Abstract
Smoking cessation treatment outcomes may be heavily influenced by genetic variations among smokers. Therefore, identifying specific variants that affect response to different pharmacotherapies is of major interest to the field. In the current study, we systematically review all studies published in or after the year 1990 which examined one or more gene–drug interactions for smoking cessation treatment. Out of 644 citations, 46 articles met the inclusion criteria for the systematic review. We summarize evidence on several genetic polymorphisms (CHRNA5-A3-B4, CYP2A6, DBH, CHRNA4, COMT, DRD2, DRD4 and CYP2B6) and their potential moderating pharamacotherarpy effects on patient cessation efficacy rates. These findings are promising and call for further research to demonstrate the effectiveness of genetic testing in personalizing treatment decision-making and improving outcome.
Keywords: : nicotine replacement therapy, pharmacogenetics, precision medicine, smoking cessation
Despite tremendous public health advances, smoking continues to be the leading preventable cause of death worldwide [1–4]. Multiple treatment options and rigorous clinical practice guidelines have been developed but cessation failure remains common [5,6]. The benefits smokers receive from individual therapies vary widely and can be partially predicted by biomarkers [7–10]. The high cost of cessation failure combined with the wide range of therapies with variable benefits lead to a need to identify treatments that are most likely to be effective for an individual smoker. Thus, efforts have been directed toward a precision medicine approach: using biomarkers to identify patients who may benefit from treatment. To date, the most investigated biomarkers for smoking cessation pharmacotherapy have been genetic or metabolic [11,12].
Heritability estimates indicate that genomic factors drive the population variability in both smoking quantity and smoking cessation [13]. Prior to the advent of genome-wide association studies (GWAS), multiple candidate gene studies evaluated the association between genetic variants and smoking behaviors [14–17]. The most robust genetic association with smoking found to date is between smoking behaviors and the nicotinic receptor subunit gene CHRNA5, first identified in a 2008 GWAS [18]. Further, GWAS meta-analyses have led to the discovery of multiple other genetic factors associated with smoking [19].
There is a growing body of studies evaluating genome-based responses to smoking cessation therapies [11,20]. Evidence has pointed to at least two genetic loci for nicotine dependence and smoking cessation. The CHRNA5-A3-B4 gene cluster on chromosome 15 has been associated with cigarettes smoked per day (CPD); with lung cancer and chronic obstructive pulmonary disease and with smoking cessation [21–32]. The cytochrome P450 2A6 and the NMR 3-hydroxycotinine/cotinine have been associated with CPD, lung and other aerodigestive cancers and smoking cessation [23,30,31,33–47]. Other candidate genes and pathways have been explored for association with smoking cessation [25,48–60].
A recent Cochrane review of clinical trials that studied smoking cessation across genotypes identified associations between some polymorphisms in the CHRNA3-A5-B4 region (rs1051730 and rs16969968) with short-term efficacy of nicotine replacement therapy (NRT), but not widespread differential treatment effects of pharmacotherapy [61]. Expanding upon this review, we conducted a systematic review of the literature that describes all studies of differential treatment effects of pharmacotherapy in smoking cessation.
Methods
We took a systematic approach to review pharmacogenetics studies of smoking cessation pharmacotherapy.
Search methods
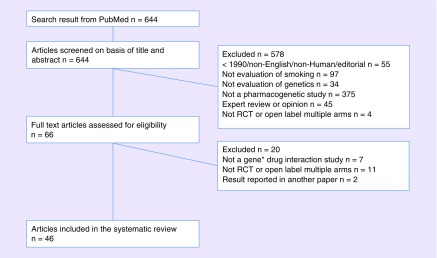
On 22 May 2017 we searched PubMed for articles published after 1 January 1990 that were related to both smoking cessation and precision medicine. The full PubMed search text is given in the Supplementary Text, which identified 644 articles. Second, we reviewed the title and abstract of 644 articles and excluded 578 articles based on the following exclusion criteria (Figure 1): published before 1990, non-English, nonhuman, editorial, no smoking content, no genetic content, not a pharmacogenetic study, expert opinion or review, not a primary research report and not a clinical trial with multiple arms. For the remaining 66 articles, we reviewed the full text and excluded the additional 20 articles based on the same exclusion criteria. A total of 46 articles were included in the systematic review.
Figure 1. . Flowchart of study inclusion.
RCT: Randomized controlled trial.
Inclusion criteria
The 46 articles were characterized based on the following study design criteria: trial design-randomized control trial (RCT) or open-label multiple treatment arms; comparison: placebo or other medication and outcome: efficacy, side effect or other outcomes. These characteristics for all included studies are outlined in Supplementary Table 1.
We then presented results in the following groups:
RCT and open-label studies with comparative treatment arms for genes with identified GWAS hits (Table 1);
RCT and open-label studies with comparative treatment arms for candidate genes based on a plausible biological rationale (Table 2).
Table 1. . Genes based on genome-wide association study hits.
| Gene | Chromosome | Medication | SNP | Number of positive studies (total n) | Number of negative studies |
|---|---|---|---|---|---|
| CHRNA5–A3–B4 | 15 | NRT | rs588765 | 2 (3776) | 2 |
| rs16969968 | 1 (328) | 4 | |||
| rs16969968*rs680244 | 1 (1073) | 0 | |||
| rs1051730 | 1 (2633) | 3 | |||
| rs2036527 | 1 (1143) | 0 | |||
| rs680244 | 0 | 1 | |||
| rs578776 | 0 | 5 | |||
| rs2229961, rs12914008, rs3813567, rs680244, rs8192475 | 0 | 1 | |||
| Bupropion | rs16969968*rs680244 | 1 (1073) | 0 | ||
| rs16969968, rs578776 | 0 | 2 | |||
| rs588765, rs2036527, rs2229961, rs1051730, rs12914008, rs3813567, rs680244, rs8192475 | 0 | 1 | |||
| Varenicline | rs16969968 | 0 | 3 | ||
| rs578776 | 0 | 2 | |||
| rs588765, haplotype rs16969968 and rs588765 | 0 | 1 | |||
| Selegiline | rs3813567 | 1 (231) | 0 | ||
| rs680244, rs16969968, rs578776, rs1051730, rs8192475, rs12914008 | 0 | 1 | |||
| CYP2A6 | 19 | NRT | Slow/Fast (genotype-based metric) | 1 (709) | 0 |
| Bupropion | Slow/Fast | 0 | 1 | ||
| CYP2A6-B6 | NRT | rs4105144, rs6474412 | 0 | 1 | |
| Varenicline | rs4105144, rs6474412 | 0 | 1 | ||
| DBH | 9 | NRT | 1368DBH A | 1 (755) | 0 |
| rs77905 | 0 | 1 | |||
| Bupropion | rs77905 | 0 | 1 | ||
| CHRNA4 | 20 | Varenicline | rs1044396 | 1 (483) | 0k |
Table includes only studies with efficacy outcome (abstinence or relapse).
