Abstract
HACE1 is an E3 ubiquitin ligase described as a tumour suppressor because HACE1-knockout mice develop multi-organ, late-onset cancers and because HACE1 expression is lost in several neoplasms, such as Wilms’ tumours and colorectal cancer. However, a search of public databases indicated that HACE1 expression is maintained in melanomas. We demonstrated that HACE1 promoted melanoma cell migration and adhesion in vitro and was required for mouse lung colonisation by melanoma cells in vivo. Transcriptomic analysis of HACE1-depleted melanoma cells revealed an inhibition of ITGAV and ITGB1 as well changes in other genes involved in cell migration. We revealed that HACE1 promoted the K27 ubiquitination of fibronectin and regulated its secretion. Secreted fibronectin regulated ITGAV and ITGB1 expression, as well as melanoma cell adhesion and migration. Our findings disclose a novel molecular cascade involved in the regulation of fibronectin secretion, integrin expression and melanoma cell adhesion. By controlling this cascade, HACE1 displays pro-tumoural properties and is an important regulator of melanoma cell invasive properties.
Introduction
HECT domain and ankyrin repeat-containing E3 ubiquitin protein ligase 1 (HACE1) is thought to be a tumour-suppressor gene because its expression is lower in Wilms’ tumours than in healthy kidneys [1] and because HACE1-knockout mice develop late-onset cancers in the breast, liver and lung [2]. HACE1 targets RAC1 for degradation, thereby controlling the level of GTP-RAC1 protein [3]. The tumour-suppressor activity of HACE1 is in agreement with its effects on RAC1, namely, reducing the level and the pro-tumourigenic functions of RAC1, which promote tumour cell migration, proliferation and survival [4]. More recently, HACE1 has been reported to reduce the tumourigenicity of lung cancer cells through the ubiquitination of optineurin and the regulation of autophagy [5].
Full exome sequencing of melanoma tumours has identified the transition (c.85 C > T) p.P29S in RAC1 as one of the most common somatic mutations in melanoma after BRAFV600 and NRASQ61. In contrast to the BRAF or NRAS mutations, the RAC1 mutation displays the hallmarks of ultraviolet B signature (C > T) [6]. The P29S mutation maintains RAC1 in a GTP-bound and active form that is endowed with oncogenic properties [7].
Although the role of RAC1 has been well studied, the role of HACE1 in melanoma is poorly understood. Taking into account the key role of HACE1 in the regulation of RAC1, a recognised driver oncogene in melanoma, we performed a comprehensive characterisation of HACE1 function in melanoma.
Unexpectedly, HACE1 silencing inhibited melanoma cell migration in vitro as well as lung colonisation in mice in vivo. DNA array analysis of 4 different melanoma cell cultures demonstrated that HACE1 silencing affected the transcriptional programme and decreased mRNA levels of beta1 and alphaV integrins. HACE1 exerted its effects through regulation of fibronectin (FN) secretion and K27 ubiquitination of FN, suggesting a causative role of K27 ubiquitination of FN in its secretion. Our data uncovered a previously unanticipated pro-oncogenic function of HACE1 in melanoma cells.
Results
HACE1 expression is maintained in melanoma cells and promotes melanoma cell migration
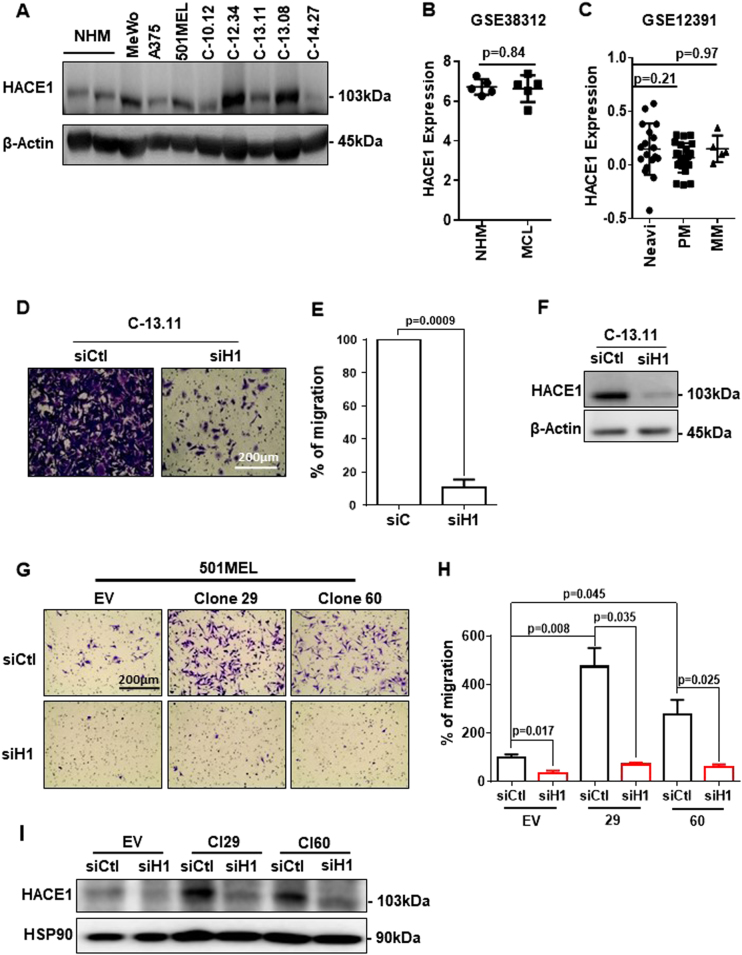
First, we analysed HACE1 expression in melanocytes (2), melanoma cell lines (3) and short-term melanoma cell cultures (5). Western blot analysis revealed that HACE1 expression did not dramatically change among the different cells analysed. Overall, the HACE1 level was maintained, with some increases in melanoma cells (Fig. 1a). Further analysis of public databases showed that HACE1 expression was not different in cutaneous melanocytes and melanoma cell cultures [8] (Fig. 1b) or nevi, primary melanoma and metastatic melanoma [9] (Fig. 1c). Therefore, in contrast to the HACE1 loss reported in breast cancer [10] and Wilms’ tumours [1], HACE1 expression is not systematically decreased during the tumourigenic transformation of melanocytes into melanomas.
Fig. 1.
