Abstract
Research has demonstrated links between adult romantic attachment and one's own physical health; little is known about links between adult attachment orientations and offspring health. Prior work has shown that parents' greater attachment anxiety and avoidance predicts less warmth toward their children. Extensive work has also shown that lower maternal warmth has negative downstream effects on offspring health. We tested the novel hypothesis that mothers' dispositional romantic attachment would be linked—via maternal warmth—to their children's expression of the glucocorticoid receptor gene NR3C1, higher expression of which is associated with healthier stress-regulation and inflammatory response. In a sample of 132 youth with asthma, we found that mothers' attachment anxiety and avoidance were both negatively associated with children's expression of NR3C1, explained by lower youth-rated maternal warmth. Effects held after adjusting for demographic and psychosocial covariates. Implications for parents' attachment influencing the health of offspring are discussed.
Keywords: Attachment, maternal warmth, gene expression, glucocorticoid receptor, NR3C1, family
Parents leave a lasting imprint on their offspring, and recent work has shown that individual differences in parents' romantic attachment orientations (Adam, Gunnar, & Tanaka, 2004) and expressions of warmth (Chen, Miller, Kobor, & Cole, 2010) predict important health-related outcomes in their offspring. Research has demonstrated links between adult romantic attachment and one's own physical health (for reviews, see Farrell & Simpson, 2017; Pietromonaco & Powers, 2015; Stanton & Campbell, 2014a); however, little is known about the links between adult attachment and the molecular mechanisms underlying the health of offspring. Advancing attachment theory and the emerging field of social genomics (cf. Slavich & Cole, 2013), the present study tested, in a sample of youth with asthma, the hypothesis that mothers' dispositional romantic attachment anxiety and avoidance would be linked with their children's expression of the glucocorticoid receptor gene NR3C1, a gene believed to play an important role in stress-regulation and glucocorticoid resistance (Bamberger et al., 1996; Bray & Cotton, 2003; Carpe et al., 2010), and explored maternal warmth as a putative mediator of this association.
According to Bowlby (1980), the attachment system encompasses patterns of cognition, emotion, and behavior that shape interpersonal experiences “from the cradle to the grave” (p. 129). Internal working models of attachment develop in early childhood and endure through adulthood (Fraley & Roisman, 2015; Simpson, W.A. Collins, Farrell, & Raby, 2015). Individual differences in attachment occur along two theoretically distinct dimensions (Fraley, Waller, & Brennan, 2000):Attachment anxiety reflects how much individuals worry and ruminate in their relationships (e.g., those scoring higher on attachment anxiety crave love while simultaneously doubting others' intentions; N.L. Collins, 1996). Conversely, attachment avoidance reflects how uncomfortable individuals are with intimacy and dependence in their relationships (e.g., those scoring higher on attachment avoidance express less warmth and are less invested in relationships; Mikulincer & Shaver, 2003). Persons scoring lower on attachment anxiety or avoidance are secure in their relationships, meaning they are not preoccupied with rejection or abandonment and are comfortable with closeness.
Recent research has demonstrated increasing evidence for links between individual differences in one's own attachment orientations and health-related outcomes. Specifically, more insecurely attached persons (i.e., those scoring higher on attachment anxiety, avoidance, or both) experience poorer health (see Farrell & Simpson, 2017; Pietromonaco & Powers, 2015; Stanton & Campbell, 2014a). For instance, both higher attachment anxiety (Jaremka et al., 2013) and higher attachment avoidance (Picardi et al., 2013) are linked to alterations in cellular immunity (e.g., T-cells). Insecure attachment is also linked to increased proinflammatory cytokines during conflict discussions (Gouin et al., 2009) and heightened cortisol reactivity to stressors (Powers, Pietromonaco, Gunlicks, & Sayer, 2006; Quirin, Pruessner, & Kuhl, 2008). Additionally, one study found that one's attachment orientations in infancy predicts susceptibility to inflammatory diseases 32 years later (Puig, Englund, Simpson, & W.A. Collins, 2013). These studies provide evidence for links between attachment and one's own health; however, extant research leaves unanswered the important question of whether adult attachment is linked to the health of offspring.
Not only does research suggest that individual differences in attachment are meaningfully associated with health-related outcomes, but these individual differences also predict parents' expression of warmth toward their children (e.g., Adam et al., 2004; Cohn, P.A. Cowan, C.P. Cowan, & Pearson, 1992; Jones, Cassidy, & Shaver, 2015). For example, more insecurely attached parents tend to be less warm and provide less structure in interactions with their children (Cohn et al., 1992). Diminished expression of warmth exerts detrimental effects on both the quality of the insecurely attached parents' relationships with their children and more distal child outcomes. Indeed, children of parents who are less warm have more behavior problems (Kochanska & Kim, 2013) and less social and academic competence as they age (Raby, Roisman, Fraley, & Simpson, 2015). Moreover, compared to those who experience greater maternal warmth, youth who experience lower maternal warmth have poorer physical health outcomes later in life (Chen et al., 2010; Miller, Lachman, et al., 2011). Guided by this research, we propose that more insecurely attached mothers' tendency to eschew expressions of warmth has deleterious effects on their offspring at a molecular level.
