Abstract
The soluble urokinase receptor (suPAR) has been implicated in the pathogenesis of chronic kidney diseases (CKD) and may function as a circulating “permeability factor” driving primary focal and segmental glomerulosclerosis (FSGS). Here we examined the mechanisms whereby suPAR causes mobilization and increased activation of Ca2+-permeable TRPC6 channels, which are also implicated in FSGS. Treatment of immortalized mouse podocytes with recombinant suPAR for 24 hr caused a marked increase in cytosolic reactive oxygen species (ROS) that required signaling through integrins. This effect was associated with increased assembly of active cell surface NADPH oxidase 2 (Nox2) complexes and was blocked by the Nox2 inhibitor apoycynin. Treatment with suPAR also evoked a functionally measurable increase in TRPC6 channels that was blocked by concurrent treatment with the ROS-quencher TEMPOL as well as by inhibition of Rac1, an essential component of active Nox2 complexes. Elevated ROS evoked by exposing cells to suPAR or H2O2 caused a marked increase in the abundance of tyrosine-phosphorylated proteins including Src, and suPAR-evoked Src activation was blocked by TEMPOL. Moreover, mobilization and increased activation of TRPC6 by suPAR or H2O2 was blocked by concurrent exposure to PP2, an inhibitor of Src family tyrosine kinases. These data suggest that suPAR induces oxidative stress in podocytes that in turn drives signaling through Src family kinases to upregulate TRPC6 channels. The combination of oxidative stress and altered Ca2+ signaling may contribute to loss of podocytes and progression of various forms of CKD.
Keywords: chronic kidney disease, TRPC6, suPAR, Src, reactive oxygen species, integrins
1. Introduction
The soluble urokinase and plasminogen activator receptor (suPAR) is the name of a class of 22–50 kD glycoproteins shed from many cell types by proteolytic cleavage or lysis of a glycosylphosphatidylinositol-anchored glycoprotein [1]. The total concentration of circulating suPAR variants is increased in a wide variety of disease states and can be considered as a general marker of inflammatory processes that can occur in almost any organ, including disorders arising from infections, immunity, and cancer [2]. suPAR has been extensively studied in the context of chronic kidney disease (CKD) [3].
Focal and segmental glomerulosclerosis (FSGS) describes a pattern of renal lesions that can occur with multiple etiologies in patients of all age groups with CKD. A glucocorticoid-resistant primary form of FSGS leads inexorable loss of renal function [4], and recurs in as many as 40% of patients who have received a kidney transplant [5]. This has long suggested that circulating factors drive the development and progression of primary FSGS by triggering pathological changes in podocytes, a population of multipolar visceral epithelial cells required for normal glomerular function. The natureof the circulating factors that evoke primary and recurrent FSGS has been an area of active investigation for decades, and several soluble molecules have been proposed to function as “glomerular permeability factors” in primary FSGS [6,7].
The initial studies of suPAR in the context of CKD suggested a role as a circulating factor driving primary and recurrent FSGS, and noted elevated serum suPAR concentrations in a subset of patients with primary FSGS, which was more pronounced in patients whose disease recurred after transplantation [8]. While subsequent studies questioned the usefulness of serum suPAR concentration as a specific biomarker for primary FSGS [9,10] several recent studies have shown that circulating and/or urinary suPAR levels may have a considerably more wide ranging clinical significance for CKD, as they can predict future declines in renal function in the general population [11], in children [12], and in the presence of other underlying conditions including cardiovascular diseases [13–15], HIV disease [16], and diabetes [17–19]. Given the association between suPAR and CKD, especially glomerular diseases, there is strong rationale for examining the actions of suPAR on glomerular cells in more detail than has been presented so far.
suPAR produces several direct effects on podocytes, including down-regulation of nephrin [20] and podocin [21], which occur after activation of αvβ3-integrin, a known receptor or co-receptor for suPAR [22]. More recently we have shown that application of suPAR markedly increases the steady-state surface expression and membrane stretch-evoked activation of TRPC6 channels in cultured podocytes [21]. This effect was mimicked by several different serum and plasma samples from patients with recurrent forms of FSGS taken during a relapse. Moreover, the effects of these blood samples could be reduced by inhibition of αvβ3-integrin or by immunoabsorption of suPAR [21]. In this regard, a wide range of evidence implicates TRPC6 in the pathogenesis of CKD. Thus, mutations of TRPC6, especially mutations that lead to a gain of function, are seen in many autosomal dominant forms of familial FSGS [23–26], and TRPC6 expression is increased in glomeruli from patients with glomerular diseases including primary FSGS [27]. Moreover, genetic inactivation of TRPC6 reduces progression of glomerular diseases in various rodent models [28] and protection from FSGS is especially robust in rats in which an essential exon in the Trpc6 gene is deleted [29]. Trpc6 knockout may also reduce the severity of glomerular lesions in mouse models of diabetes [30].
In the present study we have provided additional details regarding the transduction pathway whereby suPAR modulates podocyte function. We show that suPAR acting through αvβ3-integrin causes an increase in cytosolic reactive oxygen species (ROS) that is mediated by at least in part by NADPH-oxidase 2 (Nox2). The increase in ROS triggers activation of Src-tyrosine kinases, which results in upregulation of TRPC6 that can be seen in biochemical assays of steady-state surface expression and in functional electrophysiological measurements.
2. Materials and Methods
2.1. Podocyte cell culture.
An immortalized mouse podocyte cell line (MPC-5) was provided by Dr. Peter Mundel of Harvard Medical School and propagated and maintained as described previously [21,31].
2.2. Measurement of reactive oxygen species (ROS).
The abundance of cytosolic ROS was estimated using the cell permeable probe 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA) (Cell Biolabs Inc). This membrane-permeable dye is trapped in the cytosol after cleavage of the acetyl moieties to yield non-fluorescent 2’,7’-dichlorodihydrofluorescein (DCFH). In the presence of ROS, this molecule is oxidized to form 2′,7′-dichlorofluorescein (DCF), which emits intense green fluorescence at 530 nm upon stimulation at 480 nm, which was monitored from cultures on a microplate reader.
2.3. Immunoblot analysis, co-immunoprecipitation, and cell surface biotinylation assays.
Methods used for immunoblot analysis from podocyte lysates have been described in detail previously [32]. Filters were probed using primary antibodies, washed, incubated with horseradish peroxidase-conjugated secondary antibodies, and visualized using chemiluminescence. Methods for cell surface biotinylation assays [32] and coimmunoprecipitation [31] were described previously.
2.4. Electrophysiology.
Methods for making whole-cell recordings from cultured podocytes, including the composition of the recording electrode, and preparation of a control normosomotic bath (340 mOsm/liter), and 70% hypoosmotic bath solutions (238 mOsm/liter), were described previously [21,31]. The bath was perfused at a constant flow rate (0.5 ml/min) and outwardly rectifying currents were periodically evoked by ramp voltage commands (−80 to + 80 mV over 2.5 sec). The holding potential between ramps was −40 mV. After ensuring that the baseline current was stable in normosmotic bath solution, cells were perfused with 70% hypoosomotic stretch solution, which causes reversible activation of TRPC6 channels in podocytes. This is seen as an increase in outwardly rectifying current that reverses at 0 mV and stabilizes in 2–3 min, and responses shown here were recorded within that time. These currents are completely blocked by selective TRPC6 inhibitors [21,29].