HACE1 expression is maintained in melanoma cells and promotes melanoma cell migration. a Western blot analysis of HACE1 and actin protein levels in melanoma cell lines, (MeWo, A375, 501MEL) short-term melanoma cell cultures (C-10.12, C-12.34, C-13.11, C-13.08, C-14.27) and primary human melanocytes (NHM). b Analysis of GSE38312 data sets for HACE1 expression in pairs of cultured cutaneous melanocytes (NHM) and melanoma cells (MC). c Analysis of GSE12391 data sets for HACE1 expression in metastatic (MM) (n = 5) or primary melanomas (PM) (n = 23) compared with that in nevi (n = 18). In b and c, the mean ± SD is represented, and the statistical analysis was performed using t-test with Welch’s correction. d Boyden chamber migration assay of a short-term melanoma cell culture (C-13.11) treated with control (siCtl) or HACE1 (siH1) siRNA. Representative images are shown. e Quantification of three independent experiments. The results are expressed as the percentage of cells in control conditions (mean ± SD; n = 3). f Western blot analysis of C-13.11 cells following siRNA treatment. g Migration assay of 501MEL clones with forced expression of HACE1 (clones 29 and 60). h Quantification of three independent experiments. i Western blot analysis showing HACE1 expression
Migration is one of the main cellular functions regulated by HACE1 via the control of RAC1 stability [4]. Therefore, we studied the effect of HACE1 downregulation on melanoma cell migration. HACE1 silencing reduced the migration of short-term culture melanoma cells (C-13.11) in Boyden chambers by up to 80% (Fig. 1d, e). In these conditions, HACE1 protein levels were decreased, as shown by western blot (Fig. 1f). Similar effects on migration and HACE1 silencing were obtained in at least 3 different melanoma cell lines or short-term cultures and with 2 additional siRNAs to HACE1 (Sup. Figure 1A to I).
To confirm this unexpected effect of HACE1 silencing on migration, we transfected 501MEL cells with a plasmid encoding HACE1. Two selected HACE1-overexpressing clones (Fig. 1i) displayed an increased migratory capacity that was reverted by HACE1 silencing (Fig. 1g). Quantification of these experiments is shown Fig. 1h. Increased migration was also observed in a pool of A375 cells overexpressing HACE1 after transfection with the same plasmid (Sup. Figure 1.J, K, L).
HACE1 deficiency has been reported to predispose cells to necroptosis [11]. However, HACE1 silencing has never induced melanoma cell death in our experience (not shown). Therefore, inhibition of melanoma cell migration after HACE1 silencing cannot be ascribed to an induction of cell death.
In agreement with the literature, siRNA against RAC1 induced a decrease of both RAC1 expression and migration in 501MEL cells (Sup. Figure 2.A, B). Further, we confirmed that HACE1 controlled the degradation of GTP-bound RAC1 (Sup. Figure 2.C, D) as previously described [3].
HACE1 promotes melanoma cell adhesion and lung colonisation
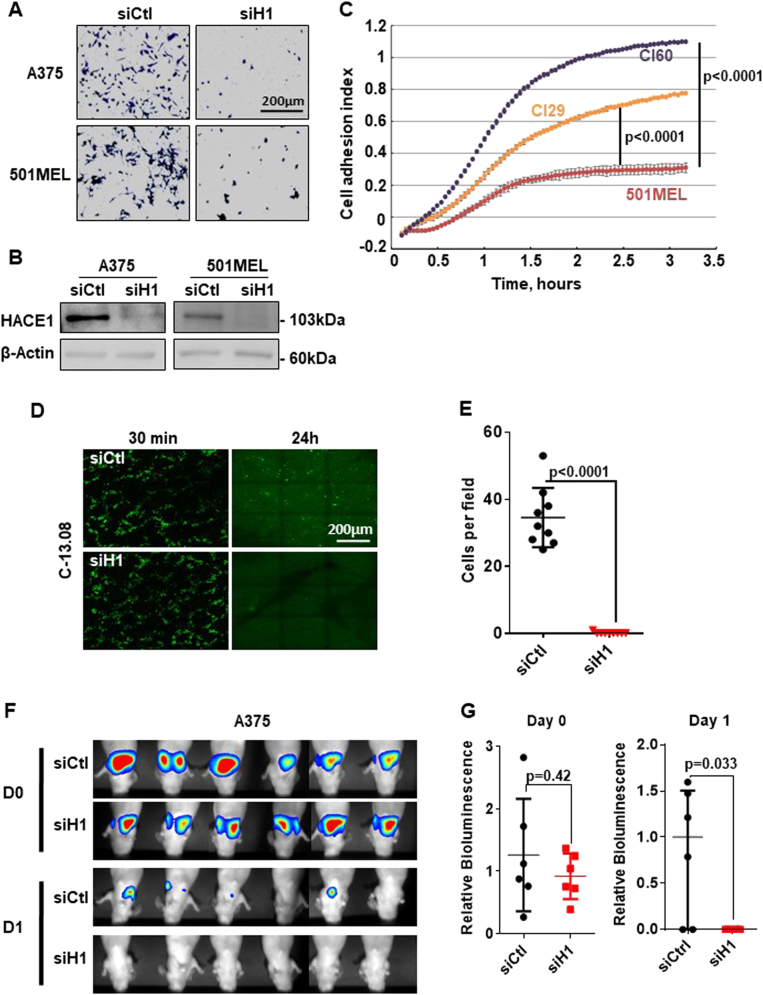
The decrease in melanoma cell migration after HACE1 silencing is the consequence of inhibiting cell adhesion. HACE1 silencing, as confirmed by western blot (Fig. 2b), severely impaired A375 and 501MEL cell adhesion (Fig. 2a) as measured by a classical adhesion assay and the xCELLigence adhesion assay using different siRNAs (Sup. Figure 3a-b). Further, 501MEL clones overexpressing HACE1 displayed increased adhesion ability as quantified by xCELLigence adhesion assays (Fig. 2c).
Fig. 2.
HACE1 promotes melanoma cell adhesion and lung colonisation. a Classical adhesion assay of A375 and 501MEL cells transfected with control (siCtl) or HACE1 (siH1) siRNA. b Immunoblotting with antibodies against HACE1. Actin was used as a loading control. c xCELLigence impedance assay with 501MEL and clones with forced expression of HACE1 (Cl29 and Cl60). The results represent the mean of cell adhesion index ± SD (n = 3) in function of time. d C-13.08 melanoma cells transfected with control (siCtl) or HACE1 (siH1) siRNA were labelled with a green fluorescent dye (CMFDA) and injected in the tail vein of nude mice. The mice were sacrificed after 30 min or 24 h, and whole lungs were analysed by fluorescence microscopy. e Quantification (mean ± SD) of lung colonisation by C-13.08 cells exposed to control (siCtl) or HACE1 (siH1) siRNA after 24 h (n = 9, 3 fields in 3 different mice). f and g Luciferase-expressing A375 melanoma cells were exposed to control (siCtl) or HACE1 (siH1) siRNA and injected in the tail vein of nude mice. At the indicated time after cell injection (30 min, D0; and 24 h, D1), the mice were injected with luciferin, and light emission was visualised (f) and quantified (mean ± SD, n = 6) (g) using a Photon Imager
Because adhesion is an obligatory step before extravasation, organ colonisation and metastases, we studied the effect of HACE1 silencing on lung colonisation and tumour development. To this end, melanoma cells isolated from a patient (C-13.08) were transfected with Ctl or HACE1 siRNA, labelled with green fluorescent vital dye and injected in the tail vain of nude mice. The mice were euthanized, and the lungs were examined by fluorescence microscopy. Thirty minutes after injection, a large number of cells reached the lung and decorated the lung alveoli in both conditions. After 24 h, almost no HACE1-silenced cells were detected in the lungs, while ten times more control cells were present in the lungs (Fig. 2d, e). Labelling of endothelial cells using Alex594-Lycopersicon esculentum lectin allowed for the visualisation of lung vasculature, demonstrating that most melanoma cells remaining in the lungs were in or near blood vessels (Sup. Figure 4.A). Similar results were obtained with C-10.01 cells (Sup. Figure 4.B) and with A375 cells using 2 different siRNAs to HACE1 (Sup. Figure 4.C). Moreover, clone 29, which overexpressed HACE1, exhibited more extensive lung colonisation after 24 h than the parental 501MEL cells (Sup. Figure 4.D).
