ABSTRACT
Bifidobacterium animalis subsp. lactis HN019 (HN019) ameliorates chronic idiopathic constipation. Our aim was to determine the efficacy and safety of 28-day supplementation with 1 × 109 or 1 × 1010 CFU of HN019/day for constipation. A total of 228 adults who were diagnosed with functional constipation according to the Rome III criteria were randomized in a double-blind and placebo-controlled trial. Colonic transit time (CTT), the primary outcome, and secondary outcomes that were measured using inventories—patient assessment of constipation symptoms (PAC-SYM) and quality of life (PAC-QoL), bowel function index (BFI), bowel movement frequency (BMF), stool consistency, degree of straining, bowel emptying, bloating, and pain severity—were assessed. Ancillary parameters and harms were also evaluated.
There were no statistically significant differences in the primary or secondary outcomes between interventions. A post hoc analysis of 65 participants with fewer than 3 bowel movements per week (BMF ≤ 3/week) showed a physiologically relevant increase in weekly BMF in the high- (+2.0) and low-dose (+1.7) HN019 groups—by RMANOVA, the HN019 groups with BMF ≤ 3/week, pooled together, had a higher BMF versus placebo (P value = 0.01). Thus, improving low stool frequency could be a target of future interventions with HN019. High-dose HN019 also decreased the degree of straining at Day 28 versus placebo in those with BMF ≤ 3/week (P value = 0.02). Three unlikely related AEs—2 with low-dose HN019 and 1 with placebo—were followed until full recovery. In conclusion, although there were no differences in the primary analysis, HN019 is well tolerated and improves BMF in adults with low stool frequency.
KEYWORDS: Bifidobacterium animalis subsp. lactis HN019, bowel movement frequency, functional constipation, probiotic, Rome III criteria
Introduction
Chronic idiopathic constipation, a gastrointestinal (GI) disorder of colonic or anorectal function, affects 14% of the population worldwide.1 Historically, health care professionals have defined constipation as fewer than 3 bowel movements per week.2 Recently, the Rome Foundation introduced a standard for classifying and diagnosing functional GI disorders. The Rome criteria were developed for global adoption and use by physicians, pharmaceutical companies, and regulatory agencies.3,4 The Rome III criteria encompass standards for diagnosing functional constipation, extending to other symptoms besides reduced bowel movement frequency.4 In addition, there are standard outcome measures of constipation, such as objective measurements of colonic transit time (CTT) by radio-opaque marker intake and x-ray count5 and the evaluation of constipation symptoms using validated inventories that are assessed by the subject or by health practitioners during medical visits.6-9
Probiotics appear to have beneficial effects on chronic idiopathic constipation,10 and a recent meta-analysis suggests that probiotic supplementation is moderately efficacious in decreasing intestinal transit times compared with control.11 This activity has been proposed to be attributed to the capacity of probiotics to alter the GI microflora,12 improve intestinal motility, and alter biochemical factors.13 These properties have been observed with Lactobacillus and Bifidobacterium species,12 and several traits of Bifidobacterium animalis subsp. lactis HN019 (HN019) have been studied with regard to its activity as a probiotic, such as its identity, safety, antipathogenic effects, immune enhancement, and intestinal colonization.14
A preliminary study has indicated that daily supplementation with HN019 for 14 days decreases CTT dose-dependently and reduces the frequency of functional GI symptoms in adults who have been diagnosed with constipation per physician-based criteria.15 Further, a mechanistic study of changes in gastrointestinal motility in isolated rat large intestine compared HN019 and prucalopride, a modulator of promotility, reporting that the enhanced motility that was induced by HN019 extract implicated the enteric neural circuitry in propulsive neurogenic colonic patterns, increasing the amplitude of propagating contractions in the colon,16 an activity that is consistent with the reduction in constipation in HN019-treated humans.
The aim of this study was to conduct a more exhaustive clinical trial to determine the effects of 28-day supplementation with HN019 over a range of doses on CTT and GI symptoms in adults who have been diagnosed with functional constipation per Rome III criteria.
Subjects and methods
Trial design
This trial was a 28-day, 3-arm parallel-group (allocation ratio 1:1:1), double-blind, randomized, placebo-controlled, monocenter study that was preceded by a 14-day run-in period. The study was conducted in full accordance with the Declaration of Helsinki and good clinical practice (GCP) standards17,18 and was registered at ClinicalTrials.gov (NCT02189707) and with French health authorities (ID-RCB 2014-A00166-41). The protocol and informed consent forms were approved by Comité de Protection des Personnes Sud-Est III (Lyon, FR) on June 24, 2014 and by Agence Nationale de Sécurité du Médicament et des Produits de Santé (ANSM) on July 3, 2014. The protocol had 2 nonsubstantial amendments for reviewing the stratification by sex, requested on November 4, 2014 and March 10, 2015, respectively. The initial stratification was 50% males and 50% females, and the final amendment stipulated the inclusion of at least 20% males (80% females by default), which, according to the enrollment rate, was the actual representation of sexes in the study population in the French region of Auvergne-Rhône-Alpes, from which the participants were recruited. The study has been reported per the CONSORT statement.19,20
Participants
Participants were eligible if they were aged 18 to 70 years inclusive, had a body mass index (BMI) of 18.5 to 34.9 kg/m2 inclusive, and had been diagnosed with functional constipation by the investigator per the Rome III criteria—in the last 3 months, with symptom onset occurring at least 6 months prior to the diagnosis4—as follows:
-
a.
Met 2 or more of the following criteria: i. Straining during at least 25% of defecations; ii. Lumpy or hard stools in at least 25% of defecations; iii. Sensation of incomplete evacuation for at least 25% of defecations; iv. Sensation of anorectal obstruction/blockage for at least 25% of defecations; v. Manual maneuvers to facilitate at least 25% of defecations (eg, digital evacuation, support of the pelvic floor); and vi. Fewer than 3 defecations per week.
-
b.
Loose stools rarely present without the use of laxatives.