2.5. Antibodies and inhibitors.
Rabbit antibodies against TRPC6 (ACC-017) were obtained from Alomone Labs (Jerusalem, Israel); antibodies against β3-integrin (sc-14009) were obtained from Santa Cruz (Santa Cruz, CA); antibodies against gp91phox and Nox4 were from Abcam; Antibodies against p47phox and pan-phosphotyrosine were from MilliporeSigma; antibodies against Src were from Abcam, and antibodies against Src-pY418 were from ThermoScientific. A recombinant form of suPAR containing domains 1, 2 and 3 was obtained from R&D Systems. H2O2, TEMPOL, apocynin, and PP2 were obtained from Sigma Aldrich. Cilengitide (CGT) and NSC23766 were obtained from Seleck Chem (Houston, TX, USA).
2.6. Rac1 Activation Assay.
Active Rac-1 was measured using the G-LISA Rac-1 Activation Assay Biochem™ kit in the colorimetric format (Cytoskeleton, Inc.) as instructed by the manufacturer. In brief, cells were treated, washed with PBS, and resuspended in kit lysis buffer. The protein concentration in each lysate was determined by the Precision Red™ Advanced Protein Assay reagent in the kits. The G-LISA kits contain a Rac1 GTP-binding protein that is immobilized on microplates. Bound active small GTPases were detected with a Rac1 primary antibody. The signal was measured at 490 nm with a microplate reader. Results were expressed as fold-increases in activity of stimulated in relation to non-stimulated controls normalized to protein content.
2.7. Statistical analyses.
All experiments on immunoblot or cell surface biotinylation assays were performed in triplicate and analyzed by densitometry using Image J™ software (Bethesda, MD). Those data are presented as fold changes in signal relative to the lowest single value observed in a control group. Electrophysiological data are presented as fold increase in current relative to basal current in the same cell measured at +80 mV (at the end of the ramp command). All bar graphs denote mean ± SD and were analyzed by Bonferroni t-test with P < 0.05 considered significant.
3. Results
3.1. Role of ROS generated by NADPH oxidase in suPAR signaling in podocytes.
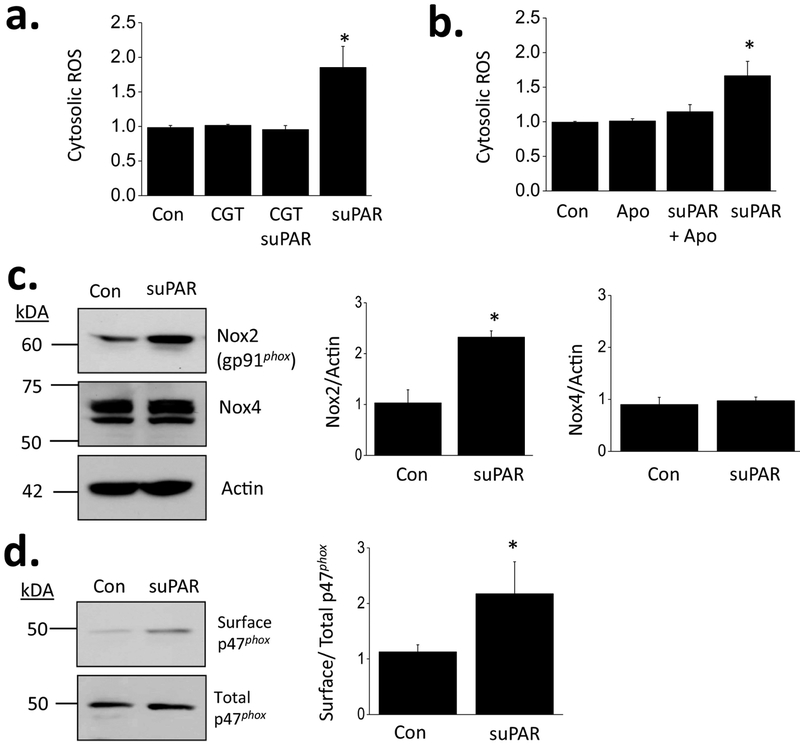
In a recent study we demonstrated that 24 hr exposure to 10 ng/ml of recombinant human suPAR increased the steady-state surface abundance of TRPC6 subunits of cultured mouse podocytes, and that this effect was blocked by cilengitide, an inhibitor of signaling through αvβ3-integrin [21]. In other studies we and others have shown that TRPC6 channels in podocytes are upregulated by processes in which there is increased generation of ROS [32–34]. Here we observed that treatment with suPAR evoked an increase in cytosolic ROS abundance that could be detected using 2’,7’-dichlorodihydrofluorescein fluorescence (Fig. 1a). This effect was blocked by concurrent exposure to 1 μM cilengitide (Fig. 1a) or 10 μM apocynin (Fig. 1b), an inhibitor of Nox2. After 24 hr treatment of suPAR, there was an increase in overall abundance of gp91phox, the heme-containing primary catalytic subunit of Nox2, whereas there was no comparable increase in the primary catalytic subunit for Nox4 (Fig. 1c). The electron transfers necessary for Nox2 activation require formation of a membrane complex that includes the regulatory subunit p47phox as well as other proteins [35,36]. Using a cell-surface biotinylation assay, we observed that suPAR treatment caused an increase in steady-state abundance of p47phox at the cell surface, consistent with activation of Nox2 (Fig. 1d). The mechanism whereby activation of αvβ3-integrin causes activation of Nox2 is not known. However we observed that both components of active Nox2 complexes can co-immunoprecipitate with β3-integrin (Supplemental Data), which might provide a basis for subsequent activation of this NADPH oxidase.
Figure 1.
Increased ROS generation in podocytes evoked by suPAR and Nox2 in mouse podocytes. (a) Fluorescence assay showing effects of recombinant suPAR (10 ng/ml for 24 hr) on cytosolic ROS abundance in immortalized mouse podocytes. The effect of suPAR was blocked by concurrent exposure to 1 μM cilengitide (CGT), an inhibitor of signaling through αVβ3 integrin. Ordinate shows fold increase in signal over control. In this and all subsequent panels, error bars denote SD. (b) The effects of suPAR on ROS abundance were blocked by concurrent exposure to 10 μM apocynin (Apo), an inhibitor of NADPH oxidase Nox2. (c) Immunnoblots showing that exposure to suPAR increases abundance of heme-containing catalytic subunits of Nox2 but not of Nox4. (d) Cell surface biotinylation assays showing that suPAR treatment increases abundance of Nox2 regulatory subunit p47phox at the cell surface. Bar graphs in (c) and (d) show results of densitometric analyses.