Additionally, while a high luminescent signal was observed emanating from the lungs of mice 24 h after injection of luciferase-expressing A375 (A375-L) cells, the pulmonary luminescent signal was dramatically reduced in mice receiving HACE1-silenced A375-L cells, thereby confirming the inhibition of lung colonisation capacity of HACE1-deficient melanoma cells (Fig. 2f, g).
Taken together, our results strongly suggested that HACE1 silencing impairs melanoma metastatic development, which is at variance with the tumour-suppressor function assigned to this E3 ligase. Of note, analysis of a publicly available data set (GSE19234) showed that patients with a high level of HACE1 have worse survival than patients with low HACE1 expression (Sup. Figure 4.E). Together with our data, this observation strengthens the pro-tumoural role of HACE1 in melanoma.
HACE1 silencing affects the transcriptional programme in melanoma cells and regulates the expression of integrins
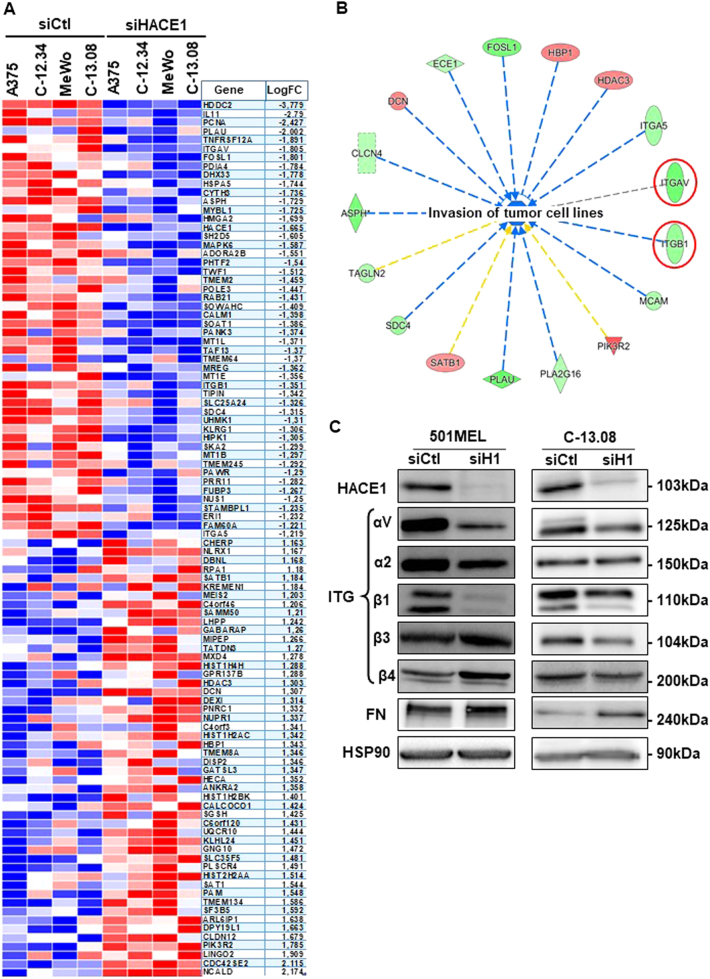
To better understand the molecular events induced by HACE1 silencing, we compared the transcriptome profiles of 4 different melanoma cell cultures after HACE1 suppression with those of parental cells.
Statistical analysis identified 93 downregulated genes and 80 upregulated genes in the 4 cell cultures (Sup. Table 1). The heat map of the top 50 down- and upregulated genes is show in Fig. 3a. Ingenuity Pathway analysis identified 22 cell function annotations inhibited by siHACE1 (Z-score < −2.62, p < 0.02). Among the top 5, 4 annotations were related to “cell movement” (Sup. Table 2), and one was related to “Invasion of tumour cell lines” (Fig. 3b), which was in agreement with our results. One annotation was related to cell proliferation, but we did not observe a significant effect on melanoma cell proliferation after 48 h of HACE1 silencing. Within the top genes inhibited by HACE1 silencing, ITGAV and ITGB1, which ranked 6 and 33, respectively, have been shown to be involved in migration processes.
Fig. 3.
HACE1 controls transcriptional programmes in melanoma cells. a Heat map showing the top 50 genes significantly affected by HACE1 silencing from the most downregulated to most upregulated. b Circular network identified by Ingenuity Analysis Pathway (Qiagen®) showing the genes regulated by HACE1 silencing and associated with “invasion of tumour cell lines”. Red denotes the genes that are upregulated, and green denotes those that are downregulated. The regulation of 14 out of 17 genes is consistent with an inhibition of “invasion of tumour cell lines” (blue dotted lines). The yellow lines indicate that the finding underlying the relationship is inconsistent with the state of the downstream node based on existing literature data. c 501MEL and C-13.08 cells were transfected with control (siCtl) or HACE1 (siH1) siRNA and analysed by western blot with antibodies against HACE1, integrins (αV, α2, β1, β3, β4), fibronectin (FN) and HSP90 (loading control)
Accordingly, western blot analysis of 501MEL and C-13.08 cells demonstrated a reproducible loss of αV and β1 integrins after transfection with siHACE1. The levels of other studied integrins were not consistently affected by HACE1 inhibition in the cells used for the study (Fig. 3c). The loss of αV integrins was confirmed by IF studies (Sup. Figure 5.A).
Further, ITGAV promoter activity was downregulated by siHACE1 in 501MEL and C-09.10 melanoma cells (Sup. Figure 5.B). This observation was confirmed in A375 cells using ITGAV or ITGB1 promoter constructs, demonstrating a transcriptional regulation of ITGB1 and ITGAV by HACE1 silencing (Sup. Figure 5.C, D).
Altogether, transcriptomic data analysis confirmed that HACE1 silencing alters cell migration processes. These effects might involve inhibition of the expression of ITGAV and ITGB1, which are known to play a key role in adhesion and migration [12, 13].
