Abstract
Objectives:
To provide the first estimate of adolescents’ abortion incidence in Uganda and to assess differences in the abortion experiences and morbidities of adolescent and nonadolescent postabortion care (PAC) patients.
Study design:
We used the age-specific Abortion Incidence Complications Method, drawing from three surveys conducted in Uganda in 2013: a nationally representative Health Facilities Survey (n=418), a Health Professionals Survey (n=147) and a Prospective Morbidity Survey of PAC patients (n=2169). Multivariable logistic and Cox proportional hazard models were used to compare adolescent and nonadolescent PAC patients on dimensions including pregnancy intention, gestational age, abortion safety, delays to care, severity of complications and receipt of postabortion family planning. We included an interaction term between adolescents and marital status to assess heterogeneity among adolescents.
Results:
Adolescent women have the lowest abortion rate among women less than 35 years of age (28.4 abortions per 1000 women 15–19) but the highest rate among recently sexually active women (76.1 abortions per 1000 women 15–19). We do not find that adolescents face greater disadvantages in their abortion care experiences as compared to older women. However, unmarried PAC patients, both adolescent and nonadolescent, have higher odds of experiencing severe complications than nonadolescent married women.
Conclusions:
The high abortion rate among sexually active adolescents highlights the critical need to improve adolescent family planning in Uganda. Interventions to prevent unintended pregnancy and to reduce unsafe abortion may be particularly important for unmarried adolescents. Rather than treating adolescents as a homogenous group, we need to understand how marriage and other social factors shape reproductive health outcomes.
Implications:
This paper provides the first estimate of the adolescent abortion rate in Uganda. Studies of adolescent abortion and reproductive health must account for sexual activity and marital status. Further, interventions to address unintended pregnancy and unsafe abortion among unmarried women of all ages in Africa should be a priority.
Keywords: Induced abortion, Abortion complications, Adolescent, Unintended pregnancy, Family planning
1. Introduction
Unintended pregnancies accounted for half of the 2.3 million pregnancies that occurred in Uganda in 2013 [1]. In 2016, a quarter of Ugandan women age 15–19 had already begun childbearing, and 46% of births to women under the age of 20 were reported as mistimed or unwanted [2]. Adolescents with unintended pregnancies may be particularly motivated to pursue abortions because of potential consequences for their lives or the circumstances around their pregnancies [3,4]. Approximately 314,300 abortions took place in 2013 [1], but it is unknown how many of these occurred among adolescents, and whether abortion care experiences and complications differ between adolescent and nonadolescent women. This paper provides the first age-specific estimates of abortion in Uganda, as well as examines differences in abortion care experiences by age and how these differences may be shaped by marital status.
The legal status of abortion in Uganda is ambiguous; the penal code permits abortion only if a woman’s life is in danger, but 2006 policy guidelines expanded legal abortion to HIV-positive women and in cases of fetal anomaly, rape or incest [5]. Though these 2006 guidelines are outdated, attempts to pass new guidelines have been unsuccessful [6]. Restrictive abortion laws like these are not associated with lower rates of abortion [7]; instead, fear of imprisonment and societal stigma surrounding abortion drive the practice underground [8–10], leading to less safe abortions [11]. Over 93,000 women (or 12 out of every 1000 women) were hospitalized for induced abortion complications in Uganda in 2013, and more than 10% of the country’s maternal deaths can be attributed to unsafe abortions [1,12,13].
Previous studies have suggested that adolescents face disadvantages in their need for, access to and experiences with abortion-related care. These disadvantages include having a higher risk of unintended pregnancy and therefore of abortion [14]; recognizing pregnancies and seeking abortions at later gestations than older women; and experiencing delays in seeking abortions and postabortion care (PAC) for social, economic and cultural reasons [4,15–18]. Provider bias and perceived negative attitudes of health workers towards adolescents seeking abortion care are additional barriers to adolescents seeking sexual and reproductive health services [19]. Delays in both having abortions and receiving care for abortion complications would be expected to result in greater morbidity and mortality among this age-group [18,20]. Adolescents are also more likely to receive abortions from untrained providers and/or providers that use inappropriate methods; given these risks, evidence suggesting adolescents experience higher rates of abortion-related cervical or uterine injury and more severe abortion-related complications is unsurprising [14,21–26].
Other studies in sub-Saharan Africa, however, highlight a more complex relationship between age and abortion. Recent analyses of national data from the Republic of Congo, Ethiopia, Gabon and Ghana found that abortions do not occur disproportionately among adolescents [27]. In addition, many adolescents are not sexually active, meaning that abortion rates may underrepresent abortion among at-risk adolescents; a recent analysis in Ethiopia, for example, found sexually active adolescents to have the highest abortion rate among all age groups [28]. Further, a national study of PAC patients in Malawi found adolescents to have similar abortion complications and gestational ages as nonadolescents [23].
