1), with no statistically significant interactions between obesity duration and each of these subgroups (all P > 0.05). The association between obesity duration and increased hs-cTnT remained significant after adjustment for current BMI (P<0.001).
Figure 1. Association Between Each 10 years Longer Duration of Obesity and Odds of Increased hs-cTnT (≥14 ng/L) Within Demographic and Clinical Subgroups.
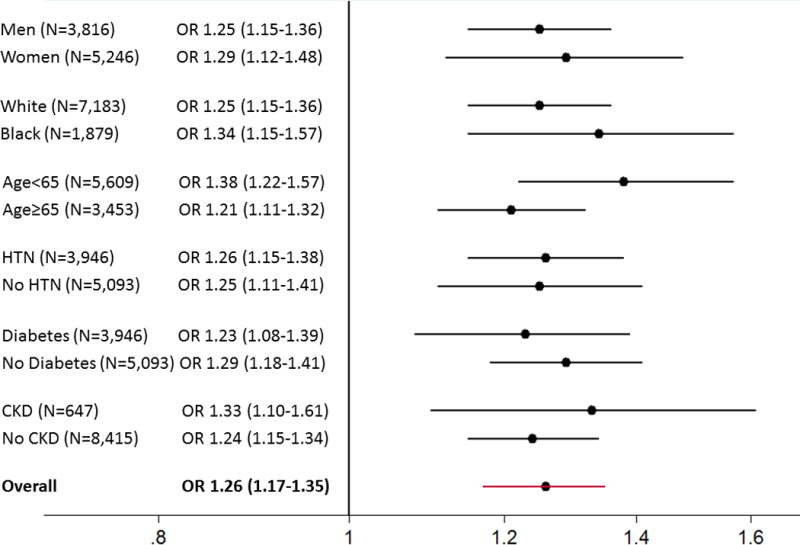
Adjusted for age, sex, race, cigarette smoking, alcohol use, LDL-C, HDL-C, triglycerides, systolic blood pressure, anti-hypertensive medication use, diabetes, NT-proBNP and estimated GFR. When stratified by a given subgroup, the covariate defining the subgroup [e.g., sex for sex-stratified analyses or estimated GFR for analyses stratified by CKD] was removed from the regression model with the exception of age modeled continuously, which was kept in all models. All P values for interactions across subgroups > 0.05.
The range of excess BMI years above 25 kg/m2 in the study population was from -274 to 1,205 kg/m2 * years. Individuals with negative values were those who had BMI values of less than 25 kg/m2 at one or more of the study time points. There was a strong, positive continuous association between excess BMI years and the likelihood of increased hs-cTnT, as shown in the restricted cubic spline in Figure 2. On average, each 100 higher excess BMI years was associated with a 21% higher odds of increased hs-cTnT (95% CI: 1.14–1.27). This association remained significant and was slightly stronger after additional adjustment for current BMI at Visit 4 (HR 1.38: 95% CI: 1.18–1.62).
Figure 2.