Researchers have begun to uncover the molecular mechanisms underlying the links between social relationships and health, paying specific attention to the social correlates of health-relevant gene expression and the downstream effects of gene expression on health outcomes (Cole et al., 2007; Slavich & Cole, 2013). The glucocorticoid receptor gene NR3C1 is viewed as an important modulator of one's inflammatory response and capacity for stress-regulation (e.g., Bet et al., 2009). Lower expression of NR3C1 has been proposed as a mechanism for glucocorticoid resistance, whereby the body's cells are unable to receive signals to terminate the inflammatory response (Carpe et al., 2010). In turn, chronic over-activation of the inflammatory response increases susceptibility to illness over time (Bray & Cotton, 2003; Cohen et al., 2012; McMahon et al., 2009). Glucocorticoid resistance is particularly problematic for those with asthma, a chronic inflammatory disease of the airways, making expression of NR3C1 important to study (see Barnes & Adcock, 2009; Miller & Chen, 2006). Prior studies have linked greater life stress (Miller & Chen, 2006) as well as targeted rejection (Murphy, Slavich, Chen, & Miller, 2015) with lower expression of the glucocorticoid receptor gene measured in the blood of youth with asthma. Other research has found associations between greater childhood abuse and lower levels of glucocorticoid receptor gene expression measured in the brain of suicide victims (McGowan et al., 2009). Additionally, it appears that lower expression of the glucocorticoid receptor gene may be related to greater glucocorticoid resistance (Carpe et al., 2010), which may play a later role in asthma complications or morbidity (cf. Meduri & Yates, 2004). Given empirical evidence suggesting that lower maternal warmth predicts greater stress reactivity and susceptibility to chronic diseases later in life (Hackman et al., 2013; Hostinar, Sullivan, & Gunnar, 2014; Miller, Chen, & Parker, 2011), in combination with evidence suggesting that adverse childhood experiences (e.g., abuse) correlate with lower glucocorticoid receptor gene expression (McGowan et al., 2009), it seems possible that lower maternal warmth may be associated with lower expression of NR3C1 in offspring.
Prior research makes a compelling case for associations between (a) mothers' attachment and their offspring's outcomes, and (b) maternal warmth and offspring health, suggesting that mothers' attachment and maternal warmth may result in downstream effects in offspring'shealth-relevant molecular pathways. However, to date no studies have systematically explored this possibility. The current study investigated how expression of the glucocorticoid receptor gene NR3C1, a key mechanism in the inflammatory response, is associated with mothers' adult attachment orientations and maternal warmth. Data for this study were collected from a sample of youth with asthma. Since asthma is a chronic inflammatory disease, and standard asthma treatment is often inhaled glucocorticoids, examining NR3C1 expression in this population is especially relevant. We hypothesized that mothers' greater attachment anxiety and avoidance would be associated with lower expression of NR3C1 in offspring, and that this relation would occur via lower levels of youth-rated maternal warmth. In other words, mothers' greater insecure attachment would be linked to lower maternal warmth, which in turn would be linked to lower expression of NR3C1. In ancillary analyses, we controlled for covariates associated with NR3C1 gene expression (i.e., demographic factors and mothers' neuroticism and depressive symptoms). The psychosocial factors were included to rule out a key potential confound—general negativity among mothers—which could explain the associations between mothers' attachment orientations and offspring's NR3C1 expression.
Method
Participants
Participants were drawn from Wave 1 of a larger longitudinal project, Asthma in the Lives of Families Today (ALOFT), a study of youth with asthma and their primary caregivers. Youth were recruited through metropolitan Detroit, Michigan hospitals, clinics, and area schools. Families were eligible for the study if youth were between the ages of 10-17 with a diagnosis of mild intermittent to severe persistent asthma. Families were excluded from participating in the study if their child was using oral steroid medication(s) during the initial screening, had a diagnosis of a chronic condition other than asthma (e.g., endocrine disorders, immunodeficiency, cardiovascular disease), or a diagnosis of a medical condition that may interfere with immune system function (e.g., pregnancy, chemotherapy, or radiotherapy in the past year). A sample of 180 families were recruited; data collection stopped at 180 families based on analyses indicating that this sample would have sufficient power to test main effects of social/health processes with effect sizes ranging from small to medium.
Given the small number of male caregivers in our study (<5%),the current analyses included only youth whose primary caregivers were mothers. Youth without gene expression data (because of, e.g., failed blood draw or assay-related problems) were not included in the current study, leaving a final sample of 132 participants (Mage= 12.78, SDage = 1.88). Approximately 18.9% of youth identified as White; 77.3% identified as Black, 1.5% identified as Latino, and 2.3% identified as multiracial. Annual maternal income ranged from the $0-$7,825 tax bracket, to the $97,926-$174,850 tax bracket, with a median range of $7,826-$31,850. Parents who were currently romantically involved reported their romantic relationship length, which ranged from 1.42 to 33.25 years (M = 16.09, SD = 7.87). Asthma diagnosis was confirmed through medical report; in the current sample, 29.9% of youth were diagnosed with severe asthma, 35.1% of youth were diagnosed with moderate to severe asthma, 11.7% of youth were diagnosed with mild to moderate and moderate asthma, and 23.4% were diagnosed with mild intermittent to mild persistent asthma. We also obtained asthma medication use across four days of the study period. Specifically, we assessed (1) inhaled beta-agonist use (yes/no), (2) inhaled corticosteroid use (yes/no), (3) inhaled combination corticosteroid and beta-agonist use (yes/no), (4) oral corticosteroid use (yes/no), and (5) leukotriene-modifying agent use (yes/no). We then created a dichotomous variable to represent asthma medication use (yes/no). If youth had a value above zero for any of the five types of asthma medication, they were given a “yes” score on the dichotomous variable; otherwise, they received a “no” score on the dichotomous variable.
Procedure
Written assent and consent were obtained from the participating youth and their caregiver(s) during a baseline laboratory visit. As part of this initial session, participants completed a series of questionnaires, including the caregivers'report of romantic attachment orientations and the youth report of maternal warmth. A peripheral blood draw was conducted on each youth after a four-day diary data collection period. For their participation, caregivers received up to US-$100.00 in cash and youth received up to US-$60.00 in gift cards depending on how much of Wave 1 of the study they completed. All study procedures were approved by the Institutional Review Board at Wayne State University.
Primary Measures
Mothers' attachment anxiety and avoidance
Mothers' romantic attachment orientations were assessed with the 36-item Experiences in Close Relationships-Revised Scale (ECR-R; Fraley et al., 2000). Attachment anxiety was measured with 18 items that tap into respondents' fear of rejection and abandonment by close others (e.g., “I worry that romantic partners won't care about me as much as I care about them”). Attachment avoidance was measured with 18 items that reflect respondents' discomfort with intimacy in close relationships (i.e. “I prefer not to show a partner how I feel deep down”). Participants indicated their agreement with each statement on a scale of 1 (strongly disagree) to 7 (strongly agree). Anxiety and avoidance scores were created by averaging responses across the 18 items of the two subscales such that higher scores indicated greater attachment anxiety (α = .93) and avoidance (α = .94).
