Abstract
Prokaryotes can provide new genetic information to eukaryotes by horizontal gene transfer (HGT), and such transfers are likely to have been particularly consequential in the era of eukaryogenesis. Since eukaryotes are highly compartmentalized, it is worthwhile to consider the mechanisms by which newly transferred proteins might reach diverse organellar destinations. Toward this goal, we have focused our attention upon the behavior of bacteria-derived tail anchors (TAs) expressed in the eukaryote Saccharomyces cerevisiae. In this study, we report that a predicted membrane-associated domain of the Escherichia coli YgiM protein is specifically trafficked to peroxisomes in budding yeast, can be found at a pre-peroxisomal compartment (PPC) upon disruption of peroxisomal biogenesis, and can functionally replace an endogenous, peroxisome-directed TA. Furthermore, the YgiM(TA) can localize to peroxisomes in mammalian cells. Since the YgiM(TA) plays no endogenous role in peroxisomal function or assembly, this domain is likely to serve as an excellent tool allowing further illumination of the mechanisms by which TAs can travel to peroxisomes. Moreover, our findings emphasize the ease with which bacteria-derived sequences might target to organelles in eukaryotic cells following HGT, and we discuss the importance of flexible recognition of organelle targeting information during and after eukaryogenesis.
Introduction
While prokaryotes can harbor compartments dedicated to specific functions and biochemical reactions1, eukaryotes are commonly characterized by a higher level of compartmentalization by membranous structures. One of these organelles, the peroxisome, is bounded by a single membrane and is often a location of fatty acid oxidation in eukaryotic cells2,3. Beyond fatty acid breakdown, peroxisomes play multiple roles among eukaryotes4,5, including sterol synthesis6, synthesis of ether lipids7, and even glycolysis8. Soluble proteins are directed to the lumen, or matrix, of peroxisomes by a conserved import machinery commonly (but not exclusively) taking advantage of a carboxyl-terminal sequence called peroxisomal targeting sequence 1 (PTS1)9,10. Membrane proteins are also targeted to peroxisomes, but mechanisms of peroxisomal membrane protein (PMP) biogenesis are not as well characterized as those processes that mediate import to the peroxisomal matrix11,12. The evolutionary origin of peroxisomes is obscure, although some evidence suggests that the core machinery required for peroxisomal assembly is derived from the endoplasmic-reticulum-associated protein degradation (ERAD) machinery13,14.
During and following eukaryogenesis, (proto-)nuclear genes were obtained by gene transfers from endosymbionts and from free-living prokaryotes, with some of these proteins subsequently targeted to organelles15–20. Beyond more ‘ancient’ gene transfers, HGT from prokaryotes to eukaryotes and conversion of endosymbionts to organelles appears to continue at present day21–24. Signals found within the polypeptide sequence of nucleus-encoded genes play a dominant role in targeting to eukaryotic organelles, and how prokaryote-derived proteins might acquire such sequences and become localized to eukaryotic organelles is a topic of intense inquiry. In a previous study directed toward the principals of organelle targeting following HGT from bacteria25, we focused our attention upon those proteins predicted to be anchored to membranes by a carboxyl-terminal hydrophobic stretch of amino acids, or tail anchor (TA). Here, we describe the trafficking of one of these bacteria-derived TAs, retrieved from the YgiM protein of E. coli. We find that the YgiM tail anchor sequence [YgiM(TA)] localizes to peroxisomes in yeast and in human cells and appears to functionally replace an endogenous, peroxisomal TA in S. cerevisiae. In mutants for which peroxisomal biogenesis is impaired, the YgiM(TA) is localized to ER or to ER-derived pre-peroxisomal compartments (PPCs), suggesting that this exogenous domain follows a trafficking pathway used by endogenously encoded peroxisomal TAs. Our work highlights the ability of eukaryotes to use prokaryotic information obtained by HGT to direct acquired proteins to distinct subcellular locations.
Results
A domain encoded by the bacterial YgiM gene is targeted to the peroxisomes of yeast cells
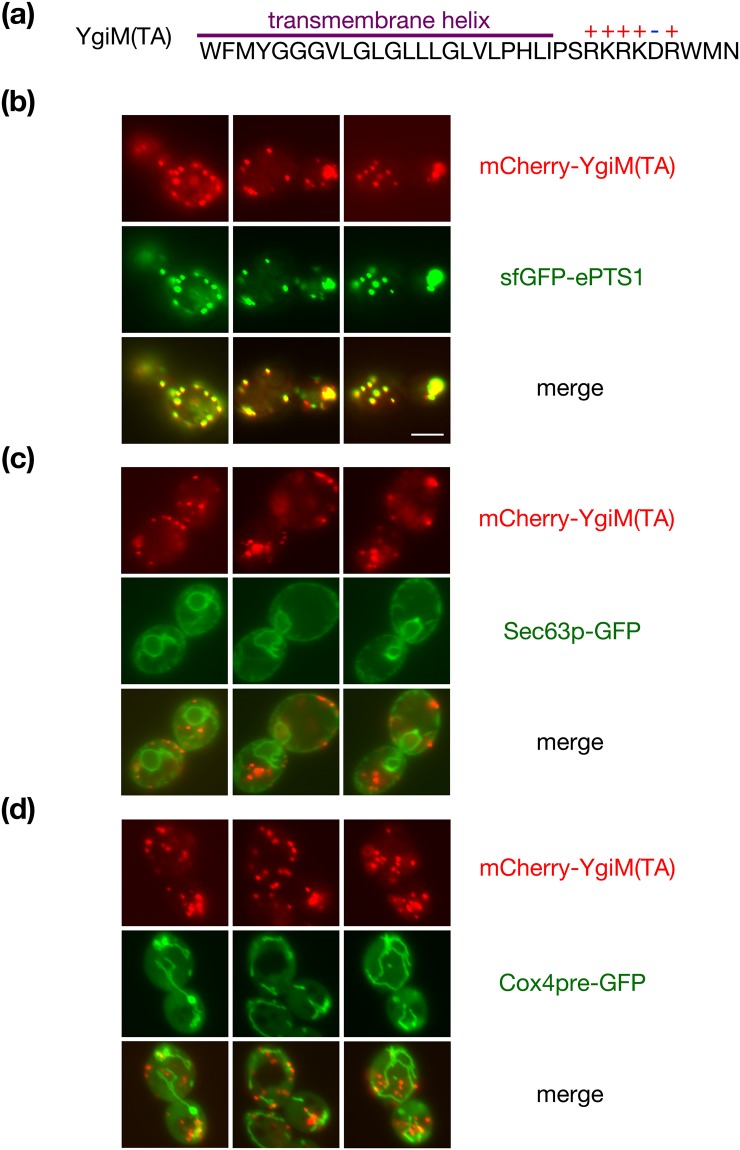
During a previous appraisal of the ability of eukaryotic cells to utilize potential targeting information encoded by prokaryotes25, we fused mCherry to the amino-terminus of predicted TAs encoded by the E. coli genome. These fluorescent fusion proteins were found at diverse locations within the cell, and we noted that mCherry fused to amino acids 173–206 of the uncharacterized YgiM protein, hereafter entitled the YgiM(TA), was found in a punctate pattern reminiscent of peroxisomes. The YgiM(TA) contains a predicted transmembrane helix followed by a positively charged lumenal tail (Fig. 1a). In order to determine whether the YgiM(TA) might indeed target to peroxisomes, we expressed mCherry-YgiM(TA) from the strong ADH1 promoter together with superfolder green fluorescent protein (sfGFP) linked to the enhanced peroxisomal targeting signal 1 (ePTS1)26. mCherry-YgiM(TA) co-localized with sfGFP-ePTS1, providing strong evidence of YgiM(TA) targeting to peroxisomes (Fig. 1b). In contrast, mCherry-YgiM(TA) was not detectable at the endoplasmic reticulum (ER) (Fig. 1c). Similarly, mCherry-YgiM(TA) was not detectable at mitochondria (Fig. 1d), even upon deletion of Msp1p (Supplementary Fig. S1), which extracts peroxisomal tail-anchored proteins mistargeted to mitochondria27,28.
