Abstract
The third-generation aromatase inhibitors (AIs), anastrozole, letrozole and exemestane, are highly effective for the treatment of estrogen receptor-positive breast cancer in postmenopausal women. AIs inhibit the aromatase (CYP19A1)-mediated production of estrogens. Most patients taking AIs achieve undetectable blood estrogen concentrations resulting in drug efficacy with tolerable side effects. However, some patients have suboptimal outcomes, which may be due, in part, to inherited germline genetic variants. This review summarizes published germline genetic associations with AI treatment outcomes including systemic AI concentrations, estrogenic response to AIs, AI treatment efficacy and AI treatment toxicities. Significant associations are highlighted with commentary about prioritization for future validation to identify pharmacogenetic predictors of AI treatment outcomes that can be used to inform personalized treatment decisions in patients with estrogen receptor-positive breast cancer.
Keywords: : aromatase inhibitors, efficacy, estrogens, GWAS, pharmacogenetics, pharmacogenomics, pharmacokinetics, review, toxicity
The third-generation aromatase inhibitors (AIs), anastrozole, letrozole and exemestane, are highly effective for the treatment of estrogen receptor-positive (ER+) breast cancer in postmenopausal patients [1] and in combination with ovarian suppression in premenopausal patients [2]. AIs work by inhibiting aromatase-mediated systemic production of estrogens, namely estrone (E1) and estradiol (E2), from their androgenic precursors androstenedione and testosterone, respectively. Suppression of circulating estrogen concentrations deprives ER+ breast cancer cells of their requisite growth factor. Most, but not all, patients on AIs achieve undetectable systemic estrogen concentrations, undergo efficacious treatment and experience tolerable side effects. However, some patients experience disease recurrence and/or bothersome toxicities, which may be due in part to germline genetic variation. The ability to predict which patients will experience suboptimal outcomes would inform optimal AI treatment decisions. This review summarizes the current state of knowledge regarding germline genetic predictors of AI pharmacokinetics and of AI treatment response including estrogenic, efficacious and toxic responses to the therapy (Figure 1). The review was conducted by searching Pubmed for combinations of relevant search terms (i.e., ‘aromatase inhibitor’, ‘anastrozole’, ‘letrozole’, ‘exemestane’, ‘pharmacogenetic’, ‘pharmacogenomics’, ‘pharmacokinetic’, among others) and searching references within the identified studies during the period 14 October 2016 to 21 December 2016. Each section highlights reported pharmacogenetic associations and attempted replications, and provides commentary on priorities for future replication and validation. Directing research toward these priorities will accelerate the translation of AI pharmacogenetics into clinical practice to improve therapeutic outcomes in patients with ER+ breast cancer.
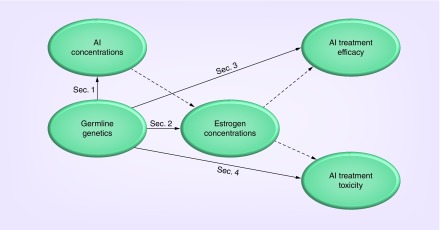
Figure 1. . Overview of review article.
Each section of the main text summarizes the literature regarding the influence of germline genetic variation on: systemic aromatase inhibitor concentrations (Sec. 1), systemic estrogen concentrations (Sec. 2), aromatase inhibitor treatment efficacy (Sec. 3) and toxicity (Sec. 4). Polymorphisms that should be prioritized for analysis are highlighted throughout. Earlier sections on endophenotypes (drug and estrogen concentrations) also include summaries regarding their putative effect on downstream clinical phenotypes (efficacy and toxicity), as denoted by dashed lines.
AI: Aromatase inhibitor; Sec: Section.
Genetic predictors of systemic AI concentrations
The AIs are structurally and pharmacologically distinct and different enzymes are involved in their metabolism (Figure 2). Many of these enzymes exhibit highly variable activity that is partially dictated by inherited genetic polymorphisms. This section summarizes the enzymatic metabolism of each AI and the studies that demonstrate differential pharmacokinetics for polymorphic gene variants. Commentary regarding the potential clinical implications of AI pharmacokinetic variability is provided in the last paragraph of the section.
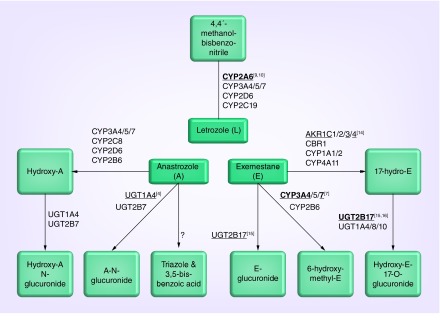
Figure 2. . Pharmacogenetics of aromatase inhibitor metabolism.
The three aromatase inhibitors have distinct metabolic pathways. Letrozole is primarily metabolized by polymorphic CYP2A6 and there is confirmed in vivo evidence that CYP2A6 genetics effects letrozole systemic concentrations. Exemestane is metabolized by several CYP, UGT and AKR enzymes, some of which have been demonstrated in vitro or in vivo to effect metabolism. Anastrozole is primarily metabolized by CYP3A4/5 and UGT1A4, with a contribution from several other CYPs and UGTs. No clinical pharmacokinetic studies have been reported for anastrozole. Polymorphic genes that have in vitro or in vivo evidence of an effect on metabolism are denoted by underlining and bolding, respectively, with superscript references.
Evidence of an effect of polymorphisms in a given gene is denoted by an underline for in vitro studies and bolding for in vivo studies, with references in superscript.
Anastrozole
Pharmacogenetic analyses of anastrozole or its metabolite concentrations in patients receiving therapy have not been reported, however, in vitro and in vivo studies have identified candidate gene SNPs that may be associated with variable drug pharmacokinetics. In vitro studies using human liver microsomes (HLM) show that the metabolism of anastrozole to hydroxyanastrozole is primarily mediated by CYP3A4/5, with a minor contribution from other CYP enzymes including CYP2C8, CYP2D6 and CYP2B6 [3]. Anastrozole and hydroxyanastrozole both undergo glucuronidation, primarily by UGT1A4. There is a strong correlation between anastrozole glucuronidation and UGT1A4 expression using in vitro assays (r = 0.99; p < 0.0001). Furthermore, HLMs derived from carriers of UGT1A4 low-expression promoter SNPs (-163G>A [rs3732218], -217T>G [UGT1A4*1g, (no rsID assigned)], and -219C>T [rs3732219]) exhibit decreased rates of anastrozole glucuronidation [4]. Subsequent analyses suggest a possible involvement of the MRP2 ATP-binding cassette transporter. In human liver samples, the expression of MRP2 and the closely related MRP3, correlated with UGT1A4 expression and SNPs in ABCC2 (MRP2; 3972C > T [rs3740066], 2366C>T [rs56220353] and -24C>T [rs717620]) were associated with anastrozole glucuronidation [5]. It is unclear from these data whether this correlation is related to altered UGT1A4 activity and whether any of these in vitro findings translate to meaningful differences in anastrozole concentrations in patients.
Several findings suggest that there could be pharmacogenetic predictors of anastrozole concentrations. The parent compound and several metabolites, including hydroxyanastrozole and hydroxyanastrozole-glucuronide, are detectable in the circulation of patients taking the medication [3]. Co-administration of fulvestrant, a selective ER degrader approved for the treatment of ER+ breast cancer, is associated with an approximately 10% decrease in systemic anastrozole concentrations [6]. It has been proposed that this drug–drug interaction is due to induction of UGT1A4 expression by fulvestrant, suggesting that the previously described UGT1A4 low-activity SNPs may be associated with higher systemic anastrozole concentrations. Other SNPs that may be causally related to systemic anastrozole concentrations include CYP3A4*22 (rs35599367), CYP3A5*3 (rs776746) and CYP3A7*1C (rs45446698), all of which have been associated with systemic concentrations of other cancer drugs including the AI exemestane (discussed below) [7,8].
Letrozole
Although letrozole is pharmacologically similar to the nonsteroidal AI anastrozole, their routes of drug metabolism and elimination differ. In vitro studies have shown that the primary route of metabolism for letrozole is via the CYP2A6 enzyme, with a minor contribution from CYP3A4 and CYP3A5 [9,10]. The CYP2A6 gene is polymorphic with known variants including an inactive gene deletion (*4; rs3892097) and others resulting in diminished catalytic activity [11]. Variants in CYP2A6 explain a substantial proportion of the variability in circulating letrozole concentrations in patients in the USA [10] and in Japan [12]. Interestingly, a population pharmacokinetic model developed in a small cohort (n = 52) of Korean patients did not detect an association for CYP2A6*4 [13], perhaps due to the limited number of heterozygous carriers (n = 9). Despite in vitro evidence showing that CYP3A4 and CYP3A5 contribute to letrozole metabolism, no clinical pharmacogenetic analyses have reported significant associations with SNPs in these two genes.
Exemestane
The steroidal AI exemestane is structurally quite distinct from the other drugs within this class. Preclinical in vitro studies have shown that CYP3A4 is the primary enzyme responsible for the metabolism of exemestane to its primary metabolite 17-hydroxyexemestane, with minor contributions from CYP1A1 and CYP4A11 [14]. Further in vitro experiments confirmed metabolic contributions from aldo–keto reductase family 1 members (AKR1) C1, C2, C3 and C4, as well as CBR1, and reported differential exemestane binding and/or metabolic activity for SNPs within AKR1C3 and AKR1C4 [15]. Finally, exemestane and its metabolites 17-hydroxyexemestane and 17-dihydroexemestane are substrates for glucuronidation via UGT2B17. HLMs with the UGT2B17 deletion polymorphism (UGT2B17*2, [no rsID assigned]) exhibit dramatically lower exemestane glucuronidation activity [16].
Clinical pharmacogenetic studies have shown that patients carrying the low-activity CYP3A4*22 allele (rs35599367) exhibit higher steady-state exemestane concentrations [7]. In addition, patients carrying the CYP3A7*1C (rs45446698) promotor region variant, which allows for adult expression of the fetal CYP3A7 enzyme, exhibit significantly decreased circulating exemestane concentrations [hertz dl et al., unpublished observations]. Associations between SNPs in other candidate genes relevant to exemestane metabolism, including CYP1A1, CYP3A5, CYP4A11, AKR1C3 and AKR1C4, have not yet been detected. The SNPs in the AKR1C genes (described above) have low minor allele frequencies, which limits the power to detect possible associations with exemestance pharmacokinetics. Recently, a study in normal healthy volunteers found that patients homozygous for the UGT2B17*2 deletion have lower 17-hydroexemestane glucuronidation [17]. This SNP has not been tested for association with drug concentrations in breast cancer patients receiving exemestane treatment.