Differences between adolescent and nonadolescent women’s abortion experiences are likely shaped by reproductive and demographic characteristics and, in particular, marriage and sexual activity. In Uganda, 46% of adolescents have ever had sex, and 20% are married or living in union [2]. Pregnancy carries different social and cultural connotations for married adolescents than it does for unmarried adolescents. While married adolescents are more likely to live in rural areas, be poorer and have less education — factors which may impact their need for and access to family planning and abortion care [29–31] — unmarried adolescents face strong social stigma around extramarital pregnancy [32]. For sexually active unmarried adolescents, this stigma is paired with higher levels of unmet need for family planning (45%) than is observed among both married adolescents (30%) and all unmarried sexually active women (32%) [2]. Marriage shapes the sexual and social experiences of adolescents and is therefore critical to understanding sexual and reproductive health differences between adolescents and nonadolescents.
This paper examines whether adolescents’ abortion experiences and morbidities differ from those of nonadolescent women and what role, if any, marital status potentially plays in shaping such differences. To answer this question, we estimate age-group differences in the incidence of abortion and unintended pregnancy, and assess how adolescent and nonadolescent PAC patients differ in their pregnancy intentions, gestational age, abortion safety, delays to care, severity of complications and receipt of postabortion family planning.
2. Methods
2.1. Data sources
To estimate the incidence of abortion among adolescent women, we used an age-specific variant of the Abortion Incidence Complications Method [28]. We indirectly estimated abortion incidence through the use of three surveys conducted in Uganda in 2013: a nationally representative Health Facilities Survey (HFS) that provided data on the number of postabortion complications cases (n=418); a Health Professionals Survey (HPS) of purposively selected, knowledgeable key informants used to assess the proportion of all abortions resulting in treated complications (n=147); and a Prospective Morbidity Survey (PMS) which collected data on PAC patients obtaining services from a nationally representative sample of health facilities (n=2169). Further details for the HFS and HPS study protocols are described in Prada et al. [1]. The Higher Degrees, Research and Ethics Committee, Makerere University, School of Public Health; the Ugandan National Council of Science and Technology; and the Guttmacher Institute’s Institutional Review Board all provided ethical clearance for the three surveys.
The facilities selected for the PMS data collection were a subsample of the facilities included in the HFS. Sampling proportions were determined based on the expected relative provision of postabortion care services. Hospitals, as the facility type expected to offer the largest proportion of PAC services, were sampled at 70%. Health center IIIs and private midwives were expected to provide the lowest relative proportion of PAC and were thus sampled at 40%. The level of PAC provision at health center IVs was expected to fall between that of hospitals and health center IIIs, which was reflected in a sampling proportion of 55%. Of 212 sampled facilities, 181 participated (an 85% response rate). The PMS was conducted for 4 weeks in nonhospital facilities and for 2 weeks in hospitals. As hospitals have larger caseloads, it was expected that these facilities would be able to collect sufficient data on PAC patients to reliably generate annual PAC caseloads in less time than lower-level health facilities. Any woman presenting for PAC was eligible for the study; a total of 2169 women participated. Selected providers from sampled facilities were trained on administration of the two PMS instruments: a survey of patients and a survey of providers. The patient survey asked women to report demographic characteristics, reproductive history and delays in seeking care. If the patient consented, additional data on the woman’s clinical diagnosis and treatment were obtained from either her provider or from her clinical records. The interviewers did not administer surveys to their own patients and instead asked another staff member in the facility to administer those surveys. Eleven patients declined to release their clinical data and were not included in the analysis.
We utilized age-specific fertility rates from the 2011 and 2016 Uganda Demographic and Health Surveys to impute rates for 2013, assuming a constant rate of change per year [2,33]. Population data by age group came from the 2013 estimate from World Population Prospects 2015 Revision [34]. We applied the 2013 age-specific fertility rates to the population to estimate live births by age group.
2.2. Age-specific abortion rates
Calculating the age-specific abortion rate requires an age-specific adaptation of the Abortion Incidence Complications Method (see mathematical Appendix A). Application of this method involves four key steps.
First, the number of PAC cases, by age, was estimated. Data on PAC caseloads were collected in the HFS, and estimates of PAC cases were calculated by Prada et al. [1]. We then calculated the age-distribution using the weighted age of PAC patients in the PMS. Weights were calculated based on the universe of health facilities that have the potential to provide PAC in Uganda, factoring in the probability of selection, nonresponse, data collection time (2 versus 4 weeks), missing or closed facilities, geographic region and facility type.
Second, we removed PAC cases due to miscarriage, by age. The Abortion Incidence Complications Method assumes that only women with late miscarriages (13–22 weeks) will go to a facility for PAC. In order to estimate the risk of late miscarriage by age, we drew on clinical data from Harlap et al. [35] to construct age-specific life tables, which are available in an appendix to Sully et al. [28]. As not all late miscarriages will result in facility-based care, we assumed that the proportion of women receiving care for late miscarriages in a facility is equal to the proportion that gives birth in a facility. Delivery care was estimated based on trends between the 2011 and 2016 Demographic and Health Surveys [2,33]. Treated miscarriages were subtracted from the estimated number of PAC cases calculated in the first step; the difference is the number of complications due to induced abortions.
Third, we estimated the number of abortions that do not result in facility-based care by calculating a multiplier, which estimates the number of women that have abortions and are not treated for complications in a health facility for every woman receiving treatment, from the HPS data [1]. Lastly, we applied the multiplier to the number of PAC cases due to induced abortions, yielding an estimated number of induced abortions by age group.
