Abstract
SETTING:
Contact tracing using pediatric index cases has not been adequately investigated in high tuberculosis (TB) and human immunodeficiency virus (HIV) prevalence settings.
OBJECTIVE:
To determine the yield of contact tracing in household contacts of pediatric TB index cases in Botswana.
DESIGN:
Index cases included all pediatric (age ⩽13 years) TB admissions from January 2009 to December 2011 to Botswana’s largest referral hospital. A contact tracing team identified cases, conducted home visits, symptom-screened contacts and referred those with ⩾1 TB symptoms. The primary outcome was newly diagnosed TB in a contact.
RESULTS:
From 163 pediatric index cases, 548 contacts were screened (median 3 contacts/case, interquartile range [IQR] 2–4). Of these, 49 (9%) were referred for positive symptoms on screening and 27/49 (55%) were evaluated for active TB. Twelve new TB cases were diagnosed (12/548, 2.2%); the median age was 31 years (IQR 23–38); 11 (92%) were smear-positive. Ten (83%) had known HIV status: 7 (70%) were HIV-positive. To find one new TB case, the number needed to contact trace (index cases/new cases) was 13.6, and the number needed to screen (contacts/new cases) was 46.
CONCLUSION:
This yield of contact tracing using pediatric index cases is similar to the traditional adult index case approach. Improving the proportion of symptomatic contacts evaluated may increase yield.
Keywords: TB case detection, TB screening
RÉSUMÉ
CONTEXTE :
Dans les contextes à haute prévalence du virus de l’immunodéficience humaine (VIH) et tuberculose (TB), la recherche des contacts à partir des cas-index pédiatriques n’a pas été investiguée adéquatement.
OBJECTIF
Déterminer au Botswana le rendement de la recherche des contacts chez les sujets en contact dans le ménage avec des cas index pédiatriques atteints de TB.
SCHÉMA
Les cas-index ont correspondu à l’ensemble de toutes les admissions TB pédiatriques (⩽13 ans) de janvier 2009 à décembre 2011 dans le plus grand hôpital de référence du Botswana. Une équipe de recherche des contacts a identifié les cas, mené des visites à domicile, dépisté les contacts sur base des symptômes et référé ceux accusant au moins un symptôme TB. Le résultat principal a été une TB nouvellement diagnostiquée chez un contact.
RÉSULTATS
A partir de 163 cas-index pédiatriques, on a dépisté 548 contacts (valeur médiane 3 contacts/cas, extrêmes interquartiles [IQR] 2–4). Parmi ceux-ci, 49 (9%) ont été référés en raison d’un dépistage positif des symptômes et 27/49 (55%) ont été évalués à la recherche d’une TB active. On a diagnostiqué 12 nouveaux cas de TB (12/548, 2,2%; âge médian 31 ans, IQR 23–38). Il y a eu 11 cas à frottis positif (92%). Dans 10 cas (83%), le statut VIH était connu : 7 (70%) étaient séropositifs pour le VIH. Pour trouver un nouveau cas TB, le nombre de cas-index nécessaires à rechercher (cas index/ nouveaux cas) a été de 13,6. Le nombre de cas-contact qu’il fallait dépister (contacts/nouveaux cas) a été de 46.
CONCLUSION
Ce rendement des recherches des contacts à partir des cas-index pédiatriques est similaire à celui de l’approche traditionnelle des cas-index adultes. Une augmentation de la proportion des contacts symptomatiques évalués pourrait augmenter ce rendement.
RÉSUMÉN
MARCO DE REFERENCIA
La investigación de contactos a partir de los casos nuevos pediátricos no se ha investigado adecuadamente en los entornos con alta prevalencia de coinfección por el virus de la inmunodeficiencia humana (VIH) y tuberculosis (TB).
OBJETIVO:
Determinar el rendimiento de la investigación de los contactos domésticos de los casos nuevos pediátricos en Botsuana.