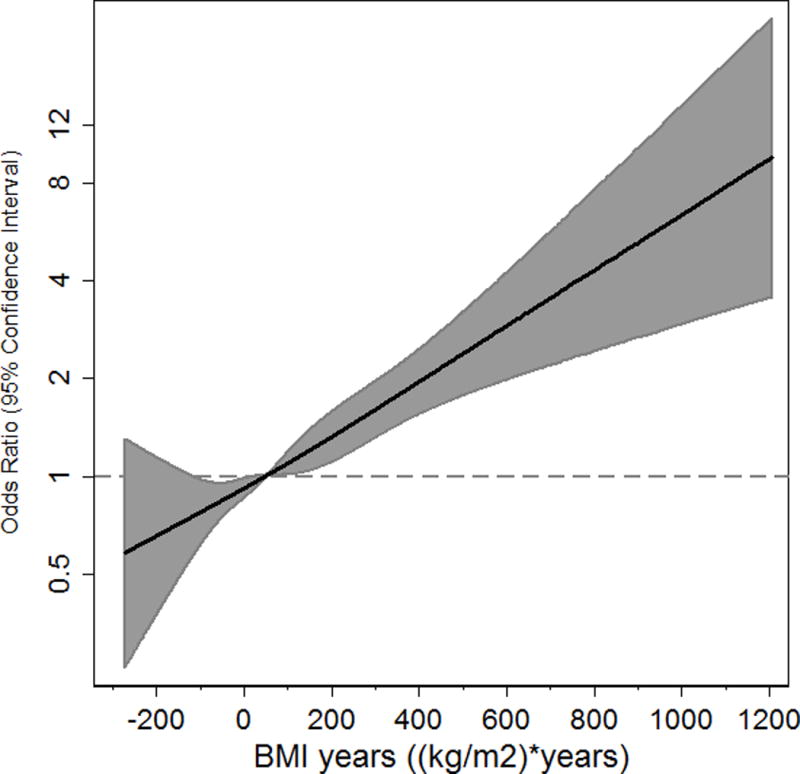
Restricted Cubic Spline for the Association between Excess BMI years and OR of Increased hs-cTnT (≥14 ng/L)
Adjusted for age, sex, race, cigarette smoking, alcohol use, LDL-C, HDL-C, triglycerides, systolic blood pressure, anti-hypertensive medication use, diabetes, NT-proBNP and estimated GFR. Restricted cubic spline centered at median value for excess BMI years.
We also found significant associations for duration of obesity and excess BMI years with increased hs-cTnT in sensitivity analyses using only measured BMI from ARIC Visits 1 through 4. In additional sensitivity analyses, we found that higher tertiles of weight at Visit 1 and Visit 4 and cross-categories of weight tertiles at both time points were significantly associated with increased hs-cTnT, whereas height tertiles were not independently associated with increased hs-cTnT at either time point.
Discussion
In this analysis of 9,062 individuals in the ARIC study, we demonstrated that weight history influences the likelihood of subclinical myocardial damage. After adjustment for demographics and comorbid conditions, increases in BMI category during middle age, and from young adulthood through late middle age, relative to stable normal weight or overweight, were associated with increased hs-cTnT. Additionally, within each category of current weight, a history of obesity was associated with increased hs-cTnT, with those individuals who were obese in both young adulthood and late middle age having the greatest overall likelihood of having increased hs-cTnT. The impact of chronic excess weight on the likelihood of myocardial damage was further demonstrated by strong associations of both obesity duration and cumulative BMI, expressed in excess BMI years, with increased odds of increased hs-c-TnT. These findings, which remained consistent across various demographic subgroups, illustrate the importance of long term weight maintenance for minimizing the likelihood of future myocardial damage.
Obesity is a potent risk factor for several forms of cardiovascular disease, but the pathways underlying these risk associations are divergent and incompletely understood(1;2;18). While the associations of obesity with coronary heart disease and stroke are largely explained by traditional mediators of cardiovascular disease, such hypertension, dyslipidemia and diabetes(19;20), the link between obesity and HF is uniquely unexplained by these traditional CVD mediators(3–6). A growing body of evidence suggests direct effects of obesity on the myocardium, which may explain the potent association between obesity and HF that is independent of comorbid conditions. Clinical studies demonstrate that obesity is independently linked to several abnormalities of myocardial structure and function that predispose to the development of HF(21–23). Laboratory studies have shown that rodents genetically predisposed to obesity demonstrate evidence of increased myocyte damage relative to wild-type mice(7;8). Clinical studies within ARIC and other cohorts demonstrate a strong and independent association between obesity and hs-cTnT among individuals without clinical cardiovascular disease(9;10). Increased hs-cTnT has been linked to increased CVD risk in prospective studies, particularly HF(15;16). Furthermore, prior work within this cohort demonstrates that the combination of severe obesity and increased hs-cTnT is associated with 9-fold higher risk of incident HF over a decade compared to those with normal weight and undetectable hs-cTnT(10). This analysis expands on prior work by demonstrating the cumulative effects of long-term obesity and increasing weight over time on the likelihood of subclinical myocardial damage.