Figure 1.
The predicted tail anchor of Escherichia coli YgiM localizes to peroxisomes in Saccharomyces cerevisiae. (a) The sequence of the YgiM(TA), as retrieved from UniProt76 record P0ADT8, is provided. The transmembrane helix is predicted using the TMHMM 2.0 server77 and charged residues are indicated. (b) The YgiM(TA) co-localizes with a protein targeted to peroxisomes. Strain BY4741, harboring plasmid b311 (sfGFP-ePTS1), was mated to strain BY4742, carrying plasmid b274 [mCherry-YgiM(TA)]. The resulting diploids were analyzed by fluorescence microscopy of live cells. (c) The YgiM(TA) does not co-localize with ER in wild-type cells. Strain BY4741, containing plasmid pJK59 (Sec63p-GFP) and strain BY4742, carrying plasmid b274 [mCherry-YgiM(TA)] were analyzed as in (b). (d) The YgiM(TA) does not co-localize with mitochondria. Strains BY4741 and BY4742, transformed with plasmids pHS1 (Cox4pre-GFP) and b274 [mCherry-YgiM(TA)], respectively, were mated and analyzed as in (b).
The YgiM tail anchor can functionally replace an endogenous, peroxisome-directed tail anchor
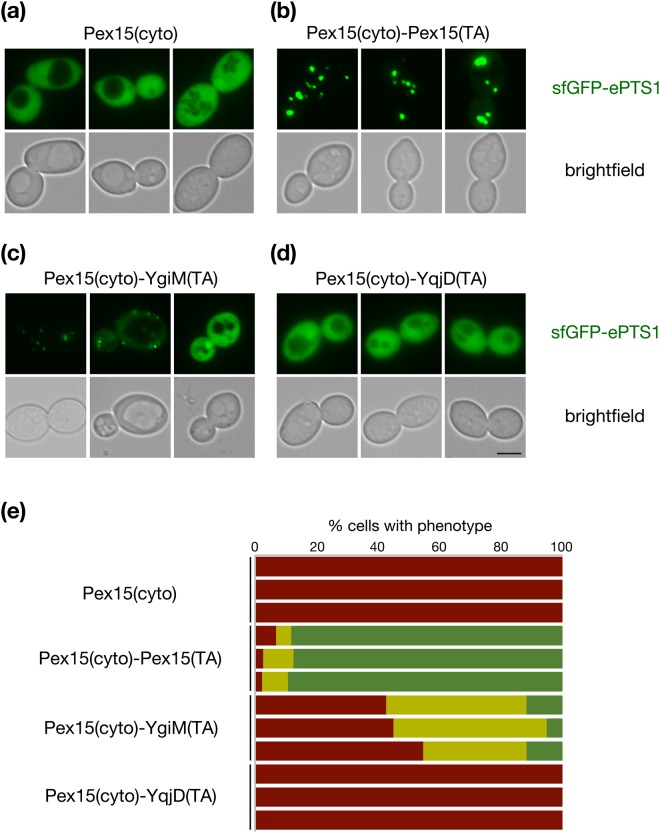
Pex15p, which participates in the import of yeast proteins to the peroxisomal matrix9,10,29, is the only S. cerevisiae protein thought to be directed specifically to peroxisomes by a TA30. A lack of Pex15p at peroxisomes leads to defective peroxisomal biogenesis and cytosolic accumulation of PTS1-directed proteins31. Previous studies have demonstrated that Pex15p is functional when its TA is replaced by that of the mammalian PEX26 protein32, suggesting that other peroxisome-inserted TAs might also support Pex15p activity. Therefore, we tested whether the YgiM(TA) might target the Pex15p cytosolic domain to peroxisomes and permit Pex15p-driven protein import.
As expected, expression of an untethered Pex15p cytosolic domain (amino acids 1–331)32 under control of the native PEX15 promoter in cells lacking a chromosomal copy of PEX15 did not allow localization of sfGFP-ePTS1 to puncta (Fig. 2a,e), while re-attachment of the Pex15(TA) to the Pex15p cytosolic domain permitted sfGFP-ePTS1 recruitment to puncta suggestive of import into the peroxisomal matrix (Fig. 2b,e). Indicating that the bacterial YgiM(TA) can provide functionality in S. cerevisiae, Pex15(1–331)-YgiM(TA) allowed punctate localization of sfGFP-ePTS1, although rescue of the pex15∆ phenotype was not absolute (Fig. 2c,e). Not all bacteria-derived TAs seem to support Pex15p function: Pex15(1–331) fused to the E. coli YqjD(TA), which was previously demonstrated25 to target predominantly to mitochondria and, to a lesser extent, the ER, failed to permit recruitment of sfGFP-ePTS1 to puncta in pex15∆ cells (Fig. 2d,e). Though a portion of the S. cerevisiae Fis1p is associated with peroxisomes33, we found no evidence that the Fis1p(TA) can allow Pex15p function (Supplementary Fig. S2).
Figure 2.
The YgiM(TA) can functionally replace the tail anchor of Pex15p. pex15∆/pex15∆ strain CDD1182, containing a counter-selectable plasmid expressing the Pex15p cytosolic domain (cyto) fused to its own TA (b354) was transformed with plasmids expressing (a) Pex15(cyto) lacking a TA (b341) (b) Pex15(cyto)-Pex15(TA) (b326) (c) Pex15(cyto) fused to the YgiM(TA) (b329) or (d) Pex15(cyto) fused to the YqjD(TA) (b330). Plasmid b354 was then removed by counter-selection with CHX, and Pex15p function was assessed by sfGFP-ePTS1 localization to puncta indicative of peroxisomes competent for import of matrix-directed proteins. (e) Reports the quantification, blinded to genotype during analysis, of three independent experiments. Red represents cells with diffuse signal in the nucleus and cytosol but no puncta, yellow represents cells with both diffuse and punctate signal, and green represents cells in which only punctate signal could be detected (n > 200 cells per sample in each experimental replicate).
