Abstract
A novel mechanism for chiroptical activity inversion based on the electronic structure of metal complexes without Λ- or Δ-type structure change was demonstrated spectroscopically and theoretically. To demonstrate the mechanism, a europium (Eu(III)) complex with chiral (+)-3-(trifluoroacetyl)camphor (+tfc) and achiral triphenylphosphine oxide (tppo) was prepared. The steric and electronic structures of the Eu(III) complex were adjusted by additional achiral tppo and coordinating acetone molecules, and were characterised by 1H NMR, photoluminescence, and emission lifetime measurements. The optical activity of the Eu(III) complex in solution was evaluated by circularly polarized luminescence (CPL) measurements. CPL sign inversion, which was independent of Λ- or Δ-type structure changes from the spectroscopic viewpoint, and a drastic CPL intensity enhancement were observed depending on the external achiral molecules around Eu(III) ion. These phenomena provide the first clarification of optical activity change associated with electronic structure rather than chiral coordination structure-type (Λ or Δ) under external environments.
Introduction
Chirality is prevalent in our life. Chirality is attributed to asymmetric molecular structure, which arises from the molecular level to the macroscopic helical scale, like a DNA double helix1,2. Chiral structures also play a critical role in chiral properties such as enzyme activity and asymmetric synthesis using chiral catalysts3–5. The chiral structures are susceptible to external environments such as solvents6, temperature7, light8, or pH9, causing chiral inversion phenomena in large variety of systems from biological macromolecules like B- or Z-form DNA10 to artificial soft matter and liquid crystals11–14. Hence, the influence of external environment is of importance to control the molecular chirality.
Generally, chiral molecules exhibit optical activities with absorption (circular dichroism: CD) or emission (circularly polarized luminescence: CPL) related to the molecular structure15. Optical activities are estimated from the different electronic transition probabilities between left- and right-handed circularly polarized light. The CD and CPL signals are dependent on the molecular chirality, which is influenced by the external field. The inversion of optical activity induced by the external field has been reported extensively16–25. Ishii and Verbiest achieved the CD signal inversion of a chiral polythiophene derivative by controlling the molecular aggregation speed16. George observed the tuneable helicity inversion of Zn(II)-coordinated naphthalenediimide assemblies with chiral adenosine phosphates using CD measurements17. Meskers and Swager described the solvent polarity-dependent CPL signal switching of a self-assembled chiral poly(p-phenylenevinylene) derivative18. The magnitude of optical activity is estimated by a dissymmetry factor (g), which is composed of electric and magnetic field components related to the electronic transition15. Their components are governed by the molecular structure in the surrounding environment.
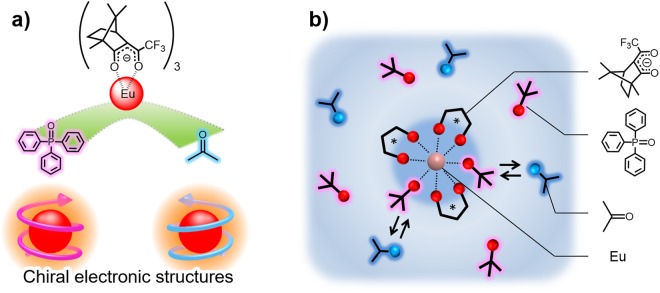
Here, we targeted chiral lanthanide complexes to clarify the influence of electric and magnetic field components on optical activities. Their sharp luminescence bands comprise electric and magnetic field components of 4f-4f transitions26. Their components in the electronic transitions are dominated by external organic molecules around the lanthanide ion. In particular, the magnetic dipole transition of the europium (Eu(III)) complex exhibits a large dissymmetry factor on the CPL (gCPL) by the introduction of chiral organic ligands; this is established by the effective contribution of the magnetic field component27–30. The optical activity signal of the lanthanide complex with chiral ligands has been evaluated by Λ- or Δ-type coordination structures previously31,32. Yuasa and Parker reported the chiroptical activity inversion of chiral Eu(III) complexes influenced by the different steric structures of achiral molecules33,34, which implied steric inversion between Λ- and Δ-type structures. Note that chiroptical activity is related to the electronic transition of the Eu(III) complex; therefore, the magnitude and sign could be also affected by the chiral electronic structure of the Eu(III) ion surrounded by external ligands (Fig. 1a).
Figure 1.
Schematic images of (a) chiral electronic structures depending on the external molecules around the Eu(III) ion and (b) the coordination structures of the Eu(III) complex depending on external achiral molecules around the Eu(III) ion in solution.
In order to demonstrate the chiroptical activity based on the chiral electronic structure in external environments, we choose the chiral Eu(III) complex with bidentate (+)-3-(trifluoroacetyl)camphor (+tfc) and monodentate triphenylphosphine oxide (tppo) as the chiral and external achiral ligands, respectively. The Eu(III) complex [Eu(+tfc)3(tppo)2] (Δ-type structure, as determined by X-ray single-crystal analysis) shows a large gCPL (−0.47) in acetone-d635. The chiral electronic structure of the Eu(III) complex in solution was adjusted by additional achiral tppo and acetone molecules (Fig. 1b). The coordination and electronic structures in liquid media were characterised using 1H NMR, photoluminescence, and emission lifetime measurements. The chiroptical activities of the Eu(III) complex under external environments were evaluated using CPL measurements. In this study, we demonstrate a mechanism for the optical activity inversion based on the chiral electronic structure of the Eu(III) complex without Λ- or Δ-type structure change spectroscopically and theoretically, for the first time.