-
c.
Insufficient criteria for irritable bowel syndrome—per Rome III criteria.4
In addition, participants had to be able (in the investigator's opinion) to comprehend the full nature and purpose of the study, including the possible risks and side effects; consent to participation in the study; be willing to comply with the study product and methods; and be covered by a health insurance system that was in compliance with national laws regarding biomedical research.
Participants were excluded if they had major GI complications (eg, Crohn disease, ulcer); had prior abdominal surgery that, in the opinion of the investigator, could create a risk for the participant or confound study results; had a clinically significant underlying systemic illness that could preclude the participant's ability to complete the trial or confound the study outcomes (eg, bowel cancer, prostate cancer, terminal illness); consumed probiotics, prebiotics, fermented milk, or yogurt other than the study products daily within 2 weeks of the screen and throughout the trial; used a laxative within 48 hours of the screen (rescue medication allowed for intolerable symptoms during the study); used any drug or dietary supplement that is known to cause constipation (eg, iron; opioids; sucralfate; misoprostol; 5-HT# antagonists; antacids with magnesium, calcium, or aluminum; antidiarrheal medication; anticholinergic agents; calcium supplements; calcium channel blockers; tricyclic antidepressants; or NSAIDs) regularly (in the investigator's opinion) within 1 month before the screen; had anticipated major changes in diet or exercise during the study; had systemic steroid use within 1 month before the screen; had an eating disorder; had any contraindication to dairy products (eg, intolerance to lactose or any substance in the study product); had a history of alcohol, drug, or medication abuse; were pregnant or lactating or planned to become pregnant during the study period; were participating in another study with any investigational product within 60 days of the screen; were believed by the investigator to be uncooperative or noncompliant; were under administrative or legal supervision; or had received more than 4500 euros as compensation for participation in biomedical research within the past 12 months, including compensation for the current study, per French regulations.
The study consisted of a screening visit (Day -14), followed by a run-in period during which participants consumed placebo while they were assessed for their capacity to follow the study procedures (ie, compliance with the completion of diaries, ≥ 80% intake of placebo, and 100% intake of radio-opaque markers)—participants were unaware that they were consuming exclusively placebo during the run-in period. Those who were eligible were randomized to 1 of the 3 arms at the second visit (Day 0) and continued treatment over the next 4 weeks until the third and last visit (Day 28) (Fig. 1). At the first visit, after signing informed consent forms, participants were assessed with regard to meeting the inclusion and exclusion criteria, were measured for anthropometric parameters, underwent a medical examination (females were tested for pregnancy), were provided with daily diaries and 24-h food recall records, and were given run-in placebo supplementation (blinded only for the participants).
Figure 1.

Study setup. AEs, adverse events; BFI, bowel function index; ICF, informed consent form; IPAQ, International Physical Activity Questionnaire; PAC-SYM, Patient Assessment of Constipation Symptoms; PAC-QoL, Patient Assessment of Constipation Quality of Life. ■, radio-opaque markers were consumed at the study site from Day -6 to Day -1 during the run-in period and from Day 22 to Day 27 during the intervention period.
From Days -6 to -1, the participants made ambulatory visits to the clinic, where a health practitioner gave them radio-opaque markers to ensure 100% intake compliance. At the second visit, participants were randomized if they had been ≥ 80% compliant with taking the run-in placebo, 100% compliant with the consumption of radio-opaque markers, and 100% compliant with daily completion of their diaries. Then, they completed constipation-specific and ancillary questionnaires, passed an abdominal x-ray, were provided with daily diaries and 24-h food recall records, and were supplemented with randomized treatments (in a double-blind manner). From Days 22 to 27, participants made ambulatory visits to the clinic, where a health practitioner gave them radio-opaque markers to ensure 100% intake compliance. At the third visit, participants were assessed for their compliance with the treatment, consumption of radio-opaque markers, and completion of records; answered constipation-specific and ancillary questionnaires; completed a product satisfaction questionnaire; were measured for weight; and underwent the final abdominal x-ray.
The study was conducted at Eurofins Optimed SAS (Gières, FR) under the supervision of Mathilde Latreille-Barbier, MD, acting as principal investigator. The x-rays were taken at Centre d'Imagerie Médicale Gustave Rivet (Grenoble, FR) by Eric Escolano, MD, a radiology specialist. The study was sponsored by DuPont Nutrition and Health (Kantvik, FI), represented by Alvin Ibarra, PhD, and Arthur C. Ouwehand, PhD, acting as study coordinators.
Interventions
Three groups were provided with a study product at the randomization (Day 0): capsules that contained the active ingredient, HN019 at 1 × 1010 CFU (high-dose group); capsules with the active ingredient, HN019 at 1 × 109 CFU (low-dose group); and placebo capsules.
Participants were instructed to open 1 capsule of the study product and mix its contents with a provided dairy product and consume the entire mixture once per day. The aim of this procedure was to mimic a typical functional food and ensure that the probiotic concentration was fixed.
The study products were manufactured by DuPont Nutrition and Health (Madison, WI, US) using hydroxypropyl methylcellulose (HPMC) capsules that were filled with a white powder, containing freeze-dried probiotic powder—or not, in the case of the placebo—in a base of microcrystalline cellulose, silicon dioxide, and potato maltodextrin. The dairy product was 100 g of Gervais Maxi (Danone, FR), which had a total microbiological count of less than 1 × 102 CFU/mL during the selection of potential vehicles prior to the study. We presumed that this low count was attributed to the dairy product first being fermented and then pasteurized during its manufacture.
Primary and secondary outcomes
The primary outcome was the effect of 28-day supplementation with HN019 over a range of doses on CTT in adults who were diagnosed with functional constipation. CTT was assessed by abdominal x-ray21 using the following equation:
where ni is the number of markers that was observed on x-ray, t is the time between the ingestion of markers in hours, and N is the total number of markers that was ingested each day. Thus, if markers are consumed at 24-h intervals and the number of markers per day is 24, CTT equals the total marker count on the x-ray.