Effect of AI concentrations on estrogen response or treatment outcomes
Maintaining systemic drug concentrations within a ‘therapeutic range’ is critically important for some drugs, but it is unclear if this is true for AIs. Clinical studies have shown that circulating estrogen suppression is less robust when doses lower than the recommended daily dose are used [18,19]; however, dose escalation above recommended doses does not further enhance estrogen suppression [20]. Additionally, in the Arimidex, Tamoxifen Alone or in Combination trial a pharmacokinetic interaction between anastrozole and tamoxifen that caused a significant decrease in anastrozole concentrations had a negligible effect on E2 suppression [21]. Analyses of drug and estrogen concentrations from other patient cohorts are similarly equivocal. As an example, Ingle et al. did not detect an association between systemic anastrozole concentrations and suppression of E2, E1 or E1 conjugates [22]. In a follow-up expansion study, the subset (5–9%) of patients whose circulating estrogen concentrations did not decrease from baseline after 1–6 months of treatment, had lower median anastrozole concentrations [23]. However, similar preliminary analyses did not detect an association between exemestane or letrozole concentrations and E2 suppression in the Exemestane and Letrozole Pharmacogenetics (ELPh) study [hertz dl et al., unpublished observations]. Finally, a pharmacokinetic–pharmacodynamic modeling analysis of 12 healthy women who were administered a single dose of exemestane suggests that exemestane pharmacokinetic variability has a negligible effect on E1-sulfate (E1-S) concentrations [24].
There is limited evidence that a patient's systemic AI concentrations are associated with their treatment side effects or breast cancer disease outcomes. In the adjuvant ELPh trial, there were no associations found between exemestane or letrozole steady-state drug concentrations and patient-reported outcomes or treatment discontinuation [25]. In terms of treatment efficacy, direct associations with AI drug concentrations have not been reported and anastrozole dose escalation, above the recommended daily dose, has not been shown to provide additional clinical benefit in patients with metastatic breast cancer [26]. Overall, there is a lack of clear evidence that systemic AI concentrations are associated with clinically relevant treatment outcomes. Unless and until an association between AI concentrations and treatment outcomes is established, therapeutic monitoring of AI drug concentrations is not warranted and additional pharmacogenetic studies to identify predictors of AI concentrations are of limited value.
Genetic predictors of estrogen suppression in response to AI treatment
The third generation AIs all dramatically suppress systemic production of E1 and E2, resulting in undetectable steroid concentrations in the vast majority of patients [27,28] despite efforts to develop ultrasensitive analytical detection assays [29]. However, in a subset of patients, estrogen concentrations remain above the level of detection and may even increase while receiving AI treatment. The CYP19A1 aromatase enzyme is responsible for the conversion of aromatic C19 androgens to C18 estrogens including E1 and E2 [30]. It has been hypothesized that germline genetic variants in the CYP19A1 gene may cause resistance to this class of enzyme inhibitors. Highly penetrant, less common CYP19A1 variants may cause a subset of patients not to have a typical estrogenic response to AI treatment or many common polymorphisms may have a marginal effect on the robustness of the estrogenic suppression. As with all pharmacogenetic associations, discovery of polymorphisms with larger effects, if they in fact exist, is less challenging and more clinically useful. In this section, we review previous studies attempting to discover CYP19A1 genetic variants responsible for variability in patient's estrogenic response to aromatase inhibition. We then survey the evidence supporting the hypotheses that an optimal level of estrogen suppression is required for treatment efficacy and that the extent of suppression is the primary determinant of treatment side effects.
A number of studies have reported associations between SNPs in and around the CYP19A1 gene with steroid hormone levels in healthy individuals or cancer patients [31–34] and with the risk of developing cancer [35]. Relatively few studies have investigated whether CYP19A1 gene variants are responsible for the lack of estrogen suppression observed in a subset of AI-treated patients (Table 1). The first attempt, by Ma et al., involved sequencing of 240 DNA samples obtained from four separate ethnic groups included in the publicly available Coriell Cell Repository [36]. They identified 88 polymorphisms, of which four nonsynonymous SNPs (Trp39Arg [rs2236722], Thr201Met [rs28757184], Arg264Cys [rs700519] and Met364Thr [rs56658716]) were selected for further in vitro mechanistic characterization. It was found that the Arg264Cys and Met364Thr variants exhibit decreased aromatase activity and that the Met364Thr variant has decreased binding affinity for androstenedione and conversion to E1. However, none of the SNPs affected letrozole or exemestane binding. A subsequent clinical pharmacogenetic analysis was conducted in 45 AI-treated patients with E1, E2 and aromatase activity measured at baseline and during treatment and targeted CYP19A1 sequencing. Two intronic SNPs (rs6493497 and rs7176005) in high-linkage disequilibrium were associated with greater decreases in tumor aromatase activity, though the association appeared to be driven by greater aromatase activity at baseline. In a cohort of 200 anastrozole-treated patients, these same SNPs were associated with greater systemic E1 and E2 concentrations prior to treatment and on average 10–13-fold higher E2 concentrations measured during treatment in variant carriers when compared with wild-type patients [37]. While the findings in this validation study are somewhat inconsistent with the initial discovery, they do support further studies testing for associations between these SNPs and estrogen suppression in additional cohorts of AI-treated patients.
Table 1. . Significant associations for CYP19A1 germline genetic variants with aromatase activity or estrogen response in in vitro or clinical studies.
| Study type | rsID | Location or AA change | n | AI | Phenotypes | Significant associations | Ref. | Nonrep |
|---|---|---|---|---|---|---|---|---|
| Invitro | rs700519 | Arg264Cys | NA | L, E | Aromatase activity, protein expression and localization, androstenedione and AI-binding affinity | Decreased aromatase activity | [36] | |
| Invitro | rs56658716 | Met364Thr | NA | L, E | Aromatase activity, protein expression and localization, androstenedione and AI-binding affinity | Decreased aromatase activity and binding of androstendione and conversion to E1 | [36] | |
| In vitro | rs6493497 and rs7176005 | Intronic | NA | NA | Tumor CYP19A1 gene expression, DNA–protein binding | Higher CYP19 expression | [37] | |
| Clinical | rs6493497 and rs7176005 | Intronic | 45 | A, L, E | Tumor E2, E1 or aromatase activity at baseline and during treatment | Greater decrease in aromatase activity from AI treatment | [37] | |
| Clinical | rs6493497 and rs7176005 | Intronic | 200 | A | E1 and E2 at baseline and on treatment | Greater E1 and E2 at baseline, 10–13-fold greater E2 on treatment | [37] | |
| Clinical | rs10046 | 3′-UTR | 390 | A, L, E | E2 and E1 during treatment | Variant carriers had higher E1 | [38] | [39] |
| Clinical | rs727479 | Intronic | 390 | A, L, E | E2 and E1 during treatment | Variant carriers had higher E1 | [38] | [39] |
| Clinical | rs11575899 | Intronic | 390 | A, L, E | E2 and E1 during treatment | Homozygous variant patients had higher E1 | [38] | |
A: Anastrozole; AI: Aromatase inhibitor; E: Exemestane; E1: Estrone; E2: Estradiol; L: Letrozole; Nonrep: Reference for unsuccessful replication attempt; Ref.: Study reference.
Other analyses have focused on CYP19A1 candidate SNPs. In a cross-sectional analysis of 390 Caucasian patients currently on AI treatment, Mao et al. reported that patients carrying the rs10046 (3′-UTR), rs727479 (intronic) or rs11575899 (intronic) SNPs had higher on-treatment E1 concentrations but no differences in E2 [38]. In a similar study of 204 patients receiving letrozole, there were no differences in E1-S concentrations at baseline or after 6 weeks, 6 months or 1 year of treatment for carriers of SNPs in the 3′-UTR (rs10046 and rs4646) or intronic regions (rs727479 and rs749292) [39]. Inconsistent findings between the two studies may be due to the different metabolites measured. Finally, in a cohort of 101 patients, the synonymous Val80Val (rs700518) and 3′-UTR (rs4646) SNPs were not associated with E2 concentrations during AI treatment [40].
Currently, there is little direct evidence that AI efficacy is related to the magnitude of estrogen suppression, though the biological and pharmacological rationales are compelling. We are not aware of any studies showing that patients with detectable circulating estrogen concentrations during AI therapy have inferior treatment outcomes. Furthermore, although letrozole has superior estrogen suppression potency compared with anastrozole [41–43], the two agents are equally effective at preventing breast cancer disease recurrence [44]. Similarly, the consensus hypothesis is that AI toxicity, particularly musculoskeletal adverse events (MS-AEs), are directly due to estrogen suppression [45,46]. This hypothesis has been supported by indirect evidence including the increased risk of MS-AEs in patients who are younger or finished menstruation more recently [47,48]. To date there has been one study published that found lower estrogen concentrations in patients who experienced AI-related MS-AEs [49]. Several other analyses have failed to validate associations between changes in, or on-treatment, estrogen concentrations and patient-reported arthralgias [50], quality of life or musculoskeletal symptoms [25], or pain sensitivity [51]. Last, side-effect improvement has been reported by switching patients to a different AI, suggesting that estrogen suppression may not be the predominant cause of treatment-related toxicities [52,53].
Genetic predictors of AI treatment efficacy
CYP19A1
The vast majority of pharmacogenetic analyses of AI treatment efficacy have focused on candidate SNPs in and around CYP19A1 (Table 2). Many of these analyses were conducted in small patient cohorts with heterogeneous clinical, disease and treatment attributes, often analyzing multiple SNPs, end points and genetic models without appropriate statistical correction for conducting multiple comparisons, making them highly susceptible to false-positives, as is typical with discovery-phase pharmacogenetics. Fortunately, several larger studies have attempted to validate previously discovered positive associations, including two well-designed analyses of approximately 1000 patients treated with single-agent letrozole on the BIG 1–98 study [54,55].