MÉTODO
Los casos nuevos consistieron en todos los pacientes pediátricos (hasta los 13 años de edad) hospitalizados por TB entre enero del 2009 y diciembre del 2011 en el principal hospital de referencia de Botsuana. Un equipo de seguimiento de contactos detectó los casos, practicó las visitas domiciliarias, la detección de los síntomas en los hogares y remitió a las personas con uno o más síntomas indicativos de TB. El criterio primario de evaluación fue el diagnóstico de un caso nuevo de TB en uno de los contactos investigados.
RESULTADOS
Se investigaron 548 contactos de 163 casos nuevos pediátricos (una mediana de tres contactos por caso nuevo, intervalo intercuartil [IQR] 2–4). De estos contactos, se remitieron 49 (9%) por una detección positiva de síntomas y en 27 de los 49 (55%) se investigó la TB activa. Se diagnosticaron 12 casos nuevos de TB (12/548 contactos; 2,2%; edad mediana 31 años, IQR 23–38); 11 casos (92%) tuvieron baciloscopias positivas. Diez personas (83%) conocían su situación frente al VIH y siete eran seropositivas (70%). A fin de detectar un caso nuevo de TB fue necesario practicar la investigación de los contactos de 13,6 casos iniciales (casos iniciales/casos nuevos); se debió practicar la detección sistemática de 46 personas (contactos/casos nuevos).
CONCLUSIÓN
Este rendimiento de la investigación de contactos de los casos nuevos pediátricos es equivalente al rendimiento de la estrategia convencional en los adultos. Se podría mejorar el rendimiento al aumentar la proporción de casos sintomáticos evaluados.
BREAKING THE CYCLE of tuberculosis (TB) infection requires both treatment and prevention. Contact tracing plays a key role in identifying new TB cases and interrupting transmission. Traditional contact tracing focuses on smear-positive adult index TB cases, as these are the most infectious.1 Children, who are less infectious due to paucibacillary disease, most often contract TB from infectious adult household contacts with active TB.2 Diagnosing a pediatric TB case therefore strongly suggests the presence of an untreated TB case at home.
In countries with high TB and human immuno-deficiency virus (HIV) burdens, as in Botswana, traditional contact tracing is a proven strategy for identifying new cases of TB. In a 2010 meta-analysis of 10 studies from low- and middle-income countries (LMICs) with an HIV prevalence of at least 5%, adult index case-based contact tracing had an average new TB case yield of 2.2%, indicating that 2.2% of household contacts had undiagnosed, active TB.3 Although contact tracing of pediatric index cases holds great potential for identifying contacts with active disease, it is unclear how case yields compare to the traditional approach. Two studies have examined contact tracing using pediatric index cases. Kubaisy et al. reported a case yield of 17.4% among contacts of children with latent tuberculous infection in Iraq; however, contact follow-up was conducted 2 years after identifi cation of the index case, indicating that the yield was not the result of contact tracing, but prevalence in the community. Eckhoff reported a case yield of 8.3% among contacts of pediatric index cases in Haiti; however, on excluding those with known TB, the yield dropped to 1.8%. Both studies are from non-African settings and were not performed using the typical contact tracing methods reported in the literature on adult TB.4,5 No study has yet examined the yield of contact tracing using pediatric index cases in a high TB-HIV prevalence setting.
Botswana has the eighth highest TB incidence worldwide (455 per 100 000 population); in 2009, children aged <15 years accounted for 12% of new cases.6,7 HIV prevalence in Botswana is 17.6% and TB-HIV co-infection is common: 68% in 2010.7 The Botswana National TB Programme (NTP) recommends contact tracing for adult smear-positive and multidrug-resistant TB cases. However, resource constraints have limited implementation. We have worked in collaboration with the NTP to improve contact tracing efforts in the capital, Gaborone.
Our objective was to describe the case yield of TB contact tracing using pediatric index cases in a high TB-HIV prevalence setting. These data will inform which contact tracing approach can most effectively identify new TB cases, an important decision in LMICs where contact tracing capacity is limited and disease incidence is high.