The precise mechanisms by which obesity leads to myocardial damage have not yet been elucidated. We demonstrated a similar association between the duration of obesity and increased hs-cTnT among individuals with and without hypertension, diabetes and CKD, demonstrating that the link between a history of excess weight and myocardial damage is not entirely explained by these comorbid conditions. Several factors have been proposed as potential contributors, including increased myocardial demand due to hemodynamic stress(21;22;24), the effects of obesity-associated adipokines and cytokines on the myocardium(6;25–28), and the toxic effects of adipose tissue accumulation in myocardial tissues(29–32).
In this analysis, we saw high odds of increased hs-cTnT for those who transitioned from obese to overweight from Visit 1 to 4, and from overweight to normal weight from Age 25 to Visit 4. The implications of this finding are uncertain, as the intentionality of weight loss within a cohort study is unknown. While speculative, it is possible that this association reflects reverse causality due to weight loss from undetected clinical comorbidities. On the other hand, prior research suggests that marked intentional weight loss occurring after bariatric surgery is associated with reduced myocardial damage, as assessed by high sensitivity cardiac troponin I concentrations(33). Further research is needed to elucidate the effects of intentional weight loss through lifestyle modification on myocardial damage. However, the rates of significant and sustained intentional weight loss remain relatively low in the general population (34;35). Therefore, understanding the pathways linking chronic obesity and increased hs-cTnT may inform novel strategies to reduce myocardial damage and the likelihood of future CVD events among those with obesity.
From a clinical standpoint, this analysis underscores the importance of long-term weight control for optimizing cardiovascular prevention. A history of excess weight was linked to a greater likelihood of myocardial damage at each level of current weight, and those individuals with both past and present obesity had the highest odds of increased hs-cTnT. These findings were even more pronounced when considering weight at age 25. Furthermore, the finding that the duration of obesity was linked to a similar likelihood of subclinical myocardial damage among individuals with and without diabetes, hypertension and chronic kidney disease indicates the limitations of traditional risk factor control alone for addressing the toxic effects of chronic obesity on the myocardium. These results indicate the importance of defining and implementing strategies to promote weight management, even from young adulthood, for reducing the likelihood of developing myocardial damage. Cumulative weight measured in excess BMI years demonstrated a strong association with increased hs-cTnT. A metric such as excess BMI years could prove useful for communicating to young and middle aged adults the risks of excess weight from early in life and the importance of maintaining a normal weight for decreasing long-term cardiovascular risk.
This analysis has some limitations. As an observational study, it is susceptible to residual confounding. Additionally, while most of the BMI values were based on measured weight and height, BMI at age 25 was based on self-report, which is subject to recall bias. Additionally, being a cohort study in which most individuals gained weight over time and the intentionality of any weight loss is uncertain, this study is poorly equipped to assess the impact of weight loss on the likelihood of myocardial damage. In addition, some survival bias is introduced by only including individuals who survived and were without CVD by Visit 4. An important consideration regarding the excess BMI years construct is its heavy dependence on age, as an older individual with the same obesity status as a younger person would have a greater number of excess BMI years. However the strong risk association between excess BMI years and myocardial damage, after adjustment for age and other confounders, also underscores the importance of maintaining a healthy weight in the context of aging. Additionally, unlike excess BMI years, the duration of obesity variable doesn’t reflect the risk associated with BMI values in the high overweight range (e.g., BMI 29 kg/m2). Nonetheless, this analysis from an extremely well-characterized cohort with repeated measures of weight across the adult lifespan provides important insights about the association between weight history and subclinical myocardial damage. Furthermore, the use of a community-based bi-racial cohort of men and women makes the results broadly generalizable.