Table 2. . Significant associations for germline genetic variants with aromatase inhibitor treatment efficacy.
| Gene | rsID | Location or AA change | n | AI | Phenotypes | Significant associations | Ref. | Nonrep |
|---|---|---|---|---|---|---|---|---|
| CYP19A1 | rs4646 | 3′-UTR | 95 | L | Clinical response, PFS | Variant carriers were more likely to be nonresponders and had a nonsignificant trend toward worse PFS | [56] | [55,57–59] |
| CYP19A1 | rs4646 | 3′-UTR | 66 | L | Clinical response, clinical benefit, TTP, OS | Variant carriers had superior TTP, clinical response and clinical benefit | [60] | |
| CYP19A1 | rs4646 | 3′-UTR | 272 | A | Clinical response, TTP, OS | Variant carriers had superior best response, clinical benefit, TTP and OS | [61] | |
| CYP19A1 | rs10046 | 3′-UTR | 22 | A, L, E | Clinical response, OS, DFS, TTP | Variant carriers had superior OS | [57] | [55–56,59–61] |
| CYP19A1 | rs10046 | 3′-UTR | 308 | A, L, E | TTF | Variant carriers had trend toward worse TTF | [58] | |
| CYP19A1 | rs700518 | Val80Val synonymous | 109 | L | Clinical response, clinical benefit, TTP | Homozygous patients had superior clinical benefit | [62] | [55,57] |
| CYP19A1 | rs4775936 | Intronic | 308 | A, L, E | TTF | Minor allele associated with prolonged TTF | [58] | |
| CYP19A1 | rs4775936 | Intronic | 109 | L | Clinical response, clinical benefit, TTP | Homozygous patients had superior clinical benefit | [62] | |
| CYP19A1 | rs10459592 | Intronic | 109 | L | Clinical response, clinical benefit, TTP | Homozygous patients had superior clinical benefit | [62] | [58] |
| CYP19A1 | rs60271534 (TTTA)n | Intronic | 308 | A, L, E | TTF | (TTTA)7 associated with prolonged TTF | [58] | |
| IL6 | rs1800796 | Intronic | 308 | A, L, E | TTF | Variant had trend toward inferior TTF | [58] | |
| ESR1 | rs9340799 (XbaI) | Intronic | 989 (3401) | L (L/T) | BCFI, DRFI | Variant carriers had reduced risk of breast cancer event in the overall study but not L arm | [54] | |
| ESR2 | rs2077647 | Synonymous | 989 (3401) | L (L/T) | BCFI, DRFI | Variant carriers had reduced distant recurrence primarily in the L arm | [54] | |
| CYP1A2 | rs762551 | *1F | 24 (201) | A, L, E | Time to breast cancer event, distant metastasis | Variant carriers had higher risk of breast cancer events within 5 years and early distant metastases | [59] | |
| AhR | rs2066853 | Arg554Lys | 24 (201) | A, L, E | Time to breast cancer event, distant metastasis | Variant carriers had higher risk for early events | [59] | |
| MIR2052HG | rs13260300 | 5′-upstream | 4658† | A, E | BCFI | SNP associated with BCFI, MIR2052HG associated with ERα expression | [63] | |
†Genome-wide association study.
A: Anastrozole; AhR: Aryl hydrocarbon receptor; AI: Aromatase inhibitor; BCFI: Breast cancer free interval; DFS: disease-free survival; DRFI: distant relapse free survival; E: Exemestane; L: Letrozole; Nonrep: Reference for unsuccessful replication attempt; NR: Not reported; OS: Overall survival; PFS: Progression-free survival; Ref.: Study reference; TTF: Time-to-treatment failure; TTP: Time to progression.
The most frequently studied CYP19A1 SNPs are two variants (rs4646 and rs10046) in the downstream 3′-UTR. As mentioned previously, rs10046, but not rs4646, was associated with E1 concentrations in AI-treated patients [38], but neither was associated with E1-S concentrations at baseline or during letrozole treatment [39]. Regardless of these equivocal findings, these SNPs have garnered much attention as potential markers of AI treatment efficacy. The rs4646 variant was associated with letrozole treatment efficacy in two small hypothesis-generating studies. Garcia-Cascado et al. (n = 95) reported that patients carrying the rs4646 variant were more likely to be nonresponders and had a nonsignificant trend toward worse progression-free survival [56]. Conversely, in the study of Colomer et al., (n = 66) patients who had metastatic breast cancer that carried the rs4646 variant and were treated with letrozole had longer time to progression, decreased hazard of progression and superior response and clinical benefit [60]. A larger study of 272 anastrozole-treated patients also reported superior tumor response, clinical benefit, time to progression and overall survival (OS) in carriers of rs4646 [61]. A ‘meta-analysis’ of the two studies that reported an improvement in time to progression suggested this could be a true association [64]; however, this analysis did not include the patient cohort in which inferior progression free survival was detected. In addition, this meta-analysis was published prior to the pharmacogenetic analysis of BIG 1–98 that found no evidence of an association of this SNP with breast cancer-free interval in 1236 letrozole treated patients (hazard ratio [HR]: 1.00; 95% CI: 0.81–1.23) [55]. Similarly conflicting results have been reported for rs10046, which is in moderate linkage disequilibrium (r2 = 0.6–0.8) with rs4646. A small, hypothesis-generating study reported improved OS in patients carrying the variant allele who were treated with an AI [57]. Suggestive supportive evidence was provided by the larger study of Ferraldeschi et al., in which patients carrying the rs10046 variant had improved time to treatment failure (HR: 1.27; 95% CI: 1.07–1.51; p = 0.007). However, larger studies of anastrozole and letrozole found no evidence of association with treatment efficacy [56,60–61], including the analysis of BIG 1–98 [55].
Reports of other exonic and intronic CYP19A1 SNPs being associated with AI efficacy have been published. In one study, patients homozygous (wild-type or variant) for the synonymous rs700518 (Val80Val) SNP experienced superior clinical benefit of letrozole treatment compared with heterozygous patients [62] although no rationale for selecting an overdominant genetic model (heterozygous vs other) or biological hypothesis supporting it were offered. However, as discussed earlier, this SNP was not associated with E2 concentrations in AI-treated patients [40] and it was not associated with drug efficacy in BIG 1–98 (HR: 1.14; 95% CI: 0.81–1.62) [55]. Other exonic SNPs of interest include the two nonsynonymous variants discussed earlier: rs700519 (Arg264Cys) and rs28757184 (Thr201Met). In vitro mechanistic studies of the Arg264Cys variant found decreased rates of aromatization of androstenedione but neither variant was shown to affect AI binding to the CYP19 enzyme. Consistent with this finding, neither SNP was associated with AI-treatment efficacy in analyses of BIG 1–98 or other large cohorts of AI treated patients [55,58].
The CYP19A1 intronic regions have also been analyzed to identify genetic predictors of AI treatment efficacy. Ferraldeschi et al. reported that patients carrying the rs4775936 variant had prolonged time to AI treatment failure (HR: 0.79; 95% CI: 0.66–0.95; p = 0.012); however, this association was attenuated (HR: 0.95; 95% CI: 0.77–1.16; p = 0.62) when adjusted for relevant clinical covariates including the number of disease sites [58]. This association is partially consistent with results from Park et al., but again their use of an overdominant genetic model substantially complicates interpretation and cross-study comparison [62]. Other reported associations for intronic CYP19A1 variants with AI efficacy include a (TTTA)7 repeat in intron 4 (rs60271534) that has been associated with longer time to treatment failure [58]. Finally, Ghimenti et al. failed to detect an association between rs7176005, which was previously reported to be associated with a 10–13-fold greater circulating E2 concentrations during anastrozole treatment, and clinical response [65]. This negative finding may be due to the limited cohort size, which included only three patients carrying the variant allele. This variant remains a leading candidate for replication studies in patient cohorts with on-treatment E2 and clinical outcomes data.
Other
Several analyses have been conducted using pharmacogenetics panels or broader lists of candidate genes, to discover SNPs in genes other than CYP19A1 that may be associated with AI efficacy. An intronic SNP (rs1800796) in IL-6, identified in a hypothesis-generating screen of 32 SNPs from 17 genes, may be associated with shorter time to AI treatment failure [58]. Other, yet to be replicated hypothesis-generating trends from the BIG 1–98 analyses, include a SNP in ESR1 (rs9340799, XbaI) that has been intensively studied as a germline cancer susceptibility variant with inconsistent results [66]. This SNP was associated with reduced breast cancer event hazard in the combined analysis of the BIG 1–98 tamoxifen, letrozole and tamoxifen/letrozole arms (n = 3401), but the association was not detected in analysis of just the letrozole monotherapy arm (n = 845; HR: 1.03; 95% CI: 0.70–1.51) [54], suggesting that this SNP is more likely to be a marker of tamoxifen than AI efficacy. More intriguingly, a synonymous SNP (rs2077647) in the gene encoding for the ER-β (ESR2) was associated with reduced hazard of distant recurrence in the BIG 1–98 combined analysis (HR: 0.69; 95% CI: 0.53–0.90), with evidence that the effect was primarily seen in the letrozole monotherapy arm (HR: 0.58; 95% CI: 0.33–1.01) [54]. Recently, an analysis in a small discovery cohort (n = 24) of AI-treated patients using the DMET™ Pharmacogenetics Panel reported a higher risk of early breast cancer events for patients carrying the CYP1A2*1F (rs76255) variant [59]. In a larger expansion cohort (n = 201), carriers of rs76255 again had a higher risk of breast cancer events within 5 years and early distant metastases. This study also reported a hypothesis-generating association between a nonsynonymous SNP in the aryl hydrocarbon receptor (AhR) gene (rs2066853 and Arg554Lys) and increased risk for early breast cancer events. SNPs in other candidate genes, including FTO, 17-β-HSD and a variety of CYP, UGT and SULT enzymes, have been analyzed in individual studies but were not associated with AI treatment outcomes [58,61].
Recently, Ingle et al. published results from a genome-wide association study of breast cancer free interval in 4658 patients on the MA.27 clinical trial, which compared the efficacy and safety of anastrozole and exemestane [63]. Although none of the 7 million SNPs analyzed were significant at accepted genome-wide thresholds (p < 5 × 10-8), the most highly associated SNPs (e.g., rs13260300) were found in and around the coding region of a long noncoding RNA, MIR2052HG and a nearby estrogen response element. In silico analysis of The Cancer Genome Atlas found that MIR2052HG and tumor ESR1 gene expression levels are modestly correlated (r = 0.370). In vitro mechanistic studies suggest that MIR2052HG regulates ER protein stability and that ER expression increases in a SNP-dependent fashion in AI-treated lymphoblastoid cells. Based on these intriguing findings, the SNPs around MIR2052HG and/or the tumor expression of MIR2052HG may predict response to AI treatment. As more somatic breast cancer gene mutations predictive of AI response [67–69] are discovered, it will be critical to determine whether these germline SNPs contribute independent prognostic or predictive information.