We calculated the age-specific abortion rate using age-group population size. As not all women are sexually active and at risk of unintended pregnancy, we also calculated the abortion rate among women who have ever had sex and among those who have had sex in the last 12 months; we used 2011 and 2016 Demographic and Health Survey data to estimate sexual activity levels for 2013 [2,33]. Lastly, we measured the proportion of all pregnancies that were unintended among each age group using this paper’s estimate of abortion, data on births by intention [2,33] and estimated numbers of miscarriages, which were calculated as 20% of births and 10% of abortions [36] (see mathematical Appendix A).
2.3. Adolescent abortion and postabortion care experiences
To determine whether adolescents’ abortion care experiences are different from older women’s, we used the PMS data to compare the demographic profiles, complications and treatment-seeking behaviors of adolescent and nonadolescent PAC patients. We operationalized six hypothesized disadvantages in abortion care experiences with the variables described in Table 1.
Table 1.
Adolescents’ hypothesized disadvantages in need for, access to and experience with abortion and postabortion care
| Abortion care experience Adolescents more likely to… |
Outcome variable Abortion care experience operationalized as… |
|---|---|
| (1) Be at risk of unintended pregnancy | Pregnancy for which a woman is seeking care was unintended (wanted later/did not want vs. wanted) |
| (2) Recognize pregnancy at later gestations | Gestational age (as reported by provider) when women presented at a facility for care (second trimester/third trimester vs. first trimester) |
| (3) Resort to less safe abortion methods | Someone other than a medical professional helped the woman interfere with her pregnancya (medical professional vs. other person/self) |
| (4) Delay reaching health facility for care | Self-reported total number of days until woman reached a facility for care (Sum of self-reported time to decide to seek care and self-reported time to reach care) |
| (5) Present with more severe complications | Severity of postabortion complications (severe vs. mild/moderate) |
| (6) Not receive postabortion family planning | Provider-reported provision of postabortion modern contraceptives by health staff (received modern method vs. no modern method received) |
Does not account for method of abortion, which is a key component of the definition of unsafe abortion [11]. For example, a trained provider could provide an unsafe abortion using an inappropriate method.
We conducted descriptive bivariate analyses for the outcome variables in Table 1 and a binary indicator for whether the PAC patient was an adolescent or not, with χ2 tests of independence. All abortion care experiences aside from delays in reaching care (Table 1) were dependent variables in multivariable logistic regression models in which we tested for differences between adolescent and nonadolescent women. For delays in care, measured in days, we used a Cox proportional hazard model to estimate differences in the hazard ratio between adolescents and nonadolescents. All models controlled for marital status, urban residence, level of education and whether the woman had experienced a previous pregnancy. Control variables were selected a priori and confirmed based on model fit. To assess how abortion care experiences may differ by marital status, we included an interaction term between the adolescent age group and marital status.
As our sample is women receiving PAC services, we cannot differentiate induced abortions from miscarriages. Complications from the two are usually clinically indistinguishable [37], and induced abortions are likely underreported by women [38]. All models other than that in which unintended pregnancy is the outcome include a control for whether the pregnancy was unintended. Pregnancy intention status is a proximate indicator of women who likely had an induced abortion; women with unintended pregnancies are potentially more likely to have had an abortion, though this group also includes some women who have unintended pregnancies that end in miscarriage.
3. Results
3.1. Adolescent abortion and unintended pregnancy
In 2013, approximately 57,000 abortions took place among adolescent women in Uganda. Adolescents have the lowest abortion rate of all women under age 35, at 28.4 per 1000 women age 15–19 (Fig. 1). However, when considering only women who have ever had sex, the adolescent abortion rate rises to 62.6 per 1000 (Fig. 1). Further restricting the denominator to adolescent women who had sex in the last 12 months, the adolescent abortion rate increases to 76.1 per 1000 women age 15–19 — the highest of any age group (though similar to women age 25–29, who have a rate of 74.4 abortions per 1000 women). Overall, half of adolescent pregnancies were unintended, and 15% ended in abortion (Fig. 2). The proportion of pregnancies ending in abortion does not vary systematically by age.
Fig. 1.
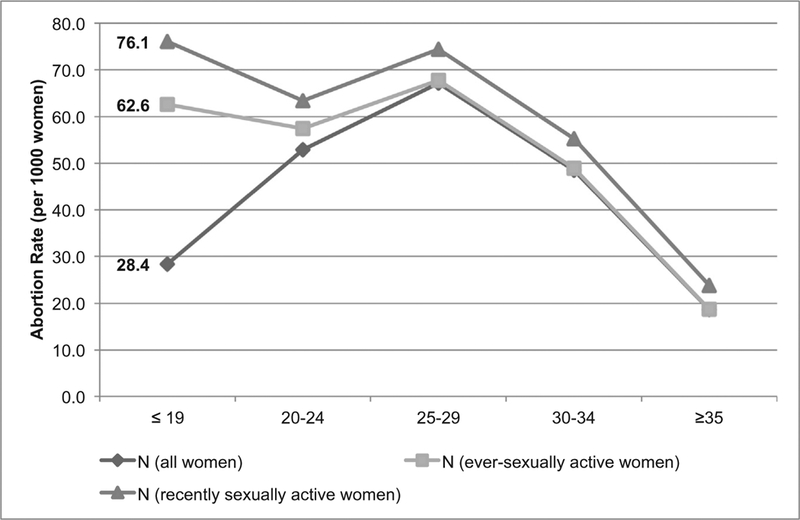
Abortion rate by age group in Uganda in 2013, including abortion rates for all women, ever-sexually active women and recently sexually active women (reported sex in the past 12 months).