Results
Coordination structures in solution
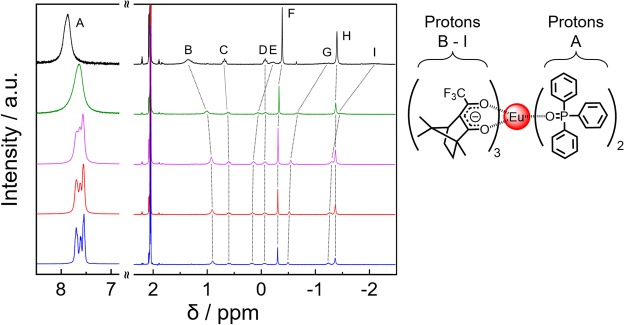
X-ray crystallography measurements indicated that the coordination structure of the Eu(III) complex in the solid is an eight-coordinated Δ-type structure composed of three chiral +tfc and two tppo ligands35. To evaluate the conformation of [Eu(+tfc)3(tppo)2] (Eu(+)) in acetone, 1H NMR spectra with additional n equivalents (n = 0, 8, 28, 48, and 98 relative to Eu(+)) of tppo molecules, namely Eu(+)-Exn, were acquired (Fig. 2 and Supplementary Table S1). In the low-magnetic-field side, protons of tppo molecules in Eu(+)-Ex0 show broad peaks in Fig. 2 (black; A). The line-broadening and chemical shifts originate from the exchange reaction and paramagnetic effect on the metal complex36,37. The paramagnetic effect of the Eu(III) ion generally induces little broadening (bandwidth; nearly 10 Hz)37. Therefore, the large broadening of tppo signals in our experiment (Fig. 2, black; A, bandwidth; nearly 300 Hz) is mainly caused by their exchange reaction in acetone-d6. The lower-magnetic-field shift in Eu(+)-Ex0 (Fig. 2, black; A) is influenced by the direct coordination of tppo ligands with the Eu(III) ion.
Figure 2.
1H NMR spectra of Eu(+)-Ex0 (black), Eu(+)-Ex8 (green), Eu(+)-Ex28 (purple), Eu(+)-Ex48 (red), and Eu(+)-Ex98 (blue) in acetone-d6 (Eu(+); 1 × 10−3 M).
NMR peaks of the chiral +tfc ligands in the Eu(III) complex were observed in the high-magnetic-field side (Fig. 2, signals B - I). Eu(+)-Ex8 (green) provided effective chemical shifts of +tfc ligands compared with those of Eu(+)-Ex0 (black). We also observed gradual shifts at the B, E, G, and I peaks of Eu(+)-Ex28 (purple), -Ex48 (red), and -Ex98 (blue). The effective shifts of the tppo and +tfc signals indicate that the Eu(III) complex with tppo molecules is rearranged by additional tppo molecules, resulting in the formation of several equilibrium states in acetone-d6.
Luminescence properties
Photophysical properties of Eu(III) complexes are affected by the coordination geometry38. The emission spectra of Eu(+)-Ex0 and -Ex498 in acetone (1 × 10−3 M) are shown in Fig. 3 (black; a, and red; b). The Eu(III) complexes show sharp emission peaks in the region of 570–630 nm, which are attributed to the 5D0 → 7FJ (J = 0, 1, and 2) transitions of Eu(III) ions. The spectra were normalised with respect to the integrated intensities of the magnetic dipole transition (5D0 → 7F1). Their spectral shapes in liquid media were different from that of [Eu(+tfc)3(tppo)2] in the solid state (Supplementary Fig. S1). The emission spectra for the 5D0 → 7F1 and 5D0 → 7F2 transitions were also changed in response to the concentration of the tppo molecules in solution. In particular, the 5D0 → 7F1 transition band is composed of three Stark sublevels under the electric field (crystal field). Eu(+)-Ex0 showed three peaks at 584.5, 588, and 593.5 nm in the 5D0 → 7F1 transition (Fig. 3 inset, black; a), whereas Eu(+)-Ex498 showed two peaks at 588 and 593 nm (Fig. 3 inset, red; b). The 5D0 → 7F1 transition of Eu(+)-Ex0 at a lower concentration (1 × 10−5 M, Fig. 3, blue; c) also showed three peaks at 584, 587.5, and 593.5 nm, which are similar to that of Eu(+)-Ex0 at a higher concentration. The small peak at 587.5 nm can be attributed to the 5D1 → 7F3 transition, which is sometimes observed in the same energy region as the 5D0 → 7F1 transition38. The emission bands at around 612 nm are attributed to hypersensitive electric dipole transitions (5D0 → 7F2), which are strongly dependent on the local symmetry of the Eu(III) ion. The change of spectral shape is influenced by the rearrangement of coordination geometries of the Eu(III) complex depending on additional tppo molecules. In case of the non-coordinating toluene solution, the emission spectra of Eu(+)-Ex0 and -Ex48 were similar in shape to that of Eu(+)-Ex498 in acetone, irrespective of the amount of additional tppo molecules (Supplementary Fig. S2). We propose that the inner coordination structure of Eu(+)-Ex498 in acetone is composed of one Eu(III) ion, three +tfc ligands, and two tppo ligands.