Genetic predictors of AI treatment related toxicity
The most serious AI treatment emergent toxicities involve bone loss, which can lead to increased risk of fracture, whereas the most common patient-reported symptoms are musculoskeletal symptoms including arthralgias, myalgias and tendinopathies. Data from observational studies demonstrate that up to 50% of patients have new or worsened musculoskeletal symptoms, which are severe enough to lead to treatment discontinuation in 20–30% of patients [52]. In addition to these symptoms, patients also commonly report vasomotor symptoms and vaginal dryness. Most of these symptoms are believed to be directly related to estrogen depletion, although as noted above, this has been difficult to verify using on-treatment estrogen concentration data.
Musculoskeletal adverse events
Likely due to the theoretical mechanistic connection to estradiol concentrations, the bulk of the candidate pharmacogenetic analyses of MS-AEs have focused on CYP19A1 SNPs (Table 3). Carriers of the intronic (TTTA)8 repeat polymorphism (rs60271534) had decreased arthralgia risk in a large (n = 390) cohort of Caucasian patients treated with AIs [38]. However, no association between this intronic repeat and treatment discontinuation due to MS-AEs was identified in the ELPh cohort, and a trend in the opposite direction was seen in the letrozole arm (HR: 1.8; 95% CI: 0.96–3.3; p = 0.065) [70]. In the BIG 1–98 analysis of 2161 patients treated with letrozole or tamoxifen/letrozole, carriers of the synonymous rs700518 SNP (Val90Val) had increased risk of MS-AEs [55]. However, this effect seemed to be confined to the tamoxifen arm and was not replicated in a smaller study of letrozole-treated patients [62]. Garcia-Garalt et al. screened 31 candidate SNPs in CYP19A1 and several other genes relevant to estrogen or vitamin D in 334 Spanish AI-treated patients from whom arthralgia severity was collected at baseline and after 3 and 12 months of treatment [71]. Patients carrying the intronic rs4775936 CYP19A1 SNP reported greater worsening of arthralgia pain, but no association with bone/joint pain was reported in a smaller study of letrozole treated patients [62]. Finally, Fontein et al. reported that patients who are homozygous for rs934635, a tag SNP of a haplotype block of unknown functional consequence downstream of CYP19A1, have greater occurrence of MS-AEs [72] but this association also awaits replication in independent patient cohorts.
Table 3. . Significant associations with aromatase inhibitor treatment related toxicities and nonreplication.
| Toxicity | Gene | rsID | AA change | n | AI | Phenotypes | Significant associations | Ref. | Nonrep |
|---|---|---|---|---|---|---|---|---|---|
| MS-AE | CYP19A1 | rs60271534 (TTTA)n | Intronic | 390 | A, L, E | Arthralgia or arthralgia-related D/C | Carriers of (TTTA)8 at decreased risk of AIAA | [38] | [70] |
| MS-AE | CYP19A1 | rs700518 | Synonymous | 989 (3401) | L (L/T) | Time to MS-AE | Variant carriers at increased risk of MS-AEs | [55] | [62] |
| MS-AE | CYP19A1 | rs4775936 | Intronic | 334 | A, L, E | Change in arthralgia pain or arthralgia-related D/C | Variant associated with worsening pain | [71] | [62] |
| MS-AE | CYP19A1 | rs934635 | Downstream | 737 | E | MS-AEs | Homozygous variant patients had more MSAEs | [72] | |
| MS-AE | RANKL | rs7984870 | Intronic | 420 | A, L | MS-AEs | Variant associated with fewer MS-AEs and higher soluble RANKL | [49] | |
| MS-AE | OPG | rs2073618 | Asn3Lys | 420 | A, L | MS-AEs | Variant associated with increased risk of MS-AEs and lower OPG expression | [49] | |
| MS-AE | OPG | rs2073618 | Asn3Lys | 154 | A, L, E | MS-AEs and severity | Variant carriers had more MS-AEs and greater pain severity | [73] | |
| MS-AE | TCL1A | rs11849538 | Downstream | 878 | A, E | Cases: grade 3+ MS-AE or D/C due to MS-AE | Higher MAF in cases | [74] | [70] |
| MS-AE | CYP17A1 | rs6163 | Synonymous | 334 | A, L, E | Change in arthralgia pain or arthralgia-related D/C | Variant associated with greater increase in arthralgia | [71] | |
| MS-AE | CYP27B1 | rs4646536 | Intronic | 334 | A, L, E | Change in arthralgia pain or arthralgia-related D/C | Variant associated with greater arthralgia-related d/c | [71] | |
| MS-AE | VDR | rs11568820 | Upstream | 334 | A, L, E | Change in arthralgia pain or arthralgia-related D/C | Variant carriers had greater increase in arthralgia pain | [71] | |
| MS-AE | ESR1 | rs2234693 | Intronic | 436 | A, L | MS-AEs | Homozygous variant patients had fewer MS-AEs | [75] | |
| MS-AE | ESR1 | rs9340799 | Intronic | 436 | A, L | MS-AEs | Variant allele associated with fewer MS-AEs | [75] | |
| MS-AE | ESR1 | rs9322336 | Intronic | 432 | L, E | Time to treatment D/C for MS-AE | Homozygous variant patients have more MS-related D/C in E arm | [70] | |
| Bone AE | CYP19A1 | rs700518 | Synonymous Val80Val | 101 | A, L, E | Change in BMD at spine, hip and femur | AA genotype had greater reduction in BMD at lumbar spine and total hip | [40] | [55] |
| Bone AE | CYP19A1 | rs936308 | intronic | 989 (3401) | L (L/T) | Time to bone AE | Variant carriers had trend toward reduced risk of bone AE | [55] | |
| Bone AE | CYP19A1 | rs6493497 | Intronic | 351 | L, E | Change in bone markers (NTX or BAP) or BMD | Homozygous variant patients had greater decrease in BMD to both drugs and E | [76] | |
| Bone AE | Gene clusters† | NA | NA | 1071‡ | A, E | Cases: fragility fracture | Different allelic frequency in cases: controls, SNP-dependent E2-induced expression of OPG. | [77] | |
| Bone AE | HTR2A | rs3742278 | Intronic | 351 | L, E | Change in bone markers (NTX or BAP) or BMD | Homozygous variant patients have greater increase in BAP in L arm | [76] | |
| Bone AE | ESR1 | rs2813543 | Downstream | 351 | L, E | Change in bone markers (NTX or BAP) or BMD | Homozygous variant patients have greater decrease in BMD in E arm | [76] | |
| Bone AE | ESR1 | rs4870061 | Intronic | 351 | L, E | Change in bone markers (NTX or BAP) or BMD | Variant associated with greater decrease in BMD in both arms, homozygous variant patients have greater decrease in BMD in L/E and L arms | [76] | |
| Bone AE | ESR1 | rs9322335 | Intronic | 351 | L, E | Change in bone markers (NTX or BAP) or BMD | Homozygous variant patients have increased BMD in L/E and L arms | [76] | |
| Bone AE | ESR2 | rs10140457 | Intronic | 351 | L, E | Change in bone markers (NTX or BAP) or BMD | Homozygous variant patients have greater increase in BMD in L arm | [76] | |
| Bone AE | ESR2 | rs2077647 | Synonymous (Ser10Ser) | 989 (3401) | L (L/T) | Bone adverse events | Variant carriers have trend toward reduced risk of bone AE in L arm | [54] | |
| Bone AE | ESR2 | rs4986938 | 3′-UTR | 989 (3401) | L (L/T) | Bone adverse events | Variant carriers have trend toward increased risk of bone AE in L arm | [54] | |
| VMS | CYP19A1 | rs10046 | 3′-UTR | 2672 | E or Tam | Early onset hot flashes/sweating | Homozygous variant patients had reduced VMS, particularly in E arm | [78] | |
| VMS | CYP19A1 | rs16964189 | Downstream | 737 | E | Sensations of heat/night sweats | Homozygous variant patients had more VMSs | [72] | |
| VMS | CYP19A1 | rs7176005 | Upstream | 737 | E | Sensations of heat/night sweats | Homozygous variant patients had more VMSs | [72] | |
| VMS | CYP19A1 | rs934635 | Downstream | 737 | E | Sensations of heat/night sweats | Homozygous variant patients had more VMSs | [72] | |
| Body mass or lipids | CYP19A1 | rs700518 | Synonymous (Val80Val) | 82 | A, L, E | Change in body mass, truncal fat or bone mineral content | GG had greater increase in trunk fat mass, decrease in fat-free mass and lean mass | [79] | |
| Body mass or lipids | CYP19A1 | rs700518 | Synonymous (Val80Val) | 303 | L, E | Change in lipids | Variant homozygous patients without LAM had greater decrease in TG | [80] | |
| Body mass or lipids | CYP19A1 | rs1008805 | Intronic | 303 | L, E | Change in lipids | Variant carriers on LAM had greater decrease in HDL | [80] | |
| Body mass or lipids | CYP19A1 | rs1062033 | Intronic | 303 | L, E | Change in lipids | Variant associated with greater decrease in HDL on LAM | [80] | |
| Body mass or lipids | CYP19A1 | rs2289105 | Intronic | 303 | L, E | Change in lipids | Homozygous variant patients without LAM had greater decrease in TG, variant associated with greater decrease in HDL on LAM | [80] | |
| Body mass or lipids | CYP19A1 | rs3759811 | Intronic | 303 | L, E | Change in lipids | Homozygous variant patients without LAM had greater decrease in TG | [80] | |
| Body mass or lipids | CYP19A1 | rs4646 | 3′-UTR | 303 | L, E | Change in lipids | Variant associated with greater decrease in HDL without LAM | [80] | |
| Body mass or lipids | CYP19A1 | rs4775936 | Intronic | 303 | L, E | Change in lipids | Homozygous variant patients without LAM had greater decrease in TG | [80] | |
| Body mass or lipids | CYP19A1 | rs749292 | Intronic | 303 | L, E | Change in lipids | Variant associated with greater decrease in TG by several models without LAM, and greater decrease in HDL by several models with LAM | [80] | |
† THIL–CTSZ–SLMO2–ATP5E, TRAM2–TMEM14A, MAP4K4.