HACE1 controls melanoma cell adhesion/migration through the regulation of FN secretion
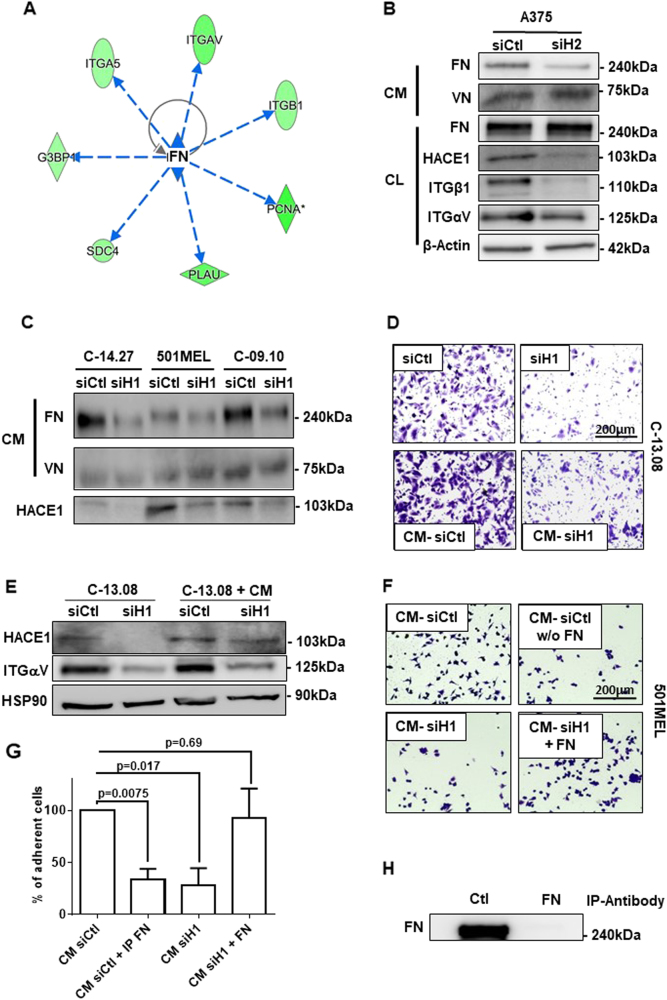
Among the possible upstream regulators that could explain the transcriptional effect of HACE1 silencing, IPA identified an inhibition of TGFβ or TNF pathways (Sup. Table 3). However, HACE1 knockdown did not affect TGFβ or NFκB reporter activities, suggesting that these pathways are not involved in the transcriptional effects evoked by HACE1 inhibition (data not shown). Inhibition of FN was also identified by IPA as a possible cause of the gene regulation induced by HACE1 silencing (Fig. 4a). Indeed, FN is the ligand of integrins, and FN plays a key role in adhesion/migration processes [14]. Neither FN messenger (DNA arrays) nor intra-cellular FN protein was affected by HACE1 silencing (Fig. 4b), but HACE1 silencing consistently decreased FN secretion in all the melanoma cells tested (Fig. 4b, c). HACE1 silencing did not affect vitronectin secretion (Fig. 4c). The decrease in FN secretion after HACE1 silencing was confirmed by ELISA in A375 and 501MEL cell lines with two different siHACE1 (Sup. Fig. 6).
Fig. 4.
HACE1 controls melanoma cell adhesion/migration through the regulation of fibronectin secretion. a Circular network identified by Ingenuity Pathway Analysis (Qiagen®) showing the relationship between fibronectin (FN) and genes that are downregulated by HACE1 silencing. b A375 cells were transfected with control (siCtl) or HACE1 (siH2) siRNA and analysed via western blotting of cell lysate (CL) using antibodies against HACE1, ITGβ1, ITGαV, FN, and actin (loading control). Conditioned medium (CM) was probed with an antibody against FN and vitronectin (VN). c Three different melanoma cell cultures were transfected with control (siCtl) or HACE1 (siH1) siRNA. The CM was analysed by western blot analysis using antibodies against FN or VN. HACE1 suppression was verified by western blot analysis of cell lysate proteins with HACE1 antibodies. d C-13.08 cells were transfected with control (siCtl) or HACE1 (siH1) siRNA or cultured in CM from cells treated with control (CM-siCtl) or HACE1 (CM-siH1) siRNA. Cells were then subjected to Boyden chamber migration assays. e The protein extracts from cells treated as described in D were analysed by western blotting with antibodies against HACE1 or ITGαV. HSP90 was used as a loading control. f 501MEL cells were seeded on plates coated with the following treatments: CM from 501MEL cells treated with siCtl (CM-siCtl) or siHACE1 (CM-siH1); fibronectin-free (immuno-depleted) CM from control 501MEL cells (CM-siCtl w/o FN); CM from HACE1-suppressed 501MEL cells supplemented with 0.5 µg/ml recombinant fibronectin (CM-siH1 + FN). g Quantification of three separate experiments as described in F. The results represent the mean ± SD of the percent of cell adhesion in the control condition. h Western blot analysis of secreted fibronectin after immuno-depletion with control (Ctl) or FN antibodies
We next verified that FN silencing by different siRNAs in two different melanoma cell lines (501MEL and WM793) decreased the expression of both αV and β1 integrins, as well as melanoma cell migration (Sup. Fig. 7.A-D). Further, we showed that exposure of untreated C-13.08 cells to conditioned medium (CM) from siHACE1-treated melanoma cells led to the inhibition of cell migration compared with that observed after exposure to CM from siControl-treated cells (Fig. 4d). In these conditions, αV integrin expression was decreased, but HACE1 levels were not affected (Fig. 4e). Similar results were obtained using 501MEL cells (Sup. Fig. 7.E). These data reinforced the notion that the effect of HACE1 suppression is mediated by a secreted factor.
Coating wells with CM from siControl-treated 501MEL promoted adhesion compared with the effects of coating with CM from HACE1-suppressed cells (Fig. 4f, left panel). Interestingly, the depletion of FN (Fig. 4f upper right panel and Fig. 4h) from control CM impaired 501MEL cell adhesion, while the addition of purified FN to CM from HACE1-depleted cells restored the adhesion of 501MEL cells (Fig. 4f, lower right panel). Quantification of these experiments is shown in Fig. 4g. Taken together, these observations demonstrated a key role of FN secretion in the regulation of adhesion, migration and expression of αV and β1 integrins induced by HACE1 silencing.