Figure 3.
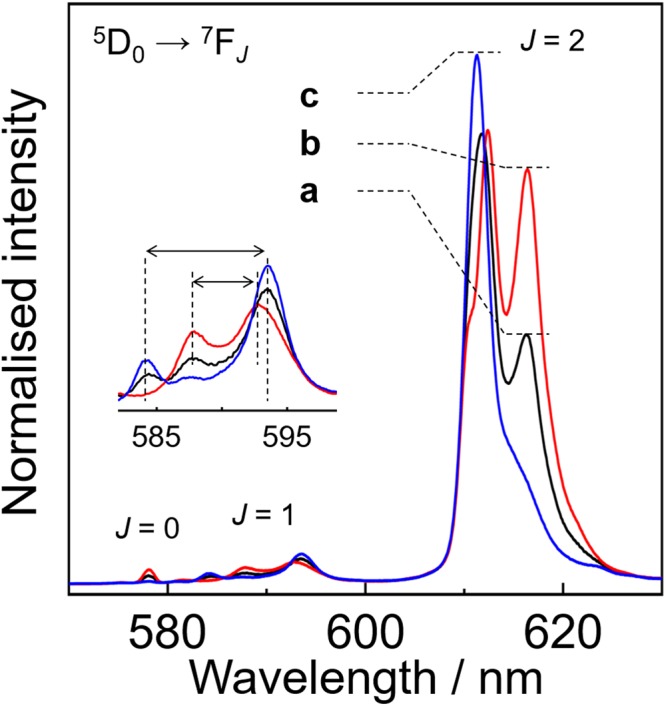
Photoluminescence spectra of (a) Eu(+)-Ex0 (1 × 10−3 M, black), (b) Eu(+)-Ex498 (1 × 10−3 M, red), and (c) Eu(+)-Ex0 (1 × 10−5 M, blue) excited at 350 nm in acetone.
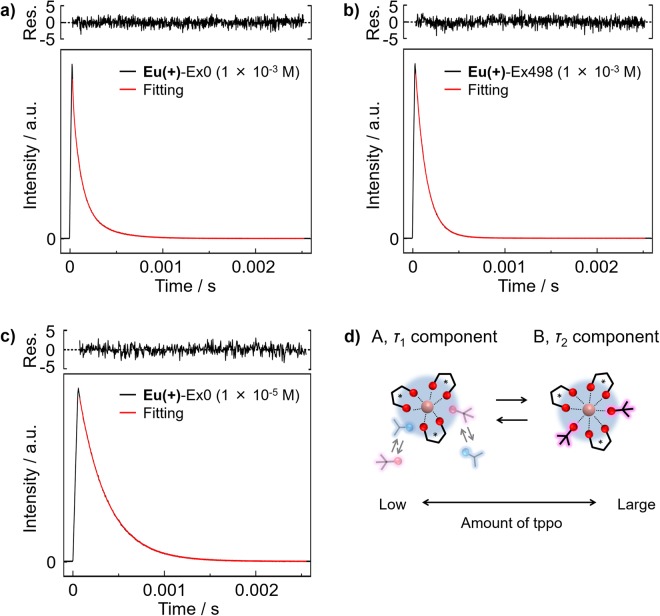
The time-resolved emission profiles of Eu(+)-Ex0 and -Ex498 in acetone (1 × 10−3 M) were measured to clarify their coordination structures. The emission lifetimes were estimated using triple (for Eu(+)-Ex0) or double (for Eu(+)-Ex498) exponential functions to analyse several conformations in solution (Fig. 4a,b). The estimated emission lifetimes are summarised in Table 1. For Eu(+)-Ex0, the emission lifetimes (τ1, τ2, and τ3) and their ratios were calculated to be 0.27 ms (50%), 0.09 ms (49%), and 0.01 ms (1%), respectively. The longer component τ1 in higher concentration (1 × 10−3 M, 0.27 ms) was similar to the single component τ1 in lower concentration (1 × 10−5 M, 0.31 ms, Fig. 4c). We consider that the τ1 component is the Eu(III) complex with coordinating acetone molecules (Fig. 4a,d). The τ1 and τ2 component ratios decreased and increased, respectively, with increasing amount of tppo molecules. The main τ2 value of Eu(+)-Ex498 was found to be 0.12 ms (97%). The lifetime τ2 in acetone was similar to the single-lifetime component in toluene (Supplementary Table S2), indicating that the Eu(III) ion is attached with three +tfc and two tppo ligands (Fig. 4b,d). We revealed that the two types of steric structures with τ1 and τ2 components were reorganized in response to the external tppo and acetone molecules.