‡Genome-wide association study.
A: Anastrozole; AI: Aromatase inhibitor; D/C: Discontinued; E: Exemestane; L: Letrozole; LAM: Lipid-altering medication; MS-AE: Musculoskeletal adverse event; Nonrep: Reference for unsuccessful replication attempt; NR: Not reported; Ref.: Study reference.
The most intriguing findings thus far may come Wang et al., who analyzed several toxicity phenotypes including occurrence of any MS-AE and decreased bone mineral density (BMD) in a study of 420 AI-treated Chinese patients [49]. Patients who experienced MS-AEs (n = 208) had higher serum concentrations of the bone turnover markers carboxy terminal telopeptide, procollagen type I N-terminal propeptide and RANKL, and higher serum ratios of RANKL/OPG (all p < 0.05). RANKL/OPG ratio was inversely correlated with BMD, decreasing linearly in patients with osteoporosis, osteopenia and normal BMD (p < 0.05). The investigators genotyped 29 tag SNPs in the RANK, RANKL and OPG genes, which are involved in the regulation of bone remodeling and osteoclast activity [81]. Patients who carried the intronic RANKL SNP rs7984870 had substantially decreased risk of MS-AEs (39.4 vs 60.6%, p = 3.07 × 10-5) and higher soluble RANKL concentrations (p < 0.05). Conversely, patients who carried the missense Asn3Lys SNP (rs2073618) in OPG, also known as TNFRSF11B, had increased risk of MS-AEs (p = 6.09 × 10-4) and significantly lower OPG expression (p < 0.05). Associations between these MS-AE risk genotypes and serum concentrations of carboxy terminal telopeptide and procollagen type I N-terminal propeptide were also reported (all p < 1 × 10-5). Unlike many of the other discoveries described in this review, successful replications of these associations have been reported. Lintermans et al. recently published an analysis of 154 AI-treated patients who were enrolled prospectively to measure MS-AE symptoms at baseline and every 3 months for the first year of treatment [82]. Similar to the findings of Wang et al., patients who carried the rs2073618 OPG SNP had increased MS pain (p = 0.003) [73]. However, this finding is somewhat controversial as the occurrence of MS-AE was actually greatest in the heterozygous group (86%), and nearly equivalent between the wild-type (63%) and homozygous variant groups (62%). This SNP was also associated with greater pain severity (p = 0.018) and higher musculoskeletal symptoms (p = 0.008) in this replication study. These SNPs in OPG and RANKL should be prioritized for further replication in independent cohorts of AI-treated patients with systematic collection of MS and bone related toxicity data.
Other groups have detected associations with MS-AEs for SNPs in genes other than CYP19A1 using panels of gene candidates or genome-wide analyses. Ingle et al. analyzed 550,000+ SNPs for association with grade 3+ MS-AEs using a nested case (n = 293)–control (n = 585) study within the MA.27 clinical trial [74]. The top hits clustered on chromosome 14 (rs7158782: p = 3.48 × 10-6), but did not surpass genome-wide significance (p < 5 × 10-8). Subsequent imputation identified a candidate SNP (rs11849538, p = 6.67 × 10-7) for further evaluation. Functional in vitro experiments using immortalized lymphoblastoid cell lines suggest that TCL1A expression is increased by E2 treatment and that cells carrying the variant allele have greater induction of TCL1A expression than wild-type cells. Further in vitro work provides additional mechanistic explanation for this association [83,84]. However, the only clinical replication attempt reported a trend toward decreased toxicity-related AI discontinuation (HR: 0.6; 95% CI: 0.4–0.9; p = 0.007), which is in the direction opposite of the original finding [70]. Other uncorrected, hypothesis-generating associations with MS-AE for SNP in CYP17A1 (rs6163), CYP27B1 (rs4646536), VDR (rs11568820) [71] and ESR1 (rs2234693, rs9340799 and rs9322336) [70,75] await replication in independent cohorts of AI-treated patients.
Bone adverse events
Bone loss is also hypothesized to be mechanistically related to estrogen suppression [85,86], thus CYP19A1 is the candidate gene of choice for most pharmacogenetic analyses. Napoli et al. conducted a longitudinal prospective observational study in which BMD was measured at baseline and after 6 and 12 months of AI treatment in 169 patients. Women homozygous for the AA genotype of the synonymous (Val80Val) rs700518 CYP19A1 SNP had a greater reduction in BMD at the lumbar spine and hip at 1 year [40]. The A allele is the minor allele in Caucasian patients but the major allele in African–Americans, but analyses stratified by or adjusted for race were not reported. An association for this SNP with time to bone AEs was not detected in the BIG 1–98 analysis [55]. In BIG 1–98 there was evidence that carrying the intronic rs936308 CYP19A1 SNP reduced risk of experiencing a bone AE during letrozole treatment. In a hypothesis-generating screen of 159 SNPs in the ELPh trial (n = 351), patients homozygous for the intronic rs6493497 variant allele treated with exemestane (-2.90 vs -0.30, p = 1.6 × 10-9) or either AI (-2.90 vs -0.37, p = 4.6 × 19-8) had greater decreases in BMD T-score after 24 months of treatment. However, this association should be viewed with skepticism as no association with changes in bone turnover markers after 3 months was detected and the association with BMD is based on only one homozygous variant patient.
A nested case–(n = 231) control (n = 840) genome-wide association study of fragility fractures was performed within the MA.27 clinical trial [77]. The most strongly associated of the 7.5 million SNPs analyzed did not surpass genome-wide statistical significance (p < 5 × 10-8). A process for detecting ‘SNP signals’ was employed to generate three clusters of six E2 responsive genes (THIL–CTSZ–SLMO2–ATP5E, TRAM2–TMEM14A and MAP4K4) for in vitro functional studies. Lymphoblastoid cell lines that are wild-type (WT) and variant at the alleles of interest were used to measure E2-dependent induction of gene expression of osteoporosis-related genes including OPG. Cell lines that were variant at SNPs in TMEM14A–TRAM2 had greater E2 induction of OPG compared with WT cells, whereas cell lines that were variant for MAP4K4 had less dramatic OPG induction than WT. These genetic regions may be associated with AI-induced bone AEs, but clinical replication of these associations, as well as others that have been identified in HTR2A, ESR1 [76] and ESR2 [54], are necessary.
Vasomotor symptoms
Vasomotor symptoms (VMSs), including hot flashes and night sweats, are a common AE of AI treatment and are often experienced during menopause, particularly in patients with lower estrogen concentrations [87]. In some large AI clinical trials VMS are associated with better AI response [88–90], again suggesting a mechanistic relationship with estrogen suppression during treatment. Pharmacogenetic predictors of VMS have recently been identified in retrospective analyses of large prospective clinical trials including the TEXT trial of patients treated with ovarian suppression and randomized to 5 years of exemestane or tamoxifen (n = 1967). Five candidate SNPs in CYP19A1 (rs10046 and rs4646) and ESR1 (rs2077647, rs2234693 and rs9340799) were analyzed for association, with appropriate covariate adjustment and statistical correction. Patients homozygous for the CYP19A1 3′-UTR rs10046 variant had a 22% reduction in the odds of vasomotor symptoms, and this association was confined to the exemestane arm, suggesting that it may be a true pharmacogenetic predictor of AI toxicity [78]. This association, detected in a large, well-controlled analysis, should be prioritized for attempted validation in independent patient cohorts. The TEAM trial, comparing exemestane versus tamoxifen followed by exemestane, is a potential cohort in which to replicate this finding. A pharmacogenetic analysis of the association of 30 CYP19A1 tag SNPs with vasomotor symptoms within the first year of exemestane treatment on TEAM has been reported (n = 737). Patients homozygous for the noncoding rs7176005 (5′), rs16964189 (3′) or rs934635 (3′) were more likely to experience vasomotor symptoms (all p < 0.05) [72]. The lists of candidate SNPs assessed within these two similar retrospective pharmacogenetic analyses of VMS during exemestane clinical trials did not overlap, and replication has not been reported for any of these reported associations.
Other treatment-related toxicities
Finally, two studies have attempted to assess whether CYP19A1 genetic variation is associated with AI-induced changes in body mass or serum lipid levels. Using a previously described cohort, Napoli et al. performed an analysis in 82 patients who underwent measurement of body mass, fat mass and bone mineral content at baseline and after 6 and 12 months of AI treatment. Patients who were homozygous for the G allele of the synonymous (Val80Val) rs700518 SNP had a significant increase in truncal fat mass index and decreased lean mass compared with carriers of the A allele [79]. Interestingly, the G allele was the minor allele in this subcohort, whereas it was the major allele in the BMD analysis of this cohort described in a previous section. These findings are somewhat consistent with those of Santa-Maria et al. who reported that patients homozygous for the same rs700518 synonymous allele treated on the letrozole arm of the ELPh trial (n = 160) had a greater decrease in triglycerides (p = 0.00019) [80]. This hypothesis-generating association, found within a statistically uncorrected analysis, requires further independent replication.
Conclusion
The third generation AIs are highly effective in improving disease outcomes for postmenopausal patients with ER+ breast cancer, and the use of AI therapy may be expanding to chemoprevention and premenopausal treatment settings. In addition, the AIs have generally tolerable side effect profiles. Despite this, some patients do not experience optimal treatment outcomes, possibly due to germline genetic variations that decrease efficacy and/or increase toxicity. Candidate gene approaches have confirmed that functional polymorphisms in CYP2A6 and CYP3A are associated with variability in letrozole and exemestane systemic drug concentrations, respectively. However, there is a lack of validation that systemic AI concentrations, or these variants, are meaningful predictors of treatment outcomes. Efficacy, and perhaps toxicity, of AI treatment is likely dependent on suppression of systemic estrogen concentrations. In vitro and clinical pharmacogenetic studies have focused on variants in the CYP19A1 aromatase enzyme. Some variants have been reported to modulate estrogenic response to AI treatment, particularly the intronic rs7176005 and rs6493497 CYP19A1 SNPs, but these findings are awaiting validation. Pharmacogenetic investigations of AI efficacy have also focused primarily on SNPs in CYP19A1, with some broader candidate or genome-wide association efforts, but reported associations have been challenging to replicate in independent patient cohorts. Similarly, pharmacogenetic predictors of AI-related toxicity have been reported but none have been adequately validated. Inconsistent findings across studies and unsuccessful replication are common in pharmacogenetics literature and are likely due to some combination of study heterogeneity, limited power and publication bias. High priority candidates for attempted replication include SNPs in RANKL and OPG that may be associated with MS-AEs and the CYP19A1 3′-UTR SNP rs10046 that has been associated with VMSs in multiple patient cohorts. Replication attempts using large cohorts of patients with systematic treatment and phenotype collection are necessary to validate these previously discovered variants and begin their translation into clinical practice to inform personalized AI treatment decisions to enhance efficacy and prevent unnecessary toxicity.