Youth-rated maternal warmth
Youth perceptions of closeness and warmth in their relationship with their mothers were assessed with 23 items from the Parental Behavior Inventory (PBI; Schaefer, 1965). The PBI is designed to assess youth perceptions of parental behavior through descriptions of specific, observable behaviors; in the current study, we examined items focusing on expressions of maternal warmth (e.g., “My mother (or female guardian) shows love for me”). Youth rated their agreement with each item on a scale of 1 (agree) and 3 (disagree). Maternal warmth scores were computed by averaging responses across items such that higher scores indicated greater youth-reported maternal warmth (α = .95).
Youth NR3C1 gene expression
Blood was drawn Mondays, Tuesdays, and Wednesdays between 7:00AM and 10:00AM (most commonly at 7:00AM before the children went to school). Each youth provided up to 6 mL of peripheral blood collected into Vacutainer Cell Preparation Tubes containing K2 EDTA (Becton Dickinson and Co., East Rutherford, NJ, USA). In order to assess messenger RNA (mRNA) levels of the glucocorticoid receptor gene NR3C1, total RNA was extracted from peripheral blood using the LeukoLOCK Total RNA Isolation System, following the manufacturer's protocol (Life Technologies, Grand Island, NY, USA). RNA integrity was assessed on an Agilent Bioanalyzer and only samples with RIN≥6.0 were included in the study. Total RNA was reverse transcribed to cDNA using Super Script III kit (Life Tech), following the manufacturer's protocol. Gene expression was quantified using TaqMan gene expression assays (Applied Biosystems) on an Applied Biosystems 7500-FAST or StepOnePlus real-time PCR thermocycler, following manufacturer's protocol. Average CT values were calculated for NR3C1 and the endogenous control (18S rRNA) across three experimental replicates for each sample. For each sample, the coefficient of variation across replicates was less than 20%. Relative gene expression values (in CT units) for NR3C1 in each sample were normalized to the endogenous control and expressed as delta CT values. A histogram of youth NR3C1 relative expression is shown in Figure 1.
Figure 1.
N = 132 youth. Histogram of youth NR3C1 relative expression.
Covariate Measures
Mothers' neuroticism
Mothers' dispositional neuroticism was included as a covariate to rule out effects of maternal attachment orientation on NR3C1 expression as a result of broader personality characteristics (e.g., highly neurotic people reporting greater levels of attachment anxiety and/or avoidance). Mothers' neuroticism was assessed with the 8-item subscale from the Big Five Inventory (BFI; John, Donahue, & Kentle, 1991) that taps into respondents' views that they are someone who experiences negativity (e.g., “is emotionally stable, not easily upset,” reverse-scored). Mothers rated the extent to which each trait description applied to them on a scale from 1 (disagree strongly) to 5 (agree strongly). Neuroticism scores were computed by averaging responses across items such that higher scores indicated greater neuroticism (α = .83).
Maternal depressive symptoms
We similarly included maternal depressive symptoms as an additional potential confound of the links between maternal attachment orientation and NR3C1 gene expression. Mothers' depressive symptoms were assessed with the Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977), which asks participants to rate the extent to which they have experienced various symptoms of depressed affect and behavior during the past week (e.g., “I felt that everything I did was an effort”). Mothers indicated the frequency to which they experienced each symptom on a scale from 0 (rarely or none of the time) to 3 (most of or all the time). A total depressive symptoms score was computed for mothers who provided valid answers on at least 16 of the 20 items for the scale by summing responses to the items such that higher scores indicated greater depressive symptoms (α = .88).
Statistical Analysis Strategy and Potential Covariates
We tested a number of covariates based on their a priori potential to influence our variables of interest; in particular, we tested the demographic covariates of youths' gender (0 = male, 1 = female), youths' ethnicity (0 = White, 1 = non-White), mothers' education (0 = less than one year of college, 1 = one year of college or more), mothers' relationship status (0 = not in relationship, 1 = in relationship), youths' and mothers' age, and youths' medication use (0 = no, 1 = yes), as well as the psychosocial covariates of mothers' neuroticism and depressive symptoms.
To address potential analytic problems related to missing data, we used the expectation maximization algorithm, which provides unbiased parameter estimates and improves statistical power of analyses (Enders, 2001; Scheffer, 2002), to replace missing values. All variables with missing data were continuous except for medication use, which was dichotomous. The expectation maximization algorithm does not allow value replacement for dichotomous data, and therefore we used mode replacement to replace those missing values.
We first explored the associations between primary study variables and demographic and psychological covariates using bivariate correlation analyses (see Table 1). We then tested the links between mothers' attachment anxiety and avoidance, youth-rated maternal warmth, and youth's expression of NR3C1 using multiple regression. Lastly, we tested if the links between mothers' attachment and youth's NR3C1 expression were explained by youth-rated maternal warmth using a bootstrapping approach (Hayes, 2013). To facilitate interpretation, all continuous predictors and potential covariates were standardized. We conducted models with and without covariates (see Table 2); for each model, covariates that correlated with the outcome variable at a significance level of p< .10 or lower (i.e., youths' ethnicity, mothers' relationship status, mothers' depressive symptoms, and mothers' neuroticism) were included.1
Table 1. Descriptive Statistics and Correlations among Study Variables.