Figure 4.
Emission decay profiles, fittings, and residuals (Res.) of (a) Eu(+)-Ex0 (1 × 10−3 M), (b) Eu(+)-Ex498 (1 × 10−3 M), and (c) Eu(+)-Ex0 (1 × 10−5 M) at 612 nm excited at 356 nm in acetone. (d) The coordination image of the τ1 and τ2 components of the Eu(III) complex in acetone.
Table 1.
Luminescence properties of Eu(+)-Exn excited at 356 nm in acetonea.
| Concentration [M] | τ1 [ms] | τ2 [ms] | τ3 [ms] | g CPL | |
|---|---|---|---|---|---|
| Eu(+)-Ex0 | 1 × 10−3 | 0.27 (50%) | 0.09 (49%) |
0.01 (1%) |
−0.44 |
| Eu(+)-Ex498 | 1 × 10−3 | 0.37 (3%) | 0.12 (97%) |
— | +0.013 |
| Eu(+)-Ex0 | 1 × 10−5 | 0.31 (100%) | — | — | −1.0 |
aEmission decay curves were analysed by multi-exponential curve fittings . The ratio of each component denotes .
Chiroptical properties
The CPL spectra and dissymmetry factors of Eu(+)-Ex0 and -Ex498 are shown in Fig. 5 and Table 1, respectively. The CPL signals for the 5D0 → 7F1 transition were composed of two peaks at 583 and 594 nm. The CPL signals at 594 nm were inverted by the addition of tppo molecules. The CPL spectrum of Eu(+)-Ex0 (1 × 10−3 M) shows a large negative peak at 594 nm, the gCPL value of which (−0.44) is similar to a previously reported gCPL value (gCPL = −0.47)35 in acetone-d6 (1 × 10−3 M). The CPL spectrum of Eu(+)-Ex498 (1 × 10−3 M, excess amount of tppo) exhibits a small positive peak (gCPL = +0.013). The enantiomer [Eu(-tfc)3(tppo)2] also exhibits CPL sign inversion at the transition (Supplementary Fig. S3). The CPL intensity of Eu(+)-Ex0 in lower concentration (1 × 10−5 M, gCPL = −1.0) is much larger than that in higher concentration (1 × 10−3 M, gCPL = −0.44). In contrast, the CPL signals of Eu(+)-Ex0 and -Ex498 at around 583 nm in the 5D0 → 7F1 transition exhibit negative CPL signals in these conditions. The CPL sign inversion behaviours depending on the external environments are summarised in Supplementary Fig. S4. Law and Dai reported similar CPL sign inversion phenomena of chiral Eu(III) complexes in the 5D0 → 7F1 transition depending on solvents (Supplementary Fig. S4b)39.
Figure 5.
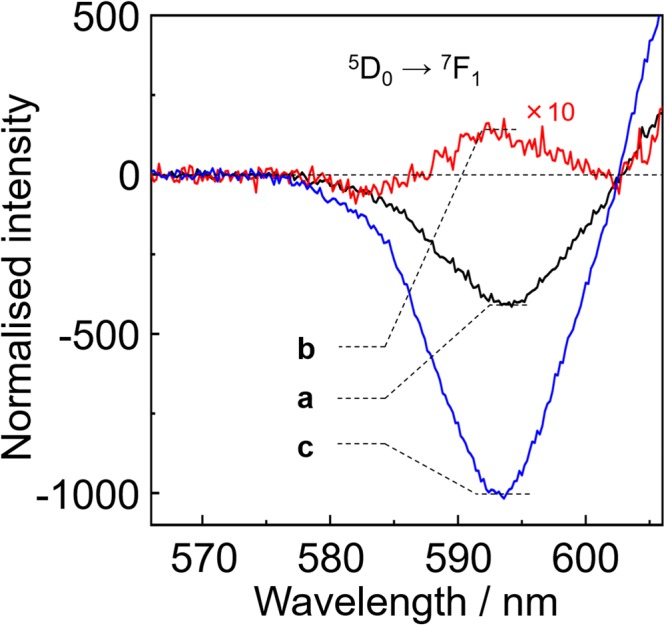
CPL spectra of (a) Eu(+)-Ex0 (1 × 10−3 M, black), (b) Eu(+)-Ex498 (1 × 10−3 M, red), and (c) Eu(+)-Ex0 (1 × 10−5 M, blue) excited at 350 nm in acetone.
Considering the presence of τ1 and τ2 components in the emission lifetime measurements, the observed CPL spectra of Eu(+)-Ex0 and -Ex498 in acetone (1 × 10−3 M) were attributed to several equilibrium states of the Eu(III) complex in acetone. The large negative gCPL of Eu(+)-Ex0 is dominated by the τ1 component related to coordinating acetone molecules (Supplementary Figs S5–S7). The small positive gCPL of Eu(+)-Ex498 is related to the τ2 component of the eight-coordinated Eu(III) complex with two inner tppo ligands; this was supported by the similar positive gCPL (+0.036) in toluene (Supplementary Fig. S8).