The YgiM tail anchor resides within a pre-peroxisomal compartment upon disruption of peroxisomal biogenesis
In yeast, many integral peroxisomal membrane proteins (PMPs) are inserted at the ER before subsequent trafficking to peroxisomes12. The tail-anchored Pex15 protein is also thought to begin its journey to the peroxisome within the ER30,34, and upon disruption of PMP trafficking, accumulates in ER-derived pre-peroxisomal compartments (PPCs)35 marked by Pex14p36–38, a component contributing to formation of the mature peroxisomal import pore39,40. To visualize Pex14p-marked PPCs, we tagged endogenous Pex14p with sfGFP. The Pex14p-sfGFP fusion protein was easily detectable, could promote peroxisomal protein import (Supplementary Fig. S3), and continued to be localized, as previously reported, in puncta representing PPCs upon disruption of peroxisomal biogenesis by removal of Pex3p or Pex19p.
We tested whether the Pex15(TA) would associate with PPCs. The Pex15(TA) with mCherry fused to its amino-terminus was indeed found to co-localize with Pex14p at peroxisomes of wild-type (WT) cells (Supplementary Fig. S4), although a notable fraction of mCherry-Pex15(TA) is also mistargeted to mitochondria. Consistent with a previous report examining the trafficking of full-length Pex15p35, we found that mCherry-Pex15(TA) could be co-localized with Pex14p-sfGFP upon disruption of PMP trafficking by removal of Pex3p or Pex19p.
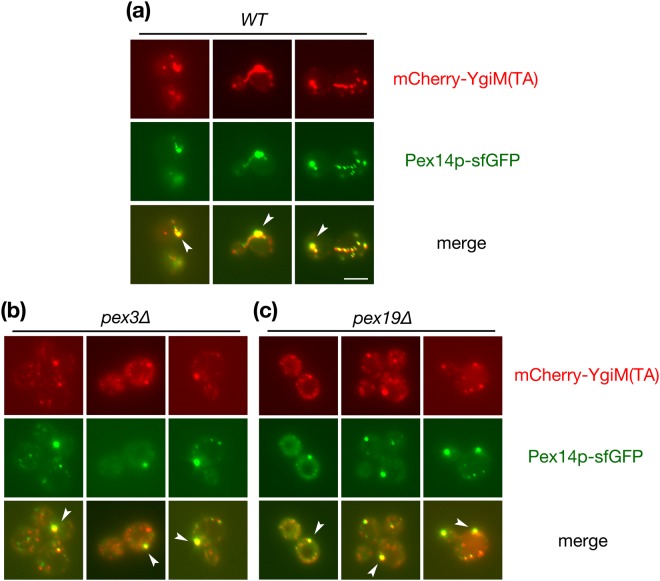
If YgiM(TA) is, like endogenous PMPs, initially targeted to ER, this domain might similarly be localized to PPCs upon disruption of PMP trafficking. mCherry-YgiM(TA) co-localized with Pex14p-sfGFP at mature peroxisomes in wild-type cells (Fig. 3a), and indeed, mCherry-YgiM(TA) continued to co-localize with Pex14p-sfGFP in pex3∆ (Fig. 3b) or pex19∆ (Fig. 3c) cells. Our findings are consistent with trafficking of the YgiM(TA) to the ER, then to PPCs, before subsequent movement to peroxisomes, and our results suggest consonance between cellular pathways handing the endogenous Pex15(TA) and the bacterial, exogenous YgiM(TA).
Figure 3.
The YgiM(TA) is associated with PPCs containing Pex14p upon blockade of PMP transit to peroxisomes. (a) WT (CDD1200) (b) pex3∆ (CDD1201) or (c) pex19∆ (CDD1202) isolates expressing mCherry-YgiM(TA) from plasmid b274 and Pex14p-sfGFP from the native PEX14 locus were examined by fluorescence microscopy of live cells. White arrows provide examples of locations at which Pex14p-sfGFP resides near mCherry-YgiM(TA).
The ER-localized Spf1 protein contributes to the trafficking of the YgiM tail anchor to peroxisomes
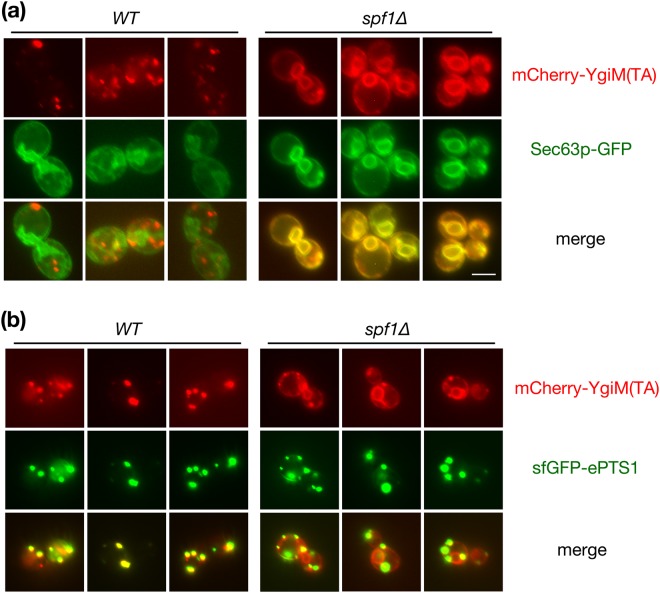
Spf1p, an ER-localized protein involved in manganese transport41, plays a role in peroxisomal biogenesis42,43, and the localization of at least two proteins capable of trafficking from ER to peroxisomes, Pex3p and Ant1p44–47, is altered by Spf1p removal48. Consequently, we investigated whether trafficking of YgiM(TA), like endogenously encoded PMPs, might be affected by Spf1p deletion. Indeed, mCherry-YgiM(TA) was significantly redistributed to ER in spf1∆ cells (Fig. 4), demonstrating a potential role for Spf1p in the targeting of peroxisome-directed TAs and consistent with initial YgiM(TA) movement through the ER. Interestingly, some mCherry-YgiM(TA) could also be found at mature peroxisomes marked by sfGFP-ePTS1 in spf1∆ cells, demonstrating that Spf1p removal does not completely abolish TA trafficking. We also note that Spf1p is apparently not required for the generation of PPCs containing Pex14p, since Pex14p-sfGFP puncta are easily visualized in spf1∆ cells, including within cells also deleted of Pex3p or Pex19p (G Lutfullahoğlu-Bal, unpublished data).
Figure 4.