STUDY POPULATION AND METHODS
Setting
Pediatric TB index cases were identified from the pediatric ward of the Princess Marina Hospital, a 500bed public referral hospital in Gaborone, Botswana.
Study population
This operational research project used written records from non-study personnel who screened household contacts of pediatric TB index cases identified between January 2009 and December 2011. Households more than 50 km from the hospital were excluded.
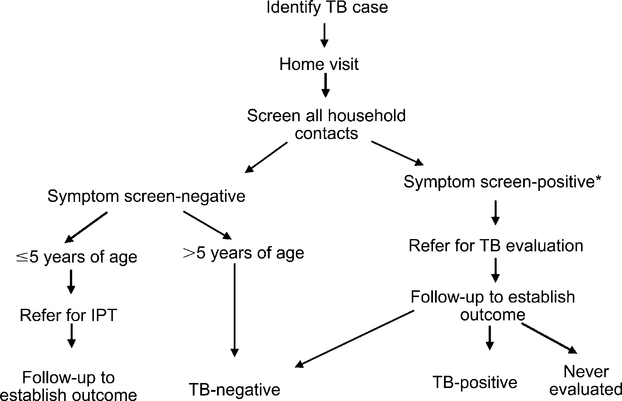
The approach used for contact tracing is summarized in Figure 1. A contact tracing team comprising a social worker and two health care assistants performed home visits for each index case, making up to three attempts to visit each household. They did not return for additional visits if at least one contact was found at home. The number of household contacts was defined as the number of contacts physically present at the time of the home visit plus child contacts (age 0–13 years) who were not present, but were reported by a primary care giver. Adults (aged >13 years) who were not present at the time of the home visit were not included. The contact tracing team screened each contact for active TB symptoms using Botswana’s National TB Contact Tracing Form, which assesses presence and duration of cough, sputum, weight loss, and previous TB evaluations. A positive symptom screen was defined as one or more of the following: cough of any duration, presence of sputum and/or weight loss. Visits occurred within 2 weeks of diagnosing the index case.
Figure 1.
Contact tracing procedure. *Defined as any household contact with one or more of the following: cough of any duration, presence of sputum, weight loss. TB = tuberculosis; IPT = isoniazid preventive therapy.
Contacts with a positive symptom screen were given a referral to a local clinic where a doctor or nurse evaluated them as per the Botswana NTP Guidelines.7 This evaluation included any of the following: clinical examination, sputum smear, chest radiography, tuberculin skin test or sputum culture.
For each referred symptomatic contact, the contact tracing team made up to three attempts to contact contacts to establish outcome: whether TB-positive, TB-negative or never evaluated. For new contact and index cases, HIV status was ascertained retrospectively from the in-patient medical record, TB register or telephone contact.
Definitions
Pediatric index cases included children aged 0– 13 years with pediatrician-diagnosed TB. ‘Household contact’ was any person, regardless of age, who had slept at least one night under the same roof as the index case within 6 months prior to the index patient initiating anti-tuberculosis treatment. A ‘new TB case’ was a household contact with newly diagnosed TB that resulted in the initiation of anti-tuberculosis treatment. Household contacts with a TB diagnosis prior to the contact tracing visit were counted as a separate group.
Outcomes
The primary outcome was yield of contact tracing, defined as the number of new TB cases diagnosed per contact screened. The number needed to screen (NNS) to find one new TB case is the number of contacts screened divided by the number of new cases diagnosed.3 The number needed to contact trace (NNCT) reflects the number of index cases who underwent contact tracing to diagnose one new TB case, calculated as the number of index cases divided by new cases. NNCT measures the yield per index case investigated, a pertinent metric for NTPs in deciding how to allocate limited funds.
Statistical methods
For new TB case yield, a point estimate and 95% confidence interval (CI) were calculated. Prevalence of new TB disease in contacts was reported as rates per 100 000 individuals screened. Sensitivity analyses explored the effect of unknown outcomes in symptomatic contacts on new TB case yield. All analyses were conducted using STATA, version 12.0 (Stata Corp, College Station, TX, USA).