Table includes genes that have GWAS hits and at least one positive gene x drug interaction study.
GWAS: Genome-wide association study; NRP: Nicotine replacement therapy.
Table 2. . Candidate genes.
| Gene | Chromosome | Medication | SNP | Number of positive studies (total n) | Number of negative studies |
|---|---|---|---|---|---|
| COMT | 22 | NRT | Val108/158Met (rs4680) | 2 (991) | 1 |
| Bupropion | rs165599 | 1 (511) | 0 | ||
| rs165599*rs737865 | 1 (511) | 0 | |||
| rs4680, rs737865 | 0 | 1 | |||
| DRD2 | 11 | NRT | Taq1A (rs1800497) | 1 (755) | 2 |
| Taq1B (rs1079597), 141C Ins/Del (rs1799732), Pro319Pro (rs6277), Exon 8 | 0 | 1 | |||
| Bupropion | Taq1A (rs1800497) | 3 (1582) | 3 | ||
| 141C ins/del (rs1799732) | 1 (414) | 0 | |||
| C957T variant, Exon III VNTR | 0 | 1 | |||
| Varenicline | Taq1A (rs1800497) | 0 | 1 | ||
| Venlafaxine | Taq1A (rs1800497) | 0 | 1 | ||
| Taq1B (rs1079597) | 0 | 1 | |||
| Rimonabant | Taq1A (rs1800497) | 1 | 0 | ||
| DRD4 | 11 | NRT | VNTR, C-521T | 0 | 1 |
| Bupropion | Exon III VNTR | 2 (1123) | 1 | ||
| CYP2B6 | 19 | Bupropion | CYP2B6*6 haplotype (rs2279343 + rs3745374) | 1 (326) | 1 |
| CYP2B6*4 (rs2279343) | 1 (478) | 0 | |||
| CYP2B6*5 (rs3211371), CYP2B6*9 (rs3745274) | 0 | 1 | |||
Table includes only studies with efficacy outcome (abstinence or relapse).
Table includes genes that have at least one SNP that has one or more replication studies and total n ≥500.
NRT: Nicotine replacement therapy; VNTR: Variable number of tandem repeat.
To note, open-label studies with one treatment arm or studies not looking at gene × drug interaction were not included. For more details about the studies included, refer to Supplementary Table 1.
Results
Genes with identified genome-wide association studies hits
CHRNA5-A3-B4
Several variants in the CHRNA5-A3-B4 gene cluster have been identified as moderators to the efficacy of NCT. A combined analysis of 8 randomized clinical trials comprising 2633 European-ancestry smokers showed that the minor alleles of rs1051730 and rs588765 were both associated with increased abstinence on NRT versus placebo at 6 months [21]. The variant rs1051730 is of particular importance because of its genome-wide significant association to smoking quantity [31]. However, rs1051730 was not found to be significantly associated with treatment outcomes in three other studies, albeit with small sample sizes [27,62,63]. Also, among 1143 African–Americans, individuals with the T-allele of rs588765 and A-allele of rs2036527 had higher and lower abstinence rates on nicotine gum versus placebo, respectively [32]. Of note, rs2036527 has been shown to be associated with genome-wide significance to CPD, in a genome-wide meta-analysis of 32,389 African-ancestry participants [24]. Another functional variant rs16969968, previously associated with smoking-related diseases such as cancer and COPD [64,65] has been shown in multiple studies to be associated with treatment response. A RCT of 1073 European–American smokers showed that individuals with high-risk haplotype, defined by rs16969968 and rs680244, had a threefold increased likelihood of responding to active treatment (NRT or bupropion) than individuals with low-risk haplotype [22]. Additional analyses performed on the NRT and placebo arms only (n = 328) revealed that individuals with the AA genotype of rs16969968 were more likely to be abstinent at the end of treatment to NRT compared with placebo [66]. Multiple studies however did not find a gene–drug interaction for this variant [32,63,67,68]. To note, the aforementioned results may have been affected by methodological limitations, unaccounted environmental moderators, small sample sizes and low statistical power. Importantly, the reported variants in CHRNA5-A3-B4 cluster are highly correlated in haplotype structure among individuals of European ancestry. For example, the correlation between rs16969968 and rs1051730 is 1.0 in individuals of European ancestry. Therefore, different variants reported in these studies indicate a common genetic profile.
To note, no individual variants in the CHRNA5-A3-B4 cluster were associated with either bupropion or varenicline (see Supplementary Table 1) [32,63,66–68]. The association of CHRNA5-A3-B4 and response to transdermal selegiline was reported in one study, where the minor C allele of rs3814567 was associated with lower abstinence rates in selegiline-versus placebo-treated smokers [69].
CYP2A6
CYP450 2A6 enzyme represents the main metabolic enzyme for the conversion of nicotine to its inactive metabolite cotinine [70]. In a large GWAS of 83,317 smokers of European ancestry for the number of CPD, the SNP rs4105144 located in the vw-B6 region was significantly associated with smoking quantity with an effect size of 0.39 (p = 2.2 × 10-12) [30]. This SNP, however, was not seen to moderate the effect of NRT or varenicline after 1 year of treatment in an open-label study of 525 Caucasian smokers, albeit showing significant association with tobacco dependence [68]. The interpretation of results is certainly limited by the absence of a placebo arm. In a placebo-controlled RCT of 709 European ancestry smokers, the effect of NRT, but not bupropion, differed with metabolism based on CYP2A6 genotype [71]. compared with placebo, NRT was effective in fast, but not slow metabolizers, whereas bupropion proved effective regardless of CYP2A6 genotype.