Fig. 2.
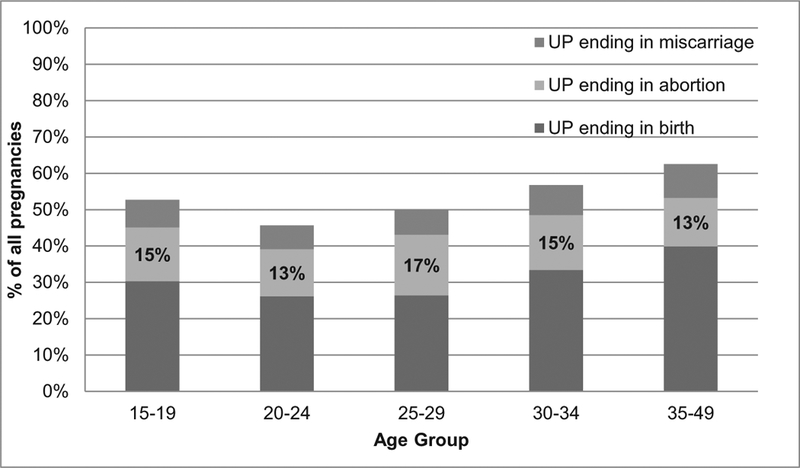
Percentage of all pregnancies in Uganda that are unintended by age group and outcome, 2013. Notes: UP = unintended pregnancy.
3.2. Adolescents’ abortion care experiences
Compared to nonadolescent PAC patients, adolescent PAC patients are more likely to be unmarried (81.1% vs. 46.1%, p<.001) and have secondary education (42.9% vs. 31.2%, p<.004) (Table 2). Adolescents are more likely to have had no prior pregnancies (62.0% vs. 11.0%, p<.000), but those who have are more likely to report a prior abortion than nonadolescents (25.0% vs. 12.1%, p<.03). Adolescents are also less likely to have been using contraception at the time of their pregnancy(24.5% vs. 36.9%, p<.02). Adolescents were more likely to report that they interfered with their pregnancy than nonadolescents (54.3% vs.30.4%, p<.002), but the majority of both adolescent and nonadolescent PAC patients who reported pregnancy interference indicated that the person who helped interfere with the pregnancy was not a medical professional.
Table 2.
Demographic and reproductive characteristics of adolescent and nonadolescent postabortion care patients, Uganda 2013
| Adolescents (N=351)c |
Nonadolescents (N=1807)c |
||||
|---|---|---|---|---|---|
| N | % | N | % | p value | |
| Marital status | .001 | ||||
| In union | 84 | 18.9 | 1034 | 53.9 | |
| Not in union | 266 | 81.1 | 770 | 46.1 | |
| Pregnancy intention | .02 | ||||
| Unintended | 215 | 71.7 | 887 | 54.9 | |
| Intended | 115 | 28.3 | 860 | 45.1 | |
| Gestation (reported by provider) | .35 | ||||
| Second trimester or later | 109 | 25.8 | 474 | 22.7 | |
| First trimester | 242 | 74.2 | 1333 | 77.3 | |
| Who helped interfere with the pregnancya | .07 | ||||
| Medical professional | 60 | 30.2 | 186 | 43.1 | |
| Other | 105 | 69.8 | 260 | 56.9 | |
|
Delay in reaching health facility (mean number of days) |
2.5 | 2.1 | .17 | ||
| Severity of complications | .12 | ||||
| Mild/moderate | 269 | 77.0 | 1451 | 81.6 | |
| Severe | 82 | 23.0 | 356 | 18.4 | |
| Received postabortion family planning | .12 | ||||
| Received modern contraceptive method | 127 | 47.4 | 595 | 40.4 | |
| No modern contraceptive method received | 209 | 52.6 | 1151 | 59.7 | |
| Residence | .67 | ||||
| Urban | 105 | 31.7 | 575 | 29.9 | |
| Rural | 246 | 68.3 | 1217 | 70.2 | |
| Highest level of education | .004 | ||||
| No education | 9 | 3.8 | 173 | 7.8 | |
| Some or completed primary | 180 | 47.8 | 770 | 45.3 | |
| Some or completed secondary | 143 | 42.9 | 528 | 31.2 | |
| Higher than secondary | 17 | 5.6 | 332 | 15.7 | |
| Had previous pregnancy | .000 | ||||
| Yes | 135 | 38.0 | 1573 | 89.0 | |
| No | 215 | 62.0 | 214 | 11.0 | |
| Using contraceptive method at time of current pregnancy | .02 | ||||
| Yes | 70 | 24.5 | 637 | 36.9 | |
| No | 281 | 75.5 | 1148 | 63.1 | |
| Interfered with pregnancy | .002 | ||||
| Yes | 169 | 54.3 | 450 | 30.4 | |
| No | 179 | 45.7 | 1342 | 69.6 | |
| Had previous abortionb | .03 | ||||
| Yes | 33 | 25.0 | 170 | 12.1 | |
| No | 102 | 75.0 | 1403 | 87.9 | |
| Total | 135 | 100.0 | 1573 | 100.0 | |
Among women who reported interference with the pregnancy.