Discussion
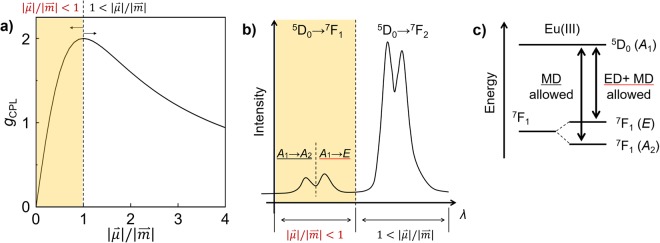
The dissymmetry factor gCPL is expressed in terms of the transition electric dipole moment and transition magnetic dipole moment as follows28,
| 1 |
where θ is the angle between and . When , equation (1) provides the largest gCPL value (=2) mathematically (Fig. 6a). In the region (Fig. 6a,b, orange regions), the Eu(III) complex with a large provides a large gCPL value. In general, the value in the 5D0 → 7F1 transition is larger than the value ()40. The intensity of in the 5D0 → 7F1 transition depends on the crystal field around the Eu(III) ion41,42. The 7F1 energy level of the Eu(III) ion in a typical eight-coordinate structure (C4v or D2d) splits into two Stark sublevels (Fig. 6b)38. The two bands at 583 and 594 nm in the CPL spectra are assigned to the A1 → A2 and A1 → E transitions42, respectively, in Fig. 6b,c. The observed CPL signal in the A1 → E transition was inverted from minus to plus, while that in the A1 → A2 transition retained the minus sign. In C4v or D2d symmetry, the direct product A2 (=A1 × A2) is expressed in terms of the electric dipole (ED) forbidden and magnetic dipole (MD) allowed transitions (Rz) on the character table in group theory (Fig. 6c, Supplementary Tables S3 and S4). On the other hand, the direct product E (=A1 × E) produces ED and MD allowed transitions ((x, y); (Rx, Ry), Fig. 6c, Supplementary Tables S3 and S4). The CPL sign at 583 nm (ED forbidden A1 → A2 transition) reflects the intrinsic Λ- or Δ-type structure, because of insensitive electronic state mixing. Considering the same CPL sign at 583 nm of the τ1 and τ2 components, the structure-type (Λ or Δ) with the τ1 component is the same as that with the τ2 component in our experiments. The coordination symmetry of τ1 component was much similar to that of τ2 component, which was supported by magnetic circular dichroism (MCD) measurements and DFT calculation (Supplementary Figs S9–S11). In contrast, the CPL sign in the ED and MD allowed A1 → E transition is sensitive to electronic state mixing even for the same chiral structure-type (Λ or Δ). The effective sign inversion and drastic intensity change of the CPL signal in the A1 → E transition should be caused by the change of based on electronic state mixing.
Figure 6.
(a) A simulated gCPL curve against ratio in equation (1) with θ = 0°. (b) An attribution of photoluminescence spectrum and (c) an energy diagram in the 5D0 → 7F1 transition of Eu(III) complex (C4v or D2d) based on the electronic structure and group theory.
In the 5D0 → 7F1 transition, is mainly altered by the J-mixing of 7F2 or 7F3 sublevels into 7F138,43. In the photoluminescence spectra, the Stark splitting energy of the τ1 component (270 cm−1, Fig. 3 inset, blue; c) was larger than that of the τ2 component (160 cm−1, Fig. 3 inset, red; b). The large Stark splitting energy suggests the large J-mixing in the A1 → E transition of the τ1 component43. The J-mixing increases the value at the ED allowed A1 → E transition relative to that at the ED forbidden A1 → A2 transition, which is consistent with relatively large emission intensity at the A1 → E transition (593.5 nm) of the τ1 component (Fig. 3 inset, blue; c). The increase leads to the large gCPL value in equation (1).
The angle θ between and of the τ1 component is larger than 90°, whereas that of the τ2 component is smaller than 90°, suggesting that the angle is established by vector change due to J-mixing. The extra-large enhancement of gCPL from +0.013 to −1.0 also indicates that J-mixing promotes the direction of and to antiparallel, leading to the large gCPL. We demonstrated that the CPL sign and intensity are strongly influenced by the chiral electronic structure depending on the under J-mixing in the same chiral structure-type.
Conclusion
We successfully observed a CPL sign inversion with a drastic gCPL change from +0.013 to −1.0 for the Eu(III) complex with the same chiral coordination structure-type. The CPL phenomena were attributed to the chiral electronic structure depending on the under J-mixing. We also achieved an extra-large gCPL (−1.3) of the Eu(III) complex with chiral +tfc ligands in DMSO, suggesting that the gCPL of the Eu(III) complex could be enhanced by J-mixing with a small 4f-5d mixing character (Supplementary Figs S12 and S13, Table S5). The results provide a novel aspect for the optical activity of metal complexes and molecular design of chiral lanthanide complex by maximising the gCPL value.