DBH
A GWAS found a significant association between DBH rs3025343 polymorphism and smoking cessation (p = 3.6 × 10-8) [31]. A placebo-controlled RCT of 755 smokers found that when considering only smokers with GA/AA of 1368 DBH and CT/TT of 32806 DRD2, subjects on the patch had an odds ratio (OR) of 3.59 of abstaining at 12 weeks compared with the subjects on placebo. This OR is reduced to 1.41 when running the same analysis on smokers with GG of 1368 DBH and CC of 32806 DRD2 (P for difference in ORs = 0.04) [72]. Results for the same DBH SNP was not replicated in an open label study of 569 Caucasian smokers in Germany treated with either NRT or bupropion, at the physician's discretion [73].
CHRNA4
The CHRNA4 gene garnered attention after being identified as a novel locus (rs2273500) for nicotine dependence in a GWAS meta-analysis of 17,074 Caucasian ever smokers [74]. CHRNA4 gene expression may significantly affect smoking behavior as well as response to treatment. A cohort study of 483 smokers from mixed ethnicities who received treatment with either varenicline, bupropion and/or NRT, found that, on one hand, for subjects with CT/TT genotypes of CHRNA4 rs1044396 polymorphism, success rate seems comparable between varenicline, varenicline plus bupropion (V+B) and bupropion plus NRT (B+NRT) (50.9, 50 and 42.2%, respectively). On the other hand, CC genotype puts subjects on varenicline at a particular disadvantage (success rate 29.5%) compared with the other two combination treatments (40% for V+B and 42.1% for B+NRT) [75].
Candidate genes based on pharmacogenetic association studies
COMT
Genes acting on the dopamine pathway are of obvious importance to the nicotine addiction pharmacogenetic studies. Namely, the gene coding for the enzyme COMT, which degrades dopamine released in the extraneuronal space. The Val108/158Met polymorphism or rs4680 of the COMT gene has been examined in multiple studies in relation to NRT but results have been inconclusive. An RCT of 741 smokers of European ancestry found greater benefit of active treatment compared with placebo on the likelihood of abstinence in the Met/Met genotype group, in comparison to the Met/Val + Val/Val group [76]. In contrast, a more recent study of 250 Asian smokers resulted in greater abstinence rates on NRT versus placebo in the group with Val/Val genotype versus the group with Met allele [77]. This may highlight the pharmacogenetic variability between different ethnicities, although the divergence in study results might simply be attributed to low power. A third RCT found no gene–drug association for this variant among 233 Caucasians smokers [62].
No gene–drug interaction was found either for rs4680 and bupropion in an analysis of two RCTs [78]. However, in a placebo-controlled RCT of 511 smokers from different ethnic backgrounds, Caucasians with at least one A allele of rs165599 had 19% abstinence on placebo and 33% on bupropion, while those with a GG genotype displayed 38% abstinence on placebo versus 22% on bupropion. In contrast, although trending towards the same results, no significant association was found among AA smokers, probably due to their small sample size [79].
DRD2
DRD2 gene is one of the most studied genes before the era of GWAS research in the pharmacogenetics of nicotine dependence, especially Taq1A, or rs1800497. This variant has been shown to alter DRD2 availability in postmortem striatal samples [80]. Among 755 smokers participating in a placebo-controlled RCT, those who possessed at least 1 T-allele had a significantly better response to NRT than placebo (OR = 2.8), while the observed OR for the response smokers with CC genotype on the patch compared with placebo was 1.41 (P for difference in ORs = 0.04) [72]. These results were not replicated however in two independent studies, an RCT and an open-label effectiveness trial [62,73].
Taq1A showed more remarkable results when studied in association with outcomes to bupropion treatment. Three independent studies, with a combined sample size of 1582 Caucasians, demonstrated consistently that smokers with Taq1A CC genotype had significantly higher rates of abstinence when treated with bupropion as opposed to placebo, while showing no difference when possessing one or two T alleles [73,81,82]. One open-label trial did not show however a gene–drug interaction with either bupropion or varenicline, perhaps owing to methodological limitations (open label, lack of placebo, mixed ethnicities) [83]. Furthermore, rs1799732, an ins/del variant of the DRD2 gene, was associated with differential response to bupropion compared with placebo in that, on bupropion, individuals with CC genotype responded better than those with at least one N allele, whereas on placebo, smoker with at least one N allele were more likely to be abstinent [84]. This result still requires replication.
DRD4
Pharmacogenetic studies have mostly focused on a variable number of tandem repeats (VNTR) polymorphism located in exon III of the DRD4 gene. A study of two pooled RCTs resulted in a significant gene–drug interaction for exon III VNTR in DRD4 and bupropion on smoking lapse rates [78]. In a more recent trial, participants with at least one L allele of this same variant had higher odds of abstinence on bupropion compared with placebo while no differential effect was seen in SS participants [85]. This result was not replicated however in a subsequent study of 416 smokers of European ancestry [86]. No association was found between exon III VNTR and NRT in a separate placebo-controlled RCT of 720 Caucasians smokers [52].
CYP2B6
CYP450 2B6 gene, CYP2B6, encodes the isoenzyme that metabolizes bupropion to hydroxybupropion [87], therefore playing an important role in bupropion treatment outcome. In an open-label study of 478 smokers receiving bupropion or varenicline (+/- NRT, treated as a covariate), CYP2B6*4 rs2279343 was observed to moderate the effect of bupropion, but not varenicline, on abstinence; wild-type AA genotype had higher success rate (48.0 %) compared with patients carrying AG or GG genotypes (35.5 %) on bupropion therapy (p = 0.05). Success rates in the varenicline sample remained virtually the same, regardless of genotype (43.4 and 43.2%, respectively) [83]. In another placebo-controlled RCT, 326 Caucasian smokers were randomized to bupropion or placebo and haplotyped for CYP2B6*6 (which comprises rs2279343 and rs3745374). A genotype by treatment interaction was found after 10 weeks of treatment, the bupropion resulted in a higher abstinence rate than placebo in the CYP2B6*6 group (CYP2B6*1/*6 or CYP2B6*6/*6 genotype), while no difference was observed between bupropion and placebo for CYP2B6*1 group (CYP2B6*1/*1) [88]. The favorable effect of bupropion on smoking cessation in the CYP2B6*6 group may result from the association of the latter on decreased bupropion metabolism in the liver [89], leading to increased bupropion plasma levels.