Ethics
Approval was given by the Institutional Review Boards of Botswana’s Ministry of Health, the Princess Marina Hospital, the US Centers for Disease Control and Prevention and the University of Pennsylvania.
RESULTS
Between January 2009 and December 2011, 163 pediatric TB index cases were traced (Table 1). The total number of pediatric TB cases admitted during this time period is unknown. The contact tracing team identified cases three times per week and included all cases living within the study area (in 2011, for example, 69.6% of pediatric TB admissions were geographically eligible). The median length of stay for pediatric TB cases at the Princess Marina Hospital is 7 days (interquartile range 3–13); it is thus likely that few cases were missed due to shorter admissions.8 HIV status was known for 83 (51%) index cases, of which 58 (70%) were HIV-positive.
Table 1.
Demographic and clinical characteristics of pediatric TB index cases
| Index cases* n (%) | |
|---|---|
| Total | 163 (100) |
| Female sex | 79 (48) |
| Age, years, median [IQR]† | 2 [1–7] |
| Range | 0.1–12.9 |
| Age ⩽5 years | 110 (67) |
| HIV status | |
| Positive | 58 (36) |
| Negative | 25 (15) |
| Unknown | 80 (49) |
| Number of household contacts, median [IQR] | 3.0 [2.0– 4.0] |
| At least one household contact ⩽5 years | 58 (36) |
| Death during hospitalization | 13 (8) |
Defined as a child aged 0–13 years with pediatrician-diagnosed TB.
Date of birth could not be obtained for five index cases.
TB = tuberculosis; IQR = interquartile range; HIV = human immunodeficiency virus.
Household contacts
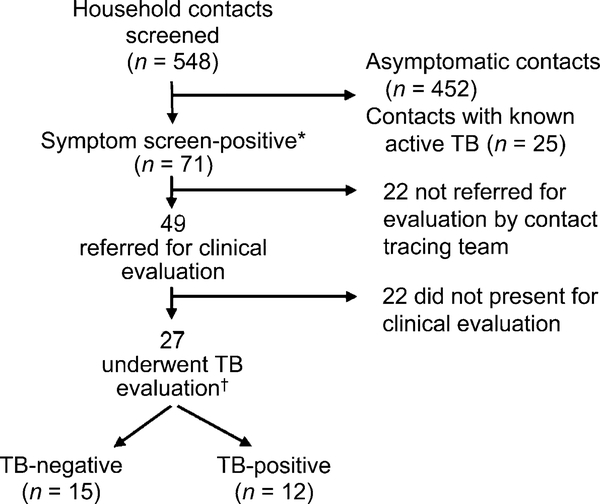
Of 548 household contacts (median 3.0 per index case) screened for active TB (Table 2, Figure 2), 62% were female; half were aged <25 years. Thirty-one (6%) contacts had completed anti-tuberculosis treatment in the past year and an additional 25 (5%) were on anti-tuberculosis treatment at the time of contact tracing. Forty-nine (9%) contacts were symptom screen-positive and were referred for clinical evaluation. In total, 71 (13%) contacts were symptom screen-positive; however, 22 were erroneously not referred by the contact tracing team. Of those not referred, 17 reported weight loss alone; 16 were care givers who attributed weight loss to stress from their child’s illness. The remaining five contacts had cough as their only symptom; of these, one had a history of asthma and one had been evaluated for TB within the last month.
Table 2.