Each participant ingested 24 radio-opaque markers (MARQUAT Génie Biomédical, Boissy-Saint-Léger, FR) daily for 6 consecutive days prior to abdominal x-rays on Days 0 and 28—radio-opaque markers were administered to participants at the site to assure compliance. The numbers of markers in the right, left, and rectosigmoid colon were summed to yield the total marker count.21 The number of radio-opaque markers in the x-rays was counted by the radiologist and confirmed by the principal investigator or a delegate.
The secondary outcomes were the effects of 28-day HN019 supplementation on other constipation-related parameters.
During the visits to the clinic on Days 0 (baseline) and 28 (end of study), participants filled out the Patient Assessment of Constipation Symptoms (PAC-SYM),7 the Patient Assessment of Constipation Quality of Life (PAC-QoL),8 and the Bowel Function Index (BFI)9 and reported their levels of adequate relief from constipation in the past week through yes/no questions.
Throughout the intervention (from Days -14 to 28), participants kept a daily diary to document their bowel movement frequency (BMF), consistency of stools per the Bristol Stool Scale (BSS),6 degree of straining (1, not at all; 2, a little bit; 3, a moderate amount; 4, a great deal; and 5, an extreme amount), self-assessment of complete bowel emptying (yes/no), and abdominal pain and bloating severity (1, none; 2, mild; 3, moderate; 4, severe; and 5, very severe). Participants also recorded their treatment consumption throughout the trial in the diary. At each follow-up visit, the participant returned the completed diary, and the investigator or research coordinator questioned the participant to corroborate and clarify the diary entries.
Participants also completed ancillary records. On Days 0 and 28, the short version of the International Physical Activity Questionnaire (IPAQ) was answered.22 Before the visits on Days 0 and 28, they supplied their 24-h food recall—total calories (Kcal), total carbohydrates (g and %Kcal), total lipids (g and %Kcal), total proteins (g and %Kcal), alcohol (g and %Kcal), and liquid (g)—and on Day 28, they reported their overall satisfaction with the products—1, not at all satisfied; 2, a little satisfied; 3, moderately satisfied; 4, quite satisfied; and 5, very satisfied.
Adverse events (AEs) that occurred and concomitant medications that were taken during the intervention were recorded using the Medical Dictionary for Regulatory Activities (MedDRA)23 and the Anatomical Therapeutic Chemical (ATC) Classification System,24 respectively.
All AEs were reported and recorded regardless of whether they were considered to be nonserious, serious, or related to the treatment. The relevant information was required in each case—ie, participant ID number, start date, description, duration, intensity (mild, moderate, or severe), seriousness, action taken, outcome and sequelae, and relationship to the test product (unrelated, unlikely relation, probable relation, and certain relation). The AE form was completed by the investigator, who identified and followed all AEs in each participant.
Sample size
Assuming a standard deviation (SD) of CTT of 30 h, the required sample size for establishing a significant difference in the mean decrease in CTT by 20 h in the HN019 groups and by 5 h in the placebo group, with 80% statistical power and a two-sided significance level (α) of 5% and allowing for 15% participant attrition, was 228 randomized participants—ie, 76 per group.
Randomization, allocation, and implementation
Participants were randomized with computer-generated block randomization lists to 1 of 3 product groups in equal proportions—3 products per block—using SAS version 9.3 (SAS Institute Inc., NC, US). The allocation was stratified by sex, allowing for at least 20% males; the treatment group was balanced per the stratification update of the protocol amendments.
All products were labeled with 5-digit numbers and additional information on the study, product storage, and emergency contacts. Tad Stuart, the representative from DuPont Nutrition and Health (Madison, WI, US), was responsible for the product manufacturing and label allocation and had no other involvement in the trial. Run-in placebo and treatment products were coded using separate sets, but only the 3 treatment products followed the set that was assigned by the randomization list. Pill boxes for the run-in period contained 20 capsules, and those for the treatment groups contained 35 capsules of the study products.
The program for generating the randomization list was written and validated by 2 statisticians at Eurofins Optimed SAS (Gières, FR). The pharmacist at Eurofins Optimed SAS was the sole researcher who kept the codes blinded during the study. Blinded codes were stored in individual envelopes.
Blinding
The personnel at the study site and representatives from the sponsor that was in charge of coordinating the study were provided only with the 5-digit numbers of the study products and remained blinded to the identities of the 3 treatment products and group allocation. Participants remained blinded to their treatment allocation, even during the run-in period. Radio-opaque marker counts were recorded by a single board-certified radiologist who remained blinded to the treatment assignments. Copies of the individual sealed envelopes containing unblinded codes for each participant were available to the principal investigator.
Investigators and study staff remained blinded to the treatment assignments until the database was locked. The treatments and placebo capsules and their contents were identical in appearance, texture, and taste.
Statistical methods
The intention-to-treat (ITT) population included all participants who were randomized at the second visit and consumed at least 1 dose of the study product, and the per-protocol (PP) subset was defined during the blind data review before the database was frozen. Participants who were eligible to be in the PP population were those who attended the end-of-study visit, received at least 80% of the assigned study product, and consumed 100% of the radio-opaque markers on time during the intervention.
A CONSORT diagram was constructed to illustrated the flow of participants through the study, beginning with the initial recruitment and eligibility assessment.19 Summary statistics of the baseline data were compiled by the study group and on all participants for each population, using means and SDs for continuous variables and frequencies and percentages for categorical variables. Compliance with consumption of the study product was evaluated by the product group.
For continuous endpoints, analysis of covariance (ANCOVA) was used to compare between-treatment groups, followed by multiple comparison tests for statistically significant analysis of variance (ANOVA) results. ANCOVA was used to analyze the effects of the study product and sex on continuous endpoints, adjusting for age and other continuous covariates (eg, endpoint at study baseline). The effect of categorical covariates on categorical endpoints was analyzed using a generalized estimating equation model. Odds ratios and their 95% confidence intervals (CIs) were determined to compare the effects of the study products and sex on categorical endpoints.