Among women who reported a previous pregnancy.
Marital status was missing for 1 adolescent and 3 nonadolescents; pregnancy intention was missing for 20 adolescents and 60 nonadolescents; who helped interfere with pregnancy was missing for 4 adolescents and 4 nonadolescents; receipt of postabortion family planning was missing for 15 adolescents and 61 nonadolescents; residence was missing for 15 nonadolescents; educational attainment was missing for 2 adolescents and 4 nonadolescents; previous pregnancy was missing for 1 adolescent and 20 nonadolescents; using contraceptive method at time of current pregnancy was missing for 22 nonadolescents; interfered with pregnancy was missing for 3 adolescents and 15 nonadolescents.
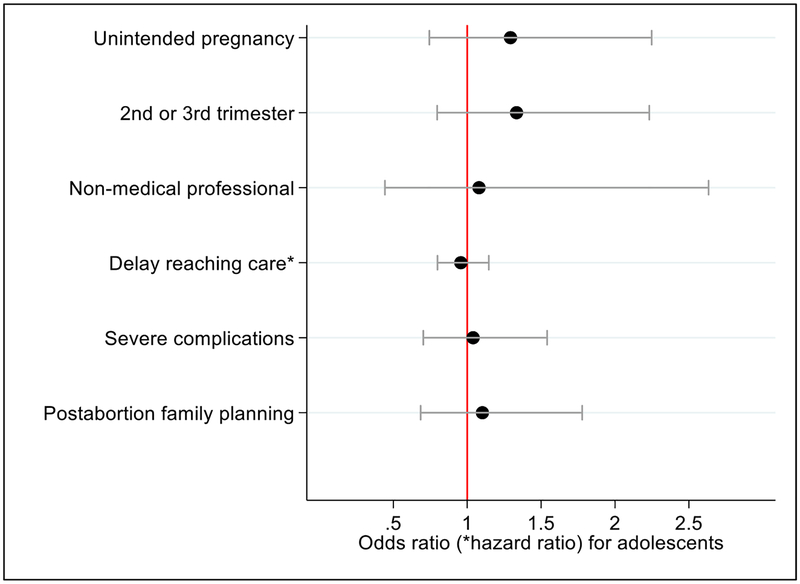
After controlling for differences in demographic and reproductive characteristics, we find that adolescents are no more likely than nonadolescent PAC patients to experience an unintended pregnancy, be at a later gestation, have a nonmedical professional help them interfere with their pregnancy, experience severe complications or receive postabortion contraception; the two groups also experience similar delays in reaching care (Fig. 3).
Fig. 3.
Odds ratios, hazard ratios and 95% confidence intervals of abortion care experiences among adolescent postabortion care patients as compared to nonadolescent postabortion care patients in Uganda, 2013. Notes: Dependent variables for each model were (1) reporting their pregnancy as unintended, (2) being past the first trimester, (3) reporting someone other than a medical professional helped them interfere with their pregnancy, (4) delays in reaching care, (5) having severe abortion complications and (6) receiving a modern postabortion family planning method (see Table 1). All models controlled for urban residence, highest level of education attained and previous pregnancy history. For all models other than unintended pregnancy, whether the pregnancy was reported as unintended was included as a control. Severe abortion complications were defined as women who received a blood transfusion, had surgery, had a septic abortion or died. Relying on someone other than a medical professional to help interfere with the pregnancy was only reported by the 611 women who self-reported that they interfered with their pregnancy and reported on who helped them. The postabortion family planning coefficient is not sensitive to the exclusion of condoms from the list of modern methods. Reference group is nonadolescent women.
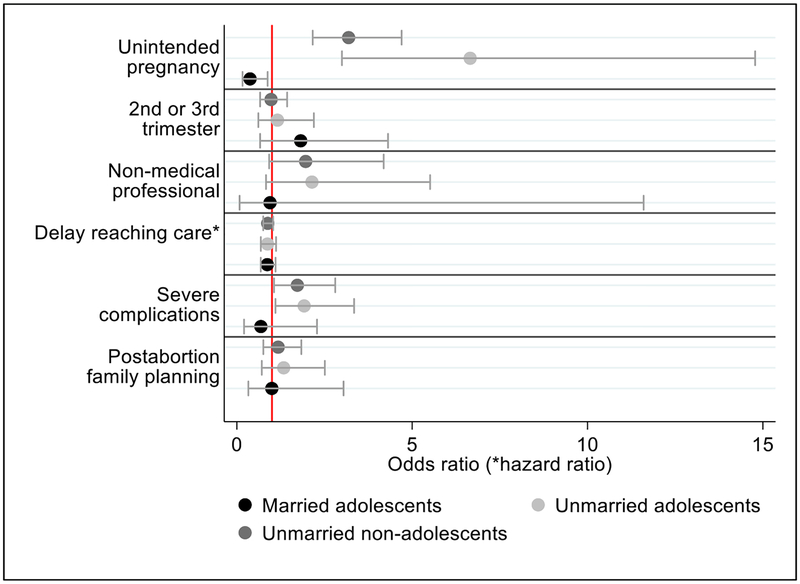
Upon disaggregating results by marital status, we find no differences in the odds of being past the first trimester, having someone other than a nonmedical professional help them interfere with the pregnancy, in the number of days to reach care or in receiving postabortion family planning for married adolescents, unmarried adolescents and unmarried nonadolescents when compared to married nonadolescents (Fig. 4). However, unmarried adolescents and unmarried nonadolescents do have significantly higher odds of reporting the current pregnancy as unintended (OR 6.7; 95% CI: 3.0–14.8 and 3.2; 95% CI: 2.2–4.7, respectively) and 90% and 70% higher odds of having severe complications as compared to married nonadolescents (OR 1.9; 95% CI: 1.1–3.3 and 1.7; 95% CI: 1.1–2.8, respectively).