Methods
Synthesis of Tris(3-trifluoroacetyl-(+)-camphorato)europium(III) bis(triphenylphosphine oxide) ([Eu(+tfc)3(tppo)2])
Europium(III) acetate n-hydrate (0.36 g) was dissolved in distilled water (150 mL), and a few drops of 28% ammonia solution were added. (+)-3-triflouroacetyl camphor (+tfc, 0.50 g, 2.0 mmol) in methanol (20 mL) was added to the solution, and the mixture was stirred for 3 h at room temperature. The obtained powder was washed with distilled water, and the powder was dried in vacuo (0.42 g). The powder (0.19 g) and triphenylphosphine oxide (tppo, 0.11 g, 0.40 mmol) were dissolved in methanol (20 mL) and refluxed for 3 h. The reaction solution was evaporated using a rotary evaporator. The obtained powder was recrystallised from a hot acetonitrile solution and gave yellow crystals. Yield: 47%. Elemental analysis: Calculated for C72H72EuF9O8P2: C, 59.63%, H, 5.00%. Found: C, 59.54%, H, 4.92%35.
Synthesis of Tris(3-trifluoroacetyl-(−)-camphorato)europium(III) bis(triphenylphosphine oxide) ([Eu(-tfc)3(tppo)2])
[Eu(-tfc)3(tppo)2] was prepared using the same method for [Eu(+tfc)3(tppo)2], starting from (−)-3-triflouroacetyl camphor, yielding yellow powder. Yield: 64%. Elemental analysis: Calculated for C72H72EuF9O8P2: C, 59.63%, H, 5.00%. Found: C, 59.49%, H, 4.94%.
Materials
Europium(III) acetate n-hydrate, acetone-d6 (99.9%), acetone (spectroscopic grade), toluene (spectroscopic grade), dimethyl sulfoxide (spectroscopic grade), and 28% ammonia solution were purchased from Wako Pure Chemical Industries Ltd. (+)-3-(trifluoroacetyl)camphor and (−)-3-(trifluoroacetyl)camphor were purchased from Sigma-Aldrich Co. Triphenylphosphine oxide was purchased from Tokyo Chemical Industry Co., Ltd. All other chemicals and solvents were of reagent grade and were used without further purification.
Apparatus
Elemental analyses were performed on an Exeter Analytical CE440. Proton nuclear magnetic resonance (1H NMR) spectra were recorded in acetone-d6 on an auto-NMR JEOL ECS 400 MHz; Acetone (δH = 2.05 ppm) was used as an internal reference. Emission spectra and emission lifetimes were measured using a Horiba/Jobin-Yvon FluoroLog-3 spectrofluorometer. CPL spectra were measured using a JASCO CPL-200 spectrofluoropolarimeter.
Electronic supplementary material
Acknowledgements
We thank professor K. Monde and assistant professor T. Taniguchi in Hokkaido University for the discussion of spectroscopic measurements. This work was partly supported by JSPS KAKENHI Grant Number JP17H06375. This work was also supported by Grants-in-Aid for Scientific Research (no. 18H02041, no. 18H04497), Young Scientists (B) (no. 17K1446707), and JSPS Research Fellow (no. 16J01325). S. Wada was also supported by The Ministry of Education, Culture, Sports Science and Technology through Program for Leading Graduate Schools (Hokkaido University ‘Ambitious Leader’s Program’).
Author Contributions
S.W. performed all the syntheses and measurements, and wrote the paper under the supervision of Y.K., T.N., K.F. and Y.H., M.G., K.T. and Y.C. supported the CPL measurements. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuichi Kitagawa, Email: y-kitagawa@eng.hokudai.ac.jp.
Yasuchika Hasegawa, Email: hasegaway@eng.hokudai.ac.jp.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-34790-0.
References
- 1.Hembury GA, Borovkov VV, Inoue Y. Chirality-sensing supramolecular systems. Chem. Rev. 2008;108:1–73. doi: 10.1021/cr050005k. [DOI] [PubMed] [Google Scholar]
- 2.Pérez-García L, Amabilino DB. Spontaneous resolution under supramolecular control. Chem. Soc. Rev. 2002;31:342–356. doi: 10.1039/B201099M. [DOI] [PubMed] [Google Scholar]
- 3.Yoon TP, Jacobsen EN. Privileged chiral catalysts. Science. 2003;299:1691–1693. doi: 10.1126/science.1083622. [DOI] [PubMed] [Google Scholar]
- 4.Saville CK, et al. Biocatalytic asymmetric synthesis of sitagliptin manufacture. Science. 2010;329:305–310. doi: 10.1126/science.1188934. [DOI] [PubMed] [Google Scholar]
- 5.Noyori R, Kitamura M, Ohkuma T. Toward efficient asymmetric hydrogenation: Architectural and functional engineering of chiral molecular catalysts. Proc. Natl. Acad. Sci. 2004;101:5356–5362. doi: 10.1073/pnas.0307928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mimura Y, et al. Solvent-sensitive sign inversion of excimer origin circularly polarized luminescence in bipyrenyl peptides. ChemistrySelect. 2017;2:7759–7764. doi: 10.1002/slct.201701315. [DOI] [Google Scholar]