The analyses descriptively compared the number and percentage of participants with specific AEs and serious AEs (SAEs) and those for withdrawals due to an AE. Thus, harms were reported descriptively, classifying nontreatment-emergent AEs (NTEAEs) that occurred during the run-in period and treatment-emergent AEs (TEAEs) that arose during the intervention. We report the results only for TEAEs and SAEs. Concomitant medications were listed by product group in the original clinical study report (not shown).
A supplementary population was defined after the first analysis. Participants from the ITT cohort who had had a BMF of ≤ 3 per week during the entire 14-day run-in period were included in this population—as suggested by Miller et al.25—and are hereafter referred to as BMF ≤ 3/week. The BMF ≤ 3/week subgroup was analyzed using the same statistical methods as for the ITT and PP populations. In addition, changes in BMF were analyzed by repeated measures ANOVA (RMANOVA). The modelization in the RMANOVA analysis considered Days 0, 7, 14, 21, and 28, calculated as mean BMF from the previous week.
All statistical analyses were performed by Laure Anne Giannone, a statistician at Eurofins Optimed SAS (Gières, FR), using SAS version 9.3 (SAS Institute Inc., NC, US).
Results
Participants
In total, 260 volunteers were screened, 228 of whom were randomized; 182 (79.8%) were females, and 46 (20.2%) were males. Fig. 2 shows the CONSORT flow diagram and the reasons for non-inclusion and the overall assignments for each population. Four subjects withdrew prematurely from the study. Two subjects withdrew due to adverse events—1 from each active group—and 2 volunteers withdrew consent—also 1 from each active group. Ultimately, 224 subjects completed all study visits.
Figure 2.
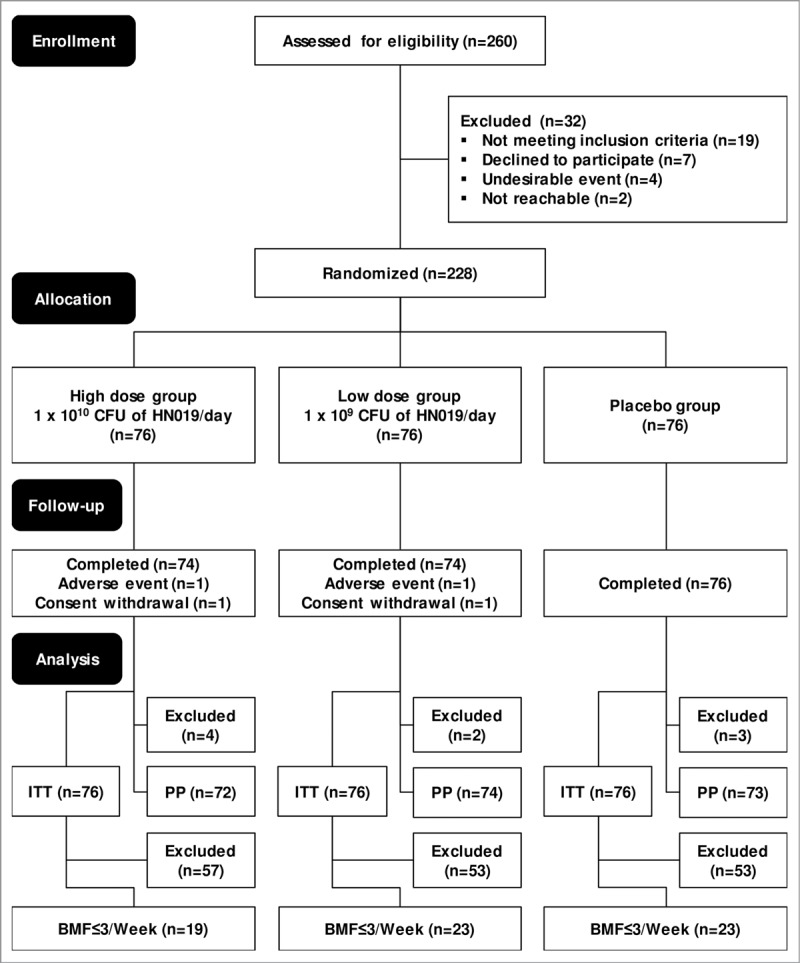
CONSORT diagram of the 28-day, 3-arm parallel-group (allocation ratio 1:1:1), double-blind, randomized, placebo-controlled, and monocenter study, preceded by a 2-week run-in period. BMF ≤ 3/week, participants from the ITT population who had fewer than or equal to 3 bowel movements per week during the entire 2-week run-in period; CONSORT, Consolidated Standards of Reporting Trials; ITT, intention-to-treat population—all participants randomized at the second visit who consumed at least 1 dose of the study product; PP, per-protocol population—participants who attended the end-of-study visit and received at least 80% of the assigned study product and consumed 100% of radio-opaque markers during the intervention on time.
The intervention took place between August 04, 2014 and April 09, 2015 and ceased after the last enrolled participant completed the final visit.
The ITT and PP populations comprised 228 and 219 randomized participants, respectively. The BMF ≤ 3/week population contained 65 randomized participants, 19 of whom were in the high-dose HN019 group; there were 23 subjects each the low-dose HN019 and placebo groups. The study had an attrition rate of 4%; therefore, the results from the ITT and PP populations were considered to be similar. Consequently, we have presented the findings only for the ITT and BMF ≤ 3/week populations. Table 1 lists the baseline characteristics of the participants, diagnosed per the Rome III criteria for functional constipation, in the ITT and BMF ≤ 3/week populations. These populations did not differ significantly with regard to baseline characteristics at the screening (Table 1) or the parameters that were measured at baseline (Day 0) between treatment groups, including placebo (Tables 2, 3, and 4).
Table 1.