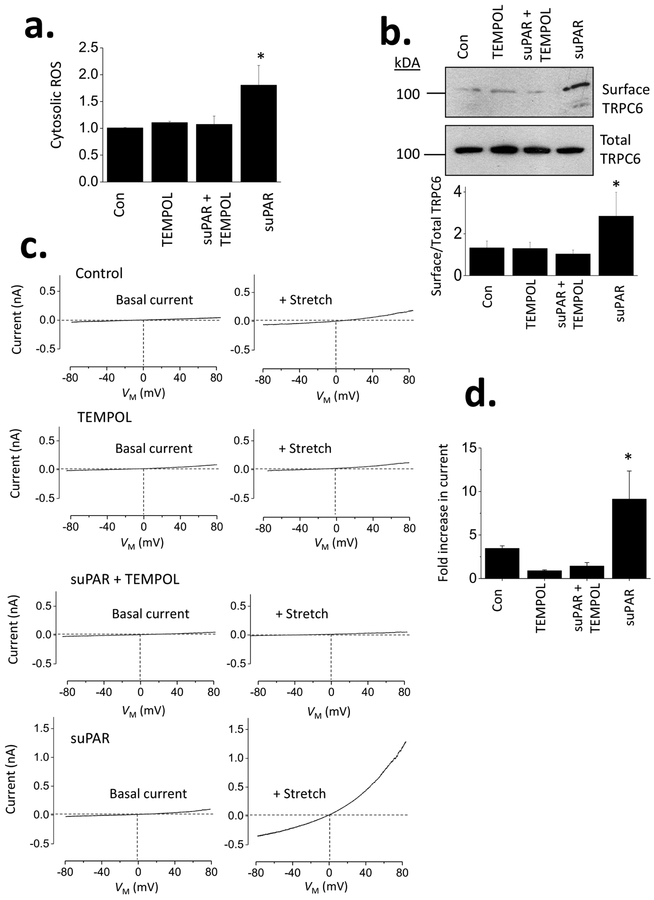
The ROS generated by suPAR exposure were entirely quenched by concurrent treatment with 100 μM 1-oxyl-2,2,6,6-tetramethyl-4-hydroxypiperidine (TEMPOL), a membrane-permeable small molecule mimetic of superoxide dismutase (Fig. 2a). Concurrent exposure to TEMPOL also prevented the increase in steady-state surface abundance of TRPC6 that is normally evoked by suPAR treatment (Fig. 2b). Thus, mobilization of TRPC6 by suPAR, which we described previously [21], requires generation of ROS, which occurs at least in part through Nox2 activation in mouse podocytes. We have previously reported that 24-hr exposure to suPAR results in increased currents through TRPC6 channels evoked by membrane stretch [21]. Here we note that this effect does not occur when cells are concurrently exposed to TEMPOL along with suPAR (Fig. 2c, d). As with our previous experiments, currents were monitored during 2.5-s ramp voltage commands (−80 mV to +80 mV) in normal external saline and in a 70% hypoosmotic external solution [31].
Figure 2.
ROS are required for TRPC6 upregulation by suPAR in mouse podocytes. (a) Increases in ROS evoked by suPAR are prevented in cells concurrently treated with 100 μM TEMPOL, a membrane-permeable ROS quencher. (b) Concurrent exposure to 100 μM TEMPOL also prevents increase in steady-state cell surface expression of TRPC6 evoked by suPAR, as measured by cell-surface biotinylation assay. (c) Examples of whole-cell recordings show that membrane stretch-evoked cationic currents (previously shown to be mediated by TRPC6) are markedly increased in cells treated with suPAR (10 ng/ml for 24 hr). However, this did not occur in cells concurrently exposed to suPAR and TEMPOL or in cells exposed to TEMPOL alone for 24 hr. (d) Bar graphs showing mean fold increases in current (measured at +80 mV) evoked by membrane stretch depending on previous 24 hr of treatment.
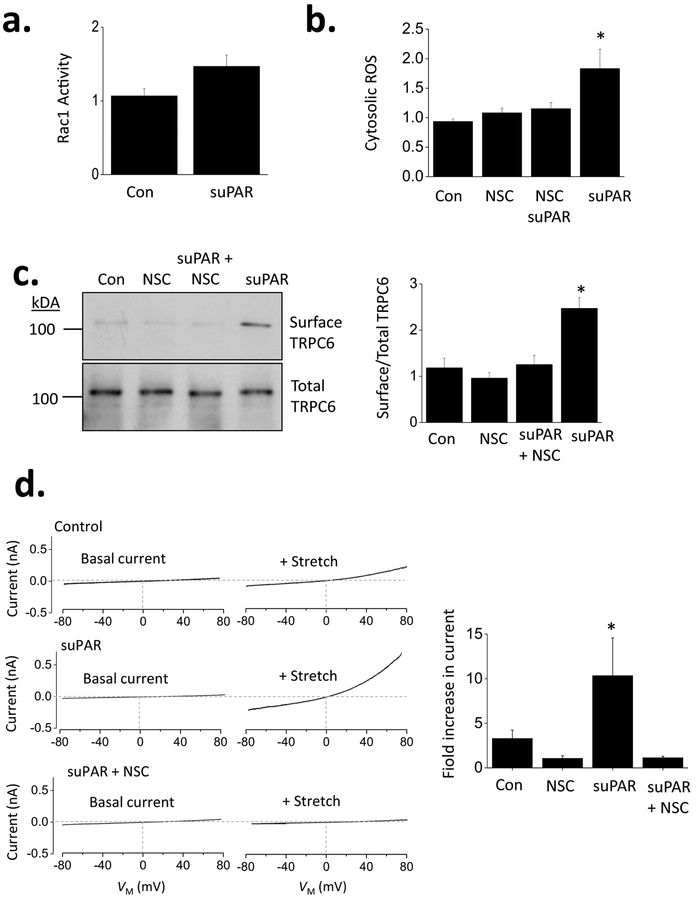
Many forms of NADPH oxidase, including Nox2, include GTP-bound Rac1 as part of the catalytically active complex [37]. Consistent with the results shown above, we noted that suPAR treatment resulted in activation of Rac1, seen here as an increase in the total abundance of GTP-bound Rac1 (Fig. 3a). Moreover, suPAR-evoked increases in total ROS were abolished by concurrent exposure to 50 μM NSC23766, an inhibitor of Rac1 that acts by preventing its interactions with guanine nucleotide exchange factors [38] (Fig. 3b). NSC23766 also blocked increases in steady-state surface expression of TRPC6 evoked by suPAR (Fig. 3c) and also the effect of suPAR to increase membrane stretch-evoked currents through TRPC6 (Fig. 3d).
Figure 3.
Effects of suPAR on TRPC6 require activation of Rac1 in mouse podocytes. (a) Treatment of suPAR increases activation of Rac1 measured by ELISA assay from podocyte lysates. (b) Increases in cytosolic ROS activation evoked by suPAR were prevented by concurrent exposure to 50 μM NSC23766 (NSC), an agent that blocks interactions of Rac1 with guanine nucleotide exchange factors, thereby preventing its activation. (c) Concurrent exposure to NSC23766 also prevented increase in steady-state cell surface expression of TRPC6 evoked by suPAR, assessed here by cell-surface biotinylation assay. (d) Increases in stretch-activated cationic currents evoked by 24 hr exposure to suPAR were prevented by concurrent exposure to NSC23766. Examples of recordings are shown to the left, and a bar graph summarizing results of this experiment are shown to the right.