| M(SD) or % | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Youth Gendera | 42.4% | — | -.02 | -.11 | .09 | .03 | .07 | .17* | -.14 | .004 | .11 | -.11 | -.002 | -.18* | .10 |
| 2 | Youth Ethnicityb | 81.1% | — | -.28** | -.25** | -.21* | .01 | -.18* | -.30** | .14 | -.17* | .20* | .20* | -.08 | -.18* | |
| 3 | Mother Educationc | 59.0% | — | .10 | .14 | -.08 | -.04 | .29** | -.05 | -.14 | -.04 | -.05 | .07 | .05 | ||
| 4 | Mother Relationship Statusd | 68.2% | — | -.01 | .05 | .06 | .06 | -.04 | .07 | -.18* | -.37** | .11 | .16+ | |||
| 5 | Youth Medication Usee | 59.1% | — | .10 | .13 | .01 | .01 | .05 | .05 | .02 | -.03 | .08 | ||||
| 6 | Youth Asthma Severity Diagnosis | 4.7(1.6) | — | .03 | -.21* | .06 | .15+ | .08 | .01 | -.11 | -.10 | |||||
| 7 | Youth Age | 12.8(1.9) | — | .30** | .05 | .07 | -.03 | .07 | -.15+ | -.04 | ||||||
| 8 | Mother Age | 40.3(7.4) | — | -.01 | .01 | -.01 | .03 | .10 | -.12 | |||||||
| 9 | Mother Depressive Symptoms | 11.3(8.8) | — | .62** | .52** | .41** | -.05 | -.16+ | ||||||||
| 10 | Mother Neuroticism | 2.6(0.9) | — | .34** | .26** | -.12 | -.16+ | |||||||||
| 11 | Mother Attachment Anxiety | 2.2(1.2) | — | .66** | -.18* | -.25** | ||||||||||
| 12 | Mother Attachment Avoidance | 2.6(1.4) | — | -.21* | -.26** | |||||||||||
| 13 | Youth-Rated Maternal Warmth | 2.7(0.4) | — | .21* | ||||||||||||
| 14 | Youth Expression of NR3C1 | 9.1(2.3) | — |
Note. M = mean; SD = standard deviation. Youth asthma severity diagnosis scores could range from 1-7. Mothers' depression scores could range from 0-60. Mothers' neuroticism scores could range from 1-5. Mothers' romantic attachment anxiety and attachment avoidance scores could range from 1-7. Youth-rated maternal warmth scores could range from 1-3.
0 = male, 1 = female.
0 = White, 1 = non-White.
0 = less than one year of college, 1 = one year of college or more.
0 = not in relationship, 1 = in relationship.
0 = no, 1 = yes.
p < .10
p < .05
p < .01
Table 2. Multiple Regression Associations between Mothers' Attachment Anxiety and Youth Expression of NR3C1.
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Coeff(SE) | 95% CI | Coeff(SE) | 95% CI | Coeff(SE) | 95% CI | Coeff(SE) | 95% CI | |
| Analysis with Mother Attachment Anxiety | ||||||||
| Mother Attachment Anxiety | -.57(.19)** | [-.95, -.19] | -.48(.20)* | [-.87, -.09] | -.52(.23)* | [-.97, -.07] | -.40(.23)+ | [-.85, .05] |
| Youth Ethnicitya | -.67(.51) | [-1.67, .34] | -.93(.54)+ | [-1.99, .13] | ||||
| Mother Relationship Statusb | .45(.43) | [-.39, 1.29] | .50(.43) | [-.35, 1.34] | ||||
| Mother Depressive Symptoms | .05(.27) | [-.48, .59] | .17(.27) | [-.37, .71] | ||||
| Mother Neuroticism | -.22(.25) | [-.71, .27] | -.41(.26) | [-.92, .10] | ||||
|
| ||||||||
| Analysis with Mother Attachment Avoidance | ||||||||
| Mother Attachment Avoidance | -.59(.19)** | [-.97, -.21] | -.49(.21)* | [-.90, -.08] | -.54(.21)* | [-.96, -.12] | -.40(.23)+ | [-.86, .05] |
| Youth Ethnicitya | -.71(.51) | [-1.71, .29] | -.96(.53)+ | [-2.02, .09] | ||||
| Mother Relationship Statusb | .24(.45) | [-.65, 1.12] | .33(.45) | [-.57, 1.22] | ||||
| Mother Depressive Symptoms | .02(.26) | [-.50, .53] | .13(.27) | [-.40, .66] | ||||
| Mother Neuroticism | -.24(.24) | [-.72, .25] | -.42(.26) | [-.93, .09] | ||||
|
| ||||||||
| Analysis with Youth-Rated Maternal Warmth | ||||||||
| Youth-Rated Maternal Warmth | .48(.19)* | [.10, .86] | .43(.19)* | [.05, .81] | .45(.19)* | [.06, .83] | .38(.19)+ | [-.01, .76] |
| Youth Ethnicitya | -.80(.50) | [-1.80, .19] | -.96(.53)+ | [-2.01, .10] | ||||
| Mother Relationship Statusb | .51(.43) | [-.33, 1.35] | .54(.42) | [-.29, 1.38] | ||||
| Mother Depressive Symptoms | -.22(.25) | [-.71, .26] | -.04(.26) | [-.54, .47] | ||||
| Mother Neuroticism | -.17(.25) | [-.66, .32] | -.38(.26) | [-.89, .13] | ||||
Note. N = 132 youth. Model 1 = original analysis with no covariates. Model 2 = analysis with demographic covariates. Model 3 = analysis with psychological covariates. Model 4 = analysis with demographic and psychological covariates. Coeff = coefficient; SE = standard error; CI = confidence interval. Continuous scores were calculated such that higher scores indicate greater standing on the variable (e.g., greater attachment anxiety). Continuous predictors and covariates were standardized.
0 = White, 1 = non-White.
0 = not in relationship, 1 = in relationship.
p < .10
p < .05
p < .01
Results
Associations between Mothers' Attachment, Maternal Warmth, and Youths' Expression of NR3C1
Similar to previous work that has used the ECR-R to assess individual differences in adult attachment (e.g., Raque-Bogdan, Ericson, Jackson, Martin, & Bryan, 2011; Sibley, Fischer, & Liu, 2005; Stanton & Campbell, 2014b), we found a strong correlation between mothers' attachment anxiety and attachment avoidance (r(132) = 0.66,p< .001); to avoid statistical issues of multicollinearity, analyses were conducted considering these two constructs separately (for a similar approach, see Raque-Bogdan et al., 2011). As shown in Table 2, Model 1, mothers' greater attachment anxietyandavoidance were associated with lower expression of NR3C1 in offspring. Youth-rated maternal warmth was positively associated with offspring's NR3C1 expression. These links remained robust when tested in models that included demographic and psychological covariates (see Table 2, Models 2-4).