Baseline characteristics of participants diagnosed with Rome III criteria for functional constipation in the ITT and BMF ≤ 3/week populations.
| Characteristics | ITT |
BMF≤3/week |
||||||||
| |
Total(n = 228) |
High dose(n = 76) |
Low dose (n = 76) |
Placebo (n = 76) |
Total (n = 65) |
High dose (n = 19) |
Low dose (n = 23) |
Placebo (n = 23) |
||
| Age (years) | 41.7 ± 14.0 | 42.9 ± 13.8 | 41.6 ± 15.3 | 40.6 ± 12.8 | 38.1±12.7 | 40.4 ± 13.2 | 34.1 ± 11.3 | 40.1 ± 13.2 | ||
| Sex | ||||||||||
| Female | 182 (79.8%) | 60 (78.9%) | 61 (80.3%) | 61 (80.3%) | 49 (75.4%) | 14 (73.7%) | 17 (73.9%) | 18 (78.3%) | ||
| Male | 46 (20.2%) | 16 (21.1%) | 15 (19.7%) | 15 (19.7%) | 16 (24.6%) | 5 (26.3%) | 6 (26.1%) | 5 (21.7%) | ||
| Weight (kg) | 68.3 ± 13.2 | 67.7 ± 13.4 | 67.8 ± 13.5 | 69.4 ± 12.8 | 69.8 ± 13.6 | 70.2 ± 1 3.5 | 69.9 ± 14.6 | 69.3 ± 13.2 | ||
| Height (cm) | 167.1 ± 8.1 | 167.5 ± 8.2 | 166.9 ± 8.6 | 166.9 ± 7.5 | 169.3 ± 8.2 | 168.9 ± 8.1 | 170.8 ± 9.5 | 168.1 ± 7.0 | ||
| BMI (kg/m2) | 24.4 ± 3.7 | 24.1 ± 3.9 | 24.2 ± 3.7 | 24.8 ± 3.6 | 24.3 ± 4.1 | 24.6 ± 4.6 | 23.9 ± 4.1 | 24.4 ± 3.8 | ||
| Rome III criteria | ||||||||||
| Criterion 1 | 220 (96.5%) | 76 (100.0%) | 70 (92.1%) | 74 (97.4%) | 65 (100.0%) | 19 (100.0%) | 23 (100.0%) | 23 (100.0%) | ||
| Criterion 2 | 222 (97.4%) | 74 (97.4%) | 73 (96.1%) | 75 (98.7%) | 64 (98.5%) | 18 (94.7%) | 23 (100.0%) | 23 (100.0%) | ||
| Criterion 3 | 218 (95.6%) | 72 (94.7%) | 71 (93.4%) | 75 (98.7%) | 61 (93.8%) | 18 (94.7%) | 21 (91.3%) | 22 (95.7%) | ||
| Criterion 4 | 199 (87.3%) | 64 (84.2%) | 67 (88.2%) | 68 (89.5%) | 56 (86.2%) | 15 (78.9%) | 21 (91.3%) | 20 (87.0%) | ||
| Criterion 5 | 49 (21.5%) | 19 (25.0%) | 14 (18.4%) | 16 (21.1%) | 11 (16.9%) | 5 (26.3%) | 2 (8.7%) | 4 (17.4%) | ||
| Criterion 6 | 127 (55.7%) | 43 (56.6%) | 39 (51.3%) | 45 (59.2%) | 42 (64.6%) | 12 (63.2%) | 14 (60.9%) | 16 (69.6%) | ||
BMF ≤ 3/week, participants from the ITT population who had fewer than or equal to 3 bowel movements per week during the entire 14-day run-in period; high-dose group, 1 × 1010 CFU of HN019/day; ITT, intention-to-treat population—all participants randomized at the second visit who consumed at least 1 dose of the study product; low-dose group 1 × 109 CFU of HN019/day; criterion 1, straining during at least 25% of defecations; criterion 2, lumpy or hard stools in at least 25% of defecations; criterion 3, sensation of incomplete evacuation for at least 25% of defecations; criterion 4, sensation of anorectal obstruction or blockage for at least 25% of defecations; criterion 5, manual maneuvers to facilitate at least 25% of defecations; and criterion 6, fewer than 3 defecations per week.
Table 2.
Parameters measured on Day 0 (baseline) and Day 28 (end of study) in the ITT and BMF≤3/week populations.
| ITT |
BMF≤3/week |
|||||||
| |
Total |
High dose |
Low dose |
Placebo |
Total |
High dose |
Low dose |
Placebo |
| Colonic Transit Time (CTT) | ||||||||
| Day 0 | 63.97 ± 35.07 | 67.55 ± 35.68 | 60.53 ± 35.40 | 63.84 ± 34.21 | 65.38 ± 34.20 | 65.00 ± 42.47 | 65.91 ± 34.94 | 65.17 ± 26.55 |
| Day 28 | 62.34 ± 34.17 | 66.70 ± 32.07 | 62.54 ± 36.68 | 57.88 ± 33.51 | 65.46 ± 31.89 | 68.37 ± 27.12 | 62.22 ± 36.64 | 66.30 ± 31.56 |
| Δ | -1.78 ± 32.38 | -1.09 ± 34.07 | 1.82 ± 33.49 | -5.96 ± 29.40 | 0.08 ± 35.54 | 3.37 ± 46.94 | -3.70 ± 31.54 | 1.13 ± 29.16 |
| Patient Assessment of Constipation Symptoms (PAC-SYM) | ||||||||