3.2. Role of Src kinases in suPAR signaling, downstream of ROS generation.
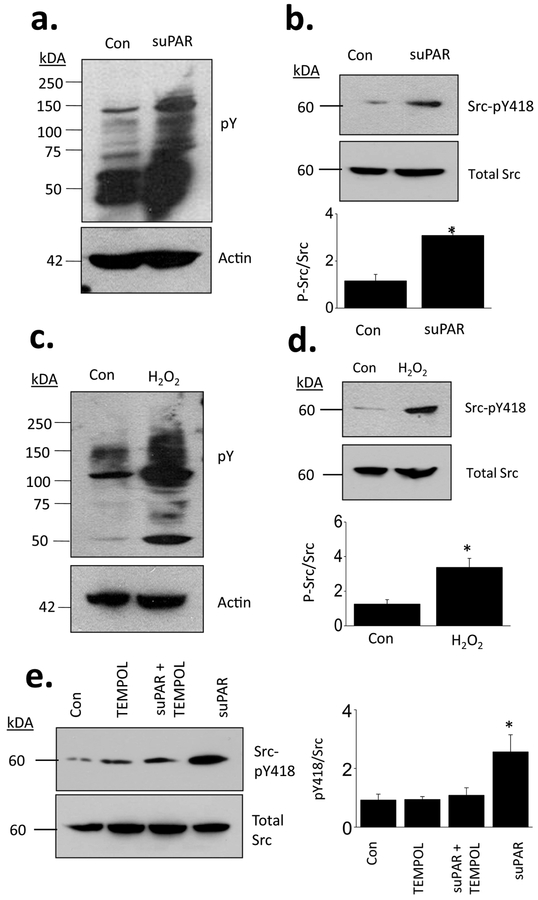
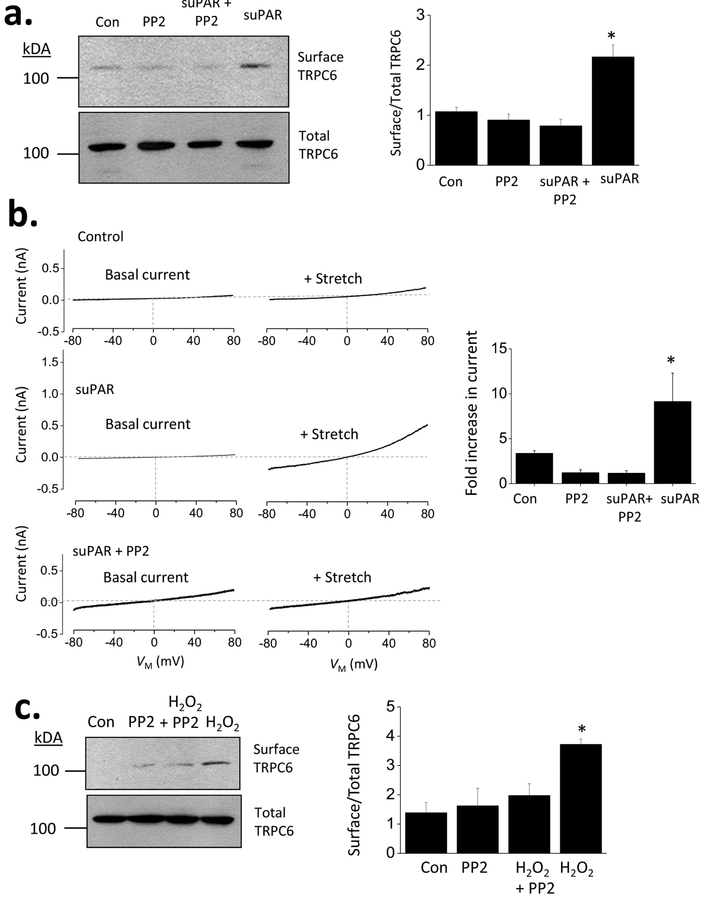
Studies in other cell types have shown that outside-in signaling through αvβ3-integrin is mediated in part by Src family tyrosine kinases [38] and indeed that Src kinases can interact with integrin β subunits [40]. This also occurs in mouse podocytes. In initial experiments we observed that suPAR treatment caused a very marked increase in the abundance of proteins phosphorylated on tyrosine residues (Fig. 4a). suPAR also increased the abundance of the active phosphorylated form of Src at tyrosine 418 (SrcpY418) (Fig. 4b). A similar pattern was evoked by simply treating podocytes with 1 mM H2O2 for 30 min in normal cell culture medium. This resulted in a large increase in the abundance of proteins phosphorylated on tyrosine residues (Fig. 4c), including a marked increase in Src-pY418 relative to total Src (Fig. 4d). Moreover, the ability of suPAR to increase Src-pY418 (and hence activation) was blocked by concurrent exposure to TEMPOL (Fig. 4e). It bears noting that Src can cause phosphorylation of glomerular TRPC6 channels in podocytes on multiple tyrosine residues, including at Y284, a residue required for expression of TRPC6 on the cell surface [41]. Moreover, Src-family protein kinases including Fyn and Src interact directly with cytosolic domains close to the carboxy and amino terminals of TRPC6 [41,42]. Here we observed reciprocal co-immunoprecipitation from podocyte lysates using antibodies that recognize TRPC6 and antibodies that recognize phosphorylated tyrosine residues. These signals appeared more robust in cells treated with suPAR, although we should note that these immunoprecipitations are unlikely to yield quantitative data. We also note that we have confirmed the critical role of Y284 for surface expression and activation of TRPC6 (Supplemental Data). More importantly, we observed that activation of Src-family kinases is required for upregulation of TRPC6 by suPAR in podocytes. Thus suPAR-evoked increases in steady-state surface abundance of podocyte TRPC6 were blocked by concurrent exposure to PP2, an inhibitor of Src-family kinases (Fig. 5a). Concurrent exposure to PP2 also blocked suPAR-evoked increases in membrane stretch-evoked activation of TRPC6 (Fig. 5b). PP2 also blocked increases in TRPC6 surface expression evoked by H2O2 (Fig. 5c), which is consistent with models in which ROS is upstream of Src activation.
Figure 4.
Exposure to suPAR increases abundance of proteins phosphorylated on tyrosine residues and causes ROS-dependent activation of Src. (a) Immunoblot analysis showing increased abundance of phosphotyrosine in podocytes treated with suPAR for 24 hr. (b) Immunoblot showing increased abundance of Src-pY418 relative to total Src in cells treated with suPAR. Exposing podocytes to medium containing 1 mM H2O2 for 30 min increased abundance of phosphortyrosine (c) and increased the abundance of Src-pY418 relative to total Src (d). (e) suPAR-evoked increases in SrcpY418 relative to total Src were blocked by concurrent exposure to TEMPOL.
Figure 5.
Upregulation of TRPC6 by suPAR or by oxidative stress requires activation of Src family kinases. (a) Cell surface biotinylation assays showing increased steady-state surface expression of TRPC6 evoked by suPAR was blocked by concurrent exposure to 5 μM PP2, an inhibitor of Src family tyrosine kinases. (b) Concurrent exposure PP2 also blocked increases in stretch-evoked cationic currents that follow 24 hr exposure to suPAR. (c) PP2 also prevented mobilization of TRPC6 channels evoked by 1 mM H2O2.