Fig. 4.
Odds ratios, hazard ratios and 95% confidence intervals of abortion care experiences among unmarried adolescent, married adolescent and unmarried nonadolescent postabortion care patients compared to married nonadolescent postabortion care patients in Uganda, 2013. Note: Dependent variables for each model were (1) reporting their pregnancy as unintended, (2) being past the first trimester, (3) reporting someone other than a medical professional helped them interfere with their pregnancy, (4) delays in reaching care, (5) having severe abortion complications and (6) receiving a modern postabortion family planning method (see Table 1). All models controlled for urban residence, highest level of education attained and previous pregnancy history. For all models other than unintended pregnancy, whether the pregnancy was reported as unintended was included as a control. Severe abortion complications were defined as women who received a blood transfusion, had surgery, had a septic abortion, or died. Relying on a nonmedical professional to help interfere with the pregnancy was only reported by the 611 women who self-reported that they interfered with their pregnancy and reported on who helped them. Reference group is married nonadolescent women.
4. Discussion
This paper provides the first estimate of the adolescent abortion rate in Uganda, finding that adolescents have the lowest abortion rate among women less than 35 years of age but the highest abortion rate among recently sexually active women. However, the proportion of unintended pregnancies that ends in abortion among adolescents is similar to that of other age groups. Together, these findings suggest that adolescent abortions are just as likely as nonadolescent abortions, and that the underlying factor driving the higher abortion rate among recently sexually active adolescents is their risk of unintended pregnancy.
We do not find evidence that adolescent PAC patients face greater disadvantages in their abortion experiences than older women: we find no differences between adolescent and nonadolescent PAC patients in their likelihood of having an unintended pregnancy, presenting for care at later gestations, having more severe complications, receiving contraceptive methods from their PAC providers, experiencing delays to care or having someone other than a medical professional help them interfere with their pregnancy. One group, however, is more likely to experience severe complications from unsafe abortion: unmarried women. Previous studies have found similar results, with a higher risk of severe complications among unmarried PAC patients documented in Zimbabwe [39] and Malawi [40]. This increased risk of more severe complications suggests that being unmarried, rather than just age, is associated with unsafe abortion.
This study has a few important limitations. First, women with abortion complications may differ from all women who have induced abortions; this study captures only women presenting in health facilities with abortion complications. Second, we do not have age-specific information on the multiplier. The multiplier is influenced by the likelihood of complications and women’s access to treatment, both of which could vary by age. This potential variance could bias the estimated abortion rate, though the direction of that bias cannot be determined without further information on complications and treatment access by age. Third, we estimated live births by age group under an assumption of a constant rate of change in age-specific fertility rates between 2011 and 2016. However, contraceptive prevalence rates changed differently for married adolescents (increased) and unmarried sexually active adolescents (decreased) during this period [2,33]. In the absence of annualized data on fertility rates, our analysis is limited by this approximation of how fertility rates changed between 2011 and 2013. Finally, our estimates of whether someone other than a medical professional helped women interfere with their pregnancies are based only on women who self-reported pregnancy interference, which are likely a select group of women who do not necessarily represent all PAC patients, let alone all women having abortions.
Though adolescents and nonadolescents have similar experiences of abortion complications, adolescent-specific needs should not be ignored in Uganda’s sexual and reproductive health policies. More effective adolescent sexual and reproductive health policies and programs are critically needed, including explicit guidance on both in-school and out-of-school adolescents’ need for and right to comprehensive sexuality education and family planning [41]. The development of guidelines must be accompanied by effective, widespread implementation and dissemination that reaches adolescents.
Preventing unintended pregnancy and reducing unsafe abortion and abortion-related complications are important for all women, and particularly unmarried women, regardless of age. We must move beyond treating adolescents as a homogenous group and understand how marriage and other social factors, rather than just age alone, may shape sexual and reproductive health experiences and outcomes. This information can be used to ensure that adolescent sexual and reproductive health programs are meeting the diverse needs of adolescents.