| Abdominal Symptoms Score | ||||||||
| Day 0 | 0.98 ± 0.69 | 0.98 ± 0.72 | 1.02 ± 0.66 | 0.94 ± 0.69 | 0.82 ± 0.64 | 0.81 ± 0.71 | 0.82 ± 0.67 | 0.85 ± 0.57 |
| Day 28 | 0.87 ± 0.61 | 0.86 ± 0.61 | 0.85 ± 0.59 | 0.88 ± 0 .64 | 0.76 ± 0.50 | 0.74 ± 0.50 | 0.74 ± 0.52 | 0.78 ± 0.49 |
| Δ | -0.10 ± 0.54 | -0.10 ± 0.59 | -0.15 ± 0.51 | -0.06 ± 0.53 | -0.07 ± 0.54 | -0.07 ± 0.58 | -0.08 ± 0.54 | -0.07 ± 0.54 |
| Rectal Symptoms Score | ||||||||
| Day 0 | 0.55 ± 0.68 | 0.51 ± 0.64 | 0.49 ± 0.63 | 0.65 ± 0.75 | 0.63 ± 0.73 | 0.48 ± 0.63 | 0.67 ± 0.80 | 0.71 ± 0.75 |
| Day 28 | 0.44 ± 0.58 | 0.47 ± 0.58 | 0.45 ± 0.59 | 0.40 ± 0.58 | 0.36 ± 0.47 | 0.39 ± 0.56 | 0.40 ± 0.49 | 0.30 ± 0.39 |
| Δ | -0.11 ± 0.63 | -0.04 ± 0.58* | -0.04 ± 0.54 | -0.25 ± 0.74 | -0.27 ± 0.59 | -0.09 ± 0.36 | -0.28 ± 0.61 | -0.42 ± 0.70 |
| Stool Symptoms Score | ||||||||
| Day 0 | 1.48 ± 0.85 | 1.42 ± 0.88 | 1.54 ± 0.84 | 1.48 ± 0.84 | 1.39 ± 0.84 | 1.08 ± 0.71 | 1.66 ± 0.91 | 1.38 ± 0.81 |
| Day 28 | 1.21 ± 0.85 | 1.22 ± 0.83 | 1.28 ± 0.91 | 1.11 ± 0.81 | 0.97 ± 0.72 | 0.99 ± 0.67 | 1.10 ± 0.75 | 0.83 ± 0.73 |
| Δ | -0.25 ± 0.66 | -0.16 ± 0.59 | -0.24 ± 0.72 | -0.37 ± 0.66 | -0.42 ± 0.70 | -0.09 ± 0.50 | -0.57 ± 0.83 | -0.56 ± 0.62 |
| Overall Score | ||||||||
| Day 0 | 1.08 ± 0.58 | 1.04 ± 0.61 | 1.10 ± 0.58 | 1.09 ± 0.57 | 1.01 ± 0.55 | 0.83 ± 0.46 | 1.13 ± 0.62 | 1.04 ± 0.53 |
| Day 28 | 0.90 ± 0.58 | 0.91 ± 0.57 | 0.93 ± 0.60 | 0.86 ± 0.57 | 0.74 ± 0.43 | 0.75 ± 0.42 | 0.80 ± 0.46 | 0.68 ± 0.43 |
| Δ | -0.17 ± 0.46 | -0.11 ± 0.44 | -0.16 ± 0.45 | -0.23 ± 0.48 | -0.27 ± 0.45 | -0.08 ±0.32 | -0.33 ± 0.54 | -0.36 ± 0.41 |
| Patient Assessment of Constipation Quality of Life (PAC-QoL) | ||||||||
| Physical Discomfort Score | ||||||||
| Day 0 | 1.17 ± 0.84 | 1.16 ± 0.84 | 1.18 ± 0.88 | 1.18 ± 0.80 | 1.11 ± 0.72 | 1.11 ± 0.62 | 1.12 ± 0.82 | 1.10 ± 0.72 |
| Day 28 | 0.91 ± 0.83 | 0.97 ± 0.82 | 0.89 ± 0.86 | 0.87 ± 0.81 | 0.80 ± 0.67 | 0.84 ± 0.54 | 0.73 ± 0.65 | 0.84 ± 0.80 |
| Δ | -0.24 ± 0.70 | -0.17 ± 0.74 | -0.26 ± 0.65 | -0.31 ± 0.69 | -0.31 ± 0.60 | -0.26 ± 0.46 | -0.39 ± 0.61 | -0.26 ± 0.70 |
| Psychosocial Discomfort Score | ||||||||
| Day 0 | 0.59 ± 0.60 | 0.52 ± 0.53 | 0.60 ± 0.59 | 0.65 ± 0.67 | 0.53 ± 0.54 | 0.63 ± 0.59 | 0.54 ± 0.61 | 0.44 ± 0.43 |
| Day 28 | 0.45 ± 0.54 | 0.40 ± 0.43 | 0.47 ± 0.60 | 0.49 ± 0.59 | 0.30 ± 0.33 | 0.34 ± 0.28 | 0.35 ± 0.46 | 0.22 ± 0.21 |
| Δ | -0.13 ± 0.47 | -0.10 ± 0.41 | -0.12 ± 0.50 | -0.16 ± 0.51 | -0.23 ± 0.47 | -0.29 ± 0.49 | -0.19 ± 0.54 | -0.22 ± 0.37 |
| Worries and Concerns Score | ||||||||
| Day 0 | 0.93 ± 0.73 | 0.88 ± 0.70 | 0.91 ± 0.75 | 1.00 ± 0.73 | 0.89 ± 0.63 | 1.03 ± 0.57 | 0.87 ± 0.74 | 0.80 ± 0.63 |
| Day 28 | 0.71 ± 0.64 | 0.67 ± 0.59 | 0.67 ± 0.62 | 0.79 ± 0.71 | 0.64 ± 0.54 | 0.68 ± 0.54 | 0.59 ± 0.52 | 0.66 ± 0.58 |
| Δ | -0.20 ± 0.48 | -0.19 ± 0.49 | -0.21 ± 0.52 | -0.20 ± 0.43 | -0.25 ± 0.42 | -0.35 ± 0.48 | -0.29 ± 0.42 | -0.14 ± 0.36 |
| Satisfaction Score | ||||||||
| Day 0 | 2.41 ± 0.77 | 2.32 ± 0.77 | 2.35 ± 0.72 | 2.56 ± 0.81 | 2.71 ± 0.71 | 2.78 ± 0.60 | 2.50 ± 0.72 | 2.87 ± 0.75 |
| Day 28 | 2.21 ± 0.79 | 2.19 ± 0.75 | 2.22 ± 0.85 | 2.22 ± 0.79 | 2.34 ± 0.79 | 2.23 ± 0.81 | 2.25 ± 0.74 | 2.51 ± 0.82 |
| Δ | -0.20 ± 0.81 | -0.13 ± 0.78 | -0.13 ± 0.83 | -0.33 ± 0.81 | -0.38 ± 0.84 | -0.55 ± 0.73 | -0.25 ± 0.89 | -0.36 ± 0.90 |
| Overall Score | ||||||||