Demographic and clinical characteristics of household contacts of pediatric TB index cases
| Household contacts n (%) | Asymptomatic contactsn (%) | Symptomatic contactsn (%) | Referred contacts n (%) | New TB casesn (%) | |
|---|---|---|---|---|---|
| Total* | 548 (100) | 452 (100) | 71 (100) | 49 (100) | 12 (100) |
| Female sex | 342 (62) | 280 (62) | 47 (66) | 32 (65) | 8 (67) |
| Age, years, median [IQR] | 25 [13–34] | 23 [11–33] | 29 [21– 40] | 28 [17–39] | 30.5 [22.5–38] |
| Range | 0.2–85 | 0.3–79 | 0.2–85 | 0.2–84 | 2–43 |
| Age ⩽5 years | 82 (15) | 72 (16) | 11 (15) | 10 (20) | 2 (17) |
| Symptoms present | |||||
| Cough | 56 (10) | 0 | 51 (72) | 46 (94) | 12 (100) |
| Sputum | 34 (61) | 0 | 29 (57) | 26 (57) | 8 (67) |
| Weight loss | 58 (11) | 0 | 47 (66) | 30 (61) | 12 (100) |
| History of TB in family | |||||
| Yes | 326 (59) | 255 (56) | 46 (65) | 31 (63) | 9 (75) |
| No | 186 (34) | 169 (37) | 17 (24) | 11 (22) | 2 (17) |
| Unknown | 36 (7) | 28 (6) | 8 (11) | 7 (14) | 1 (8) |
| Personal TB history | |||||
| On anti-tuberculosis treatment | 25 (5) | 0 | 0 | 0 | 0 |
| Completed anti-tuberculosis treatment | 31 (6) | 23 (5) | 8 (11) | 6 (12) | 3 (25) |
Household contacts (n = 25) who were on anti-tuberculosis treatment at the time of contact tracing are excluded from the final four columns of this table.
TB = tuberculosis; IQR = interquartile range.
Figure 2.
Study flow diagram. * Defined as any household contact with one or more of the following: cough of any duration, presence of sputum, weight loss. † Consisting of a clinical history and examination as well as sputum smear, chest radiography, tuberculin skin test, and/or sputum culture as determined by a clinic nurse or doctor. TB = tuberculosis.
Symptomatic contacts
The characteristics of the symptomatic contacts are summarized in Table 3. Of 49 contacts referred for positive symptom screen, 27 (55%) underwent clinical evaluation.
Table 3.
Characteristics of symptomatic contacts who were both referred and evaluated
| Referred symptomatic contactsn (%) | Referred and screenedsymptomatic contactsn (%) | |
|---|---|---|
| Total | 49 (100) | 27 (100) |
| Time between index case identification and contact screening | ||
| <1 week | 12 (24) | 12 (44) |
| 1 week–<1 month | 9 (18) | 9 (33) |
| 1–3 months | 1 (2) | 1 (4) |
| >3 months | 3 (6) | 3 (11) |
| Unknown | 2 (4) | 2 (7) |
| Never screened | 22 (45) | 0 |
| Sputum results | ||
| Positive | 11 (22) | 11 (41) |
| Negative | 11 (22) | 11 (41) |
| Unknown | 5 (10) | 5 (19) |
| Not performed | 22 (45) | 0 |
| Mantoux results | ||
| Positive | 0 | 0 |
| Negative | 4 (8) | 4 (15) |
| Not performed | 45 (90) | 23 (85) |
| Culture performed | 0 | 0 |
| Clinical diagnosis only | 1 (2) | 1 (4) |
| Final diagnosis | ||
| TB | 12 (24) | 12 (44) |
| No TB | 15 (31) | 15 (56) |
| Unknown | 22 (45) | 0 |
TB = tuberculosis.
New tuberculosis cases
Overall, 12 new TB cases were diagnosed among the 548 contacts screened (yield 2.2%, prevalence 2190/100 000). Eleven (92%) had smear-positive TB, and one (8%) was diagnosed clinically. The NNS to find one new TB case was 46, while the NNCT was 13.6.