In conclusion, we found that a past history of excess weight and increasing weight over time are associated with a greater likelihood of future myocardial damage. These findings are also seen when considering past weights from young adulthood, which underscores the importance of weight management across the entire adult lifespan for the lowest likelihood of myocardial damage and related cardiovascular events.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
The views expressed here are those of the authors and do not necessarily reflect those of the Department of Veterans Affairs.
Sources of Funding:
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I, HHSN2682017000021). This work was supported by the Robert E. Meyerhoff Professorship, a Johns Hopkins University Catalyst Award, a Robert Wood Johnson Amos Medical Faculty Development Award and an NIH/NHLBI grant (K23HL12247) awarded to Dr. Ndumele. Dr. Selvin was supported by NIH/NIDDK grants K24DK106414 and R01DK089174.
Reference List
- 1.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 2.Zalesin KC, Franklin BA, Miller WM, Peterson ED, McCullough PA. Impact of obesity on cardiovascular disease. Med Clin North Am. 2011;95:919–37. doi: 10.1016/j.mcna.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Del Gobbo LC, Kalantarian S, Imamura F, Lemaitre R, Siscovick DS, Psaty BM, Mozaffarian D. Contribution of Major Lifestyle Risk Factors for Incident Heart Failure in Older Adults: The Cardiovascular Health Study. JACC Heart Fail. 2015;3:520–8. doi: 10.1016/j.jchf.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 5.Morkedal B, Vatten LJ, Romundstad PR, Laugsand LE, Janszky I. Risk of myocardial infarction and heart failure among metabolically healthy but obese individuals: HUNT (Nord-Trondelag Health Study), Norway. J Am Coll Cardiol. 2014;63:1071–8. doi: 10.1016/j.jacc.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 6.Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. Obesity and risk of incident heart failure in older men with and without pre-existing coronary heart disease: does leptin have a role? J Am Coll Cardiol. 2011;58:1870–7. doi: 10.1016/j.jacc.2011.06.057. [DOI] [PubMed] [Google Scholar]
- 7.Barouch LA, Gao D, Chen L, Miller KL, Xu W, Phan AC, et al. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ Res. 2006;98:119–24. doi: 10.1161/01.RES.0000199348.10580.1d. [DOI] [PubMed] [Google Scholar]
- 8.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–9. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McEvoy JW, Lazo M, Chen Y, Shen L, Nambi V, Hoogeveen RC, et al. Patterns and determinants of temporal change in high-sensitivity cardiac troponin-T: The Atherosclerosis Risk in Communities Cohort Study. Int J Cardiol. 2015;187:651–7. doi: 10.1016/j.ijcard.2015.03.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ndumele CE, Coresh J, Lazo M, Hoogeveen RC, Blumenthal RS, Folsom AR, et al. Obesity, subclinical myocardial injury, and incident heart failure. JACC Heart Fail. 2014;2:600–7. doi: 10.1016/j.jchf.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alpert MA, Terry BE, Mulekar M, Cohen MV, Massey CV, Fan TM, et al. Cardiac morphology and left ventricular function in normotensive morbidly obese patients with and without congestive heart failure, and effect of weight loss. Am J Cardiol. 1997;80:736–40. doi: 10.1016/s0002-9149(97)00505-5. [DOI] [PubMed] [Google Scholar]
- 12.Reis JP, Allen N, Gunderson EP, Lee JM, Lewis CE, Loria CM, et al. Excess body mass index- and waist circumference-years and incident cardiovascular disease: the CARDIA study. Obesity (Silver Spring) 2015;23:879–85. doi: 10.1002/oby.21023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alpert MA, Lambert CR, Panayiotou H, Terry BE, Cohen MV, Massey CV, et al. Relation of duration of morbid obesity to left ventricular mass, systolic function, and diastolic filling, and effect of weight loss. Am J Cardiol. 1995;76:1194–7. doi: 10.1016/s0002-9149(99)80338-5. [DOI] [PubMed] [Google Scholar]