Discussion
With this systematic review, we identify the potential of genetic markers, identified by the GWAS discoveries, in predicting the efficacy of smoking cessation pharmacotherapy, based on the review of 46 studies. Multiple pharmacodynamics (e.g., CHRNA5, DBH, CHRNA4) and pharmacokinetic (e.g., CYP2A6) markers may predict efficacy of NRT, although both positive and negative studies exist. These discrepant results may be influenced by the sample ascertainment, study power, concurrent nonpharmacological therapy and other potential confounders. One of the most studied gene clusters is CHRNA5-A3-B4, which has been heavily linked to smoking characteristics including nicotine dependence [29], smoking quantity [90] and biomarkers of smoking [91,92].
Other GWAS-identified genes that were also shown to be associated with treatment efficacy included CYP2A6, a highly polymorphic gene that has been associated with multiple smoking phenotypes [36,45], DBH that catalyzes the conversion of dopamine to norepinephrine, hence playing an important role in the addictive properties of smoking and CHRNA4 which encodes one of the subunits that form the nicotinic acetylcholine receptors, the activation of which leads to downstream dopamine release. We also reviewed 30 pharmacogenetic studies for a treatment by genotype effect of candidate genes, not yet identified by GWAS and show inconsistent results; however, multiple candidate genes (e.g., COMT, DRD2, DRD4 and CYP2B6) may predict efficacy of bupropion.
In addition, we find that study design is crucial in the linkage of evidence and clinical applications. Randomization is key in determining whether the difference is based on the medication or other selecting factors and 30% of the studies categorized under pharmacogenetics do not have a randomization design. Having a placebo-controlled arm is another key in determining whether the medication efficacy (defined as medication vs placebo) varies by genetic markers. Many studies (60%) have no placebo control or use a different medication as control.
This systematic review also highlights the highly variable level of replication and nonreplication across these gene–drug pairs examined in the RCTs. For example, for CHRNA5, there are 4 reports from 11 studies showing positive pharmacogenetics associations and seven reports from 14 studies showing negative associations. Replication and sample size are both important in determining the strength of the evidence. In addition, few pharmacogenetics studies (10%) examined individuals of non-European ancestry. This clearly indicates a research gap because the effect and distribution of genetic markers may differ across diverse populations. For example, the frequency distribution of risk haplotypes in CHRNA5-A3-B4 varied significantly across individuals of European, African and Asian ancestry [93]. Therefore, the validity and utility of these pharmacogenetics findings may vary across populations.
This review needs to be interpreted with several limitations in mind. First, findings that are not published or indexed in PubMed before 5/22/2017 are not included. However, PubMed is the primary database that indexes the medical literature including pharmacogenetics findings. Second, our focus is on pharmacogenetics (does the genetic marker predict a superior or inferior efficacy of medication compared with placebo or a different medication?), therefore we focused on papers with study design to answer the pharmacogenetic question (RCT with placebo control, RCT with comparative arms and open-label trial with comparative arms). This paper does not cover the other related questions (e.g., Does the genetic marker predict medication efficacy among individuals taking the same specific medication? Does the genetic marker predict smoking cessation among individuals taking the placebo or no medication?). Other reviews on genetics of smoking cessation [94,95] and a Cochrane review of different pharmacogenetics studies are available to provide insights into these related questions [61].
Conclusion
In conclusion, pharmacogenetics of smoking cessation is a rapidly growing field and likely to benefit from these scientific efforts: Larger GWAS of smoking behaviors [19,74] are likely to reveal exponentially increasing number of promising genetic markers for translational investigation; we need more clinical trials with genetic markers in diverse populations beyond European ancestry; meta-analyses of existing studies with careful adjudication are necessary because of the limited sample size in treatment trials and the need to compare and combine different pharmacotherapy arms. Replication and sufficient study power are only possible with large collaborative efforts and necessary for clinical translation and anticipate future use of polygenic predictors in predicting cessation success, smoking-related health outcomes, efficacy and side effects of pharmacotherapy. Smoking cessation pharmacotherapy such as NRT, bupropion and varenicline are moderately effective, yet have side effects. Identifying genes predicting efficacy and side effects may lead to improved treatment algorithms that further the precision treatment to help smokers quit successfully.
Future perspective
With increasing use of genetic markers in medicine, we anticipate future use of polygenic predictors for patients in predicting cessation success, smoking-related health outcomes and for providers in choice of cessation pharmacotherapy for maximized efficacy and minimized side effects.
Executive summary.
Methods
We conducted a systematic review of pharmacogenetics in smoking cessation.
We searched the PubMed, identified 644 articles and included a total of 46 articles based on predefined criteria.
Genes with genome-wide association studies hits
CHRNA5-A3-B4 gene cluster is the most extensively studied in smoking cessation pharmacogenetics. The following single nucleotide polymorphisms (rs1051730, rs588765, rs2036527 and rs16969968) have all shown significant interaction with nicotine replacement therapy (NRT) outcomes. However, several studies have not been able to replicate the results for rs16969968.
CYP2A6 genotype variation moderates the outcome to NRT but not bupropion.
GA/AA of 1368 DBH and CT/TT of 32806 DRD2 moderate the comparative effect of NRT to placebo.
CHRNA4 rs1044396 polymorphism moderates the effect of varenicline when compared with combined varenicline and bupropion or NRT and bupropion.
Candidate genes
In COMT, rs4680 moderates the effect of NRT whereas rs165599 moderates the effect of bupropion.
Moderating effects of DRD2 Taq1A and DRD4 exon III VNTR on bupropion treatment outcome have been replicated in several studies.