HACE1 promotes K27 branched ubiquitination of FN
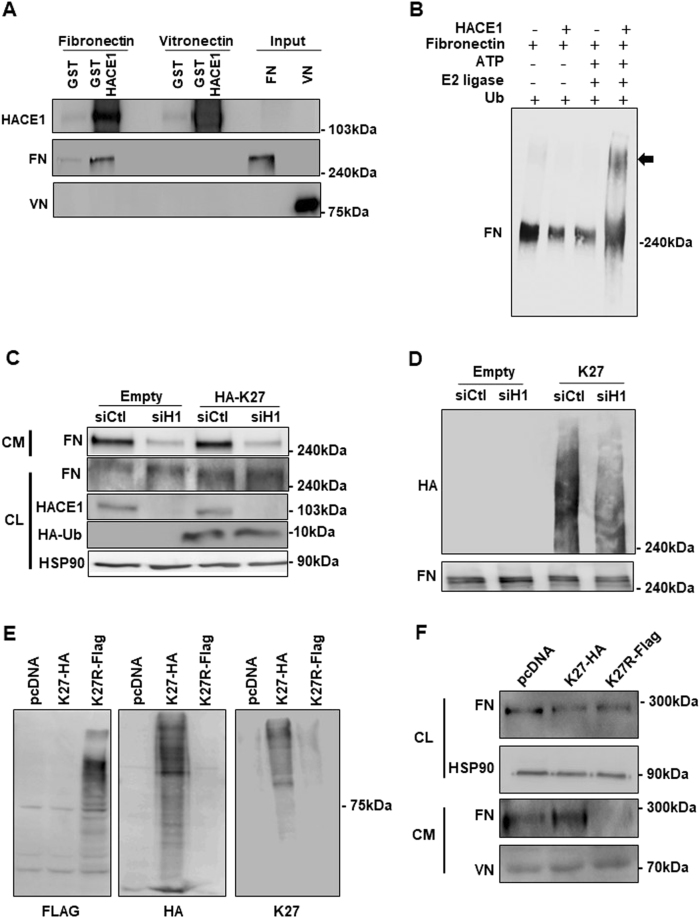
We next searched for a direct molecular link between HACE1 and FN. Taking into account the E3 ligase activity of HACE1, we hypothesised that HACE1 might interact with and promote FN ubiquitination. GST pull-down assays with melanoma cell lysates (CLs) or with CM showed that recombinant GST-tagged HACE1, but not GST, interacted with FN (Sup. Fig. 8.A). This interaction might be mediated by a third partner present in the cell lysate or in the CM of melanoma cells. However, an additional experiment using purified FN demonstrated a direct interaction of FN with GST-HACE1, but not with GST (Fig. 5a). Further, no interaction with vitronectin was detected in these experiments. The interaction between HACE1 and FN was also demonstrated in intact cells under endogenous conditions (Sup. Fig. 8.B).
Fig. 5.
HACE1 interacts with and promotes fibronectin ubiquitination. a GST or GST-HACE1 was incubated with reconstituted fibronectin (FN) or vitronectin (VN). Bound proteins were analysed by western blotting using antibodies against FN or VN. b Purified FN (1 µM) was incubated with ATP, E2 ligase, ubiquitin and recombinant HACE1 (0.4 µM) as indicated and then analysed by western blotting using an antibody against FN. c COS cells were transfected with an empty vector or vectors encoding HA-tag-K27 and control (siCtl) or HACE1 (siH1) siRNA. Total cell extracts (CL) were analysed by western blotting using antibodies against FN, HACE1, HA or HSP90 (loading control). The secreted proteins (CM) were analysed by western blotting using an antibody against FN. d Cellular proteins from cells treated as in C were subjected to immunoprecipitation with a FN antibody and analysed by western blotting using antibodies against HA-tag or FN. e Cells were transfected with an empty vector (EV) or plasmid encoding for HA-tagged K27 ubiquitin (K27-HA) or Flag-tagged K27R ubiquitin (K27R-Flag). Cell lysates were analysed by western blotting using antibodies against Flag, HA or K27 ubiquitin. f The cell lysates (CL) as in (e) were analysed by western blotting using antibodies against FN or HSP90 (loading control). Conditioned media (CM) from the same cells were analysed by western blotting using antibodies against FN or VN (loading control)
We then investigated whether HACE1 promotes FN ubiquitination. An in vitro ubiquitination assay indicated that HACE1 modified FN in the presence of ATP, E2 ligase and ubiquitin (Fig. 5b). Therefore, these data suggested that HACE1 catalyses FN ubiquitination. We next aimed to identify which type of ubiquitin chain linkage on FN is promoted by HACE1. COS cells were transfected with plasmids encoding HA-tagged ubiquitin mutated on all lysines expected, lysine 27 (HA-K27), lysine 63 (HA-K63) and lysine 48 (HA-K48). Analysis of the total cellular lysate by western blot showed an equivalent amount of FN, as detected by an anti-FN antibody, as well as an equivalent amount of ubiquitin, as detected with an anti-HA antibody, in all conditions (Sup. Fig. 8.C, left panel). Immunoprecipitation using an antibody against FN showed that FN was mainly modified by K27 ubiquitination as revealed with an anti-HA antibody. Ubiquitination was barely detectable in cells transfected with HA-K63 and HA-K48 (Sup. Fig. 8.C, right panel). Western blot with anti-FN showed that equivalent amounts of FN were precipitated in all conditions. No FN or ubiquitination was detected after immunoprecipitation with a non-relevant antibody (IP-Myc) (Sup. Fig. 8.C, middle panel). The above data indicated that FN interacts with HACE1 and undergoes K27 ubiquitination.
We next tested the effect of HACE1 silencing on the K27 ubiquitination of FN. COS cells were co-transfected with empty or HA-tagged ubiquitin K27 (HA-K27) plasmids and control or HACE1 siRNA (Fig. 5c, d). HACE1 siRNA efficiently inhibited HACE1 expression (Fig. 5c). HACE1 silencing inhibited FN levels in the CM, but did not affect FN levels in the CL. Moreover, an equal amount of ubiquitin K27 was expressed in both control and HACE1 siRNA-treated cells. FN was immunoprecipitated and analysed by western blotting. Anti-FN antibodies revealed an equal amount of immunoprecipitated FN in all conditions. Anti-HA antibodies detected ubiquitination only in cells transfected with HA-K27. However, a clear decrease in the ubiquitination of FN was observed in HACE1-silenced cells, demonstrating that HACE1 promotes the K27 ubiquitination of FN (Fig. 5d). Taken together, these findings suggested that HACE1 controls FN secretion through K27 ubiquitination. To confirm this hypothesis, the cells were transfected with HA-tagged K27 ubiquitin or Flag-tagged K27R ubiquitin, which cannot support K27 branching (Fig. 5e). A decrease in the FN level in the CM from cells expressing K27R ubiquitin (Fig. 5f) provided further support for the key role of FN K27 ubiquitination in the regulation of its secretion.
Discussion
In contradiction of the expected anti-tumoural role ascribed to HACE1, we demonstrated in this study that HACE1 favoured melanoma cell adhesion, migration and lung colonisation. HACE1 expression was also maintained in melanoma cells, as shown by western blot analyses and the mining of publicly available transcriptomic data sets, indicating that the loss of HACE1 was not a prerequisite for melanoma development in contrast to what has been reported for Wilms’ tumours or colorectal cancers [1, 15].
In agreement with the role of HACE1 in the control of RAC1 level and the key role of RAC1 in melanoma, it might be surprising that HACE1 displayed pro-tumoural functions in melanoma cells. However, the RAC1 level is controlled by other E3 ligases, such as IAPs [16, 17] and FBXL19 [18]. The respective roles of HACE1, IAPS and FBXL19 in RAC1 degradation in melanoma are not known. A RAC1 activation assay demonstrated that HACE1 silencing prevented the CNF-induced degradation of GTP-RAC1, but had no effect on basal levels of RAC1. Therefore, the residual level of GTP-RAC1 that persists in HACE1-expressing melanoma cells might be sufficient to sustain cell adhesion and migration. As HACE1 silencing inhibited melanoma cell tumourigenic properties, other pro-tumourigenic functions of HACE1 must overcome the HACE1-induced degradation of active RAC1.