The YgiM(TA) is mislocalized to the endoplasmic reticulum upon deletion of the Spf1 protein. WT (BY4742) or spf1∆ (CDD949) cells expressing mCherry-YgiM(TA) from plasmid b274 and either (a) Sec63p-GFP from plasmid pJK59 or (b) sfGFP-ePTS1 from plasmid b311 were examined by live-cell fluorescence microscopy.
We tested whether a construct anchored by the TA of the endogenous Pex15p would, like the YgiM(TA), be found predominantly at the ER upon deletion of Spf1p. Indeed, like mCherry-YgiM(TA), mCherry-Pex15(TA) was localized abundantly to ER in spf1∆ cells but not in WT cells (Supplementary Fig. S5), again suggesting congruence between trafficking mechanisms used by the exogenous YgiM(TA) and the endogenous Pex15(TA).
Expression of the YgiM(TA) does not disturb peroxisomal biogenesis
Successful study of a biological process often requires that the system under investigation is not perturbed by the chosen experimental approach. Since overexpression of full-length Pex15p, the only endogenous, peroxisome-specific TA, is known to perturb peroxisomal biogenesis30, we asked whether expression of only the TA domains of YgiM or Pex15p, driven by the strong ADH1 promoter, would have a detrimental effect on peroxisome assembly. Toward this goal, the behavior of sfGFP-ePTS1 was assessed in cells expressing mCherry-YgiM(TA) or mCherry-Pex15(TA). Peroxisomal biogenesis was indeed disrupted by mCherry-Pex15(TA) overexpression (Fig. 5), with an average of 8% of cells lacking discernable peroxisomes across three independent experiments. Moreover, partial nucleocytoplasmic accumulation of sfGFP-ePTS1 was visible in nearly twice as many cells expressing mCherry-Pex15(TA) as those expressing empty vector. However, mCherry-YgiM(TA) expression had no effect on sfGFP-ePTS1 localization when compared to cells harboring empty vector; distinct peroxisomes could be visualized in all cells. Therefore, overexpression of the YgiM(TA), unlike overexpression of an endogenous peroxisome-directed TA, does not appear to readily disrupt peroxisomal biogenesis.
Figure 5.
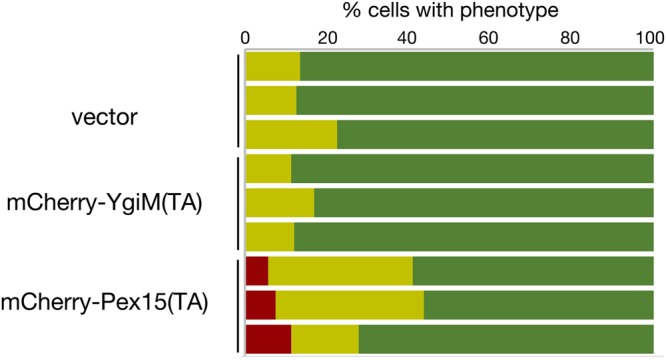
Expression of the YgiM(TA) does not perturb peroxisomal biogenesis. WT cells (BY4742) expressing sfGFP-ePTS1 from plasmid b311 along with mCherry-YgiM(TA) from plasmid b274, mCherry-Pex15(TA) from plasmid b365, or empty vector pRS315 were examined as in Fig. 2e.
The YgiM(TA) is localized to the peroxisomes of mammalian cells
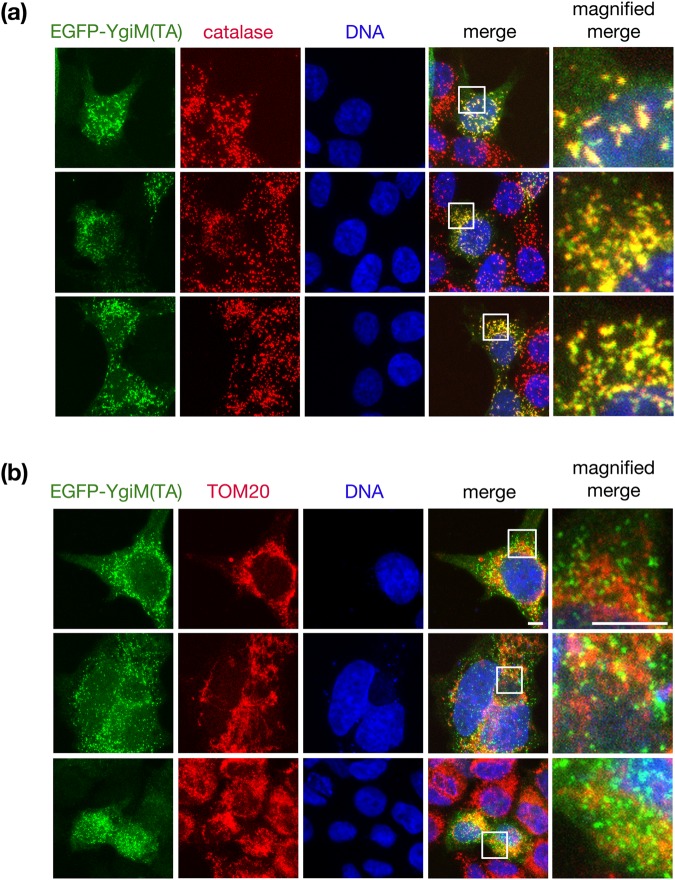
Finally, we investigated whether YgiM(TA) might target to peroxisomes in mammalian cells, since the mechanism by which tail-anchored proteins are delivered to peroxisomes may differ between yeast and mammals9,12,49. Upon transient transfection of a construct in which enhanced green fluorescent protein (EGFP) is fused to the YgiM(TA), punctate structures suggesting peroxisomal localization were visualized in HEK293T cells (Fig. 6a). This localization was confirmed by co-localization with catalase, a marker of mature peroxisomes. As in yeast, EGFP-YgiM(TA) was not trafficked to mitochondria, as revealed by scant co-localization between EGFP-YgiM(TA) and the mitochondrial TOM20 protein (Fig. 6b).
Figure 6.
The YgiM(TA) localizes to peroxisomes in mammalian cells. Plasmid b374, expressing EGFP-YgiM(TA), was transiently transfected into HEK293T cells, and cultures were processed for immunofluorescence. Anti-GFP antibodies were used to detect EGFP-YgiM(TA), and DAPI was used to stain cellular DNA. (a) Anti-catalase antibodies were used to label mature peroxisomes, or (b) anti-TOM20 antibodies were used to label mitochondria. A white box defines the region of the image magnified in the right-most panel set.
Discussion
What features of YgiM(TA) allow targeting to peroxisomes?