Future perspective
In the next 5–10 years, the use of AIs will continue to expand to additional patient populations. These advances in our clinical understanding will be based on results of large clinical trials, which are ideal for pharmacogenetic discovery and validation. Enhanced appreciation of the importance of systematically collecting germline DNA and patient-reported outcomes data will improve pharmacogenetic analyses. Similarly, the intrinsic subtyping of breast cancer into more homogeneous molecular subtypes will benefit efforts to discover germline predictors of treatment efficacy. However, the expanded use of combination therapy in cancer, such as recent evidence that the addition of palbociclib to letrozole for metastatic breast cancer improves progression-free survival [91], may complicate pharmacogenetic efforts. Nevertheless, recent analyses have identified intriguing candidate SNPs for validation that could be integrated into prospective AI clinical trials within the next 10 years to exclude patients unlikely to respond to AIs or highly likely to experience AI treatment-related toxicity.
Executive summary.
Third-generation aromatase inhibitors (AIs), anastrozole, letrozole and exemestane, are highly effective in estrogen receptor-positive breast cancer.
AIs work by suppressing systemic estrogen concentrations via inhibition of CYP19A1 (aromatase) enzyme-mediated conversion of androgens to estrogens.
Treatment outcomes including efficacy and toxicity, and endophenotypes including circulating AI drug and estrogen concentrations during treatment, are highly variable and may be associated with germline genetic variation.
Genetic predictors of systemic AI concentrations
Clinical pharmacogenetic analyses of anastrozole-treated patient cohorts have not been reported, but should focus on candidate SNPs in the genes that encode for the CYP3A and UGT1A4 enzymes.
Clinical pharmacogenetic analyses have confirmed that functional polymorphisms in CYP2A6 and CYP3A4 are predictive of letrozole and exemestane concentrations, respectively.
The clinical utility of these genetic predictors is not yet clear as systemic drug concentrations have not been validated to predict treatment outcomes.
Genetic predictors of estrogen response to AI treatment
Polymorphisms in CYP19A1 have been linked with steroid hormonal levels and cancer risk, but less is known about whether estrogenic suppression during AI treatment is affected by variation in this enzyme.
In vitro and clinical follow-up studies suggest that the intronic CYP19A1 SNPs rs6493497 and rs7176005 may be predictive of AI-related estrogenic suppression.
The putative associations between magnitude of estrogen suppression and treatment-related efficacy or toxicities have not been validated, therefore, the clinical relevance of SNPs that affect estrogenic response to AI treatment is unclear.
Genetic predictors of AI treatment efficacy
SNPs within and proximal to the CYP19A1 gene have been analyzed repeatedly for associations with AI treatment efficacy, but replication of reported associations has been challenging.
Pharmacogenetic discovery using broader SNP panels and genome-wide chips have also identified candidates for attempted replication.
Genetic predictors of AI treatment-related toxicity
Pharmacogenetic analyses have focused primarily on AI-related musculoskeletal, vasomotor and bone symptoms.
Recently reported associations for SNPs in RANKL (rs7984870) and OPG (rs2073618) with AI-related musculoskeletal adverse events should be prioritized for validation studies.
Other SNP associations identified in candidate and genome-wide analyses require additional replication and validation prior to translation into clinical practice.
Footnotes
Financial & competing interests disclosure
Funding has been provided by The Breast Cancer Research Foundation (BCRF; N003173 to JM Rae), the NIH (1RO1GM099143 to JM Rae). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Early Breast Cancer Trialists’ Collaborative Group. Dowsett M, Forbes JF, et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–1352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 2.Regan MM, Francis PA, Pagani O, et al. Absolute benefit of adjuvant endocrine therapies for premenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative early breast cancer: TEXT and SOFT Trials. J. Clin. Oncol. 2016;34(19):2221–2231. doi: 10.1200/JCO.2015.64.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamdem LK, Liu Y, Stearns V, et al. In vitro and in vivo oxidative metabolism and glucuronidation of anastrozole. Br. J. Clin. Pharmacol. 2010;70(6):854–869. doi: 10.1111/j.1365-2125.2010.03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edavana VK, Dhakal IB, Williams S, et al. Potential role of UGT1A4 promoter SNPs in anastrozole pharmacogenomics. Drug Metab. Dispos. 2013;41(4):870–877. doi: 10.1124/dmd.112.048157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edavana VK, Penney RB, Yao-Borengasser A, Starlard-Davenport A, Dhakal IB, Kadlubar S. Effect of MRP2 and MRP3 polymorphisms on anastrozole glucuronidation and MRP2 and MRP3 gene expression in normal liver samples. Int. J. Cancer Res. Mol. Mech. 2015;1(3) doi: 10.16966/2381-3318.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hertz DL, Barlow WE, Kidwell KM, et al. Fulvestrant decreases anastrozole drug concentrations when taken concurrently by patients with metastatic breast cancer treated on SWOG study S0226. Br. J. Clin. Pharmacol. 2016 doi: 10.1111/bcp.12904. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hertz DL, Kidwell KM, Seewald NJ, et al. Polymorphisms in drug-metabolizing enzymes and steady-state exemestane concentration in postmenopausal patients with breast cancer. Pharmacogenomics J. 2016 doi: 10.1038/tpj.2016.60. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Confirmed association for CYP3A4*22 and steady-state exemestane concentrations.
- 8.Egbelakin A, Ferguson MJ, Macgill EA, et al. Increased risk of vincristine neurotoxicity associated with low CYP3A5 expression genotype in children with acute lymphoblastic leukemia. Pediatr. Blood Cancer. 2011;56(3):361–367. doi: 10.1002/pbc.22845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murai K, Yamazaki H, Nakagawa K, Kawai R, Kamataki T. Deactivation of anti-cancer drug letrozole to a carbinol metabolite by polymorphic cytochrome P450 2A6 in human liver microsomes. Xenobiotica. 2009;39(11):795–802. doi: 10.3109/00498250903171395. [DOI] [PubMed] [Google Scholar]
- 10.Desta Z, Kreutz Y, Nguyen AT, et al. Plasma letrozole concentrations in postmenopausal women with breast cancer are associated with CYP2A6 genetic variants, body mass index, and age. Clin. Pharmacol. Ther. 2011;90(5):693–700. doi: 10.1038/clpt.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Confirmed association between genotype-predicted CYP2A6 activity and steady-state letrozole concentrations.
- 11.Ingelman-Sundberg M, Daly AK, Nebert DW. Home page of the human cytochrome P450 (CYP) allele nomenclature database. 2008. www.cypalleles.ki.se/cyp2d6.htm 2010(Web Page).