Indirect Associations of Mothers' Attachment with Youths'NR3C1 Expression via Maternal Warmth
We used the PROCESS macro for SPSS (Hayes, 2013)to estimate the indirect associations of mothers' attachment anxiety(Figure 2, Panel A) and avoidance (Figure 2, Panel B) with youths' expression of NR3C1 via youth-rated maternal warmth. Bias-corrected bootstrap confidence intervals for indirect associations were estimated based on 10,000 bootstrap samples. As displayed in Figure 2A, analyses indicated that there was a significant indirect association of mothers' attachment anxiety through maternal warmth with youths'NR3C1 gene expression. This indirect association was significant in a model without covariates (Model 1 95% CI:[-.21, -.01]), but also remained significant when controlling for demographic factors (Model 2 95% CI:[-.19, -.002]) and when controlling for mothers' neuroticism and depressive symptoms (Model 3 95% CI:[-.24, -.01]), but not when controlling for demographic factors, neuroticism, and depressive symptoms all in the same model (Model 4 95% CI:[-.20, .001]).
Figure 2.
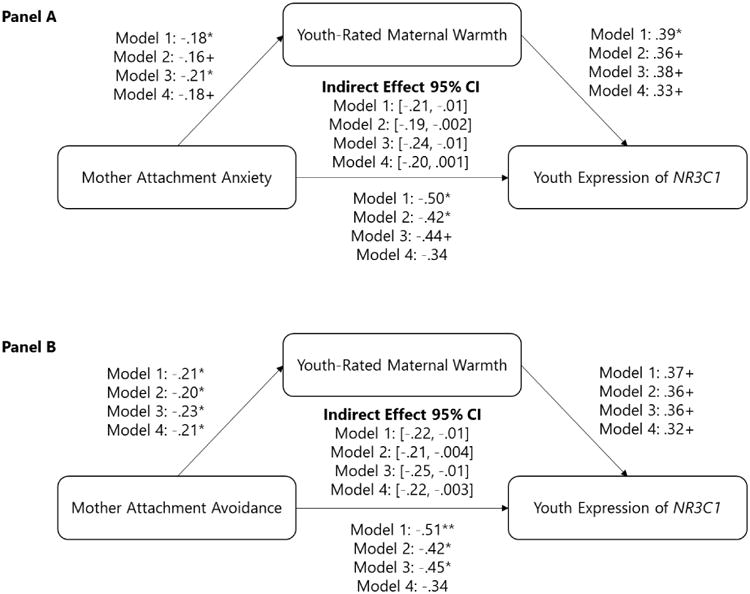
N = 132 youth. Direct and indirect associations between mother romantic attachment anxiety (Panel A), mother romantic attachment avoidance (Panel B), youth-rated maternal warmth, and youth expression of NR3C1. CI = confidence interval. Model 1 = original analysis with no covariates. Model 2 = analysis with demographic covariates. Model 3 = analysis with psychological covariates. Model 4 = analysis with demographic and psychological covariates. +p< .10, *p< .05, **p< .01
Turning to Figure 2B, analyses indicated that that there was a significant indirect association of mothers' attachment avoidance through maternal warmth with youth NR3C1 gene expression. This indirect association was significant in a model without covariates (Model 1 95% CI:[-.22, -.01]), but also remained significant when controlling for demographic factors (Model 2 95% CI:[-.21, -.004]), when controlling for mothers' neuroticism and depressive symptoms (Model 3 95% CI:[-.25, -.01]), and when controlling for demographic factors, neuroticism and depressive symptoms all in the same model (Model 4 95% CI: [-.22, -.003]). Taken together, these findings indicate that mothers' insecure attachment orientations—as assessed by both attachment anxiety and attachment avoidance—are linked with their children's lower levels of expression of the glucocorticoid receptor gene NR3C1 and that these links are driven, at least in part, through lower maternal warmth reported by their children.
Discussion
The present study tested links among mothers' romantic attachment orientations, maternal warmth, and offspring's expression of the glucocorticoid receptor gene NR3C1 in a sample of youth with asthma. We found that mothers' greater attachment anxiety and avoidance were both negatively linked with youth gene expression of NR3C1, explained by lower youth-rated maternal warmth. That is, more anxious and more avoidant mothers were perceived to be less warm by their offspring, and this was associated with youths'lower levels of NR3C1 expression. To our knowledge, this research is the first demonstration of individual differences in mothers' romantic attachment orientations predicting their offspring' santi-inflammatory gene expression. Further, we identified a plausible mediator—maternal warmth—already known to be associated with important youth health outcomes (e.g., Chen et al., 2010).
Our findings support the effects that parents' attachment can have on offspring (see Fraley & Roisman, 2015). Prior studies guided by attachment theory have demonstrated that parents with greater insecure attachment tend to eschew expressing warmth and provide less structure for offspring (e.g., Adam et al., 2004; Cohn et al., 1992), but no studies to our knowledge have tested the effects of parents' insecure attachment on the health-related outcomes of offspring. Our results suggest that mothers' adult attachment orientations influence children's expression of a gene relevant to both adaptive stress-regulation and the inflammatory response. The present research additionally lends insight into a molecular pathway that may help explain why those who experience lower maternal warmth in childhood tend to have poorer health in adulthood (cf. Miller, Lachman, et al., 2011). The links between NR3C1 and the body's ability to regulate its immune response are well-established (Bamberger et al., 1996; Bray & Cotton, 2003; Carpe et al., 2010), as are the associations between maternal warmth in early life and later health problems (Chen et al., 2011; Hackman et al., 2013; Miller, Chen, et al., 2011; Repetti et al., 2002). By demonstrating a meaningful association between mothers' attachment and youths' NR3C1expression via maternal warmth, we illuminate a potentially importantmolecularpredictorof physical health outcomes later in life.