CYP2B6*4 and CYP2B6*6 were both shown to be associated with bupropion treatment outcomes when compared with varenicline and placebo respectively.
Conclusion
Multiple pharmacodynamics marker (e.g., CHRNA5, DBH and CHRNA4) and pharmacokinetic marker (e.g., CYP2A6) may predict efficacy of NRT.
Multiple candidate genes (e.g., COMT, DRD2, DRD4 and CYP2B6) may predict efficacy of bupropion.
Discrepant results may be influenced by the sample ascertainment, study power, concurrent nonpharmacological therapy and other potential confounders.
Study design is crucial, particularly the use of randomization and a placebo-controlled arm.
Meta-analyses of existing studies with careful adjudication are necessary to generate pharmacogenetic evidence before clinical translation.
Supplementary Material
Acknowledgments
The authors thank Nina Smock for her assistance in project coordination and editing/preparing the manuscript. Salloum NC and Trapp N were affiliated with Washington University in St Louis when the major part of the work was completed. Salloum NC is currently affiliated with Massachusetts General Hospital/Harvard Medical School and Trapp N is affiliated with the University of Iowa.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: https://www.futuremedicine.com/doi/suppl/10.2217/pgs-2018-0023
Financial & competing interests disclosure
This research was supported by R01 DA038076, and K08 DA030398 (Chen LS) from the National Institute on Drug Abuse, P30 CA091842-16S2 from the National Caner Institute, U54 MD010724 (David SP), and sub-award KL2 RR024994 (Chen LS) from the National Center for Research Resources. The authors have no other relevant affiliations or financialinvolvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Schroeder SA. New evidence that cigarette smoking remains the most important health hazard. N. Engl. J. Med. 2013;368(4):389–390. doi: 10.1056/NEJMe1213751. [DOI] [PubMed] [Google Scholar]
- 2.Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N. Engl. J. Med. 2013;368(4):351–364. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whiteford HA, Baxter AJ. The global burden of disease 2010 study: what does it tell us about mental disorders in Latin America? Rev. Bras. Psiquiatr. 2013;35(2):111–112. doi: 10.1590/1516-4446-2012-3502. [DOI] [PubMed] [Google Scholar]
- 4.Knopik VS, Maccani MA, Francazio S, McGeary JE. The epigenetics of maternal cigarette smoking during pregnancy and effects on child development. Dev. Psychopathol. 2012;24(4):1377–1390. doi: 10.1017/S0954579412000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.2008 PHS Guideline Update Panel La and Staff. Treating tobacco use and dependence: 2008 Update US Public Health Service Clinical Practice Guideline executive summary. Respir. Care. 2008;53(9):1217–1222. [PubMed] [Google Scholar]
- 6.Schlam TR, Baker TB. Interventions for tobacco smoking. Annu. Rev. Clin. Psychol. 2013;9:675–702. doi: 10.1146/annurev-clinpsy-050212-185602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gold AB, Lerman C. Pharmacogenetics of smoking cessation: role of nicotine target and metabolism genes. Hum. Genet. 2012 doi: 10.1007/s00439-012-1143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rose JE, Behm FM, Drgon T, Johnson C, Uhl GR. Personalized smoking cessation: interactions between nicotine dose, dependence and quit-success genotype score. Mol. Med. 2010;16(7-8):247–253. doi: 10.2119/molmed.2009.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uhl GR, Drgon T, Johnson C, Ramoni MF, Behm FM, Rose JE. Genome-wide association for smoking cessation success in a trial of precessation nicotine replacement. Mol. Med. 2010;16(11–12):513–526. doi: 10.2119/molmed.2010.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGeary JE, Knopik VS, Hayes JE, Palmer RH, Monti PM, Kalman D. Predictors of relapse in a bupropion trial for smoking cessation in recently-abstinent alcoholics: preliminary results using an aggregate genetic risk score. Subst. Abuse. 2012;6:107–114. doi: 10.4137/SART.S8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bough KJ, Lerman C, Rose JE, et al. Biomarkers for smoking cessation. Clin. Pharmacol. Ther. 2013;93(6):526–538. doi: 10.1038/clpt.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saccone NL, Baurley JW, Bergen AW, et al. The value of biosamples in smoking cessation trials: a review of genetic, metabolomic, and epigenetic findings. Nicotine Tob. Res. 2017;20(4):403–413. doi: 10.1093/ntr/ntx096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xian H, Scherrer JF, Madden PA, et al. The heritability of failed smoking cessation and nicotine withdrawal in twins who smoked and attempted to quit. Nicotine Tob. Res. 2003;5(2):245–254. [PubMed] [Google Scholar]
- 14.Gelernter J. Genetics of complex traits in psychiatry. Biol. Psychiatry. 2015;77(1):36–42. doi: 10.1016/j.biopsych.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kendler KS, Chen X, Dick D, et al. Recent advances in the genetic epidemiology and molecular genetics of substance use disorders. Nat. Neurosci. 2012;15(2):181–189. doi: 10.1038/nn.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loukola A, Hällfors J, Korhonen T, Kaprio J. Genetics and smoking. Curr. Addict. Rep. 2014;1(1):75–82. doi: 10.1007/s40429-013-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bierut LJ. Genetic vulnerability and susceptibility to substance dependence. Neuron. 2011;69(4):618–627. doi: 10.1016/j.neuron.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saccone SF, Hinrichs AL, Saccone NL, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum. Mol. Genet. 2007;16(1):36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hancock DB, Guo Y, Reginsson GW, et al. Genome-wide association study across European and African American ancestries identifies a SNP in DNMT3B contributing to nicotine dependence. Mol. Psychiatry. 2017 doi: 10.1038/mp.2017.193. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mamoun M, Bergen AW, Shieh J, Wiggins A, Brody AL. Biomarkers of response to smoking cessation pharmacotherapies: progress to date. CNS Drugs. 