- 12.Tanii H, Shitara Y, Horie T. Population pharmacokinetic analysis of letrozole in Japanese postmenopausal women. Eur. J. Clin. Pharmacol. 2011;67(10):1017–1025. doi: 10.1007/s00228-011-1042-3. [DOI] [PubMed] [Google Scholar]
- 13.Jin SJ, Jung JA, Cho SH, et al. The pharmacokinetics of letrozole: association with key body mass metrics. Int. J. Clin. Pharmacol. Ther. 2012;50(8):557–565. doi: 10.5414/CP201709. [DOI] [PubMed] [Google Scholar]
- 14.Kamdem LK, Flockhart DA, Desta Z. In vitro cytochrome P450-mediated metabolism of exemestane. Drug Metab. Dispos. 2011;39(1):98–105. doi: 10.1124/dmd.110.032276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Platt A, Xia Z, Liu Y, Chen G, Lazarus P. Impact of nonsynonymous single nucleotide polymorphisms on in vitro metabolism of exemestane by hepatic cytosolic reductases. Pharmacogenet. Genomics. 2016;26(8):370–380. doi: 10.1097/FPC.0000000000000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun D, Chen G, Dellinger RW, Sharma AK, Lazarus P. Characterization of 17-dihydroexemestane glucuronidation: potential role of the UGT2B17 deletion in exemestane pharmacogenetics. Pharmacogenet. Genomics. 2010;20(10):575–585. doi: 10.1097/FPC.0b013e32833b04af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen SM, Atchley DH, Murphy MA, Gurley BJ, Kamdem LK. Impact of UGT2B17 gene deletion on the pharmacokinetics of 17-hydroexemestane in healthy volunteers. J. Clin. Pharmacol. 2016;56(7):875–884. doi: 10.1002/jcph.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bajetta E, Zilembo N, Noberasco C, et al. The minimal effective exemestane dose for endocrine activity in advanced breast cancer. Eur. J. Cancer. 1997;33(4):587–591. doi: 10.1016/s0959-8049(96)00494-7. [DOI] [PubMed] [Google Scholar]
- 19.Zilembo N, Noberasco C, Bajetta E, et al. Endocrinological and clinical evaluation of exemestane, a new steroidal aromatase inhibitor. Br. J. Cancer. 1995;72(4):1007–1012. doi: 10.1038/bjc.1995.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geisler J, King N, Dowsett M, et al. Influence of anastrozole (Arimidex), a selective, non-steroidal aromatase inhibitor, on in vivo aromatisation and plasma oestrogen levels in postmenopausal women with breast cancer. Br. J. Cancer. 1996;74(8):1286–1291. doi: 10.1038/bjc.1996.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dowsett M, Cuzick J, Howell A, Jackson I ATAC Trialists’ Group. Pharmacokinetics of anastrozole and tamoxifen alone, and in combination, during adjuvant endocrine therapy for early breast cancer in postmenopausal women: a sub-protocol of the ‘Arimidex and tamoxifen alone or in combination’ (ATAC) trial. Br. J. Cancer. 2001;85(3):317–324. doi: 10.1054/bjoc.2001.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingle JN, Buzdar AU, Schaid DJ, et al. Variation in anastrozole metabolism and pharmacodynamics in women with early breast cancer. Cancer Res. 2010;70(8):3278–3286. doi: 10.1158/0008-5472.CAN-09-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingle JN, Kalari KR, Buzdar AU, et al. Estrogens and their precursors in postmenopausal women with early breast cancer receiving anastrozole. Steroids. 2015;99(Pt A):32–38. doi: 10.1016/j.steroids.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valle M, Di Salle E, Jannuzzo MG, et al. A predictive model for exemestane pharmacokinetics/pharmacodynamics incorporating the effect of food and formulation. Br. J. Clin. Pharmacol. 2005;59(3):355–364. doi: 10.1111/j.1365-2125.2005.02335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kadakia KC, Snyder CF, Kidwell KM, et al. Patient-reported outcomes and early discontinuation in aromatase inhibitor-treated postmenopausal women with early stage breast cancer. Oncologist. 2016;21(5):539–546. doi: 10.1634/theoncologist.2015-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buzdar A, Jonat W, Howell A, et al. Anastrozole, a potent and selective aromatase inhibitor, versus megestrol acetate in postmenopausal women with advanced breast cancer: results of overview analysis of two Phase III trials. Arimidex Study Group. J. Clin. Oncol. 1996;14(7):2000–2011. doi: 10.1200/JCO.1996.14.7.2000. [DOI] [PubMed] [Google Scholar]
- 27.Geisler J, Haynes B, Anker G, Dowsett M, Lonning PE. Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J. Clin. Oncol. 2002;20(3):751–757. doi: 10.1200/JCO.2002.20.3.751. [DOI] [PubMed] [Google Scholar]
- 28.Geisler J, King N, Anker G, et al. In vivo inhibition of aromatization by exemestane, a novel irreversible aromatase inhibitor, in postmenopausal breast cancer patients. Clin. Cancer Res. 1998;4(9):2089–2093. [PubMed] [Google Scholar]
- 29.Santen RJ, Demers L, Ohorodnik S, et al. Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids. 2007;72(8):666–671. doi: 10.1016/j.steroids.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Simpson ER, Mahendroo MS, Means GD, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr. Rev. 1994;15(3):342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 31.Dunning AM, Dowsett M, Healey CS, et al. Polymorphisms associated with circulating sex hormone levels in postmenopausal women. J. Natl Cancer Inst. 2004;96(12):936–945. doi: 10.1093/jnci/djh167. [DOI] [PubMed] [Google Scholar]
- 32.Vandenput L, Ohlsson C. Genome-wide association studies on serum sex steroid levels. Mol. Cell. Endocrinol. 2014;382(1):758–766. doi: 10.1016/j.mce.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Chen Z, Tao S, Gao Y, et al. Genome-wide association study of sex hormones, gonadotropins and sex hormone-binding protein in Chinese men. J. Med. Genet. 2013;50(12):794–801. doi: 10.1136/jmedgenet-2013-101705. [DOI] [PubMed] [Google Scholar]
- 34.Prescott J, Thompson DJ, Kraft P, et al. Genome-wide association study of circulating estradiol, testosterone, and sex hormone-binding globulin in postmenopausal women. PLoS ONE. 2012;7(6):e37815. doi: 10.1371/journal.pone.0037815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson DJ, O'Mara TA, Glubb DM, et al. CYP19A1 fine-mapping and Mendelian randomization: estradiol is causal for endometrial cancer. Endocr. Relat. Cancer. 2016;23(2):77–91. doi: 10.1530/ERC-15-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma CX, Adjei AA, Salavaggione OE, et al. Human aromatase: gene resequencing and functional genomics. Cancer Res. 2005;65(23):11071–11082. doi: 10.1158/0008-5472.CAN-05-1218. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Ellsworth KA, Moon I, et al. Functional genetic polymorphisms in the aromatase gene CYP19 vary the response of breast cancer patients to neoadjuvant therapy with aromatase inhibitors. Cancer Res. 2010;70(1):319–328. doi: 10.1158/0008-5472.CAN-09-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• CYP19A1 sequencing analysis followed by in vitro and clinical assessments reporting two linked intronic CYP19A1 SNPs that may be associated with aromatase activity and response to aromatase inhibitors (AIs).
- 38.Mao JJ, Su HI, Feng R, et al. Association of functional polymorphisms in CYP19A1 with aromatase inhibitor associated arthralgia in breast cancer survivors. Breast Cancer Res. 2011;13(1):R8. doi: 10.1186/bcr2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lunardi G, Piccioli P, Bruzzi P, et al. Plasma estrone sulfate concentrations and genetic variation at the CYP19A1 locus in postmenopausal women with early breast cancer treated with letrozole. Breast Cancer Res. 2013;137(1):167–174. doi: 10.1007/s10549-012-2306-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Napoli N, Rastelli A, Ma C, et al. Genetic polymorphism at Val80 (rs700518) of the CYP19A1 gene is associated with aromatase inhibitor associated bone loss in women with ER + breast cancer. Bone. 2013;55(2):309–314. doi: 10.1016/j.bone.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Folkerd EJ, Dixon JM, Renshaw L, A'Hern RP, Dowsett M. Suppression of plasma estrogen levels by letrozole and anastrozole is related to body mass index in patients with breast cancer. J. Clin. Oncol. 2012;30(24):2977–2980. doi: 10.1200/JCO.2012.42.0273. [DOI] [PubMed] [Google Scholar]
- 42.Dixon JM, Renshaw L, Young O, et al. Letrozole suppresses plasma estradiol and estrone sulphate more completely than anastrozole in postmenopausal women with breast cancer. J. Clin. Oncol. 2008;26(10):1671–1676. doi: 10.1200/JCO.2007.13.9279. [DOI] [PubMed] [Google Scholar]
- 43.Geisler J, Helle H, Ekse D, et al. Letrozole is superior to anastrozole in suppressing breast cancer tissue and plasma estrogen levels. Clin. Cancer Res. 2008;14(19):6330–6335. doi: 10.1158/1078-0432.CCR-07-5221. [DOI] [PubMed] [Google Scholar]
- 44.O'Shaughnessy J, Yardley D, Burris H, et al. Abstract PD2-01: randomized Phase 3 trial of adjuvant letrozole versus anastrozole in postmenopausal patients with hormone receptor positive, node positive early breast cancer: final efficacy and safety results of the Femara versus Anastrozole Clinical Evaluation (Face) trial. Cancer Res. 2016;76(Suppl. 4):PD2-01–PD02-01. doi: 10.1200/JCO.2016.69.2871. [DOI] [PubMed] [Google Scholar]
- 45.Henry NL, Giles JT, Stearns V. Aromatase inhibitor-associated musculoskeletal symptoms: etiology and strategies for management. Oncology (Williston Park) 2008;22(12):1401–1408. [PubMed] [Google Scholar]
- 46.Lintermans A, Neven P. Pharmacology of arthralgia with estrogen deprivation. Steroids. 2011;76(8):781–785. doi: 10.1016/j.steroids.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 47.Kanematsu M, Morimoto M, Honda J, et al. The time since last menstrual period is important as a clinical predictor for non-steroidal aromatase inhibitor-related arthralgia. BMC Cancer. 2011;11:436. doi: 10.1186/1471-2407-11-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mao JJ, Stricker C, Bruner D, et al. Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer. 2009;115(16):3631–3639. doi: 10.1002/cncr.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, Lu K, Song Y, et al. RANKL and OPG polymorphisms are associated with aromatase inhibitor-related musculoskeletal adverse events in Chinese han breast cancer patients. PLoS ONE. 2015;10(7):e0133964. doi: 10.1371/journal.pone.0133964. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Initial discovery that SNPs in RANKL and OPG may be predicors of AI-associated musculoskeletal (MS) and bone adverse events (AEs).
- 50.Lintermans A, Vanderschueren D, Verhaeghe J, et al. Arthralgia induced by endocrine treatment for breast cancer: a prospective study of serum levels of insulin like growth factor-I, its binding protein and oestrogens. Eur. J. Cancer. 2014;50(17):2925–2931. doi: 10.1016/j.ejca.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 51.Henry NL, Conlon A, Kidwell KM, et al. Effect of estrogen depletion on pain sensitivity in aromatase inhibitor-treated women with early-stage breast cancer. J. Pain. 2014;15(5):468–475. doi: 10.1016/j.jpain.2014.01.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Henry NL, Azzouz F, Desta Z, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J. Clin. Oncol. 2012;30(9):936–942. doi: 10.1200/JCO.2011.38.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Briot K, Tubiana-Hulin M, Bastit L, Kloos I, Roux C. Effect of a switch of aromatase inhibitors on musculoskeletal symptoms in postmenopausal women with hormone-receptor-positive breast cancer: the ATOLL (articular tolerance of letrozole) study. Breast Cancer Res. Treat. 2010;120(1):127–134. doi: 10.1007/s10549-009-0692-7. [DOI] [PubMed] [Google Scholar]
- 54.Leyland-Jones B, Gray KP, Abramovitz M, et al. ESR1 and ESR2 polymorphisms in the BIG 1–98 trial comparing adjuvant letrozole versus tamoxifen or their sequence for early breast cancer. Breast Cancer Res. Treat. 2015;154(3):543–555. doi: 10.1007/s10549-015-3634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leyland-Jones B, Gray KP, Abramovitz M, et al. CYP19A1 polymorphisms and clinical outcomes in postmenopausal women with hormone receptor-positive breast cancer in the BIG 1–98 trial. Breast Cancer Res. Treat. 2015;151(2):373–384. doi: 10.1007/s10549-015-3378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Attempted replication of associations for SNPs in CYP19A1 with AI efficacy and toxicity in the large, prospective BIG 1–98 clinical trial.