4. Discussion
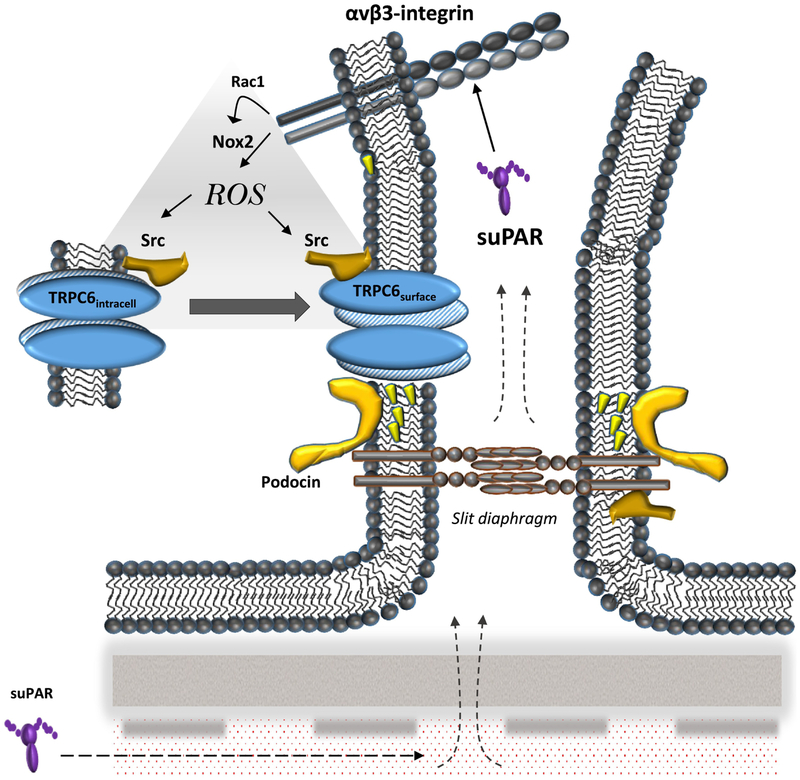
The TRPC6 channel and suPAR have both been implicated in the progression of various forms of CKD, and in a previous study we demonstrated that recombinant suPAR caused up-regulation of podocyte TRPC6 channels, and contributed to similar activity in plasma of some patients with recurrent forms of primary FSGS [21]. Under certain conditions, i.e. in the presence of other pro-inflammatory factors, suPAR could also induce surface expression of TRPC5 [21], which has also been suggested to play a role in CKD [43] and also appears to be a redox-sensitive channel [44]. In the present study we have elucidated some of the mechanisms whereby suPAR upregulates functional TRPC6. We have observed that suPAR, by acting on a cilengitide-sensitive αV-containing integrin, evokes an increase in ROS generation mediated by mobilization of an active Nox2 complex at the cell surface, which requires activation of the small GTPase Rac1. The increase in bulk cytosolic ROS in turn causes an increase in protein tyrosine phosphorylation that requires Src-family protein kinases, which are known to phosphorylate TRPC6 and modulate its expression on the cell surface [41]. Src itself is among the proteins that is tyrosine phosphorylated in response to suPAR, and this effect also required ROS. A schematic summarizing these observations is shown in Fig. 6.
Figure 6.
Schematic diagram summarizing results of this study. Circulating suPAR, which is increased in some patients, including a subset of patients with primary or recurrent FSGS, causes outside-in activation of αVβ3 integrins in podocytes. This results in Rac1-dependent activation of Nox2, which results in production of ROS. Those in turn lead to increased surface expression of TRPC6, which can be seen as an increase in cationic currents evoked by mechanical stimuli. The modulation of TRPC6 requires Src activation, which is in a position to cause a variety of effects on other proteins in slit diaphragm domains.
Circulating suPAR is elevated in a wide range of conditions, and the serum levels of this glycoprotein cannot be considered to be a specific biomarker for any particular kidney disease. suPAR is widely regarded as a marker for chronic systemic inflammation or infection [2]. Notably, serum suPAR concentration can predict CKD from a variety of causes, in some cases many years before clinical presentation [11–19]. Given this, there is value in understanding its effects at a cellular level. In this study we have focused on podocytes, a particularly vulnerable and essential renal cell type implicated in many renal pathologies. However, it is possible that many of the effects noted here are conserved in other cell types.
The starting point of the cascade presented here is the generation of ROS in response to suPAR, which appears to be mediated by Nox2. To our knowledge, increased ROS generation in response to suPAR exposure has not been described in other cell types. However, an increase in ROS generation in response to activation of β3-containing integrins has been described previously in vascular smooth muscle, where it is thought to mediate myogenic vasoconstriction [45], a process that entails activation of TRP channels including TRPC6 [46] and other redox-sensitive TRP channels [44]. Integrin-dependent ROS generation also occurs in podocytes in response to soluble syndecan 4 [47]. Other integrins are reported to mediate activation of NADPH oxidases [48–51] including Nox2 [52]. Consistent with this, we observed that ROS generation and TRPC6 mobilization required Rac1 activation, which was readily detected after suPAR treatment. Note that Nox2 only becomes catalytically active after GTP-bound Rac1 becomes part of the complex [35–37].
We also observed that the increase in bulk cytosolic ROS observed after suPAR exposure, or after exposing podocytes to H2O2, drove an increase in Src activation and overall cell tyrosine phosphorylation. The relationship between cellular redox status and Src regulation has been extensively studied in a wide range of cell types [53]. There are a number of members in the Src family of non-receptor tyrosine kinases subjected to this kind of regulation. Src is regulated by phosphorylation of tyrosine 418 (in the rat variant) [54] and can autophosphorylate at that residue [55]. Notably, the ability of Src to autophosphorylate is enhanced after oxidation of cysteine residues within SH2 and kinase domains enhances the catalytic activity of the enzyme towards other substrates [56]. The degree to which a regulatory protein is phosphorylated reflects a balance between the activity of kinases and phosphatases and it is notable that active sites of a host of tyrosine phosphatases are redox-sensitive [57]. It is worth noting that the interaction between Src and NADPH oxidases may be bidirectional. Thus, an earlier study has shown that Src can stimulate activation of NADPH oxidases in vascular smooth muscle exposed to angiotensin II [58]. If similar dynamics occur in podocytes it could set up a positive feedback loop in which activity of both Src and Nox2 becomes sustained, which could contribute to ongoing disease processes.
We observed that the ability of suPAR to upregulate functional TRPC6 was blocked by PP2, an agent that inhibits Src family kinases. This drug also blocked TRPC6 upregulation by H2O2, which strongly suggests that Src kinases lie downstream of ROS generation in this pathway. It bears noting that TRPC6 is phosphorylated by Src on at least two different tyrosine residues [41] and that one of these (Y284) appears necessary for expression of TRPC6 on the cell surface and activation [41], and observation that we have confirmed [Supplemental Data]. We observed that TRPC6 channels in podocytes are phosphorylated on tyrosine residues. This was easier to observe in suPAR-treated cells, especially when the precipitation was carried out with anti-TRPC6.