2015;29(5):359–369. doi: 10.1007/s40263-015-0243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergen AW, Javitz HS, Krasnow R, et al. Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenet. Genomics. 2013;23(2):94–103. doi: 10.1097/FPC.0b013e32835cdabd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen LS, Baker TB, Piper ME, et al. Interplay of genetic risk factors (CHRNA5-CHRNA3-CHRNB4) and cessation treatments in smoking cessation success. Am. J. Psychiatry. 2012;169(7):735–742. doi: 10.1176/appi.ajp.2012.11101545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen LS, Bloom AJ, Baker TB, et al. Pharmacotherapy effects on smoking cessation vary with nicotine metabolism gene (CYP2A6) Addiction. 2014;109(1):128–137. doi: 10.1111/add.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.David SP, Hamidovic A, Chen GK, et al. Genome-wide meta-analyses of smoking behaviors in African Americans. Transl. Psychiatry. 2012;2:e119. doi: 10.1038/tp.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King DP, Paciga S, Pickering E, et al. Smoking cessation pharmacogenetics: analysis of varenicline and bupropion in placebo-controlled clinical trials. Neuropsychopharmacology. 2012;37(3):641–650. doi: 10.1038/npp.2011.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu JZ, Tozzi F, Waterworth DM, et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat. Genet. 2010;42(5):436–440. doi: 10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munafo MR, Johnstone EC, Walther D, Uhl GR, Murphy MF, Aveyard P. CHRNA3 rs1051730 genotype and short-term smoking cessation. Nicotine Tob. Res. 2011;13(10):982–988. doi: 10.1093/ntr/ntr106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saccone NL, Culverhouse RC, Schwantes-An TH, et al. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genetics. 2010;6(8):e1001053. doi: 10.1371/journal.pgen.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorgeirsson TE, Geller F, Sulem P, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452(7187):638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorgeirsson TE, Gudbjartsson DF, Surakka I, et al. Sequence variants at CHRNB3–CHRNA6 and CYP2A6 affect smoking behavior. Nat. Genet. 2010;42(5):448–453. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat. Genet. 2010;42(5):441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu AZ, Zhou Q, Cox LS, et al. Association of CHRNA5-A3-B4 SNP rs2036527 with smoking cessation therapy response in African American smokers. Clin. Pharmacol. Ther. 2014;96(2):256–265. doi: 10.1038/clpt.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bloom AJ, Harari O, Martinez M, et al. Use of a predictive model derived from in vivo endophenotype measurements to demonstrate associations with a complex locus, CYP2A6 . Hum. Mol. Genet. 2012;21(13):3050–3062. doi: 10.1093/hmg/dds114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canova C, Hashibe M, Simonato L, et al. Genetic associations of 115 polymorphisms with cancers of the upper aerodigestive tract across 10 European countries: the ARCAGE project. Cancer Res. 2009;69(7):2956–2965. doi: 10.1158/0008-5472.CAN-08-2604. [DOI] [PubMed] [Google Scholar]
- 35.Dempsey D, Tutka P, Jacob P, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin. Pharmacol. Ther. 2004;76(1):64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Fujieda M, Yamazaki H, Saito T, et al. Evaluation of CYP2A6 genetic polymorphisms as determinants of smoking behavior and tobacco-related lung cancer risk in male Japanese smokers. Carcinogenesis. 2004;25(12):2451–2458. doi: 10.1093/carcin/bgh258. [DOI] [PubMed] [Google Scholar]
- 37.Gemignani F, Landi S, Szeszenia-Dabrowska N, et al. Development of lung cancer before the age of 50: the role of xenobiotic metabolizing genes. Carcinogenesis. 2007;28(6):1287–1293. doi: 10.1093/carcin/bgm021. [DOI] [PubMed] [Google Scholar]
- 38.Ho MK, Mwenifumbo JC, Al Koudsi N, et al. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African American light smokers. Clin. Pharmacol. Ther. 2009;85(6):635–643. doi: 10.1038/clpt.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lerman C, Tyndale R, Patterson F, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin. Pharmacol. Ther. 2006;79(6):600–608. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Lerman C, Jepson C, Wileyto EP, et al. Genetic variation in nicotine metabolism predicts the efficacy of extended-duration transdermal nicotine therapy. Clin. Pharmacol. Ther. 2010;87(5):553–557. doi: 10.1038/clpt.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu T, David SP, Tyndale RF, et al. Associations of CYP2A6 genotype with smoking behaviors in southern China. Addiction. 2011;106(5):985–994. doi: 10.1111/j.1360-0443.2010.03353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patterson F, Schnoll RA, Wileyto EP, et al. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin. Pharmacol. Ther. 2008;84(3):320–325. doi: 10.1038/clpt.2008.57. [DOI] [PubMed] [Google Scholar]
- 43.Rotunno M, Yu K, Lubin JH, et al. Phase I metabolic genes and risk of lung cancer: multiple polymorphisms and mRNA expression. PLoS ONE. 2009;4(5):e5652. doi: 10.1371/journal.pone.0005652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacol. Biochem. Behav. 2009;92(1):6–11. doi: 10.1016/j.pbb.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14(9):615–626. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Tamaki Y, Arai T, Sugimura H, et al. Association between cancer risk and drug-metabolizing enzyme gene (CYP2A6, CYP2A13, CYP4B1, SULT1A1, GSTM1, and GSTT1) polymorphisms in cases of lung cancer in Japan. Drug Metab. Pharmacokinet. 2011;26(5):516–522. doi: 10.2133/dmpk.dmpk-11-rg-046. [DOI] [PubMed] [Google Scholar]
- 47.Topcu Z, Chiba I, Fujieda M, et al. CYP2A6 gene deletion reduces oral cancer risk in betel quid chewers in Sri Lanka. Carcinogenesis. 2002;23(4):595–598. doi: 10.1093/carcin/23.4.595. [DOI] [PubMed] [Google Scholar]