One implication of this work dovetails nicely with prior research demonstrating the capacity for adult attachment orientations to change over time (see Arriaga, Kumashiro, Finkel, VanderDrift, & Luchies, 2014; Simpson, Rholes, Campbell, & Wilson, 2003). Notably, research on attachment change has been conducted mostly in the context of romantic relationships; the present study raises interesting questions regarding how reducing attachment anxiety and avoidance may promote positive outcomes for offspring. If causal pathways between mothers' attachment and youth's health outcomes are established by future longitudinal studies, a potential clinical implication of this work involves designing interventions that enhance attachment security and mothers' expression of warmth toward their offspring.
Our research provides a foundation from which future studies can advance the role of attachment theory in the emerging field of social genomics. For example, future research may benefit from an exploration of how maternal warmth and parents' attachment influence biological processes of their children over time. Prior research has demonstrated that greater maternal warmth can “override” the negative health effects associated with stressful experiences (e.g., childhood poverty, Miller, Lachman, et al., 2011). Studies endeavoring to understand when maternal warmth is most important for forming a protective buffer (e.g., infancy, early childhood, youth/adolescence), or how other relationship-relevant processes (e.g., parents' relationship quality) may influence offspring health outcomes, would have further benefits for clinical research. Further investigations of how NR3C1 expression may influence asthma pathogenesis over time will also be important in moving forward with this work.
Before concluding, we note that the present research is somewhat limited by possible confounding genetic effects that may correlate with the environment (gene-environment correlations) as well as potential gene-environment interactions. Indeed, parenting behavior may be influenced not only by environmental factors, but also by genetic factors (cf. Kendler, 1996). Additionally, previous studies have identified genetic variants that are associated with variation in gene expression across individuals (e.g., GTEx Consortium). Finally, variation in gene expression response to glucocorticoids may also be modulated by genetic variation (Maranville, Baxter, Witonsky, Chase, & Di Rienzo, 2013; Maranville et al., 2011; Moyerbrailean et al., 2016). Each of these may thus represent an additional mechanism by which children respond to parental behavior. Our study did not involve genotyping for parents and children, making us unable to account for these possibilities. Future studies, nevertheless, should endeavor to disentangle genetic and parenting factors when considering gene expression in offspring.
We also acknowledge that our findings highlight a thin slice of the effects of maternal attachment and warmth on offspring's molecular outcomes, and longitudinal investigations of these effects would offer a more comprehensive view of these links, especially in terms of how changes in caregivers' attachment and warmth over time might be associated with glucocorticoid receptor gene expression. Longitudinal studies may also lend interesting insight into how variable gene expression is over time in relation to differing environmental factors. Additionally, attachment anxiety and avoidance were strongly correlated in the current study, leading us to examine the constructs in separate statistical models (cf. Raque-Bogdan et al., 2011); thus, multicollinearity creates another potential limitation. Lastly, our study lacked a control group of healthy youth to examine if and how maternal attachment and warmth may uniquely (or perhaps similarly) affect youth with asthma compared to healthy controls.
The present research identified, for the first time, the influence that mothers' romantic attachment and warmth may have on their offspring's health-related gene expression. Mothers who were more anxiously or avoidantly attached were rated as less warm by their offspring, and this lower warmth was negatively linked to youth's expression of the glucocorticoid receptor gene NR3C1, a gene linked to glucocorticoid resistance and, therefore, important health outcomes. This study highlights the capacity for primary caregivers' attachment orientations to have downstream effects not only on offspring's perceptions of their care (e.g., viewing the parent as less warm) but also on the genetic building blocks of offspring's health-related biology. More broadly, these findings deepen our understanding of the links between adult attachment and health outcomes. Given this meaningful way parents leave an imprint on their children, establishing the causal effects of parents' attachment on offspring outcomes, as well as longitudinal investigations of these effects, are the next logical steps for future research.
Supplementary Material
Acknowledgments
This research was supported by NIH grant R01HL114097, awarded to Richard B. Slatcher. The authors gratefully acknowledge Daniel Saleh and the Wayne State University Close Relationships Lab for their assistance in data collection.
Biographies
Sarah C.E. Stanton is a lecturer in the Department of Psychology at the University of Edinburgh. She uses a social psychological approach to understand links between close relationships and health and well-being.
Samuele Zilioli is an assistant professor in the Department of Psychology and the Department of Family Medicine and Public Health Sciences at Wayne State University. His research examines the biopsychological mechanisms by which social status differences and stressors affect health and behavior.
Julia L. Briskin is a doctoral student in the Department of Psychology at Wayne State University. Her research investigates how relationship processes and self-regulation processes interact to influence health in romantic couples.
Ledina Imami is a doctoral student in the Department of Psychology at Wayne State University. Her research explores how distinct social contexts and family relationships influence health and well-being.
Erin T. Tobin is a postdoctoral fellow at the Henry Ford Health System. Her research focuses on the psychological, biological, and health consequences of close relationships and environmental adversity.
Derek E. Wildman is a professor in the Institute for Genomic Biology at the University of Illinois Urbana-Champaign. His research seeks to determine the genomic underpinnings of human phenotypes.
Henriette Mair-Meijers is a research assistant in the Center for Molecular Medicine and Genetics at Wayne State University. Her research explores links between social relationships, biological processes, and physical health.
Francesca Luca is an assistant professor in the Center for Molecular Medicine and Genetics and the Department of Obstetrics and Gynecology at Wayne State University. Her work investigates the genetic and molecular basis of inter-individual and inter-population differences in complex phenotypes.
Heidi S. Kane is an assistant professor in the School of Behavioral and Brain Sciences at the University of Texas at Dallas. Her research examines stress, coping, and social support processes in couples.
Richard B. Slatcher is an associate professor in the Department of Psychology at Wayne State University. His work focuses on basic social processes in close relationships and their links to health and well-being.