In the present study we used membrane stretch to activate podocyte TRPC6 channels. It is well established that TRPC6 can be activated by this stimulus in multiple cell types [31,46,59–63] although the mechanisms whereby this occurs are not understood and are probably cell type-specific. In podocytes this mode of TRPC6 activation does not require activation of any G protein [31], in marked contrast to TRPC6 activation by ATP or angiotensin II [33,34]. Hypoosmotic stimuli are widely used in studies on mechanical activation of ionic channels because they are very reproducible and do not require any specialized apparatus. In most cells, however, this is not a physiological stimulus. Podocytes in situ are exposed to a variety of different mechanical stimuli, including pulsation of the glomerular capillary [64], as well as fluid shear forces associated with hydrodynamic movements over the cell surface [65], and possibly expansion of the sub-podocyte space that would occur when single-nephron glomerular filtration rates are increased [66]. Consequently, a stimulus such as suPAR that causes a sustained upregulation in TRPC6 activation by mechanical stimuli would presumably result in Ca2+ overload in foot processes, the cell body, or both. Within the foot process this could lead to changes in cytoskeletal arrangements, whereas in the cell body this could lead to calcineurin- and NFATc1-dependent changes in gene expression [67], including, in time, the upregulation of TRPC6 itself [68]. Similar chronic activation of TRPC6 explain why genetic ablation of functional TRPC6 channels is protective in animal models of acquired glomerular disease [29]. In any case, it bears noting that TRPC6 channels may have a quite general role in connecting cellular Ca2+ dynamics to overall redox status.
5. Conclusions
We have shown that suPAR acting though αV-containing integrins causes functionally significant mobilization of TRPC6 through a pathway that entails Nox2-dependent generation of ROS. The accumulation of ROS subsequently increases activity of Src-family tyrosine kinases which are required for increased mobilization and stretch-activation of TRPC6 evoked by suPAR. These results suggest several potential therapeutic targets that could conceivably reduce the loss of podocytes during CKD.
Supplementary Material
Highlights.
suPAR increases TRPC6 mobilization to the podocyte cell surface through stimulation of integrins.
The effect of suPAR on TRPC6 requires reactive oxygen species produced by NADPH oxidase 2
Increases in reactive oxygen species lead to Src activation in podocytes, which is required for TRPC6 upregulation by suPAR and by H2O2.
TRPC6 is phosphorylated on tyrosine residues in response to suPAR and H2O2.
Acknowledgements
We are grateful to Dr. Peter Mundel, formerly of Harvard Medical School (Boston, MA) for providing the MPC-5 immortalized podocyte cell line used in these studies. Parisa Yazdizadeh Shotorbani provided technical assistance. We are grateful to Dr. Kyoungjae Choi for preparing constructs encoding TRPC6 Y31A, Y284A, and Y31A/Y284A.
This work was supported by National Institutes of Health grant R01-DK104708
Abbreviations
- Apo
apocynin
- CGT
cilengitide
- CKD
chronic kidney disease
- FSGS
focal and segmental glomerulosclerosis
- gp91phox
catalytic subunit of NADPH oxidase 2
- Nox2
NADPH oxidase 2
- Nox4
NADPH oxidase 4
- NSC23766
N6-[2-[[4-(Diethylamino)-1-methylbutyl]amino]-6-methyl-4-pyrimidinyl]-2-methyl-4,6-quinolinediamine trihydrochloride
- p47phox
regulatory subunit of NADPH oxidase 2
- PP2
4-amino-5-(4-chlorophenyl)-7-(dimethylethyl)pyrazolo[3,4-d] pyrimidine
- ROS
reactive oxygen species
- suPAR
soluble urokinase receptor
- TEMPOL
1-oxyl-2,2,6,6-tetramethyl-4-hydroxypiperidine
- TRPC5
canonical transient receptor potential-5 channel
- TRPC6
canonical transient receptor potential-6 channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- [1].Montuori N, Carriero MV, Salzano S, Rossi G, Ragno P. The cleavage of the urokinase receptor regulates its multiple functions. J BiolChem. 2002. 277(49):46932–9. [DOI] [PubMed] [Google Scholar]
- [2].Desmedt S, Desmedt V, Delanghe JR, Speeckaert R, Speeckaert MM. The intriguing role of soluble urokinase receptor in inflammatory diseases. Crit Rev Clin Lab Sci. 2017. 54(2):117–133. [DOI] [PubMed] [Google Scholar]
- [3].Zeier M, Reiser J. suPAR and chronic kidney disease-a podocyte story. Pflugers Arch. 2017. 469(7–8):1017–1020. [DOI] [PubMed] [Google Scholar]
- [4].Rosenberg AZ, Kopp JB. Focal Segmental Glomerulosclerosis. Clin J Am Soc Nephrol. 2017. 12(3):502–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Blosser CD, Bloom RD. Recurrent glomerular disease after kidney transplantation. Curr Opin Nephrol Hypertens. 2017. 26(6):501–508. [DOI] [PubMed] [Google Scholar]
- [6].Davin JC. The glomerular permeability factors in idiopathic nephrotic syndrome. Pediatr Nephrol. 2016. 31(2):207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Königshausen E, Sellin L. Circulating permeability factors in primary focal segmental glomerulosclerosis: A review of proposed candidates. Biomed Res Int. 2016:3765608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M, Zhang Q, Nikolic B, Chaudhuri A, Daftarian P, Salido E, Torres A, Salifu M, Sarwal MM, Schaefer F, Morath C, Schwenger V, Zeier M, Gupta V, Roth D, Rastaldi MP, Burke G, Ruiz P, Reiser J. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. NatMed. 2011. 17(8):952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Maas RJ, Deegens JK, Wetzels JF. Serum suPAR in patients with FSGS: trash or treasure? Pediatr Nephrol. 2013. 28(7):1041–8. [DOI] [PubMed] [Google Scholar]
- [10].Kronbichler A, Saleem MA, Meijers B, Shin JI. Soluble urokinase receptors in focal segmental glomerulosclerosis: A review on the scientific point of view. J Immunol Res. 2016:2068691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schulz CA, Persson M, Christensson A, Hindy G, Almgren P, Nilsson PM, Melander O, Engström G, Orho-Melander M. Soluble urokinase-type plasminogen activator receptor (suPAR) and impaired kidney function in the population-based Malmö diet and cancer study. Kidney Int Rep. 2017. 2(2):239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schaefer F, Trachtman H, Wühl E, Kirchner M, Hayek SS, Anarat A, Duzova A, Mir S, Paripovic D, Yilmaz A, Lugani F, Arbeiter K, Litwin M, Oh J, Matteucci MC, Gellermann J, Wygoda S, Jankauskiene A, Klaus G, Dusek J, Testa S, Zurowska A, Caldas Afonso A, Tracy M, Wei C, Sever S, Smoyer W, Reiser J; ESCAPE trial consortium and the 4C study group. Association of serum soluble urokinase receptor levels with progression of kidney disease in children. JAMA Pediatr. 2017. 