- 14.Reis JP, Allen N, Gibbs BB, Gidding SS, Lee JM, Lewis CE, et al. Association of the degree of adiposity and duration of obesity with measures of cardiac structure and function: the CARDIA study. Obesity (Silver Spring) 2014;22:2434–40. doi: 10.1002/oby.20865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–76. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 18.Aune D, Sen A, Norat T, Janszky I, Romundstad P, Tonstad S, Vatten LJ. Body Mass Index, Abdominal Fatness, and Heart Failure Incidence and Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Circulation. 2016;133:639–49. doi: 10.1161/CIRCULATIONAHA.115.016801. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383:970–83. doi: 10.1016/S0140-6736(13)61836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ndumele CE, Matsushita K, Lazo M, Bello N, Blumenthal RS, Gerstenblith G, et al. Obesity and Subtypes of Incident Cardiovascular Disease. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88:389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321:225–36. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Turkbey EB, McClelland RL, Kronmal RA, Burke GL, Bild DE, Tracy RP, et al. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA) JACC Cardiovasc Imaging. 2010;3:266–74. doi: 10.1016/j.jcmg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;1:93–102. doi: 10.1016/j.jchf.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Frankel DS, Vasan RS, D’Agostino RB, Sr, Benjamin EJ, Levy D, Wang TJ, Meigs JB. Resistin, adiponectin, and risk of heart failure the Framingham offspring study. J Am Coll Cardiol. 2009;53:754–62. doi: 10.1016/j.jacc.2008.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karas MG, Benkeser D, Arnold AM, Bartz TM, Djousse L, Mukamal KJ, et al. Relations of plasma total and high-molecular-weight adiponectin to new-onset heart failure in adults >/=65 years of age (from the Cardiovascular Health study) Am J Cardiol. 2014;113:328–34. doi: 10.1016/j.amjcard.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McManus DD, Lyass A, Ingelsson E, Massaro JM, Meigs JB, Aragam J, et al. Relations of circulating resistin and adiponectin and cardiac structure and function: the Framingham Offspring Study. Obesity (Silver Spring) 2012;20:1882–6. doi: 10.1038/oby.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Negi SI, Jeong EM, Shukrullah I, Raicu M, Dudley SC., Jr Association of low plasma adiponectin with early diastolic dysfunction. Congest Heart Fail. 2012;18:187–91. doi: 10.1111/j.1751-7133.2011.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szczepaniak LS, Dobbins RL, Metzger GJ, Sartoni-D’Ambrosia G, Arbique D, Vongpatanasin W, et al. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med. 2003;49:417–23. doi: 10.1002/mrm.10372. [DOI] [PubMed] [Google Scholar]
- 30.van der Meer RW, Rijzewijk LJ, Diamant M, Hammer S, Schar M, Bax JJ, et al. The ageing male heart: myocardial triglyceride content as independent predictor of diastolic function. Eur Heart J. 2008;29:1516–22. doi: 10.1093/eurheartj/ehn207. [DOI] [PubMed] [Google Scholar]
- 31.Wende AR, Abel ED. Lipotoxicity in the heart. Biochim Biophys Acta. 2010;1801:311–9. doi: 10.1016/j.bbalip.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–9. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyngbakken MN, Omland T, Nordstrand N, Norseth J, Hjelmesaeth J, Hofso D. Effect of weight loss on subclinical myocardial injury: A clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. Eur J Prev Cardiol. 2016;23:874–80. doi: 10.1177/2047487315618796. [DOI] [PubMed] [Google Scholar]
- 34.Kraschnewski JL, Boan J, Esposito J, Sherwood NE, Lehman EB, Kephart DK, Sciamanna CN. Long-term weight loss maintenance in the United States. Int J Obes (Lond) 2010;34:1644–54. doi: 10.1038/ijo.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacLean PS, Wing RR, Davidson T, Epstein L, Goodpaster B, Hall KD, et al. NIH working group report: Innovative research to improve maintenance of weight loss. Obesity (Silver Spring) 2015;23:7–15. doi: 10.1002/oby.20967. [DOI] [PMC free article] [PubMed] [Google Scholar]