- 48.Benowitz NL, Zhu AZ, Tyndale RF, Dempsey D, Jacob P. Influence of CYP2B6 genetic variants on plasma and urine concentrations of bupropion and metabolites at steady state. Pharmacogenet Genomics. 2013;23(3):135–141. doi: 10.1097/FPC.0b013e32835d9ab0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu AZ, Cox LS, Nollen N, et al. CYP2B6 and bupropion's smoking-cessation pharmacology: the role of hydroxybupropion. Clin. Pharmacol. Ther. 2012;92(6):771–777. doi: 10.1038/clpt.2012.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bergen AW, Javitz HS, Krasnow R, et al. Organic cation transporter variation and response to smoking cessation therapies. Nicotine Tob. Res. 2014;16(12):1638–1646. doi: 10.1093/ntr/ntu161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balfour DJ. The neuronal pathways mediating the behavioral and addictive properties of nicotine. Handb. Exp. Pharmacol. 2009;192:209–233. doi: 10.1007/978-3-540-69248-5_8. [DOI] [PubMed] [Google Scholar]
- 52.David SP, Munafò MR, Murphy MF, et al. Genetic variation in the dopamine D4 receptor (DRD4) gene and smoking cessation: follow-up of a randomized clinical trial of transdermal nicotine patch. Pharmacogenomics J. 2008;8(2):122–128. doi: 10.1038/sj.tpj.6500447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hadjiconstantinou M, Neff NH. Nicotine and endogenous opioids: neurochemical and pharmacological evidence. Neuropharmacology. 2011;60(7–8):1209–1220. doi: 10.1016/j.neuropharm.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 54.Marteau TM, Aveyard P, Munafò MR, et al. Effect on adherence to nicotine replacement therapy of informing smokers their dose is determined by their genotype: a randomised controlled trial. PLoS ONE. 2012;7(4):e35249. doi: 10.1371/journal.pone.0035249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Munafò MR, Elliot KM, Murphy MF, Walton RT, Johnstone EC. Association of the mu-opioid receptor gene with smoking cessation. Pharmacogenomics J. 2007;7(5):353–361. doi: 10.1038/sj.tpj.6500432. [DOI] [PubMed] [Google Scholar]
- 56.Munafò MR, Johnstone EC, Aveyard P, Marteau T. Lack of association of OPRM1 genotype and smoking cessation. Nicotine Tob. Res. 2013;15(3):739–744. doi: 10.1093/ntr/nts174. [DOI] [PubMed] [Google Scholar]
- 57.Quaak M, van Schayck CP, Postma DS, Wagena EJ, van Schooten FJ. Genetic variants in the serotonin transporter influence the efficacy of bupropion and nortriptyline in smoking cessation. Addiction. 2012;107(1):178–187. doi: 10.1111/j.1360-0443.2011.03534.x. [DOI] [PubMed] [Google Scholar]
- 58.Ray R, Jepson C, Wileyto EP, et al. Genetic variation in mu-opioid-receptor-interacting proteins and smoking cessation in a nicotine replacement therapy trial. Nicotine Tob. Res. 2007;9(11):1237–1241. doi: 10.1080/14622200701648367. [DOI] [PubMed] [Google Scholar]
- 59.Verhagen M, Kleinjan M, Engels RC. A systematic review of the A118G (Asn40Asp) variant of OPRM1 in relation to smoking initiation, nicotine dependence and smoking cessation. Pharmacogenomics. 2012;13(8):917–933. doi: 10.2217/pgs.12.76. [DOI] [PubMed] [Google Scholar]
- 60.Wang J, Li MD. Common and unique biological pathways associated with smoking initiation/progression, nicotine dependence, and smoking cessation. Neuropsychopharmacology. 2010;35(3):702–719. doi: 10.1038/npp.2009.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schuit E, Panagiotou OA, Munafo MR, Bennett DA, Bergen AW, David SP. Pharmacotherapy for smoking cessation: effects by subgroup defined by genetically informed biomarkers. Cochrane Database Syst. Rev. 2017;9 doi: 10.1002/14651858.CD011823.pub2. CD011823. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.De Ruyck K, Nackaerts K, Beels L, et al. Genetic variation in three candidate genes and nicotine dependence, withdrawal and smoking cessation in hospitalized patients. Pharmacogenomics. 2010;11(8):1053–1063. doi: 10.2217/pgs.10.75. [DOI] [PubMed] [Google Scholar]
- 63.Sarginson JE, Killen JD, Lazzeroni LC, et al. Markers in the 15q24 nicotinic receptor subunit gene cluster (CHRNA5-A3-B4) predict severity of nicotine addiction and response to smoking cessation therapy. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011;156B(3):275–284. doi: 10.1002/ajmg.b.31155. [DOI] [PubMed] [Google Scholar]
- 64.Hung RJ, McKay JD, Gaborieau V, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452(7187):633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 65.Cho MH, McDonald ML, Zhou X, et al. Risk loci for chronic obstructive pulmonary disease: a genome-wide association study and meta-analysis. Lancet Respir. Med. 2014;2(3):214–225. doi: 10.1016/S2213-2600(14)70002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen LS, Baker TB, Jorenby D, et al. Genetic variation (CHRNA5), medication (combination nicotine replacement therapy vs. varenicline), and smoking cessation. Drug Alcohol Depend. 2015;154:278–282. doi: 10.1016/j.drugalcdep.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tyndale RF, Zhu AZ, George TP, et al. Lack of associations of CHRNA5-A3-B4 genetic variants with smoking cessation treatment outcomes in Caucasian smokers despite associations with baseline smoking. PLoS ONE. 2015;10(5):e0128109. doi: 10.1371/journal.pone.0128109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hubacek JA, Pankova A, Stepankova L, et al. SNPs within CHRNA5-A3-B4 and CYP2A6/B6 are associated with smoking dependence but not with tobacco dependence treatment outcomes in the Czech population. Gene. 2017;606:35–38. doi: 10.1016/j.gene.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 69.Sarginson JE, Killen JD, Lazzeroni LC, et al. Response to transdermal selegiline smoking cessation therapy and markers in the 15q24 chromosomal region. Nicotine Tob. Res. 2015;17(9):1126–1133. doi: 10.1093/ntr/ntu273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Messina ES, Tyndale RF, Sellers EM. A major role for CYP2A6 in nicotine C-oxidation by human liver microsomes. J. Pharmacol. Exp. Ther. 1997;282(3):1608–1614. [PubMed] [Google Scholar]