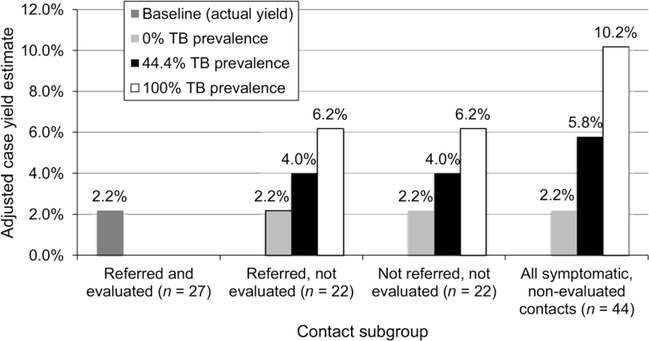
Sensitivity analysis
Sensitivity analysis (Figure 3) indicated that the range of yield for new TB cases, when taking into account the 44 non-evaluated symptomatic contacts, ranged from 2.2% to 10.2%. If the percentage of nonevaluated symptomatic contacts diagnosed with TB were equal to the subset of 27 contacts who underwent evaluation, 20 additional cases would have been diagnosed, giving a case yield of 5.8%.
Figure 3.
One-way sensitivity analysis of TB prevalence among non-evaluated, symptomatic contacts to determine adjusted TB case yields. ‘Baseline yield’ refers to the TB prevalence detected and reported among contacts in this study; ‘44.4% TB prevalence’ assumes the same TB prevalence among symptomatic contacts who were not evaluated and contacts who were; ‘100% TB prevalence’ assumes 100% TB prevalence among symptomatic contacts who were not evaluated.
Overall burden of tuberculosis disease by household
Of 548 contacts, 37 (6.5%, prevalence 6457/100 000) had a current TB diagnosis, which included new cases identified via contact tracing and those on anti-tuberculosis treatment at the time of contact tracing. Of 163 households, 33 (20%) had at least one contact with a current TB diagnosis (Table 4). When also including those households with an individual who had completed anti-tuberculosis treatment in the past year, 51/163 (31%) households were affected. Of these households, 11/51 (22%) had two or more contacts with TB.
Table 4.
TB burden by household
| Households n (%) | |
|---|---|
| Total number of households | 163 (100) |
| Households with 1 or more new TB cases* | 11 (7) |
| Households with 1 or more active TB cases† | 33 (20) |
| Households with 1 or more recent TB cases‡ | 51 (31) |
| Number of recent TB cases per household | |
| 1 | 40 (78) |
| 2 | 8 (16) |
| 3 | 3 (6) |
New TB cases defined as household contacts diagnosed with active TB as a result of contact tracing.
Active TB cases includes both new TB cases and persons diagnosed with TB prior to the contact tracing visit and who were on anti-tuberculosis treatment at the time of contact tracing.
Recent TB includes all active TB cases and persons who had had active TB within the past year and completed anti-tuberculosis treatment.
TB = tuberculosis.
DISCUSSION
The TB case detection rate (2190/100 000) from contact tracing using pediatric index cases in Botswana’s high TB-HIV prevalent setting was four times that of the country’s TB notification rate (455/100 000). In total, 20% of households had at least one additional person with newly diagnosed TB or currently on antituberculosis treatment, emphasizing the sizeable burden of TB. These findings demonstrate that using pediatric index cases as an entry point for screening families for TB in this setting is feasible and a strategy that can be used to identify undiagnosed TB cases.
Traditional contact tracing uses adult smear-positive index cases, as these are the most infectious and are assumed to be responsible for the majority of new TB cases among household contacts.1,9 The current study challenges this notion, suggesting that contact tracing of pediatric cases is as good as the traditional approach. We postulate that because newly diagnosed pediatric TB generally reflects TB transmission within the past year,10 often from a household contact,2,11 a pediatric TB case will frequently signify the presence of an undiagnosed infectious case in the household. Furthermore, pediatric cases may have been infected in the community and represent source cases at home, responsible for child-to-adult and child-to-child transmission.12,13 In settings with poor health care access, these cases have the potential to spread disease in both the family and the community. In Botswana, a middle-income country with free health care for all citizens, it is notable that 31% of the households investigated had at least one additional person affected by TB in the last year, many of whom were undiagnosed prior to contact tracing using pediatric index cases.