- 7.Yao J, et al. Temperature-driven planar chirality switching of a pillar[5]arene-based molecular universal joint. Angew. Chem. Int. Ed. 2017;56:6869–6873. doi: 10.1002/anie.201702542. [DOI] [PubMed] [Google Scholar]
- 8.Kim J, et al. Induction and control of supramolecular chirality by light in self-assembled helical nanostructures. Nat. Commun. 2015;6:1–8. doi: 10.1038/ncomms7959. [DOI] [PubMed] [Google Scholar]
- 9.Lin R, et al. pH-switchable inversion of the metal-centered chirality of metallabenzenes: Opposite stereodynamics in reactions of ruthenabenzene with L- and D-cysteine. Chem. Eur. J. 2011;17:2420–2427. doi: 10.1002/chem.201001867. [DOI] [PubMed] [Google Scholar]
- 10.Choi J, Majima T. Conformational changes of non-B DNA. Chem. Soc. Rev. 2011;40:5893–5909. doi: 10.1039/c1cs15153c. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Qin L, Wang X, Cao H, Liu M. Supramolecular Chirality in Self-Assembled Soft Materials: Regulation of Chiral Nanostructures and Chiral Functions. Adv. Mater. 2014;26:6959–6964. doi: 10.1002/adma.201305422. [DOI] [PubMed] [Google Scholar]
- 12.Mateos-Timoneda MA, Crego-Calama M, Reinhoudt DN. Supramolecular chirality of self-assembled systems in solution. Chem. Soc. Rev. 2004;33:363–372. doi: 10.1039/b305550g. [DOI] [PubMed] [Google Scholar]
- 13.Katsonis N, Lacaze E, Ferrarini A. Controlling chirality with helix inversion in cholesteric liquid crystals. J. Mater. Chem. 2012;22:7088–7097. doi: 10.1039/c2jm15962g. [DOI] [Google Scholar]
- 14.Pieraccini S, Masiero S, Ferrarini A, Piero Spada G. Chirality transfer across length-scales in nematic liquid crystals: Fundamentals and applications. Chem. Soc. Rev. 2011;40:258–271. doi: 10.1039/B924962C. [DOI] [PubMed] [Google Scholar]
- 15.Mason, S. F. Molecular optical activity and the chiral discriminations. (Cambridge University Press, 1982).
- 16.Hattori S, Vandendriessche S, Koeckelberghs G, Verbiest T, Ishii K. Evaporation rate-based selection of supramolecular chirality. Chem. Commun. 2017;53:3066–3069. doi: 10.1039/C6CC09842H. [DOI] [PubMed] [Google Scholar]
- 17.Kumar M, et al. A dynamic supramolecular polymer with stimuli-responsive handedness for in situ probing of enzymatic ATP hydrolysis. Nat. Commun. 2014;5:1–8. doi: 10.1038/ncomms6793. [DOI] [PubMed] [Google Scholar]
- 18.Satrijo A, Meskers SCJ, Swager TM. Probing a conjugated polymer’s transfer of organization-dependent properties from solutions to films. J. Am. Chem. Soc. 2006;128:9030–9031. doi: 10.1021/ja063027c. [DOI] [PubMed] [Google Scholar]
- 19.Okazaki M, et al. Solvent-controlled sign inversion of circularly polarized luminescent binaphthylacetic acid derivative. J. Photochem. Photobiol. A. 2016;331:115–119. doi: 10.1016/j.jphotochem.2016.04.010. [DOI] [Google Scholar]
- 20.Kimoto T, et al. Control of solid-state circularly polarized luminescence of binaphthyl organic fluorophores through environmental changes. Asian J. Org. Chem. 2013;2:404–410. doi: 10.1002/ajoc.201300034. [DOI] [Google Scholar]
- 21.Suzuki N, Fujiki M, Kimpinde-Kalunga R, Koe JR. Chiroptical inversion in helical Si-Si bond polymer aggregates. J. Am. Chem. Soc. 2013;135:13073–13079. doi: 10.1021/ja405570q. [DOI] [PubMed] [Google Scholar]
- 22.Guo S, Suzuki N, Fujiki M. Oligo- and polyfluorenes meet cellulose alkyl esters: Retention, inversion, and racemization of circularly polarized luminescence (CPL) and circular dichroism (CD) via intermolecular C-H/O = C interactions. Macromolecules. 2017;50:1778–1789. doi: 10.1021/acs.macromol.6b02762. [DOI] [Google Scholar]
- 23.Nishikawa T, Nagata Y, Suginome M. Poly(quinoxaline-2,3-diyl) as a multifunctional chiral scaffold for circularly polarized luminescent materials: Color tuning, energy transfer, and switching of the CPL handedness. ACS Macro Lett. 2017;6:431–435. doi: 10.1021/acsmacrolett.7b00131. [DOI] [PubMed] [Google Scholar]
- 24.Sheng Y, et al. Reversal circularly polarized luminescence of AIE-active chiral binaphthyl molecules from solution to aggregation. Chem. Eur. J. 2015;21:13196–13200. doi: 10.1002/chem.201502193. [DOI] [PubMed] [Google Scholar]