171(11):e172914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hayek SS, Sever S, Ko YA, Trachtman H, Awad M, Wadhwani S, Altintas MM, Wei C, Hotton AL, French AL, Sperling LS, Lerakis S, Quyyumi AA, Reiser J. Soluble urokinase receptor and chronic kidney disease. N Engl JMed. 2015. 373(20):1916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hayek SS, Ko YA, Awad M, Ahmed H, Gray B, Hosny KM, Aida H, Tracy MJ, Wei C, Sever S, Reiser J, Quyyumi AA. Cardiovascular disease biomarkersand suPAR in predicting decline in renal function: A prospective cohort study. Kidney Int Rep. 2017. 2(3):425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mossanen JC, Pracht J, Jansen TU, Buendgens L, Stoppe C, Goetzenich A, Struck J, Autschbach R, Marx G, Tacke F. Elevated soluble urokinase plasminogen activator receptor and proenkephalin serum levels predict the development of acute kidney injury after cardiac surgery. Int J Mol Sci. 2017. 18(8). pii: E1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kirkegaard-Klitbo DM, Langkilde A, Mejer N, Andersen O, Eugen-Olsen J, Benfield T. Soluble urokinase plasminogen activator receptor is a predictor of incident non-AIDS comorbidity and all-cause mortality in human immunodeficiency virus type 1 infection. J Infect Dis. 2017. 216(7):819–823. [DOI] [PubMed] [Google Scholar]
- [17].Theilade S, Lyngbaek S, Hansen TW, Eugen-Olsen J, Fenger M, Rossing P, Jeppesen JL. Soluble urokinase plasminogen activator receptor levels are elevated and associated with complications in patients with type 1 diabetes. J Intern Med. 2015. 277(3):362–371. [DOI] [PubMed] [Google Scholar]
- [18].Dande RR, Peev V, Altintas MM, Reiser J. Soluble urokinase receptor and the kidney response in diabetes mellitus. J Diabetes Res. 2017:3232848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Guthoff M, Wagner R, Randrianarisoa E, Hatziagelaki E, Peter A, Häring HU, Fritsche A, Heyne N. Soluble urokinase receptor (suPAR) predicts microalbuminuria in patients at risk for type 2 diabetes mellitus. Sci Rep. 2017. 7:40627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Alfano M, Cinque P, Giusti G, Proietti S, Nebuloni M, Danese S, D’Alessio S, Genua M, Portale F, Lo Porto M, Singhal PC, Rastaldi MP, Saleem MA, Mavilio D, Mikulak J. Full-length soluble urokinase plasminogen activator receptor down-modulates nephrin expression in podocytes. Sci Rep. 2015. 5:13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kim EY, Roshanravan H, Dryer SE. Changes in podocyte TRPC channels evoked by plasma and sera from patients with recurrent FSGS and by putative glomerular permeability factors. Biochim Biophys Acta. 2017. 1863(9):2342–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tarui T, Mazar AP, Cines DB, Takada Y. Urokinase-type plasminogen activator receptor (CD87) is a ligand for integrins and mediates cell-cell interaction. J Biol Chem. 2001. 276(6):3983–90. [DOI] [PubMed] [Google Scholar]
- [23].Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005. 308(5729):1801–4. [DOI] [PubMed] [Google Scholar]
- [24].Reiser J, Polu KR, Möller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005. 37(7):739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Heeringa SF, Möller CC, Du J, Yue L, Hinkes B, Chernin G, Vlangos CN, Hoyer PF, Reiser J, Hildebrandt F. A novel TRPC6 mutation that causes childhood FSGS. PLoS One. 2009. 4(11):e7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Riehle M, Büscher AK, Gohlke BO, Kaßmann M, Kolatsi-Joannou M, Bräsen JH, Nagel M7, Becker JU, Winyard P, Hoyer PF, Preissner R, Krautwurst D, Gollasch M, Weber S, Harteneck C. TRPC6 G757D loss-of-function mutation associates with FSGS. J Am Soc Nephrol. 2016. 27(9):2771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Möller CC, Wei C, Altintas MM, Li J, Greka A, Ohse T, Pippin JW, Rastaldi MP, Wawersik S, Schiavi S, Henger A, Kretzler M, Shankland SJ, Reiser J. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol. 2007. 18(1):29–36. [DOI] [PubMed] [Google Scholar]
- [28].Wang L, Jirka G, Rosenberg PB, Buckley AF, Gomez JA, Fields TA, Winn MP, Spurney RF. Gq signaling causes glomerular injury by activating TRPC6. J Clin Invest. 2015. 125(5):1913–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kim EY, Yazdizadeh Dhotorbani Y, Dryer SE. Trpc6 inactivation confers protection in a model of severe nephrosis in rats. J Mol Med (Berlin). 2018. 96(7): 631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ilatovskaya DV, Blass G, Palygin O, Levchenko V, Pavlov TS, Grzybowski MN, Winsor K, Shuyskiy LS, Geurts AM, Cowley AW Jr, Birnbaumer L, Staruschenko A. A NOX4/TRPC6 pathway in podocyte calcium regulation and renal damage in diabetic kidney disease. J Am Soc Nephrol. 2018. 29(7):1917–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Anderson M, Kim EY, Hagmann H, Benzing T, Dryer SE. Opposing effects of podocin on the gating of podocyte TRPC6 channels evoked by membrane stretch or diacylglycerol. Am J Physiol Cell Physiol. 2013. 305(3):C276–89. [DOI] [PubMed] [Google Scholar]
- [32].Kim EY, Anderson M, Dryer SE. Insulin increases surface expression of TRPC6 channels in podocytes: role of NADPH oxidases and reactive oxygen species. Am J Physiol Renal Physiol. 2012. 302(3):F298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Anderson M, Roshanravan H, Khine J, Dryer SE. Angiotensin II activation of TRPC6 channels in rat podocytes requires generation of reactive oxygen species. J Cell Physiol. 2014. 229(4):434–42. [DOI] [PubMed] [Google Scholar]
- [34].Roshanravan H, Dryer SE. ATP acting through P2Y receptors causes activation of podocyte TRPC6 channels: role of podocin and reactive oxygen species. Am J Physiol Renal Physiol. 2014. 306(9):F1088–97. [DOI] [PubMed] [Google Scholar]
- [35].Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007. 87(1):245–313. [DOI] [PubMed] [Google Scholar]
- [36].El-Benna J, Dang PM, Gougerot-Pocidalo MA, Marie JC, Braut-Boucher F. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Exp Mol Med. 2009. 41(4):217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sumimoto H, Miyano K, Takeya R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem Biophys Res Commun. 2005. 338(1):677–86. [DOI] [PubMed] [Google Scholar]
- [38].Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A. 2004. 101(20):7618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Courter DL, Lomas L, Scatena M, Giachelli CM. Src kinase activity is required for integrin alphaVbeta3-mediated activation of nuclear factor-kappaB. J Biol Chem. 2005. 280(13):12145–51. [DOI] [PubMed] [Google Scholar]