The E. coli YgiM(TA) was detected at peroxisomes, with no microscopic or functional evidence of mitochondrial localization in yeast or in human cells. Conversely, TAs found within two other proteins encoded by the same organism, YqjD and ElaB, targeted to mitochondria and ER, with no evidence of peroxisomal localization25 (and this study). Other tail-anchored proteins can target to both mitochondria and peroxisomes. For example, human FIS150,51, yeast Fis1p33, and human MFF are localized to both organelles52. The parameters that allow TAs to discriminate between peroxisomes, mitochondria, and other organelles are not understood, but may be related to the hydrophobicity of the membrane associated domain, along with the number and specific location of charges within the TA53,54. When considering the recent development of a classifier for peroxisome-directed mammalian TAs54, the GRAVY hydrophobicity score (1.7)55 of the YgiM transmembrane domain, denoting more limited hydrophobicity, together with the net charge (+4.1) of the proposed lumenal tail at neutral pH (Protein Calculator v3.4, http://protcalc.sourceforge.net/) do, in fact, predict peroxisomal localization of the YgiM(TA). We note that the biogenesis of YgiM in bacteria has not been investigated, and indeed full-length YgiM contains a predicted signal sequence at its amino-terminus56, indicating co-translational insertion and suggesting that any predicted TA would not drive initial membrane targeting in E. coli.
Heterologous expression of YgiM(TA) may reveal mechanisms of tail-anchor trafficking to peroxisomes
Only two peroxisome-directed proteins harboring TAs are encoded by S. cerevisiae: Pex15p and Fis1p. Both associate with other peroxisomal proteins: Pex15p is found in a complex with Pex3p57, and Fis1p cooperates with Pex11p58. Based on these findings, one might propose a scenario in which both tail-anchored polypeptides obtain their final peroxisomal location solely through their functional assembly with other proteins not harboring a TA, and that no pathway with a specific role in directing tail-anchored client proteins to peroxisomes exists in budding yeast. However, the YgiM(TA), separated from eukaryotes by billions of years of evolutionary distance, appears to localize specifically to peroxisomes in both yeast and human cells. This exogenously expressed domain would not bind to any endogenous interaction partners in order to carry out a cellular function, yet makes its way to peroxisomes nonetheless, supporting the presence of a more generalized mechanism that allows trafficking of tail-anchored proteins to peroxisomes in S. cerevisiae.
Importantly, expression of native proteins at incorrect stoichiometry can perturb cellular functions that may be under investigation59,60, as illustrated by disruption of peroxisomal biogenesis upon overexpression of full-length Pex15p30. In this study, we found that the TA of Pex15p could also disrupt peroxisomal protein import, attenuating its value as an experimental substrate for studies of TA targeting in yeast. In contrast, YgiM(TA) expression did not affect peroxisomal assembly, and unlike overexpressed Pex15(TA), appears specifically targeted to peroxisomes. Moreover, the YgiM(TA) is also relatively short when compared to several other peroxisome-directed TAs, providing the opportunity for facile mutational analyses of YgiM(TA). Therefore, we suggest that heterologously expressed YgiM(TA) is likely to be a preferred substrate for further exploration of the mechanisms by which TAs reach peroxisomes.
Why is protein targeting to eukaryotic organelles so permissive?
In this study, we have demonstrated that a predicted membrane insertion sequence obtained from a prokaryote can be directed to the peroxisomes of eukaryotic cells. Our findings expand upon earlier studies in which protein sequence derived from prokaryotes could traffic to eukaryotic organelles, such as ER and mitochondria25,61–65. We propose that the ability to direct prokaryotic protein sequences to eukaryotic organelles, even though these regions were not previously selected for targeting prowess in eukaryotes, might have been of general benefit to eukaryotes over evolutionary time. Specifically, failure to allow degeneracy among organelle targeting sequences66 would potentially have limited the utility of genes acquired from the proto-mitochondrial endosymbiont or from neighboring microorganisms near the dawn of eukaryogenesis, potentially slowing or forbidding the emergence of the eukaryotic cell67. In addition, the ability of eukaryotes to take advantage of genes acquired by HGT at present day23,68,69 could similarly be hampered by a strict sequence requirement, rather than lax structural requirements, for recognition of organelle-targeting signals contained within polypeptides. Additionally, sequestration at an organelle might avoid detrimental effects of aggregation or chaperone sequestration, and thereby avoid selection against an otherwise advantageous gene transfer, and this aspect of organelle targeting may be particularly important when considering hydrophobic regions like the TA examined in this study.
Encompassing the specific use of HGT-acquired genetic information would be a more general need for eukaryotic cells to harbor permissive organelle translocation machineries that allow recognition of degenerate import signals. Given that organelles are maintained in order to compartmentalize biochemical pathways and other cellular activities, it follows that multiple polypeptides will often act together as a module within a given organelle. Strict sequence requirements for organelle import would make it highly improbable that multiple proteins once cooperating at one cellular location, such as the cytosol, could later find themselves simultaneously localized together in a different cellular compartment. Conversely, more relaxed structural determinants of organelle-targeting regions of a protein that might be recognized by permissive substrate receptors, such as hydrophobicity and charge, would allow proteins already encoded by a cell to sample novel compartments. Eventually, as previously proposed by Martin70, organelle sampling by polypeptides, followed by further mutational tinkering with organelle targeting sequences, could lead to increased fitness through the localization of an entire cellular pathway to a new location. Moreover, genes can evolve de novo71, and the ability of newly generated polypeptides to test different organelle environments may also contribute to fitness or the exploration of a new ecological niche. Ultimately, then, the question of how the protein translocation machineries of organelles recognize targeting information of client proteins, obtained by HGT or as the outcome of other genetic processes, becomes a question of ‘evolvability’, or the advantageous capacity of a pedigree of organisms to more easily sample genotypic and phenotypic space72.
Methodology
Yeast strains, plasmids, and culture conditions
Culture conditions are as described in73. All experiments with S. cerevisiae have been performed at 30 °C. Plasmids, strains, and oligonucleotides used in this study can be located in the Supplementary Dataset.
Assessment of Pex15 functionality
Diploid strain CDD1182, deleted of chromosomal PEX15, expressing peroxisome-targeted sfGFP from plasmid b311, and carrying a fully-functional fusion between the cytosolic and TA domains of Pex15p from plasmid b354 driven by the PEX15 promoter, was transformed with plasmids expressing variants of Pex15p in which the cytosolic domain was fused to test TAs by a linker region consisting of Fis1p amino acids 119–128, a stretch of amino acids not necessary or sufficient for organelle targeting74,75. Strains were cultured overnight in supplemented minimal medium (SMM) lacking uracil and histidine (-Ura-His). Cells were then transferred to SMM-Ura-His containing 3 mg/L cycloheximide (CHX) and cultured overnight before fluorescence microscopy in the logarithmic phase of proliferation. Counter-selection of plasmid b354 was confirmed by lack of proliferation on medium lacking tryptophan.