Even though most reports on HACE1 have indicated that it behaves as an anti-oncogene, several studies do not agree with this viewpoint. HACE1 is involved in Golgi biogenesis [19], which is required for normal cell functioning. A report has indicated that HACE1 is not a tumour suppressor in NK-cell neoplasms [20]. It should also be noted that HACE1 deficiency causes an autosomal recessive neurodevelopmental syndrome, but no cancer predisposition is observed in homozygous or heterozygous mutation carriers [21]. Taken together, these observations are not in agreement with the universal tumour-suppressor role ascribed to HACE1.
In the original publication showing that HACE1 may function as a tumour suppressor, the effects of HACE1 KO are barely observable in a p53WT background. Two pigmented lesions, identified by the authors as a melanoma, were observed among the 252 mice analysed [2]. However, because the mice had a ubiquitous heterozygous or homozygous HACE1 KO, the development of melanoma and some other tumours might have been due to alterations in immune cell biology and tumour cell immune surveillance.
Our data do not challenge the clearly established tumour-suppressor role of HACE1 in Wilms’ tumour and in breast cancer [1, 10], but our study discloses a cell-specific tumourigenic function of HACE1 in melanoma that deserves to be evaluated in other neoplasms.
Concerning the molecular mechanisms involved in the pro-tumourgenic function of HACE1, we demonstrated that HACE1 silencing induced a modification of the transcriptional programme in melanoma cells that was compatible with a decrease in cell movement and migration. Among the genes associated with these effects, ITGAV and ITGB1 were downregulated at the transcriptional level, and their involvement in cell migration has been extensively described [12, 22]. Other genes, such as PLAU and SDC4, which promote cell invasiveness [23, 24], were also downregulated by HACE1 silencing.
All of these genes (ITGAV, ITGB1, PLAU and SDC4) have been reported to be regulated by FN [25, 26, 27, 28], but neither FN1 expression nor intra-cellular FN level was affected by HACE1 silencing. However, our data revealed that FN secretion was markedly affected by HACE1 silencing. As FN must be secreted to interact with integrins, the decrease in extracellular FN will lead to the inhibition of FN signalling and biological effects.
Little is known regarding FN secretion. A recent report has demonstrated that caspase 3 controls FN secretion without affecting FN1 expression [29]. The authors hypothesised that caspase 3 controls the ER-Golgi transport or vesicle trafficking. Of note, caspase 3 was downregulated upon HACE1 suppression in three out of four cell lines analysed, but this decrease did reach statistical significance. We cannot rule out that the regulation of caspase 3 might also be involved in the inhibition of FN secretion in some melanoma cells.
We demonstrated in this report that HACE1 interacts with FN, promotes its K27 ubiquination and regulates its secretion. In agreement with our data, HACE1 has been recently reported to promote K27 ubiquitination of YB1 and to regulate its secretion [30]. Because FN is a large protein, we have not been able to identify the site of K27 ubiquitination, but the inhibition of FN secretion by the forced expression of K27R ubiquitin strongly supports the hypothesis that HACE1 regulates FN secretion through its K27 ubiquitination.
Secreted FN interacts with integrins and activates canonical signalling pathways, such as ERK or PI3 kinase pathways [31, 32], which in turn regulate the expression of integrins [26, 33], thus promoting cell adhesion and migration. This molecular cascade must be assessed in other neoplasms to clarify the function of HACE1 in cell-specific contexts.
Nevertheless, our data revealed a pivotal role of HACE1 in the regulation of a feed forward loop between integrins and FN that plays a key role in the adhesion, migration and metastasis ability of melanoma cells, thereby making HACE1 a new player in melanoma biology.
Materials and methods
Antibodies, siRNA and other reagents
An antibody against HACE1 (Ab80651) was purchased from Abcam, and antibodies against FN (#610077BD), αV integrin and β1 integrin were obtained from BD Biosciences. Antibodies against Myc and HSP90 were purchased from Santa Cruz Biotechnologies.
Horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibodies were obtained from Dakopatts (Glostrup, Denmark).
A siRNA pool targeting HACE1 (siH1) was purchased from Santa Cruz (#sc-95301), and single siRNAs against HACE1 were purchased from Qiagen (siH2 #SI00127680, siH3 #SI00127694 and siH4 #SI03062227). A siRNA pool targeting FN (M-009853-01) was purchased from Dharmacon, and a single siRNA against FN was purchased from Qiagen (SI02663997). Scramble siRNAs were used as a control. The HA-HACE1 plasmid was a gift from Dr. Emmanuel Lemichez. In brief, human HACE1 cDNA was obtained from ATCC (clone I.M.A.G.E. 4838835; GenBank accession no. BC034982), amplified by PCR and cloned via BamHI/EcoRI into pXJ-HA.
Cell culture
Cells were grown in RPMI-1640 (A375 and short-term cultures) or DMEM (501MEL and MeWo) supplemented with 7% foetal bovine serum and penicillin/streptomycin (100 U/ml:50 μg/ml) in a humidified atmosphere containing 5% CO2 at 37 °C. The cells overexpressing HACE1 were obtained by transfection with pXJ-HA-HACE1 and pBABE-Puro (10/1 ratio). Two days after transfection, puromycin selection was applied (1 µg/ml) for 3–4 weeks. Individual clones were amplified and analysed for HACE1 expression by western blot or qPCR analysis.
RNAi transfection
Transfection of siRNAs (25 nM) was performed using Lipofectamine RNAiMAX (Invitrogen) in OptiMEM (Invitrogen) as previously described. Cells were analysed 3 days post transfection.
Western blot, immunoprecipitation and pull-down experiment
Cells were washed with cold PBS and lysed with ice-cold buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl and 1% Triton X-100 supplemented with protease and phosphatase inhibitors. Soluble proteins were then analysed by SDS-PAGE and transferred onto polyvinylidene fluoride membranes (Millipore) using a semi-dry apparatus (Trans-blot SD; Bio-Rad). For immunodetection, the membranes were blocked in a blocking buffer solution and then exposed to appropriate antibodies. Western blot analyses shown are representative of at least three independent experiments.
For immunoprecipitation assays, whole CLs were incubated with 2 µg of specific antibody bound to PAG magnetic beads (Ademtech). After three washes in lysis buffer (0.1% Triton X-100), bead-bound proteins were subjected to western blot analysis using the appropriate antibodies.
GST and GST-HACE1 recombinant proteins were purified from bacteria after IPTG induction. These proteins were collected from bacteria lysates using GSH-Sepharose beads.
Transwell migration assay
Tumour cell migration assays were performed in transwell chambers. Boyden chambers (8.0-μm pores, Transwell; Corning, Inc.) were placed into 24-well chambers containing medium supplemented with 7% FBS. Cells were re-suspended in FBS-starved medium and loaded into the top chamber. Several hours later (depending on the cells used), the cells adhered to the underside of the filters were fixed with 4% paraformaldehyde (PFA) and stained with 0.4% crystal violet, and five random fields at 20 × magnification were counted. The results represent the mean ± SD of triplicate samples from 3 independent experiments.