Footnotes
Our original research question concerned only NR3C1 expression as a plausible molecular mechanism that potentially impacts multiple biological systems, rather than multiple specific measures of biological outcomes. However, we thank an anonymous reviewer for suggesting we also explore the links between mothers' attachment, youth-rated maternal warmth, and glucocorticoid resistance. We report these additional analyses in online supplemental material.
References
- Adam EK, Gunnar MR, Tanaka A. Adult attachment, parent emotion, and observed parenting behavior: Mediator and moderator models. Child Development. 2004;75:110–122. doi: 10.1111/j.1467-8624.2004.00657x. [DOI] [PubMed] [Google Scholar]
- Arriaga XB, Kumashiro M, Finkel EJ, VanderDrift LE, Luchies LB. Filling the void: Bolstering attachment security in committed relationships. Social Psychological and Personality Science. 2014;5:398–406. doi: 10.1177/1948550613509287. [DOI] [Google Scholar]
- Bamberger CM, Schulte HM, Chrousos GP. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocrine Reviews. 1996;17:245–261. doi: 10.1210/edrv-17-3-245. doi:N/A. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. The Lancet. 2009;373:1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- Bet PM, Penninx BWJH, Bochdanovits Z, Uitterlinden AG, Beekman ATF, van Schoor NM, et al. Hoogendijk WJG. Glucocorticoid receptor gene polymorphisms and childhood adversity are associated with depression: New evidence for a gene-environment interaction. American Journal of Medical Genetics B: Neuropsychiatric Genetics. 2009;150B:660–669. doi: 10.1002/ajmg.b.30886. [DOI] [PubMed] [Google Scholar]
- Biosystems. Using TaqMan endogenous control assays to select an endogenous control for experimental studies. Life Technologies Application Note. 2005 doi:N/A. [Google Scholar]
- Bowlby J. Attachment and loss: Vol 3 Loss. New York, NY: Basic Books; 1980. doi:N/A. [Google Scholar]
- Bray PJ, Cotton RGH. Variations of the human glucocorticoid receptor gene (NR3C1): Pathological and in vitro mutations and polymorphisms. Human Mutation. 2003;21:557–568. doi: 10.1002/humu.10213. [DOI] [PubMed] [Google Scholar]
- Carpe N, Mandeville I, Ribeiro L, Ponton A, Martin JG, Kho AT, et al. Kaplan F. Genetic influences on asthma susceptibility in the developing lung. American Journal of Respiratory Cell and Molecular Biology. 2010;43:720–730. doi: 10.1165/rcmb.2009-0412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular Psychiatry. 2010;16:729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, et al. Turner RB. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proceedings of the National Academy of Sciences. 2012;109:5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn DA, Cowan PA, Cowan CP, Pearson J. Mothers' and fathers' working models of childhood attachment relationships, parenting styles, and child behavior. Development and Psychopathology. 1992;4:417–431. doi: 10.1017/S0954579400000870. [DOI] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biology. 2007;8:R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins NL. Working models of attachment: Implications for explanation, emotion, and behavior. Journal of Personality and Social Psychology. 1996;71:810–832. doi: 10.1037/0022-3514.71.4.810. [DOI] [PubMed] [Google Scholar]
- Enders CK. A primer on maximum likelihood algorithms available for use with missing data. Structural Equation Modeling. 2001;8:128–141. doi: 10.1207/S15328007SEM0801_7. [DOI] [Google Scholar]
- Farrell AK, Simpson JA. Effects of relationship functioning on the biological experience of stress and physical health. Current Opinion in Psychology. 2017;13:49–53. doi: 10.1016/j.copsyc.2016.04.014. [DOI] [PubMed] [Google Scholar]
- Fraley RC, Roisman GI. Do early caregiving experiences leave an enduring or transient mark on developmental adaptation? Current Opinion in Psychology. 2015;1:101–106. doi: 10.1016/j.copsyc.2014.11.007. [DOI] [Google Scholar]
- Fraley RC, Waller NG, Brennan KA. An item response theory analysis of self-report measures of adult attachment. Journal of Personality and Social Psychology. 2000;78:350–365. doi: 10.1037/0022-3514.78.2.350. [DOI] [PubMed] [Google Scholar]
- Gouin JP, Glaser R, Loving TJ, Malarkey WB, Stowell J, Houts C, et al. Kiecolt-Glaser JK. Attachment avoidance predicts inflammatory responses to marital conflict. Brain, Behavior, and Immunity. 2009;23:898–904. doi: 10.1016/j.bbi.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GTEx Consortium. The genotype-tissue expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science. 2015;348:648–660. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Betancourt LM, Brodsky NL, Kobrin L, Hurt H, Farah MJ. Selective impact of early parental responsivity on adolescent stress reactivity. PLoS ONE. 2013;8:e58250. doi: 10.1371/journal.pone.0058250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford Press; 2013. doi:N/A. [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the HPA axis: A review of animal models and human studies across development. Psychological Bulletin. 2014;140:256–282. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaremka LM, Glaser R, Loving TJ, Malarkey WB, Stowell JR, Kiecolt-Glaser JK. Attachment anxiety is linked to alterations in cortisol production and cellular immunity. Psychological Science. 2013;24:272–279. doi: 10.1177/0956797612452571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John OP, Donahue EM, Kentle RL. The Big Five Inventory—Versions 4a and 54. Berkeley, CA: University of California, Berkeley, Institute of Personality and Social Research; 1991. [Google Scholar]