Acknowledgments
We would like to thank Elena Prada, Christopher Garimoi Orach and Simon Kasasa, who were members of the study team that collected the data. We would also like to thank all of the data collectors and supervisors who worked to gather these data and to the respondents who gave their time. We are also grateful to Ann Biddlecom of the Guttmacher Institute for her feedback. We are thankful to our colleagues at the Population Association of America annual meeting in Washington, DC, in April 2016 and the International Population Conference in Cape Town, South Africa, in October 2017 for their feedback on the presentations of preliminary results and in particular Shireen Jejeebhoy, who discussed our paper.
Funding:
This study was made possible by the Swedish International Development Cooperation Agency, Sweden, the Dutch Ministry of Foreign Affairs, Netherlands and UK Aid, United Kingdom from the UK Government. Additional support was provided by the Guttmacher Center for Population Research Innovation and Dissemination (National Institutes of Health, United States grant 5 R24 HD074034). The findings and conclusions contained in the study are those of the authors and do not necessarily reflect the positions and policies of the donors.
Footnotes
Appendix A. Mathematical appendix
Supplementary data to this article can be found online at https://doi/org/10.1016/j.contraception.2018.07.135.
References
- [1].Prada E, Atuyambe LM, Blades NM, Bukenya JN, Orach CG, Bankole A. Incidence of induced abortion in Uganda, 2013: new estimates since 2003. PLoS ONE 2016;11: e0165812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Uganda Bureau of Statistics (UBOS), ICF. Uganda demographic and health survey 2016. Kampala, Uganda and Rockville, Maryland, USA: UBOS and ICF; 2018. [Google Scholar]
- [3].Kaye DK, Mirembe F, Bantebya G, Johansson A, Ekstrom AM. Reasons, methods used and decision-making for pregnancy termination among adolescents and older women in Mulago hospital, Uganda. East Afr Med J 2005;82:579–85. [DOI] [PubMed] [Google Scholar]
- [4].Olukoya AA, Kaya A, Ferguson BJ, AbouZahr C. Unsafe abortion in adolescents. Int J Gynaecol Obstet 2001;75:137–47. [DOI] [PubMed] [Google Scholar]
- [5].Reproductive Health Division, Department of Community Health, Ministry of Health, Republic of Uganda. The national policy guidelines and service standards for sexual and reproductive health and rights. Kampala, Uganda: Ministry of Health, Uganda; 2006. [Google Scholar]
- [6].Mulumba M, Kiggundu C, Nassimbwa J, Nakibuuka NM. Access to safe abortion in Uganda: leveraging opportunities through the harm reduction model. Int J Gynaecol Obstet 2017;138:231–6. [DOI] [PubMed] [Google Scholar]
- [7].Sedgh G, Bearak J, Singh S, Bankole A, Popinchalk A, Ganatra B, et al. Abortion incidence between 1990 and 2014: global, regional, and subregional levels and trends. Lancet 2016;388:258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mbonye AK. Abortion in Uganda: magnitude and implications. Afr J Reprod Health 2000;4:104–8. [Google Scholar]
- [9].Paluku JL, Kalisoke S, Julius W, Kiondo P. Knowledge and attitudes about induced abortions among female youths attending Naguru Teenage Information and Health Centre, Kampala, Uganda. J Public Health Epidemiol 2013;5: 178–85. [Google Scholar]
- [10].Prada E, Mirembe F, Ahmed FH, Nalwadda R, Kiggundu C. Abortion and postabortion care in Uganda: a report from health care professionals and health facilities. The Alan Guttmacher Institute: New York, NY; 2005. [Google Scholar]
- [11].Ganatra B, Gerdts C, Rossier C, Johnson BR, Tunçalp Ö, Assifi A, et al. Global, regional, and subregional classification of abortions by safety, 2010–14: estimates from a Bayesian hierarchical model. Lancet 2017;390:2372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mbonye AK, Asimwe JB, Kabarangira J, Nanda G, Orinda V. Emergency obstetric care as the priority intervention to reduce maternal mortality in Uganda. Int J Gynaecol Obstet 2007;96:220–5. [DOI] [PubMed] [Google Scholar]
- [13].Ministry of Health, Republic of Uganda. Investment case for reproductive, maternal, newborn, child and adolescent health sharpened plan for Uganda: 2016/17–2019/20; 2016.