Adhesion assay
Cells were seeded in a 96-well plate and incubated at 37 °C in a CO2 incubator for the indicated period. The plates were then washed with PBS, and the remaining cells were fixed with 4% paraformaldehyde for 15 min and then stained with crystal violet for 15 min. For the adhesion assay, using previously coated wells, the plates were first incubated with the appropriate CM and washed with PBS before plating cells. Representative images were captured and adherent cells were counted. The results represent the mean ± SD of triplicate samples from three independent experiments.
xCELLigence system
Real-time monitoring of live cells was performed using the xCELLigence RTCA DP instrument (ACEA Biosciences), which was placed in a humidified incubator at 37 °C and 5% CO2. Cell adhesion experiments were performed using 16-well plates (ACEA Biosciences, Ref 00300600880). Initially, 100 μL of culture medium was added to each well, and a background measurement step was performed as a background signal generated by cell-free medium. To initiate the experiment, siCtl- or siH1-transfected cells (501MEL EV, 501MEL C29 and C60) were counted and seeded at a similar concentration. Each condition was performed in triplicate with a programmed signal detection scheduled once every minute for several hours. The results represent the average of triplicate samples from three independent experiments.
Mouse experiments
For short-term lung colonisation assays, melanoma cells were transfected with Ctl or HACE1 siRNA for 72 h. The cells were detached using HyQtase and labelled with 10 µM Cell Tracker Green CMFDA for 30 min, and an equal number of cells was injected separately into the tail veins of nude mice. The mice were euthanized after 30 min or 24 h, and whole lungs were examined by immunofluorescence microscopy.
Alternatively, 3 × 106 L-A375 cells were injected into the tail veins of 6-week-old nude mice. Prior to imaging, the mice were administered by intraperitoneal injection 150 mg/kg XenoLight D-Luciferin (from Perkin Elmer) and anaesthetised. Bioluminescent images were acquired using a Photon Imager system (Biospace Lab). ROI optical images were analysed with M3 Vision Software.
Ubiquitination assay
An in vitro ubiquitination assay was conducted using the Ubiquitin Conjugating kit purchased from R&D systems (K-982). The experiment was performed according to the manufacturer’s specifications. HA-Ubiquitin plasmids (HA-K27, HA-K48 and HA-K63) were purchased from Addgene. The GST-HACE1-His plasmid for pull-down assays was a generous gift from Dr. Cory Hu (Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences). The plasmid encoding Flag-tagged K27R ubiquitin was provided by Pr. D. Alessi (University of Dundee).
Gene expression profiling
Total RNA from 4 different melanoma cell cultures (melanoma cell lines, A375 and MeWo, and short-term melanoma cell cultures, C-12.34 and C-13.08) was extracted using the RNeasy kit (Qiagen, Hilden, Germany). Integrity of RNA was assessed using an Agilent BioAnalyzer 2100 (Agilent Technologies) (RIN greater than 9). RNA samples were then labelled with Cy3 dye using the Low RNA Input QuickAmp kit (Agilent) as recommended by the supplier. Briefly, 825 ng of labelled cRNA probe was hybridised onto 8 × 60 K high-density SurePrint G3 gene expression mouse Agilent microarrays. Normalisation and statistical analysis of microarray data were performed using the Limma package available from Bioconductor (http://www.bioconductor.org). Inter-slide normalisation was performed using quantile methods. Differentially expressed genes were selected based on AbsLogFC > 1 and Log Odds-ratio > 0. The data from expression microarrays were analysed for enrichment in biological themes (Gene Ontology, molecular function and canonical pathways) using Ingenuity Pathway Analysis software (http://www.ingenuity.com/).
Statistical methods
Unless specified in figure legends, all data were subjected to two-tailed t-test analysis using Prism 5 (GraphPad Software, San Diego, CA, USA). The results are presented as the mean ± SD.
Electronic supplementary material
Acknowledgements
This work was supported by Institut National du Cancer (INCA) grants (2013-070 and 2013-1-MELA-04) and the “Association pour la Recherche sur le Cancer”, Equipe labellisée ARC 2015. Labex SIGNALIFE, ANR-11 LABEX-0028-01, la Ville de Nice. NEH is a recipient of “Fondation pour la Recherche Medicale” and LABEX. NH is a recipient of the ARC. We thank Dr. Emmanuel Lemichez for providing the HACE1 expression vector, Dr. Cory Hu for providing the GST-HACE1 plasmid and Pr. Alessi for providing the K27R plasmid. Frédéric Rénier, Centre Méditerranéen de Médecine Moléculaire, Bioinformatic Unit and Nicolas Nottet are supported by The Cancéropôle PACA. The authors thank the C3M animal and imaging facilities.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
The online version of this article (10.1038/s41418-018-0090-y) contains supplementary material, which is available to authorised users.
References
- 1.Anglesio MS, Evdokimova V, Melnyk N, Zhang L, Fernandez CV, Grundy PE, et al. Differential expression of a novel ankyrin containing E3 ubiquitin-protein ligase, Hace1, in sporadic Wilms’ tumor versus normal kidney. Hum Mol Genet. 2004;13:2061–74. doi: 10.1093/hmg/ddh215. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Anglesio MS, O’Sullivan M, Zhang F, Yang G, Sarao R, et al. The E3 ligase HACE1 is a critical chromosome 6q21 tumor suppressor involved in multiple cancers. Nat Med. 2007;13:1060–9. doi: 10.1038/nm1621. [DOI] [PubMed] [Google Scholar]
- 3.Torrino S, Visvikis O, Doye A, Boyer L, Stefani C, Munro P, et al. The E3 ubiquitin-ligase HACE1 catalyzes the ubiquitylation of active Rac1. Dev Cell. 2011;21:959–65. doi: 10.1016/j.devcel.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Castillo-Lluva S, Tan CT, Daugaard M, Sorensen PH, Malliri A. The tumour suppressor HACE1 controls cell migration by regulating Rac1 degradation. Oncogene. 2013;32:1735–42. doi: 10.1038/onc.2012.189. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z, Chen P, Gao H, Gu Y, Yang J, Peng H, et al. Ubiquitylation of autophagy receptor Optineurin by HACE1 activates selective autophagy for tumor suppression. Cancer Cell. 2014;26:106–20. doi: 10.1016/j.ccr.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–63. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halaban R. RAC1 and melanoma. Clin Ther. 2015;37:682–5. doi: 10.1016/j.clinthera.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mischiati C, Natali PG, Sereni A, Sibilio L, Giorda E, Cappellacci S, et al. cDNA-array profiling of melanomas and paired melanocyte cultures. J Cell Physiol. 2006;207:697–705. doi: 10.1002/jcp.20610. [DOI] [PubMed] [Google Scholar]