- 71.Chen LS, Bloom AJ, Baker TB, et al. Pharmacotherapy effects on smoking cessation vary with nicotine metabolism gene (CYP2A6) Addiction. 2014;109(1):128–137. doi: 10.1111/add.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnstone EC, Yudkin PL, Hey K, et al. Genetic variation in dopaminergic pathways and short-term effectiveness of the nicotine patch. Pharmacogenetics. 2004;14(2):83–90. doi: 10.1097/00008571-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 73.Breitling LP, Twardella D, Hoffmann MM. Prospective association of dopamine-related polymorphisms with smoking cessation in general care. Pharmacogenomics. 2010;11(4):527–536. doi: 10.2217/pgs.10.1. [DOI] [PubMed] [Google Scholar]
- 74.Hancock DB, Reginsson GW, Gaddis NC, et al. Genome-wide meta-analysis reveals common splice site acceptor variant in CHRNA4 associated with nicotine dependence. Transl. Psychiatry. 2015;5:e651. doi: 10.1038/tp.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rocha Santos J, Tomaz PR, Issa JS, et al. CHRNA4 rs1044396 is associated with smoking cessation in varenicline therapy. Front. Genet. 2015;6:46. doi: 10.3389/fgene.2015.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnstone EC, Elliot KM, David SP, Murphy MF, Walton RT, Munafò MR. Association of COMT Val108/158Met genotype with smoking cessation in a nicotine replacement therapy randomized trial. Cancer Epidemiol. Biomarkers Prev. 2007;16(6):1065–1069. doi: 10.1158/1055-9965.EPI-06-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun H, Guo S, Chen D, et al. Association of functional COMT Val108/Met polymorphism with smoking cessation in a nicotine replacement therapy. J. Neural. Transm. 2012;119(12):1491–1498. doi: 10.1007/s00702-012-0841-8. [DOI] [PubMed] [Google Scholar]
- 78.David SP, Strong DR, Leventhal AM, et al. Influence of a dopamine pathway additive genetic efficacy score on smoking cessation: results from two randomized clinical trials of bupropion. Addiction. 2013;108(12):2202–2211. doi: 10.1111/add.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berrettini WH, Wileyto EP, Epstein L, et al. Catechol-O-methyltransferase (COMT) gene variants predict response to bupropion therapy for tobacco dependence. Biol. Psychiatry. 2007;61(1):111–118. doi: 10.1016/j.biopsych.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 80.Blum K, Noble EP, Sheridan PJ, et al. Allelic association of human dopamine D2 receptor gene in alcoholism. JAMA. 1990;263(15):2055–2060. [PubMed] [Google Scholar]
- 81.David SP, Strong DR, Munafò MR, et al. Bupropion efficacy for smoking cessation is influenced by the DRD2 Taq1A polymorphism: analysis of pooled data from two clinical trials. Nicotine Tob. Res. 2007;9(12):1251–1257. doi: 10.1080/14622200701705027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.David SP, Brown RA, Papandonatos GD, et al. Pharmacogenetic clinical trial of sustained-release bupropion for smoking cessation. Nicotine Tob. Res. 2007;9(8):821–833. doi: 10.1080/14622200701382033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tomaz PR, Santos JR, Issa JS, et al. CYP2B6 rs2279343 polymorphism is associated with smoking cessation success in bupropion therapy. Eur. J. Clin. Pharmacol. 2015;71(9):1067–1073. doi: 10.1007/s00228-015-1896-x. [DOI] [PubMed] [Google Scholar]
- 84.Lerman C, Jepson C, Wileyto EP, et al. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: results of two randomized clinical trials. Neuropsychopharmacology. 2006;31(1):231–242. doi: 10.1038/sj.npp.1300861. [DOI] [PubMed] [Google Scholar]
- 85.Leventhal AM, David SP, Brightman M, et al. Dopamine D4 receptor gene variation moderates the efficacy of bupropion for smoking cessation. Pharmacogenomics J. 2012;12(1):86–92. doi: 10.1038/tpj.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bergen AW, Javitz HS, Su L, et al. The DRD4 exon III VNTR, bupropion, and associations with prospective abstinence. Nicotine Tob. Res. 2013;15(7):1190–1200. doi: 10.1093/ntr/nts245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Faucette SR, Hawke RL, Lecluyse EL, et al. Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab. Dispos. 2000;28(10):1222–1230. [PubMed] [Google Scholar]
- 88.Lee AM, Jepson C, Hoffmann E, Epstein L, Hawk LW, Lerman C, et al. CYP2B6 genotype alters abstinence rates in a bupropion smoking cessation trial. Biol. Psychiatry. 2007;62(6):635–641. doi: 10.1016/j.biopsych.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 89.Hesse LM, He P, Krishnaswamy S, et al. Pharmacogenetic determinants of interindividual variability in bupropion hydroxylation by cytochrome P450 2B6 in human liver microsomes. Pharmacogenetics. 2004;14(4):225–238. doi: 10.1097/00008571-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 90.Saccone NL, Culverhouse RC, Schwantes-An TH, et al. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 2010;6(8):pii:e1001053. doi: 10.1371/journal.pgen.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Keskitalo K, Broms U, Heliovaara M, et al. Association of serum cotinine level with a cluster of three nicotinic acetylcholine receptor genes (CHRNA3/CHRNA5/CHRNB4) on chromosome 15. Hum. Mol. Genet. 2009;18(20):4007–4012. doi: 10.1093/hmg/ddp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu AZ, Renner CC, Hatsukami DK, et al. Allelic association of human dopamine D2 receptor gene in alcoholism. Addiction. 2013;108(10):1818–1828. [Google Scholar]
- 93.Chen LS, Saccone NL, Culverhouse RC, et al. Smoking and genetic risk variation across populations of European, Asian, and African American ancestry: a meta-analysis of chromosome 15q25. Genet. Epidemiol. 2012;36(4):340–351. doi: 10.1002/gepi.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen LS, Horton A, Bierut L. Pathways to precision medicine in smoking cessation treatments. Neurosci Lett. 2016;669:92. doi: 10.1016/j.neulet.2016.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen LS, Zawertailo L, Piasecki TM, et al. Leveraging genomic data in smoking cessation trials in the era of precision medicine: why and how. Nicotine Tob. Res. 2017;20(4):424. doi: 10.1093/ntr/ntx097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.