- 25.Hutin M, Nitschke J. Solvent-tunable inversion of chirality transfer from carbon to copper. Chem. Commun. 2006;2:1724–1726. doi: 10.1039/b601012a. [DOI] [PubMed] [Google Scholar]
- 26.Bünzli J-CG. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015;293–294:19–47. doi: 10.1016/j.ccr.2014.10.013. [DOI] [Google Scholar]
- 27.Richardson F. Selection rules for lanthanide optical activity. Inorg. Chem. 1980;19:2806–2812. doi: 10.1021/ic50211a063. [DOI] [Google Scholar]
- 28.Francesco Z, Di Bari L. Lanthanide circularly polarized luminescence: Bases and applications. Chirality. 2015;27:1–13. doi: 10.1002/chir.22382. [DOI] [PubMed] [Google Scholar]
- 29.Lunkley JL, Shirotani D, Yamanari K, Kaizaki S, Muller G. Chiroptical spectra of a series of tetrakis((+)-3-heptafluorobutylyrylcamphorato)lanthanide(III) with an encapsulated alkali metal ion: circularly polarized luminescence and absolute chiral structures for the Eu(III) and Sm(III) complexes. Inorg. Chem. 2011;50:12724–12732. doi: 10.1021/ic201851r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar J, Marydasan B, Nakashima T, Kawai T, Yuasa J. Chiral supramolecular polymerization leading to eye differentiable circular polarization in luminescence. Chem. Commun. 2016;52:9885–9888. doi: 10.1039/C6CC05022K. [DOI] [PubMed] [Google Scholar]
- 31.Carr R, Evans NH, Parker D. Lanthanide complexes as chiral probes exploiting circularly polarized luminescence. Chem. Soc. Rev. 2012;41:7673–7686. doi: 10.1039/c2cs35242g. [DOI] [PubMed] [Google Scholar]
- 32.Kono Y, Hara N, Shizuma M, Fujiki M, Imai Y. Complexes of Eu(III)(hfa)3 with a planar chiral P(III) ligand (Phanephos): solvent-sensitive sign inversion of circularly polarised luminescence. Dalton. Trans. 2017;46:5170–5174. doi: 10.1039/C7DT00741H. [DOI] [PubMed] [Google Scholar]
- 33.Yuasa J, Ueno H, Kawai T. Sign reversal of a large circularly polarized luminescence signal by the twisting motion of a bidentate ligand. Chem. Eur. J. 2014;20:8621–8627. doi: 10.1002/chem.201402268. [DOI] [PubMed] [Google Scholar]
- 34.Smith DG, Pal R, Parker D. Measuring equilibrium bicarbonate concentrations directly in cellular mitochondria and in human serum using europium/terbium emission intensity ratios. Chem. Eur. J. 2012;18:11604–11613. doi: 10.1002/chem.201201738. [DOI] [PubMed] [Google Scholar]
- 35.Harada T, et al. Circularly polarized luminescence of Eu(III) complexes with point- and axis-chiral ligands dependent on coordination structures. Inorg. Chem. 2009;48:11242–11250. doi: 10.1021/ic901663w. [DOI] [PubMed] [Google Scholar]
- 36.Kleckner IR, Foster MP. An introduction to NMR based approaches for measuring protein dynamics. Biochim. Biophys. Acta. 2011;1814:942–968. doi: 10.1016/j.bbapap.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cockerill AF, Davies GLO, Harden RC, Rackham DM. Lanthanide shift reagents in nuclear magnetic resonance spectroscopy. Chem. Rev. 1976;73:553–588. doi: 10.1021/cr60286a001. [DOI] [Google Scholar]
- 38.Binnemans K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015;295:1–45. doi: 10.1016/j.ccr.2015.02.015. [DOI] [Google Scholar]
- 39.Dai L, Lo WS, Coates ID, Pal R, Law GL. New class of bright and highly stable chiral cyclen europium complexes for circularly polarized luminescence applications. Inorg. Chem. 2016;55:9065–9070. doi: 10.1021/acs.inorgchem.6b01546. [DOI] [PubMed] [Google Scholar]
- 40.Richardson FS, Berry MT, Reid MF. Ligand polarization contributions to lanthanide 4f → 4f magnetic dipole transition moments and rotatory strengths. Mol. Phys. 1986;58:929–945. doi: 10.1080/00268978600101681. [DOI] [Google Scholar]
- 41.Görller-Walrand C, Fluyt L, Ceulemans A, Carnall WT. Magnetic dipole transitions as standards for Judd-Ofelt parametrization in lanthanide spectra. J. Chem. Phys. 1991;95:3099–3106. doi: 10.1063/1.460867. [DOI] [Google Scholar]
- 42.Forsberg JH. Complexes of lanthanide (III) ions with nitrogen donor ligands. Coord. Chem. Rev. 1973;10:195–226. doi: 10.1016/S0010-8545(00)80235-0. [DOI] [Google Scholar]
- 43.Ma CG, Brik MG, Kiisk V, Kangur T, Sildos I. Spectroscopic and crystal-field analysis of energy levels of Eu3+ in SnO2 in comparison with ZrO2 and TiO2. J. Alloys Compd. 2011;509:3441–3451. doi: 10.1016/j.jallcom.2010.12.071. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.