Mammalian cell culture and transfection
Cells were maintained at 37 °C and 5% CO2 and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 U/ml penicillin/streptomycin, and 50 μg/ml uridine. HEK293T cells were plated overnight before transfection in 500 μl of complete growth medium at a cell density of 1 × 105 cells/ml in a 24-well plate containing glass coverslips. Transfection was performed using TransIT-2020 (Mirus Bio) reagent, and transfection mixture contained: 250 ng of plasmid b374, 50 μl of cell culture medium, and 1 μl of transfection reagent. The mixture was incubated at room temperature for 20 min, and transfection mixture was added drop-wise to the cells. Cells were fixed for immunofluorescence analysis 24 hr after transfection.
Microscopy
Microscopy on yeast cells was performed using logarithmic phase cultures. Live-cell epifluorescence microscopy was performed using either a Nikon Eclipse 80i microscope equipped with a 100X Plan Fluor objective and DS-Qi1Mc camera or a Zeiss Axio Imager.M2 fixed with a 63X Plan-Apochromat/1.40 Oil DIC objective and AxioCam HR R3 camera. mCherry fusions are driven by the ADH1 promoter and contain Fis1p amino acids 119–128 linking mCherry to each carboxyl-terminal organelle targeting sequence.
To carry out indirect immunofluorescence experiments on mammalian cells, transfected HEK293T cells were fixed using 4% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.4 for 10 min at room temperature. Cells were washed three times with PBS for 5 min, then blocked for 1 hr using PBS containing 0.3% Triton X and 1% bovine serum albumin. Cells were then incubated overnight in primary antibodies (listed in Supplementary Dataset) diluted in blocking solution at 4 °C. Next, cells were washed 3x with PBS and incubated with secondary antibodies in the blocking solution for 1 h in the dark. After secondary antibody incubation, 4′,6-diamidino-2-phenylindole (DAPI) was added to a final concentration of 1 μg/ml for 10 min. Cells were again washed 3x with PBS, and coverslips were mounted using 80% glycerol prepared in 20 mM Tris-HCl pH 8.0. Coverslips were sealed and stored at 4 °C before microscopy. Imaging was performed using a Zeiss LSM700 Axio Imager.M2 confocal microscope equipped with an LCI Plan-Neofluar 63x/1.30 Imm Corr objective. Scale bars provided with yeast and mammalian cell microscopy images correspond to 5 µm.
Electronic supplementary material
Acknowledgements
We thank Pekka Katajisto (University of Helsinki) for anti-catalase antibodies, and we thank Gülayşe İnce Dunn (University of Helsinki and Koç University) for HEK293T cells. In addition, we thank Maya Schuldiner, Ville Paavilainen, Anı Akpınar, and Gülayşe İnce Dunn for helpful comments on this manuscript. This work was supported by a European Research Council Starting Grant (637649-RevMito) to C.D.D., by a Turkish Academy of Sciences Outstanding Young Scientist Award (TÜBA-GEBİP) to C.D.D., by an EMBO Installation Grant (2138) to C.D.D., and by Koç University. These funding bodies had no role in the design of the study, data collection, data analysis, data interpretation, or manuscript preparation.
Author Contributions
C.D.D. designed the study, wrote the manuscript, and performed experiments. G.L.B. and A.B.S. performed experiments and generated reagents. A.K. and E.A. generated reagents. All authors read and approved the final manuscript.
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-34646-7.
References
- 1.Surovtsev IV, Jacobs-Wagner C. Subcellular Organization: A Critical Feature of Bacterial Cell Replication. Cell. 2018;172:1271–1293. doi: 10.1016/j.cell.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammer SK, Avalos JL. Harnessing yeast organelles for metabolic engineering. Nat. Chem. Biol. 2017;13:823–832. doi: 10.1038/nchembio.2429. [DOI] [PubMed] [Google Scholar]
- 3.Schlüter A, Real-Chicharro A, Gabaldón T, Sánchez-Jiménez F, Pujol A. PeroxisomeDB 2.0: an integrative view of the global peroxisomal metabolome. Nucleic Acids Res. 2010;38:D800–5. doi: 10.1093/nar/gkp935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabaldón T. Peroxisome diversity and evolution. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2010;365:765–73. doi: 10.1098/rstb.2009.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Islinger M, Cardoso MJR, Schrader M. Be different-The diversity of peroxisomes in the animal kingdom. Biochim. Biophys. Acta - Mol. Cell Res. 2010;1803:881–897. doi: 10.1016/j.bbamcr.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Faust PL, Kovacs WJ. Cholesterol biosynthesis and ER stress in peroxisome deficiency. Biochimie. 2014;98:75–85. doi: 10.1016/j.biochi.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Dean JM, Lodhi IJ. Structural and functional roles of ether lipids. Protein Cell. 2017;9:1–11. doi: 10.1007/s13238-017-0423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsons MG. Parasites and the divergence of peroxisomal purpose. Mol. Microbiol. 2004;53:717–724. doi: 10.1111/j.1365-2958.2004.04203.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim PK, Hettema EH. Multiple pathways for protein transport to peroxisomes. J. Mol. Biol. 2015;427:1176–1190. doi: 10.1016/j.jmb.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erdmann RA. maintenance and dynamics of peroxisomes. Biochim. Biophys. Acta - Mol. Cell Res. 2016;1863:787–789. doi: 10.1016/j.bbamcr.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Giannopoulou EA, Emmanouilidis L, Sattler M, Dodt G, Wilmanns M. Towards the molecular mechanism of the integration of peroxisomal membrane proteins. Biochim. Biophys. Acta - Mol. Cell Res. 2016;1863:863–869. doi: 10.1016/j.bbamcr.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayerhofer PU. Targeting and insertion of peroxisomal membrane proteins: ER trafficking versus direct delivery to peroxisomes. Biochim. Biophys. Acta - Mol. Cell Res. 2016;1863:870–880. doi: 10.1016/j.bbamcr.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 13.Schlüter A, et al. The evolutionary origin of peroxisomes: An ER-peroxisome connection. Mol. Biol. Evol. 2006;23:838–845. doi: 10.1093/molbev/msj103. [DOI] [PubMed] [Google Scholar]
- 14.Gabaldón T, et al. Origin and evolution of the peroxisomal proteome. Biol. Direct. 2006;1:1–14. doi: 10.1186/1745-6150-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Duve C. The origin of eukaryotes: a reappraisal. Nat. Rev. Genet. 2007;8:395–403. doi: 10.1038/nrg2071. [DOI] [PubMed] [Google Scholar]
- 16.Esser C, et al. A genome phylogeny for mitochondria among α-proteobacteria and a predominantly eubacterial ancestry of yeast nuclear genes. Mol. Biol. Evol. 2004;21:1643–1660. doi: 10.1093/molbev/msh160. [DOI] [PubMed] [Google Scholar]