- [40].Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil SJ. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc Natl Acad Sci U S A. 2003. 100(23):13298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kanda S, Harita Y, Shibagaki Y, Sekine T, Igarashi T, Inoue T, Hattori S. Tyrosine phosphorylation-dependent activation of TRPC6 regulated by PLC-γ1 and nephrin: effect of mutations associated with focal segmental glomerulosclerosis. Mol Biol Cell. 2011. 22(11):1824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kawasaki BT, Liao Y, Birnbaumer L. Role of Src in C3 transient receptor potential channel function and evidence for a heterogeneous makeup of receptor- and store-operated Ca2+ entry channels. Proc Natl Acad Sci U S A 2006. 103:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhou Y, Castonguay P, Sidhom EH, Clark AR, Dvela-Levitt M, Kim S, Sieber J, Wieder N, Jung JY, Andreeva S, Reichardt J, Dubois F, Hoffmann SC, Basgen JM, Montesinos MS, Weins A, Johnson AC, Lander ES, Garrett MR, Hopkins CR, Greka A. A small-molecule inhibitor of TRPC5 ion channels suppresses progressive kidney disease in animal models. Science. 2017. 358:1332–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ogawa N, Korokawa T, Mori Y. Sensing of redox status by TRP channels. Cell Calcium. 2016. 60:115–22. [DOI] [PubMed] [Google Scholar]
- [45].Staiculescu MC, Ramirez-Perez FI, Castorena-Gonzalez JA, Hong Z, Sun Z, Meininger GA, Martinez-Lemus LA. Lysophosphatidic acid induces integrin activation in vascular smooth muscle and alters arteriolar myogenic vasoconstriction. Front Physiol. 2014. 5:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res. 2002. 90(3):248–50. [DOI] [PubMed] [Google Scholar]
- [47].Kim EY, Roshanravan H, Dryer SE. Syndecan-4 ectodomain evokes mobilization of podocyte TRPC6 channels and their associated pathways: An essential role for integrin signaling. Biochim Biophys Acta. 2015. 1853(10 Pt A):2610–20. [DOI] [PubMed] [Google Scholar]
- [48].Chiarugi P, Pani G, Giannoni E, Taddei L, Colavitti R, Raugei G, Symons M, Borrello S, Galeotti T, Ramponi G. Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J Cell Biol. 2003. 161(5):933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].McMillan SJ, Sharma RS, Richards HE, Hegde V, Crocker PR. Siglec-E promotes β2-integrin-dependent NADPH oxidase activation to suppress neutrophil recruitment to the lung. J Biol Chem. 2014. 289(29):20370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wyssenbach A, Quintela T, Llavero F, Zugaza JL, Matute C, Alberdi E. Amyloid β-induced astrogliosis is mediated by β1-integrin via NADPH oxidase 2 in Alzheimer’s disease. Aging Cell. 2016. 15: 10.1111/acel.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hou L, Bao X, Zang C, Yang H, Sun F, Che Y, Wu X, Li S, Zhang D, Wang Q. Integrin CD11b mediates α-synuclein-induced activation of NADPH oxidase through a Rho-dependent pathway. Redox Biol. 2018. 14:600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Moraes JA, Frony AC, Dias AM, Renovato-Martins M, Rodrigues G, Marcinkiewicz C, Assreuy J, Barja-Fidalgo C. Alpha1beta1 and integrin-linked kinase interact and modulate angiotensin II effects in vascular smooth muscle cells. Atherosclerosis. 2015. 243(2):477–85. [DOI] [PubMed] [Google Scholar]
- [53].Giannoni E, Chiarugi P. Redox circuitries driving Src regulation. Antioxid Redox Signal. 2014. 20(13):2011–25. [DOI] [PubMed] [Google Scholar]
- [54].Kmiecik TE, Shalloway D. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell. 1987. 49(1):65–73. [DOI] [PubMed] [Google Scholar]
- [55].Sun G, Sharma AK, Budde RJ. Autophosphorylation of Src and Yes blocks their inactivation by Csk phosphorylation. Oncogene. 1998. 17(12):1587–95. [DOI] [PubMed] [Google Scholar]
- [56].Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol. 2005. 25(15):6391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell. 2005. 121(5):667–70. [DOI] [PubMed] [Google Scholar]
- [58].Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002. September 6;91(5):406–13. [DOI] [PubMed] [Google Scholar]
- [59].Dyachenko V, Husse B, Rueckschloss U, Isenberg G. Mechanical deformation of ventricular myocytes modulates both TRPC6 and Kir2.3 channels. Cell Calcium. 2009. 45(1):38–54. [DOI] [PubMed] [Google Scholar]
- [60].Inoue R, Jensen LJ, Jian Z, Shi J, Hai L, Lurie AI, Henriksen FH, Salomonsson M, Morita H, Kawarabayashi Y, Mori M, Mori Y, Ito Y. Synergistic activation of vascular TRPC6 channel by receptor and mechanical stimulation via phospholipase C/diacylglycerol and phospholipase A2/omega-hydroxylase/20-HETE pathways. Circ Res. 2009. 104(12):1399–409. [DOI] [PubMed] [Google Scholar]
- [61].Quick K, Zhao J, Eijkelkamp N, Linley JE, Rugiero F, Cox JJ, Raouf R, Gringhuis M, Sexton JE, Abramowitz J, Taylor R, Forge A, Ashmore J, Kirkwood N, Kros CJ, Richardson GP, Freichel M, Flockerzi V, Birnbaumer L, Wood JN. TRPC3 and TRPC6 are essential for normal mechanotransduction in subsets of sensory neurons and cochlear hair cells. Open Biol. 2012. 2(5):120068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Seo K, Rainer PP, Lee DI, Hao S, Bedja D, Birnbaumer L, Cingolani OH, Kass DA. Hyperactive adverse mechanical stress responses in dystrophic heart are coupled to transient receptor potential canonical 6 and blocked by cGMP-protein kinase G modulation. Circ Res. 2014. (5):823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Yamaguchi Y, Iribe G, Nishida M, Naruse K. Role of TRPC3 and TRPC6 channels in the myocardial response to stretch: Linking physiology and pathophysiology. Prog Biophys Mol Biol. 2017. 130(Pt B):264–272. [DOI] [PubMed] [Google Scholar]
- [64].Brenner BM, Troy JL, Daugharty TM. The dynamics of glomerular ultrafiltration in the rat. J Clin Invest. 1971. 50(8):1776–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kriz W, Lemley KV. Potential relevance of shear stress for slit diaphragm and podocyte function. Kidney Int. 2017. 91(6):1283–1286. [DOI] [PubMed] [Google Scholar]
- [66].Neal CR, Muston PR, Njegovan D, Verrill R, Harper SJ, Deen WM, Bates DO. Glomerular filtration into the subpodocyte space Am J Physiol Renal Physiol. 2007. 293(6):F1787–98. [DOI] [PubMed] [Google Scholar]
- [67].Schlöndorff J, Del Camino D, Carrasquillo R, Lacey V, Pollak MR. TRPC6 mutations associated with focal segmental glomerulosclerosis cause constitutive activation of NFAT-dependent transcription. Am J Physiol Cell Physiol. 2009. 296(3):C558–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Nijenhuis T, Sloan AJ, Hoenderop JG, Flesche J, van Goor H, Kistler AD, Bakker M, Bindels RJ, de Boer RA, Möller CC, Hamming I, Navis G, Wetzels JF, Berden JH, Reiser J, Faul C, van der Vlag J. Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am J Pathol. 2011. 179(4):1719–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.