| Day 0 | 1.13 ± 0.56 | 1.07 ± 0.54 | 1.12 ± 0.57 | 1.20 ± 0.57 | 1.15 ± 0.47 | 1.24 ± 0.42 | 1.10 ± 0.55 | 1.11 ± 0.44 |
| Day 28 | 0.93 ± 0.52 | 0.91 ± 0.48 | 0.92 ± 0.54 | 0.97 ± 0.55 | 0.87 ± 0.41 | 0.88 ± 0.41 | 0.84 ± 0.37 | 0.89 ± 0.45 |
| Δ | -0.19 ± 0.41 | -0.15 ± 0.40 | -0.17 ± 0.42 | -0.23 ± 0.39 | -0.28 ± 0.36 | -0.36 ± 0.35 | -0.27 ± 0.33 | -0.22 ± 0.39 |
| Bowel Function Index (BFI) | ||||||||
| Day 0 | 56.57 ± 25.73 | 52.69 ± 24.74 | 58.54 ± 27.92 | 58.49 ± 24.27 | 62.90 ± 25.03 | 53.16 ± 18.57 | 66.23 ± 29.69 | 67.62 ± 23.29 |
| Day 28 | 48.17 ± 25.97 | 47.77 ± 27.36 | 47.74 ± 25.21 | 49.00 ± 25.64 | 47.25 ± 22.58 | 47.49 ± 22.84 | 46.01 ± 18.18 | 48.28 ± 26.90 |
| Δ | -8.03 ± 24.35 | -4.45 ± 23.03 | -10.12 ± 25.83 | -9.50 ± 24.06 | -15.66 ± 24.60 | -5.67 ± 22.86 | -20.22 ± 24.29 | -19.35 ± 24.92 |
BMF ≤ 3/week, participants from the ITT population who had fewer than or equal to 3 bowel movements per week during the entire 14-day run-in period; high-dose group, 1 × 1010 CFU of HN019/day; ITT, intention-to-treat population—all participants randomized at the second visit who consumed at least 1 dose of the study product; low-dose group 1 × 109 CFU of HN019/day; Δ, change from Day 0 to Day 28. Values are mean ± standard deviation. *P value<0.05 compared with the placebo group.
Table 3.
Parameters assessed with participants' diaries in the ITT and BMF≤3/week populations. Results are expressed weekly: Day 0 = Day -7 to Day -1, Day 28 = Day 21 to Day 27.
| ITT |
BMF≤3/week |
||||||||
| |
Total |
High dose |
Low dose |
Placebo |
Total |
High dose |
Low dose |
Placebo |
|
| Bowel Movement Frequency (BMF) | |||||||||
| Day 0 | 4.59 ± 2.20 | 4.89 ± 2.42 | 4.76 ± 2.43 | 4.13 ± 1.60 | 2.75 ± 0.83 | 2.68 ± 0.82 | 2.78 ± 0.85 | 2.78 ± 0.85 | |
| Day 28 | 5.16 ± 2.18 | 5.56 ± 2.45 | 5.39 ± 2.20 | 4.54 ± 1.70 | 4.20 ± 2.11 | 4.68 ± 1.89 | 4.43 ± 2.61 | 3.57 ± 1.59 | |
| Δ | 0.57 ± 1.53 | 0.67 ± 1.69 | 0.62 ± 1.67 | 0.41 ± 1.18 | 1.45 ± 2.19 | 2.00 ± 1.97 | 1.65 ± 2.72 | 0.78 ± 1.62 | |
| Bristol Stool Scale (BSS) | |||||||||
| Day 0 | 3.30 ± 1.09 | 3.34 ± 1.12 | 3.27 ± 1.11 | 3.30 ± 1.06 | 3.36 ± 1.30 | 3.88 ± 1.55 | 2.87 ± 1.11 | 3.42 ± 1.09 | |
| Day 28 | 3.51 ± 1.08 | 3.4 6± 1.11 | 3.52 ± 1.19 | 3.56 ± 0.96 | 3.67 ± 1.27 | 3.80 ± 1.31 | 3.48 ± 1.23 | 3.74 ± 1.32 | |
| Δ | 0.21 ± 0.87 | 0.12 ± 0.89 | 0.26 ± 0.82 | 0.25 ± 0.91 | 0.31 ± 1.39 | -0.08 ± 1.65 | 0.61 ± 1.11 | 0.32 ± 1.40 | |
| Degree of Straining | |||||||||
| Day 0 | 2.43 ± 0.73 | 2.33 ± 0.73 | 2.41 ± 0.74 | 2.54 ± 0.72 | 2.55 ± 0.91 | 2.22 ± 0.87 | 2.61 ± 0.92 | 2.78 ± 0.89 | |
| Day 28 | 2.13 ± 0.68 | 2.11 ± 0.72 | 2.08 ± 0.70 | 2.20 ± 0.61 | 2.10 ± 0.77 | 1.70 ± 0.64* | 2.18 ± 0.79 | 2.35 ± 0.77 | |
| Δ | -0.29 ± 0.60 | -0.21 ± 0.64 | -0.31 ± 0.57 | -0.33 ± 0.59 | -0.46 ± 0.95 | -0.52 ± 0.72 | -0.43 ± 1.06 | -0.44 ± 1.03 | |
| Complete Bowel Emptying | |||||||||
| Day 0 | 0.44 ± 0.31 | 0.47 ± 0.31 | 0.39 ± 0.30 | 0.47 ± 0.31 | 0.51 ± 0.39 | 0.52 ± 0.43 | 0.56 ± 0.41 | 0.44 ± 0.36 | |
| Day 28 | 0.54 ± 0.33 | 0.58 ± 0.34 | 0.49 ± 0.33 | 0.55 ± 0.31 | 0.62 ± 0.36 | 0.68 ± 0.37 | 0.59 ± 0.35 | 0.61 ± 0.38 | |
| Δ | 0.09 ± 0.25 | 0.11 ± 0.26 | 0.09 ± 0.24 | 0.08 ± 0.25 | 0.12 ± 0.42 | 0.16 ± 0.40 | 0.03 ± 0.42 | 0.18 ± 0.44 | |
| Abdominal Pain | |||||||||
| Day 0 | 1.70 ± 0.63 | 1.66 ± 0.56 | 1.76 ± 0.71 | 1.70 ± 0.63 | 1.56 ± 0.66 | 1.45 ± 0.52 | 1.65 ± 0.88 | 1.57 ± 0.51 | |