- 17.Nowack Eva C.M., Weber Andreas P.M. Genomics-Informed Insights into Endosymbiotic Organelle Evolution in Photosynthetic Eukaryotes. Annual Review of Plant Biology. 2018;69(1):51–84. doi: 10.1146/annurev-arplant-042817-040209. [DOI] [PubMed] [Google Scholar]
- 18.Embley TM, Martin W. Eukaryotic evolution, changes and challenges. Nature. 2006;440:623–30. doi: 10.1038/nature04546. [DOI] [PubMed] [Google Scholar]
- 19.Rivera MC, Lake JA. The ring of life provides evidence for a genome fusion origin of eukaryotes. Nature. 2004;431:152–5. doi: 10.1038/nature02848. [DOI] [PubMed] [Google Scholar]
- 20.Gray MW. Mosaic nature of the mitochondrial proteome: Implications for the origin and evolution of mitochondria. Proc. Natl. Acad. Sci. 2015;112:10133–10138. doi: 10.1073/pnas.1421379112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roger Andrew J. Reply to 'Eukaryote lateral gene transfer is Lamarckian'. Nature Ecology & Evolution. 2018;2(5):755–755. doi: 10.1038/s41559-018-0522-6. [DOI] [PubMed] [Google Scholar]
- 22.Husnik F, McCutcheon JP. Functional horizontal gene transfer from bacteria to eukaryotes. Nat. Rev. Microbiol. 2017;16:67–79. doi: 10.1038/nrmicro.2017.137. [DOI] [PubMed] [Google Scholar]
- 23.Singer A, et al. Massive Protein Import into the Early-Evolutionary-Stage Photosynthetic Organelle of the Amoeba Paulinella chromatophora. Curr. Biol. 2017;27:2763–2773.e5. doi: 10.1016/j.cub.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Lacroix B, Citovsky V. Beyond Agrobacterium-Mediated Transformation: Horizontal Gene Transfer from Bacteria to Eukaryotes. Curr. Top. Microbiol. Immunol. 2018;351:139–57. doi: 10.1007/82_2018_82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lutfullahoğlu-Bal G, Keskin A, Seferoğlu AB, Dunn CD. Bacterial tail anchors can target to the mitochondrial outer membrane. Biol. Direct. 2017;12:1–9. doi: 10.1186/s13062-017-0187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeLoache WC, Russ ZN, Dueber JE. Towards repurposing the yeast peroxisome for compartmentalizing heterologous metabolic pathways. Nat. Commun. 2016;7:1–11. doi: 10.1038/ncomms11152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okreglak V, Walter P. The conserved AAA-ATPase Msp1 confers organelle specificity to tail-anchored proteins. Proc. Natl. Acad. Sci. 2014;111:8019–8024. doi: 10.1073/pnas.1405755111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y-C, et al. Msp1/ATAD1 maintains mitochondrial function by facilitating the degradation of mislocalized tail-anchored proteins. EMBO J. 2014;33:1548–1564. doi: 10.15252/embj.201487943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francisco T, et al. Protein transport into peroxisomes: Knowns and unknowns. BioEssays. 2017;39:1–8. doi: 10.1002/bies.201700047. [DOI] [PubMed] [Google Scholar]
- 30.Elgersma Y, et al. Overexpression of Pex15p, a phosphorylated peroxisomal integral membrane protein required for peroxisome assembly in S. cerevisiae, causes proliferation of the endoplasmic reticulum membrane. EMBO J. 1997;16:7326–7341. doi: 10.1093/emboj/16.24.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elgersma Y, V den Berg M, Tabak HF, Distel B. An efficient positive selection procedure for the isolation of peroxisomal import and peroxisome assembly mutants of Saccharomyces cerevisiae. Genetics. 1993;135:731–740. doi: 10.1093/genetics/135.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buentzel J, Vilardi F, Lotz-Havla A, Gartner J, Thoms S. Conserved targeting information in mammalian and fungal peroxisomal tail-anchored proteins. Sci. Rep. 2015;5:1–14. doi: 10.1038/srep17420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuravi K. Dynamin-related proteins Vps1p and Dnm1p control peroxisome abundance in Saccharomyces cerevisiae. J. Cell Sci. 2006;119:3994–4001. doi: 10.1242/jcs.03166. [DOI] [PubMed] [Google Scholar]
- 34.Schuldiner M, et al. The GET Complex Mediates Insertion of Tail-Anchored Proteins into the ER Membrane. Cell. 2008;134:634–645. doi: 10.1016/j.cell.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wróblewska JP, et al. Saccharomyces cerevisiae cells lacking Pex3 contain membrane vesicles that harbor a subset of peroxisomal membrane proteins. Biochim. Biophys. Acta - Mol. Cell Res. 2017;1864:1656–1667. doi: 10.1016/j.bbamcr.2017.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agrawal G, Fassas SN, Xia ZJ, Subramani S. Distinct requirements for intra-ER sorting and budding of peroxisomal membrane proteins from the ER. J. Cell Biol. 2016;212:335–348. doi: 10.1083/jcb.201506141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joshi AS, et al. A family of membrane-shaping proteins at ER subdomains regulates pre-peroxisomal vesicle biogenesis. J. Cell Biol. 2016;215:515–529. doi: 10.1083/jcb.201602064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knoops K, et al. Preperoxisomal vesicles can form in the absence of Pex3. J. Cell Biol. 2014;204:659–668. doi: 10.1083/jcb.201310148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meinecke M, et al. The peroxisomal importomer constitutes a large and highly dynamic pore. Nat. Cell Biol. 2010;12:273–277. doi: 10.1038/ncb2027. [DOI] [PubMed] [Google Scholar]
- 40.Montilla-Martinez M, et al. Distinct Pores for Peroxisomal Import of PTS1 and PTS2 Proteins. Cell Rep. 2015;13:2126–34. doi: 10.1016/j.celrep.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 41.Cohen Y, et al. The yeast P5 type ATPase, Spf1, regulates manganese transport into the endoplasmic reticulum. PLoS One. 2013;8:1–14. doi: 10.1371/journal.pone.0085519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lockshon D, Surface LE, Kerr EO, Kaeberlein M, Kennedy BK. The sensitivity of yeast mutants to oleic acid implicates the peroxisome and other processes in membrane function. Genetics. 2007;175:77–91. doi: 10.1534/genetics.106.064428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marelli M, et al. Quantitative mass spectrometry reveals a role for the GTPase Rho1p in actin organization on the peroxisome membrane. J. Cell Biol. 2004;167:1099–1112. doi: 10.1083/jcb.200404119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kragt Astrid, Voorn-Brouwer Tineke, van den Berg Marlene, Distel Ben. Endoplasmic Reticulum-directed Pex3p Routes to Peroxisomes and Restores Peroxisome Formation in aSaccharomyces cerevisiae pex3Δ Strain. Journal of Biological Chemistry. 2005;280(40):34350–34357. doi: 10.1074/jbc.M505432200. [DOI] [PubMed] [Google Scholar]
- 45.Tam YYC, Fagarasanu A, Fagarasanu M, Rachubinski RA. Pex3p initiates the formation of a preperoxisomal compartment from a subdomain of the endoplasmic reticulum in Saccharomyces cerevisiae. J. Biol. Chem. 2005;280:34933–34939. doi: 10.1074/jbc.M506208200. [DOI] [PubMed] [Google Scholar]