When comparing contact tracing using the traditional and the pediatric index case-based approaches, detection of new cases was similar. A meta-analysis of traditional contact tracing in settings with similar TB and HIV prevalence described a median new case yield of 2.2% (range 0.01–14.5) and an NNS of 45 (range 7–10 000).3 Among the 10 studies included, two focused on multidrug-resistant index cases, which had significantly higher yield. A similar meta-analysis of 23 studies demonstrated a pooled yield of 2.3% (2.1–2.5%, heterogeneity: P < 0.001, I2 = 96.6%).14 Our yield of 2.2% (95%CI 1.1–3.8) is comparable, emphasizing that contact tracing using pediatric index cases should be used to identify new TB cases.
The HIV infection rate among those with known HIV status was 70% among index cases and new cases. These numbers are similar to Botswana’s national TB-HIV coinfection statistics, suggesting that HIV status did not affect the yield of contact tracing in this setting, although HIV-infected persons were more likely to have paucibacillary disease.7 Although index cases were drawn from a single hospital, this figure suggests that, at least from an HIV prevalence standpoint, the cohort did not differ from national TB numbers. The impact of HIV infection on contact tracing yield, however, needs to be confirmed in larger multicenter studies.
Although diagnostic methods for index cases were not captured, pediatric TB cases in Botswana are generally diagnosed clinically, without bacteriological confirmation, and account for 10–12% of the national TB case burden.7 As TB diagnostics for pediatric populations improve, contact tracing of pediatric cases may see a substantial increase in yield— assuming that when relying on a clinical diagnosis at least some proportion of index cases were misdiagnosed with TB and should not have undergone contact tracing, as they are unlikely to have new cases at home.
Our study had several limitations. The contact tracing team screened each household only once, limiting contacts who underwent screening to those present. As an operational research project, our results reflect what can be achieved if some resources are dedicated to contact tracing, giving a realistic view of what can be achieved when contact tracing is implemented in a high TB-HIV prevalence setting.
Our yield is likely an underestimate of detectable new TB cases using this single home visit approach. As described, of 71 symptomatic contacts, 22 were erroneously not referred for evaluation. Of the 49 who were referred, 22 did not undergo clinical evaluation. Among the 27 contacts evaluated, 12 (44%) were diagnosed with TB. Sensitivity analysis indicated that the yield for new TB cases would have been 5.8% when assuming a TB diagnosis in a comparable 44% of the 44 unevaluated symptomatic contacts.
Using symptom screening for only cough, sputum and weight loss may have missed infected pediatric contacts who have a wider variation of disease presentation. In our cohort, 36% of index cases had at least one contact aged <5 years at home. Although using a modified symptom screen is adequate in HIV-negative children, data are limited in HIV-infected children.15 A detailed investigation of children, including adjusted symptom screening and diagnostic testing, might have resulted in more children being diagnosed. Several pediatric contacts were symptom screen-negative, referred for isoniazid preventive therapy (IPT) and diagnosed with TB at their IPT appointment, which highlights the challenge of diagnosing TB in children.
The data used for our study were limited to those captured by the NTP’s contact tracing form and those retrospectively extracted from electronic medical records. A number of possible secondary benefits of contact tracing were therefore not measured— cases prevented by education and IPT referral, transmission prevented by bringing infectious cases to treatment, community TB education and decreases in morbidity and mortality for new cases by linking them to care.
CONCLUSIONS
In a high TB-HIV burden setting, we report that the yield of contact tracing using pediatric index cases is similar to the traditional adult index case approach. Future studies should focus on improving the proportion of symptomatic subjects who are evaluated for TB, which will likely increase new case detection, and on quantifying the additional potential benefits of this approach.
Acknowledgements
The authors thank the tuberculosis (TB) contact tracing team health care assistants (E Rankae and S Kekwaletswe), doctors, nurses, TB coordinators and staff at the Princess Marina Hospital Pediatric Ward who assisted with this project. They also greatly appreciate the extensive assistance of the staff of the Botswana-UPenn Partnership with carrying out this project.