- 56.Garcia-Casado Z, Guerrero-Zotano A, Llombart-Cussac A, et al. A polymorphism at the 3′-UTR region of the aromatase gene defines a subgroup of postmenopausal breast cancer patients with poor response to neoadjuvant letrozole. BMC Cancer. 2010;10:36–2407. 2410–2436. doi: 10.1186/1471-2407-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miron L, Negura L, Peptanariu D, Marinca M. Research on aromatase gene (CYP19A1) polymorphisms as a predictor of endocrine therapy effectiveness in breast cancer. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2012;116(4):997–1004. [PubMed] [Google Scholar]
- 58.Ferraldeschi R, Arnedos M, Hadfield KD, et al. Polymorphisms of CYP19A1 and response to aromatase inhibitors in metastatic breast cancer patients. Breast Cancer Res. Treat. 2012;133(3):1191–1198. doi: 10.1007/s10549-012-2010-z. [DOI] [PubMed] [Google Scholar]
- 59.Simonsson M, Veerla S, Markkula A, Rose C, Ingvar C, Jernstrom H. CYP1A2 – a novel genetic marker for early aromatase inhibitor response in the treatment of breast cancer patients. BMC Cancer. 2016;16:256. doi: 10.1186/s12885-016-2284-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Colomer R, Monzo M, Tusquets I, et al. A single-nucleotide polymorphism in the aromatase gene is associated with the efficacy of the aromatase inhibitor letrozole in advanced breast carcinoma. Clin. Cancer Res. 2008;14(3):811–816. doi: 10.1158/1078-0432.CCR-07-1923. [DOI] [PubMed] [Google Scholar]
- 61.Liu L, Bai YX, Zhou JH, et al. A polymorphism at the 3′-UTR region of the aromatase gene is associated with the efficacy of the aromatase inhibitor, anastrozole, in metastatic breast carcinoma. Int. J. Mol. Sci. 2013;14(9):18973–18988. doi: 10.3390/ijms140918973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park IH, Lee YS, Lee KS, et al. Single nucleotide polymorphisms of CYP19A1 predict clinical outcomes and adverse events associated with letrozole in patients with metastatic breast cancer. Cancer Chemother. Pharmacol. 2011;68(5):1263–1271. doi: 10.1007/s00280-011-1615-y. [DOI] [PubMed] [Google Scholar]
- 63.Ingle JN, Xie F, Ellis MJ, et al. Genetic polymorphisms in the long noncoding RNA MIR2052HG offer a pharmacogenomic basis for the response of breast cancer patients to aromatase inhibitor therapy. Cancer Res. 2016 doi: 10.1158/0008-5472.CAN-16-1371. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Genome-wide association study of the MA.27 clinical identifying assocaitions of SNPs near MIR2052HG with breast cancer free interval from AI treatment.
- 64.Artigalas O, Vanni T, Hutz MH, Ashton-Prolla P, Schwartz IV. Influence of CYP19A1 polymorphisms on the treatment of breast cancer with aromatase inhibitors: a systematic review and meta-analysis. BMC Med. 2015;13:139. doi: 10.1186/s12916-015-0373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghimenti C, Mello-Grand M, Grosso E, et al. Regulation of aromatase expression in breast cancer treated with anastrozole neoadjuvant therapy. Exp. Ther. Med. 2013;5(3):902–906. doi: 10.3892/etm.2012.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Zhang M, Yuan X, et al. Association between ESR1 PvuII, XbaI, and P325P polymorphisms and breast cancer susceptibility: a meta-analysis. Med. Sci. Monit. 2015;21:2986–2996. doi: 10.12659/MSM.894010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gellert P, Segal CV, Gao Q, et al. Impact of mutational profiles on response of primary oestrogen receptor-positive breast cancers to oestrogen deprivation. Nat. Commun. 2016;7:13294. doi: 10.1038/ncomms13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ellis MJ, Ding L, Shen D, et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature. 2012;486(7403):353–360. doi: 10.1038/nature11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robinson DR, Wu YM, Vats P, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat. Genet. 2013;45(12):1446–1451. doi: 10.1038/ng.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henry NL, Skaar TC, Dantzer J, et al. Genetic associations with toxicity-related discontinuation of aromatase inhibitor therapy for breast cancer. Breast Cancer Res. Treat. 2013;138(3):807–816. doi: 10.1007/s10549-013-2504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garcia-Giralt N, Rodriguez-Sanz M, Prieto-Alhambra D, et al. Genetic determinants of aromatase inhibitor-related arthralgia: the B-ABLE cohort study. Breast Cancer Res. Treat. 2013;140(2):385–395. doi: 10.1007/s10549-013-2638-3. [DOI] [PubMed] [Google Scholar]
- 72.Fontein DB, Houtsma D, Nortier JW, et al. Germline variants in the CYP19A1 gene are related to specific adverse events in aromatase inhibitor users: a substudy of Dutch patients in the TEAM trial. Breast Cancer Res. Treat. 2014;144(3):599–606. doi: 10.1007/s10549-014-2873-2. [DOI] [PubMed] [Google Scholar]; • Attemped discovery of SNPs associated with AI associated MS and vasomotor AEs in the prospective TEAM clinical trial.
- 73.Lintermans A, Van Asten K, Jongen L, et al. Genetic variant in the osteoprotegerin gene is associated with aromatase inhibitor-related musculoskeletal toxicity in breast cancer patients. Eur. J. Cancer. 2016;56:31–36. doi: 10.1016/j.ejca.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 74.Ingle JN, Schaid DJ, Goss PE, et al. Genome-wide associations and functional genomic studies of musculoskeletal adverse events in women receiving aromatase inhibitors. J. Clin. Oncol. 2010;28(31):4674–4682. doi: 10.1200/JCO.2010.28.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Genome-wide association study of the MA.27 clinical trial identifying association of SNPs in TCL1A with AI-related MS-AEs.
- 75.Wang J, Lu K, Song Y, et al. Indications of clinical and genetic predictors for aromatase inhibitors related musculoskeletal adverse events in Chinese Han women with breast cancer. PLoS ONE. 2013;8(7):e68798. doi: 10.1371/journal.pone.0068798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oesterreich S, Henry NL, Kidwell KM, et al. Associations between genetic variants and the effect of letrozole and exemestane on bone mass and bone turnover. Breast Cancer Res. Treat. 2015;154(2):263–273. doi: 10.1007/s10549-015-3608-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu M, Goss PE, Ingle JN, et al. Aromatase inhibitor-associated bone fractures: a case–cohort GWAS and functional genomics. Mol. Endocrinol. 2014;28(10):1740–1751. doi: 10.1210/me.2014-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johansson H, Gray KP, Pagani O, et al. Impact of CYP19A1 and ESR1 variants on early-onset side effects during combined endocrine therapy in the TEXT trial. Breast Cancer Res. 2016;18(1):110. doi: 10.1186/s13058-016-0771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Attemped discovery of SNPs associated with AI associated vasomotor symptoms in the prospective TEXT clinical trial.
- 79.Napoli N, Rastelli A, Ma C, Colleluori G, Vattikuti S, Armamento-Villareal R. Genetic polymorphism at Val80 (rs700518) of the CYP19A1 gene is associated with body composition changes in women on aromatase inhibitors for ER (+) breast cancer. Pharmacogenet. Genomics. 2015;25(8):377–381. doi: 10.1097/FPC.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Santa-Maria CA, Blackford A, Nguyen AT, et al. Association of variants in candidate genes with lipid profiles in women with early breast cancer on adjuvant aromatase inhibitor therapy. Clin. Cancer Res. 2016;22(6):1395–1402. doi: 10.1158/1078-0432.CCR-15-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baud'huin M, Duplomb L, Ruiz Velasco C, Fortun Y, Heymann D, Padrines M. Key roles of the OPG–RANK–RANKL system in bone oncology. Expert Rev. Anticancer Ther. 2007;7(2):221–232. doi: 10.1586/14737140.7.2.221. [DOI] [PubMed] [Google Scholar]
- 82.Lintermans A, Van Asten K, Wildiers H, et al. A prospective assessment of musculoskeletal toxicity and loss of grip strength in breast cancer patients receiving adjuvant aromatase inhibitors and tamoxifen, and relation with BMI. Breast Cancer Res. Treat. 2014;146(1):109–116. doi: 10.1007/s10549-014-2986-7. [DOI] [PubMed] [Google Scholar]
- 83.Ho MF, Bongartz T, Liu M, et al. Estrogen, SNP-dependent chemokine expression and selective estrogen receptor modulator regulation. Mol. Endocrinol. 2016;30(3):382–398. doi: 10.1210/me.2015-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu M, Wang L, Bongartz T, et al. Aromatase inhibitors, estrogens and musculoskeletal pain: estrogen-dependent T-cell leukemia 1A (TCL1A) gene-mediated regulation of cytokine expression. Breast Cancer Res. 2012;14(2):R41. doi: 10.1186/bcr3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heshmati HM, Khosla S, Robins SP, O'Fallon WM, Melton LJ, 3rd, Riggs BL. Role of low levels of endogenous estrogen in regulation of bone resorption in late postmenopausal women. J. Bone Miner. Res. 2002;17(1):172–178. doi: 10.1359/jbmr.2002.17.1.172. [DOI] [PubMed] [Google Scholar]
- 86.Chapurlat RD, Bauer DC, Cummings SR. Association between endogenous hormones and sex hormone-binding globulin and bone turnover in older women: study of osteoporotic fractures. Bone. 2001;29(4):381–387. doi: 10.1016/s8756-3282(01)00584-1. [DOI] [PubMed] [Google Scholar]
- 87.Erlik Y, Meldrum DR, Judd HL. Estrogen levels in postmenopausal women with hot flashes. Obstetrics Gynecol. 1982;59(4):403–407. [PubMed] [Google Scholar]
- 88.Cuzick J, Sestak I, Cella D, Fallowfield L ATAC Trialists Group. Treatment-emergent endocrine symptoms and the risk of breast cancer recurrence: a retrospective analysis of the ATAC trial. Lancet Oncol. 2008;9(12):1143–1148. doi: 10.1016/S1470-2045(08)70259-6. [DOI] [PubMed] [Google Scholar]
- 89.Fontein DB, Seynaeve C, Hadji P, et al. Specific adverse events predict survival benefit in patients treated with tamoxifen or aromatase inhibitors: an international tamoxifen exemestane adjuvant multinational trial analysis. J. Clin. Oncol. 2013;31(18):2257–2264. doi: 10.1200/JCO.2012.45.3068. [DOI] [PubMed] [Google Scholar]
- 90.Stearns V, Chapman JA, Ma CX, et al. Treatment-associated musculoskeletal and vasomotor symptoms and relapse-free survival in the NCIC CTG MA.27 adjuvant breast cancer aromatase inhibitor trial. J. Clin. Oncol. 2015;33(3):265–271. doi: 10.1200/JCO.2014.57.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Turner NC, Ro J, Andre F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl. J. Med. 2015;373(3):209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]