- 46.Hoepfner D, Schildknegt D, Braakman I, Philippsen P, Tabak HF. Contribution of the endoplasmic reticulum to peroxisome formation. Cell. 2005;122:85–95. doi: 10.1016/j.cell.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 47.van der Zand A, Braakman I, Tabak HF. Peroxisomal membrane proteins insert into the endoplasmic reticulum. Mol. Biol. Cell. 2010;21:2057–65. doi: 10.1091/mbc.e10-02-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen Y, et al. Peroxisomes are juxtaposed to strategic sites on mitochondria. Mol. BioSyst. 2014;10:1742–1748. doi: 10.1039/C4MB00001C. [DOI] [PubMed] [Google Scholar]
- 49.Veenhuis M, van derKlei IJ. A critical reflection on the principles of peroxisome formation in yeast. Front. Physiol. 2014;5:110. doi: 10.3389/fphys.2014.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delille HK, Schrader M. Targeting of hFis1 to peroxisomes is mediated by Pex19p. J. Biol. Chem. 2008;283:31107–31115. doi: 10.1074/jbc.M803332200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koch A, Yoon Y, Bonekamp NA, McNiven MA, Schrader M. A role for Fis1 in both mitochondrial and peroxisomal fission in mammalian cells. Mol. Biol. Cell. 2005;16:5077–86. doi: 10.1091/mbc.e05-02-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gandre-Babbe S, van der Bliek AM. The Novel Tail-anchored Membrane Protein Mff Controls Mitochondrial and Peroxisomal Fission in Mammalian Cells. Mol. Biol. Cell. 2008;19:2402–2412. doi: 10.1091/mbc.e07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chio US, Cho H, Shan S. Mechanisms of Tail-Anchored Membrane Protein Targeting and Insertion. Annu. Rev. Cell Dev. Biol. 2017;33:417–438. doi: 10.1146/annurev-cellbio-100616-060839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Costello JL, et al. Predicting the targeting of tail-anchored proteins to subcellular compartments in mammalian cells. J. Cell Sci. 2017;130:1675–1687. doi: 10.1242/jcs.200204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982;157:105–32. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 56.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods. 2011;8:785–6. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 57.Weir NR, Kamber RA, Martenson JS, Denic V. The AAA protein Msp1 mediates clearance of excess tail-anchored proteins from the peroxisomal membrane. Elife. 2017;6:1–28. doi: 10.7554/eLife.28507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joshi S, Agrawal G, Subramani S. Phosphorylation-dependent Pex11p and Fis1p interaction regulates peroxisome division. Mol. Biol. Cell. 2012;23:1307–1315. doi: 10.1091/mbc.e11-09-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987;329:219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- 60.Meeks-Wagner D, Hartwell LH. Normal stoichiometry of histone dimer sets is necessary for high fidelity of mitotic chromosome transmission. Cell. 1986;44:43–52. doi: 10.1016/0092-8674(86)90483-6. [DOI] [PubMed] [Google Scholar]
- 61.Baker A, Schatz G. Sequences from a prokaryotic genome or the mouse dihydrofolate reductase gene can restore the import of a truncated precursor protein into yeast mitochondria. Proc. Natl. Acad. Sci. 1987;84:3117–3121. doi: 10.1073/pnas.84.10.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lemire BD, Fankhauser C, Baker A, Schatz G. The mitochondrial targeting function of randomly generated peptide sequences correlates with predicted helical amphiphilicity. J. Biol. Chem. 1989;264:20206–20215. [PubMed] [Google Scholar]
- 63.Lucattini R, Likić VA, Lithgow T. Bacterial Proteins Predisposed for Targeting to Mitochondria. Mol. Biol. Evol. 2004;21:652–658. doi: 10.1093/molbev/msh058. [DOI] [PubMed] [Google Scholar]
- 64.Hall J, Hazlewood GP, Surani MA, Hirst BH, Gilbert HJ. Eukaryotic and prokaryotic signal peptides direct secretion of a bacterial endoglucanase by mammalian cells. J. Biol. Chem. 1990;265:19996–19999. [PubMed] [Google Scholar]
- 65.Walther DM, Papic D, Bos MP, Tommassen J, Rapaport D. Signals in bacterial beta-barrel proteins are functional in eukaryotic cells for targeting to and assembly in mitochondria. Proc. Natl. Acad. Sci. USA. 2009;106:2531–2536. doi: 10.1073/pnas.0807830106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.von Heijne G. Signal sequences. The limits of variation. J. Mol. Biol. 1985;184:99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]
- 67.Koonin EV. Horizontal gene transfer: essentiality and evolvability in prokaryotes, and roles in evolutionary transitions. F1000Research. 2016;5:1805. doi: 10.12688/f1000research.8737.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang J. Horizontal gene transfer in eukaryotes: The weak-link model. BioEssays. 2013;35:868–875. doi: 10.1002/bies.201200182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nowack ECM, et al. Gene transfers from diverse bacteria compensate for reductive genome evolution in the chromatophore of Paulinella chromatophora. Proc. Natl. Acad. Sci. 2016;113:12214–12219. doi: 10.1073/pnas.1608016113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martin W. Evolutionary origins of metabolic compartmentalization in eukaryotes. Philos. Trans. R. Soc. B Biol. Sci. 2010;365:847–855. doi: 10.1098/rstb.2009.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schlötterer C. Genes from scratch - the evolutionary fate of de novo genes. Trends Genet. 2015;31:215–219. doi: 10.1016/j.tig.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kirschner M, Gerhart J. Perspective Evolvability. Proc. Natl. Acad. Sci. USA. 1998;95:8420–8427. doi: 10.1073/pnas.95.15.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Keskin A, Akdoğan E, Dunn CD. Evidence for amino acid snorkeling from a high-resolution, in vivo analysis of Fis1 tail-anchor insertion at the mitochondrial outer membrane. Genetics. 2017;205:691–705. doi: 10.1534/genetics.116.196428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mozdy AD, McCaffery JM, Shaw JM. Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J. Cell Biol. 2000;151:367–379. doi: 10.1083/jcb.151.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Förtsch J, Hummel E, Krist M, Westermann B. The myosin-related motor protein Myo2 is an essential mediator of bud-directed mitochondrial movement in yeast. J. Cell Biol. 2011;194:473–488. doi: 10.1083/jcb.201012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bateman A, et al. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2017;45:D158–D169. doi: 10.1093/nar/gkw1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krogh A, Larsson B, Von Heijne G, Sonnhammer ELL. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.