Funding for the contact tracing team was provided by the President’s Emergency Fund for AIDS Relief; additional funding was provided by the Doris Duke Charitable Foundation (ID: 200787). SP is a recipient of the Clinical Research Fellowship at the University of Pennsylvania from the Doris Duke Charitable Foundation. In addition, this publication was made possible through core services and support from the Penn Center for AIDS Research, a National Institute of Health-funded program (P30 AI 045008). The funding bodies played no role in the study design, data collection, analysis and interpretation, in the writing of the manuscript or in the decision to submit the manuscript for publication.
Footnotes
Conflict of interest: none declared.
References
- 1.Toman K Case detection In: Frieden TR, ed. Toman’s tuberculosis: case detection, treatment, and monitoring. 2nd ed. Geneva, Switzerland: World Health Organization, 2004. [Google Scholar]
- 2.Marks SM, Taylor Z, Qualls NL, Shrestha-Kuwahara RJ, Wilce MA, Nguyen CH. Outcomes of contact investigations of infectious tuberculosis patients. Am J Respir Crit Care Med 2000; 162: 2033–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kranzer K, Houben RM, Glynn JR, Bekker LG, Wood R, Lawn SD. Yield of HIV-associated tuberculosis during intensified case finding in resource-limited settings: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10: 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckhoff CT Evaluation of a clinical index among adult contacts of children with tuberculosis in rural Haiti. Int J Tuberc Lung Dis 2000; 4: 1143–1148. [PubMed] [Google Scholar]
- 5.Al Kubaisy W, Al Dulayme A, Hashim DS. Active tuberculosis among Iraqi schoolchildren with positive skin tests and their household contacts. Eastern Mediterr Health J 2003; 9: 675–688. [PubMed] [Google Scholar]
- 6.World Health Organization. Global tuberculosis report, 2012. WHO/HTM/TB/2012.6. Geneva, Switzerland: WHO, 2012. [Google Scholar]
- 7.Ministry of Health, Republic of Botswana. National Tuberculosis Programme manual. 7th ed. Gaborone, Botswana: Republic of Botswana Ministry of Health, 2011. [Google Scholar]
- 8.Steenhoff AP, Mazhani L, Shah SS, Kung SJ. Pneumonia out-comes in HIV-exposed uninfected and HIV-negative children in Gaborone, Botswana. Poster at 42nd Union World Conference on Lung Health in Lille, France, 26–30th October 2011. Int J Tuberc Lung Dis 2011; 15 (Suppl 3): S307 [Abstract no. PC-752–30] [Google Scholar]
- 9.Sinfield R, Nyirenda M, Haves S, Molyneux EM, Graham SM. Risk factors for TB infection and disease in young childhood contacts in Malawi. Ann Trop Paediatr 2006; 26: 205–213. [DOI] [PubMed] [Google Scholar]
- 10.Marais BJ, Schaaf HS. Childhood tuberculosis: an emerging and previously neglected problem. Infect Dis Clin North Am 2010; 24: 727–749. [DOI] [PubMed] [Google Scholar]
- 11.Wood R, Johnstone-Robertson S, Uys P, et al. Tuberculosis transmission to young children in a South African community: modeling household and community infection risks. Clin Infect Dis 2010; 51: 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaaf HS, Michaelis IA, Richardson M, et al. Adult-to-child transmission of tuberculosis: household or community contact? Int J Tuberc Lung Dis 2003; 7: 426–431. [PubMed] [Google Scholar]
- 13.Batra S, Ayaz A, Murtaza A, Ahmad S, Hasan R, Pfau R. Child-hood tuberculosis in household contacts of newly diagnosed TB patients. PloS ONE 2012; 7: e40880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis 2008; 8: 359–368. [DOI] [PubMed] [Google Scholar]
- 15.Kruk A, Gie RP, Schaaf HS, Marais BJ. Symptom-based screening of child tuberculosis contacts: improved feasibility in resource-limited settings. Pediatrics 2008; 121: e1646–1652. [DOI] [PubMed] [Google Scholar]