- [14].Woog V, Singh S, Browne A, Philbin J. Adolescent women’s need for and use of sexual and reproductive health services in developing countries. New York, NY: Guttmacher Institute; 2015. [Google Scholar]
- [15].Kalyanwala S, Jejeebhoy SJ, Francis Zavier AJ, Kumar R. Experiences of unmarried young abortion-seekers in Bihar and Jharkhand, India. Cult Health Sex 2012;14: 241–55. [DOI] [PubMed] [Google Scholar]
- [16].Sowmini CV. Delay in termination of pregnancy among unmarried adolescents and young women attending a tertiary hospital abortion clinic in Trivandrum, Kerala, India. Reprod Health Matters 2013;21:243–50. [DOI] [PubMed] [Google Scholar]
- [17].Munasinghe S, van den Broek N. Abortions in adolescents. Trop Doct 2005;35:133–6. [DOI] [PubMed] [Google Scholar]
- [18].Mutua MM, Maina BW, Achia TO, Izugbara CO. Factors associated with delays in seeking post abortion care among women in Kenya. BMC Pregnancy Childbirth 2015;15(241):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Atuyambe LM, Kibira SPS, Bukenya J, Muhumuza C, Apolot RR, Mulogo E. Understanding sexual and reproductive health needs of adolescents: evidence from a formative evaluation in Wakiso district, Uganda. Reprod Health 2015;12(35):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Grimes DA, Benson J, Singh S, Romero M, Ganatra B, Okonofua FE, et al. Unsafe abortion: the preventable pandemic. Lancet 2006;368:1908–19. [DOI] [PubMed] [Google Scholar]
- [21].Olukoya P Reducing maternal mortality from unsafe abortion among adolescents in Africa. Afr J Reprod Health 2004;8:57–62. [PubMed] [Google Scholar]
- [22].Sundaram A, Juarez F, Bankole A, Singh S. Factors associated with abortion-seeking and obtaining a safe abortion in Ghana. Stud Fam Plann 2012;43:273–86. [DOI] [PubMed] [Google Scholar]
- [23].Levandowski BA, Pearson E, Lunguzi J, Katengeza HR. Reproductive health characteristics of young Malawian women seeking post-abortion care. Afr J Reprod Health 2012;16:253–62. [PubMed] [Google Scholar]
- [24].Varga CA. Pregnancy termination among South African adolescents. Stud Fam Plann 2002;33:283–98. [DOI] [PubMed] [Google Scholar]
- [25].Neal S, Mahendra S, Bose K, Camacho AV, Mathai M, Nove A, et al. The causes of maternal mortality in adolescents in low and middle income countries: a systematic review of the literature. BMC Pregnancy Childbirth 2016;16(352):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ziraba AK, Izugbara C, Levandowski BA, Gebreselassie H, Mutua M, Mohamed SF, et al. Unsafe abortion in Kenya: a cross-sectional study of abortion complication severity and associated factors. BMC Pregnancy Childbirth 2015;15(34):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chae S, Desai S, Crowell M, Sedgh G, Singh S. Characteristics of women obtaining induced abortions in selected low- and middle-income countries. PLoS ONE 2017;12: e0172976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sully E, Dibaba Y, Fetters T, Blades N, Bankole A. Playing it safe: legal and clandestine abortions among adolescents in Ethiopia. J Adolesc Health 2018;62:729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chae S Timing of orphanhood, early sexual debut, and early marriage in four sub-Saharan African countries. Stud Fam Plann 2013;44:123–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Council Population. The adolescent experience in-depth: using data to identify and reach the most vulnerable young people: Uganda 2006; 2009. [New York: ]. [Google Scholar]
- [31].Palermo T, Peterman A. Are female orphans at risk for early marriage, early sexual debut, and teen pregnancy? Evidence from sub-Saharan Africa. Stud Fam Plann 2009;40:101–12. [DOI] [PubMed] [Google Scholar]
- [32].Maly C, McClendon KA, Baumgartner JN, Nakyanjo N, Ddaaki WG, Serwadda D, et al. Perceptions of adolescent pregnancy among teenage girls in Rakai, Uganda. Glob Qual Nurs Res 2017;4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Uganda Bureau of Statistics (UBOS), ICF International Inc. Uganda demographic and health survey 2011; 2012. [Kampala, Uganda and Calverton, Maryland: ]. [Google Scholar]
- [34].United Nations, Department of Economic and Social Affairs, Population Division. World population prospects: the 2015 revision, Key Findings and Advance Tables; 2015.
- [35].Harlap S, Shiono PH, Ramcharan S. A life table of spontaneous abortions and the effects of age, parity and other variables. In: Porter I, Hook E, editors. Human embryonic and fetal death. New York: Academic Press; 1980. p. 145–58. [Google Scholar]
- [36].Leridon H Human fertility: the basic component. Chicago: University of Chicago Press; 1977. [Google Scholar]
- [37].Singh S, Prada E, Juarez F. The abortion incidence complications method: a quantitative technique Methodologies for estimating abortion incidence and abortion-related morbidity: a review. New York, NY: Guttmacher Institute; 2010. p. 71–98. [Google Scholar]
- [38].Sedgh G, Singh S, Shah IH, Ahman E, Henshaw SK, Bankole A. Induced abortion: incidence and trends worldwide from 1995 to 2008. Lancet 2012;379:625–32. [DOI] [PubMed] [Google Scholar]
- [39].Madziyire MG, Polis CB, Riley T, Sully EA, Owolabi O, Chipato T. Severity and management of postabortion complications among women in Zimbabwe, 2016: a cross-sectional study. BMJ Open 2018;8:e019658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kalilani-Phiri L, Gebreselassie H, Levandowski BA, Kuchingale E, Kachale F, Kangaude G. The severity of abortion complications in Malawi. Int J Gynaecol Obstet 2015;128: 160–4. [DOI] [PubMed] [Google Scholar]
- [41].Reproductive Health Division, Department of Community Health, Ministry of Health, Republic of Uganda. Adolescent health policy guidelines and service standards. Kampala, Uganda: Ministry of Health, Uganda; 2012. [Google Scholar]