- Jones JD, Cassidy J, Shaver PR. Parents' self-reported attachment styles: A review of links with parenting behaviors, emotions, and cognitions. Personality and Social Psychology Review. 2015;19:44–76. doi: 10.1177/1088868314541858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS. Parenting: A genetic-epidemiologic perspective. American Journal of Psychiatry. 1996;153:11–20. doi: 10.1176/ajp.153.1.11. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Kim S. Early attachment organization with both parents and future behavior problems: From infancy to middle childhood. Child Development. 2013;84:283–296. doi: 10.1111/j.1467-8624.2012.01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranville JC, Baxter SS, Witonsky DB, Chase MA, Di Rienzo A. Genetic mapping with multiple levels of phenotypic information reveals determinants of lymphocyte glucocorticoid sensitivity. American Journal of Human Genetics. 2013;93:735–743. doi: 10.1016/j.ajhg.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranville JC, Luca F, Richards AL, Wen X, Witonsky DB, Baxter S, et al. Di Rienzo A. Interactions between glucocorticoid treatment and cis-regulatory polymorphisms contribute to cellular response phenotypes. PLoS Genetics. 2011;7(7):e1002162. doi: 10.1371/journal.pgen.1002162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SK, Pretorius CJ, Ungerer JPJ, Salmon NJ, Conwell LS, Pearen MA, et al. Batch JA. Neonatal complete generalized glucocorticoid resistance and growth hormone deficiency caused by a novel homozygous mutation in helix 12 of the ligand binding domain of the glucocorticoid receptor gene (NR3C1) Journal of Clinical Endocrinology and Metabolism. 2010;95:297–302. doi: 10.1210/jc.2009-1003. [DOI] [PubMed] [Google Scholar]
- Mikulincer M, Shaver PR. The attachment behavioral system in adulthood: Activation, psychodynamics, and interpersonal processes. In: Zanna MP, editor. Advances in experimental social psychology. Vol. 35. New York, NY: Academic Press; 2003. pp. 53–152. [DOI] [Google Scholar]
- Miller GE, Chen E. Life stress and diminished expression of genes encoding glucocorticoid receptor and β2-adrenergic receptor in children with asthma. Proceedings of the National Academy of Sciences. 2006;103:5496–5501. doi: 10.1073/pnas.0506312103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Lachman ME, Chen E, Gruenewald TL, Karlamangla AS, Seeman TE. Pathways to resilience: Maternal nurturance as a buffer against the effects of childhood poverty on metabolic syndrome at midlife. Psychological Science. 2011;22:1591–1599. doi: 10.1177/0956797611419170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyerbrailean GA, Richards AL, Kurtz D, Kalita CA, Davis GO, Harvey CT, et al. Luca F. High-throughput allele-specific expression across 250 environmental conditions. Genome Research. 2016 doi: 10.1101/gr.209759.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MLM, Slavich GM, Chen E, Miller GE. Targeted rejection predicts decreased anti-inflammatory gene expression and increased symptom severity in youth with asthma. Psychological Science. 2015;26:111–121. doi: 10.1177/0956797614556320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picardi A, Miglio R, Tarsitani L, Battisti F, Baldassari M, Copertaro A, et al. Biondi M. Attachment style and immunity: A 1-year longitudinal study. Biological Psychology. 2013;92:353–358. doi: 10.1016/j.biopsycho.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Pietromonaco PR, Powers SI. Attachment and health-related physiological stress processes. Current Opinion in Psychology. 2015;1:34–39. doi: 10.1016/j.copsyc.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SI, Pietromonaco PR, Gunlicks M, Sayer A. Dating couples' attachment styles and patterns of cortisol reactivity and recovery in response to a relationship conflict. Journal of Personality and Social Psychology. 2006;90:613–628. doi: 10.1037/0022-3514.90.4.613. [DOI] [PubMed] [Google Scholar]
- Puig J, Englund MM, Simpson JA, Collins WA. Predicting adult physical iillness from infant attachment: A prospective longitudinal study. Health Psychology. 2013;32:409–417. doi: 10.1037/a0028889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirin M, Pruessner JC, Kuhl J. HPA system regulation and adult attachment anxiety: Individual differences in reactive and awakening cortisol. Psychoneuroendocrinology. 2008;33:581–590. doi: 10.1016/j.psyneuen.2008.01.013. doi:j.psyneuen.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Raby KL, Roisman GI, Fraley RC, Simpson JA. The enduring predictive significance of early maternal sensitivity: Social and academic competence through age 32 years. Child Development. 2015;86:695–708. doi: 10.1111/cdev.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Raque-Bogdan TL, Ericson SK, Jackson J, Martin HM, Bryan NA. Attachment and mental and physical health: Self-compassion and mattering as mediators. Journal of Counseling Psychology. 2011;58:272–278. doi: 10.1037/a0023041. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–366. doi: 10.1037/0033-2909.128.2.330. [DOI] [PubMed] [Google Scholar]
- Schaefer ES. Children's reports of parental behavior: An inventory. Child Development. 1965;36:413–424. doi: 10.2307/1126465. [DOI] [PubMed] [Google Scholar]
- Scheffer J. Dealing with missing data. Research Letters in the Information and Mathematical Sciences. 2002;3:153–160. doi:N/A. [Google Scholar]
- Sibley CG, Fischer R, Liu JH. Reliability and validity of the Revised Experiences in Close Relationships (ECR-R) self-report measure of adult romantic attachment. Personality and Social Psychology Bulletin. 2005;31:1524–1536. doi: 10.1177/0146167205276865. [DOI] [PubMed] [Google Scholar]
- Simpson JA, Collins WA, Farrell AK, Raby KL. Attachment and relationships across time: An organizational-developmental perspective. In: Zayas V, Hazan C, editors. Bases of adult attachment: Linking brain, mind and behavior. New York, NY: Springer; 2015. pp. 61–78. [DOI] [Google Scholar]
- Simpson JA, Rholes WS, Campbell L, Wilson CL. Changes in attachment orientations across the transition to parenthood. Journal of Experimental Social Psychology. 2003;39:317–331. doi: 10.1016/S0022-1031(03)00030-1. [DOI] [Google Scholar]
- Slavich GM, Cole SW. The emerging field of human social genomics. Clinical Psychological Science. 2013;1:331–348. doi: 10.1177/2167702613478594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton SCE, Campbell L. Psychological and physiological predictors of health in romantic relationships: An attachment perspective. Journal of Personality. 2014a;82:528–538. doi: 10.1111/jopy.12056. [DOI] [PubMed] [Google Scholar]
- Stanton SCE, Campbell L. Perceived social support moderates the link between attachment anxiety and health outcomes. PLoS ONE. 2014b;9:e95358. doi: 10.1371/journal.pone.0095358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