- 9.Scatolini M, Grand MM, Grosso E, Venesio T, Pisacane A, Balsamo A, et al. Altered molecular pathways in melanocytic lesions. Int J Cancer. 2010;126:1869–81. doi: 10.1002/ijc.24899. [DOI] [PubMed] [Google Scholar]
- 10.Goka ET, Lippman ME. Loss of the E3 ubiquitin ligase HACE1 results in enhanced Rac1 signaling contributing to breast cancer progression. Oncogene. 2015;34:5395–405. doi: 10.1038/onc.2014.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tortola L, Nitsch R, Bertrand MJ, Kogler M, Redouane Y, Kozieradzki I, et al. The tumor suppressor Hace1 is a critical regulator of TNFR1-mediated cell fate. Cell Rep. 2016;16:3414. doi: 10.1016/j.celrep.2016.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, et al. Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell. 2007;13:496–510. doi: 10.1016/j.devcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Morgan MR, Byron A, Humphries MJ, Bass MD. Giving off mixed signals--distinct functions of alpha5beta1 and alphavbeta3 integrins in regulating cell behaviour. IUBMB Life. 2009;61:731–8. doi: 10.1002/iub.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Digiacomo G, Tusa I, Bacci M, Cipolleschi MG, Dello Sbarba P, Rovida E. Fibronectin induces macrophage migration through a SFK-FAK/CSF-1R pathway. Cell Adh Migr. 2017;11:1–11. doi: 10.1080/19336918.2016.1221566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hibi K, Sakata M, Sakuraba K, Shirahata A, Goto T, Mizukami H, et al. Aberrant methylation of the HACE1 gene is frequently detected in advanced colorectal cancer. Anticancer Res. 2008;28:1581–4. [PubMed] [Google Scholar]
- 16.Oberoi-Khanuja TK, Rajalingam K. Ubiquitination of Rac1 by inhibitors of apoptosis (IAPs) Methods Mol Biol. 2014;1120:43–54. doi: 10.1007/978-1-62703-791-4_4. [DOI] [PubMed] [Google Scholar]
- 17.Oberoi TK, Dogan T, Hocking JC, Scholz RP, Mooz J, Anderson CL, et al. IAPs regulate the plasticity of cell migration by directly targeting Rac1 for degradation. EMBO J. 2012;31:14–28. doi: 10.1038/emboj.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao J, Mialki RK, Wei J, Coon TA, Zou C, Chen BB, et al. SCF E3 ligase F-box protein complex SCF(FBXL19) regulates cell migration by mediating Rac1 ubiquitination and degradation. FASEB J. 2013;27:2611–9. doi: 10.1096/fj.12-223099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang D, Xiang Y, De Renzis S, Rink J, Zheng G, Zerial M, et al. The ubiquitin ligase HACE1 regulates Golgi membrane dynamics during the cell cycle. Nat Commun. 2011;2:501. doi: 10.1038/ncomms1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sako N, Dessirier V, Bagot M, Bensussan A, Schmitt C. HACE1, a potential tumor suppressor gene on 6q21, is not involved in extranodal natural killer/T-cell lymphoma pathophysiology. Am J Pathol. 2014;184:2899–907. doi: 10.1016/j.ajpath.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Hollstein R, Parry DA, Nalbach L, Logan CV, Strom TM, Hartill VL, et al. HACE1 deficiency causes an autosomal recessive neurodevelopmental syndrome. J Med Genet. 2015;52:797–803. doi: 10.1136/jmedgenet-2015-103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Horst G, Bos L, van der Mark M, Cheung H, Heckmann B, Clement-Lacroix P, et al. Targeting of alpha-v integrins reduces malignancy of bladder carcinoma. PLoS ONE. 2014;9:e108464. doi: 10.1371/journal.pone.0108464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carriero MV, Franco P, Votta G, Longanesi-Cattani I, Vento MT, Masucci MT, et al. Regulation of cell migration and invasion by specific modules of uPA: mechanistic insights and specific inhibitors. Curr Drug Targets. 2011;12:1761–71. doi: 10.2174/138945011797635777. [DOI] [PubMed] [Google Scholar]
- 24.Elfenbein A, Simons M. Syndecan-4 signaling at a glance. J Cell Sci. 2013;126:3799–804. doi: 10.1242/jcs.124636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hapke S, Kessler H, Arroyo de Prada N, Benge A, Schmitt M, Lengyel E, et al. Integrin alpha(v)beta(3)/vitronectin interaction affects expression of the urokinase system in human ovarian cancer cells. J Biol Chem. 2001;276:26340–8. doi: 10.1074/jbc.M100181200. [DOI] [PubMed] [Google Scholar]
- 26.Hofmann G, Bernabei PA, Crociani O, Cherubini A, Guasti L, Pillozzi S, et al. HERG K + channels activation during beta(1) integrin-mediated adhesion to fibronectin induces an up-regulation of alpha(v)beta(3) integrin in the preosteoclastic leukemia cell line FLG 29.1. J Biol Chem. 2001;276:4923–31. doi: 10.1074/jbc.M005682200. [DOI] [PubMed] [Google Scholar]
- 27.Landowski TH, Olashaw NE, Agrawal D, Dalton WS. Cell adhesion-mediated drug resistance (CAM-DR) is associated with activation of NF-kappa B (RelB/p50) in myeloma cells. Oncogene. 2003;22:2417–21. doi: 10.1038/sj.onc.1206315. [DOI] [PubMed] [Google Scholar]
- 28.Milner R, Campbell IL. The extracellular matrix and cytokines regulate microglial integrin expression and activation. J Immunol. 2003;170:3850–8. doi: 10.4049/jimmunol.170.7.3850. [DOI] [PubMed] [Google Scholar]
- 29.Brentnall M, Weir DB, Rongvaux A, Marcus AI, Boise LH. Procaspase-3 regulates fibronectin secretion and influences adhesion, migration and survival independently of catalytic function. J Cell Sci. 2014;127:2217–26. doi: 10.1242/jcs.135137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palicharla VR, Maddika S. HACE1 mediated K27 ubiquitin linkage leads to YB-1 protein secretion. Cell Signal. 2015;27:2355–62. doi: 10.1016/j.cellsig.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Jin YJ, Park I, Hong IK, Byun HJ, Choi J, Kim YM, et al. Fibronectin and vitronectin induce AP-1-mediated matrix metalloproteinase-9 expression through integrin alpha(5)beta(1)/alpha(v)beta(3)-dependent Akt, ERK and JNK signaling pathways in human umbilical vein endothelial cells. Cell Signal. 2011;23:125–34. doi: 10.1016/j.cellsig.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Matsuo M, Sakurai H, Ueno Y, Ohtani O, Saiki I. Activation of MEK/ERK and PI3K/Akt pathways by fibronectin requires integrin alphav-mediated ADAM activity in hepatocellular carcinoma: a novel functional target for gefitinib. Cancer Sci. 2006;97:155–62. doi: 10.1111/j.1349-7006.2006.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Lu H, Dazin P, Kapila Y. Functional differences between integrin alpha4 and integrins alpha5/alphav in modulating the motility of human oral squamous carcinoma cells in response to the V region and heparin-binding domain of fibronectin. Exp Cell Res. 2004;295:48–58. doi: 10.1016/j.yexcr.2003.12.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.