| Day 28 | 1.58 ± 0.62 | 1.57 ± 0.62 | 1.63 ± 0.71 | 1.55 ± 0.52 | 1.46 ± 0.51 | 1.32 ± 0.49 | 1.48 ± 0.54 | 1.55 ± 0.49 | |
| Δ | -0.12 ± 0.46 | -0.09 ± 0.49 | -0.13 ± 0.50 | -0.14 ± 0.37 | -0.11 ± 0.59 | -0.13 ± 0.56 | -0.17 ± 0.71 | -0.03 ± 0.49 | |
| Bloating | |||||||||
| Day 0 | 1.81 ± 0.69 | 1.81 ± 0.69 | 1.81 ± 0.70 | 1.80 ± 0.69 | 1.75 ± 0.78 | 1.62 ± 0.61 | 1.90 ± 0.99 | 1.70 ± 0.67 | |
| Day 28 | 1.67 ± 0.68 | 1.68 ± 0.70 | 1.68 ± 0.76 | 1.65 ± 0.58 | 1.54 ± 0.61 | 1.38 ± 0.45 | 1.56 ± 0.77 | 1.64 ± 0.55 | |
| Δ | -0.14 ± 0.48 | -0.12 ± 0.49 | -0.13 ± 0.54 | -0.15 ± 0.39 | -0.21 ± 0.62 | -0.25 ± 0.66 | -0.34 ± 0.67 | -0.05 ± 0.52 | |
BMF≤3/week, participants from the ITT population who had fewer than or equal to 3 bowel movements per week during the entire 14-day run-in period; high-dose group, 1 × 1010 CFU of HN019/day; ITT, intention-to-treat population—all participants randomized at the second visit who consumed at least 2 dose of the study product; low-dose group 1 × 109 CFU of HN019/day; Δ, change from Day 0 to Day 28. Values are mean ± standard deviation. *P value < 0.05 compared with the placebo group.
Table 4.
Adequate Relief of Constipation—measured at Day 0 (baseline) and Day 28 (end of study)—in the ITT and BMF≤3/week populations.
| ITT |
BMF≤3/week |
|||||||
| |
Total |
High dose |
Low dose |
Placebo |
Total |
High dose |
Low dose |
Placebo |
| Day 0 | ||||||||
| No | 112 (49.1%) | 34 (44.7%) | 34 (44.7%) | 44 (57.9%) | 45 (69.2%) | 12 (63.2%) | 15 (65.2%) | 18 (78.3%) |
| Yes | 116 (50.9%) | 42 (55.3%) | 42 (55.3%) | 32 (42.1%) | 20 (30.8%) | 7 (36.8%) | 8 (34.8%) | 5 (21.7%) |
| Day 28 | ||||||||
| Missing | 4 (1.8%) | 2 (2.6%) | 2 (2.6%) | — | — | — | — | — |
| No | 78 (34.2%) | 29 (38.2%) | 27 (35.5%) | 22 (28.9%) | 26 (40.0%) | 7 (36.8%) | 10 (43.5%) | 9 (39.1%) |
| Yes | 146 (64.0%) | 45 (59.2%) | 47 (61.8%) | 54 (71.1%) | 39 (60.0%) | 12 (63.2%) | 13 (56.5%) | 14 (60.9%) |
| Δ | ||||||||
| Missing | 4 (1.8%) | 2 (2.6%) | 2 (2.6%) | — | — | — | — | — |
| Improvement | 55 (24.1%) | 14 (18.4%) | 17 (22.4%) | 24 (31.6%) | 22 (33.8%) | 5 (26.3%) | 8 (34.8%) | 9 (39.1%) |
| No change | 146 (64.0%) | 50 (65.8%) | 46 (60.5%) | 50 (65.8%) | 40 (61.5%) | 14 (73.7%) | 12 (52.2%) | 14 (60.9%) |
| Worsening | 23 (10.1%) | 10 (13.2%) | 11 (14.5%) | 2 (2.6%) | 3 (4.6%) | — | 3 (13.0%) | — |
BMF≤3/week, participants from the ITT population who had fewer than or equal to 3 bowel movements per week during the entire 14-day run-in period; high-dose group, 1 × 1010 CFU of HN019/day; ITT, intention-to-treat population—all participants randomized at the second visit who consumed at least 1 dose of the study product; low-dose group 1 × 109 CFU of HN019/day. ‘-‘, no value. Values are number of participants (%).
In the ITT population, 228 participants were analyzed for harms during the run-in period as NTEAEs and during the intervention period as TEAEs.
Colonic transit time
All participants consumed 100% of radio-opaque markers, so no adjustments were needed in the CTT calculations.21 There were no significant differences in CTT between treatments in the ITT or BMF ≤ 3/week population (see Table 2). The only notable finding in both populations was a significant difference in CTT between sexes (P value<0.05)—females had higher CTT values—and baseline values (P value < 0.01)—overall, in cases of a reduction, those who had higher versus lower baseline CTTs experienced a greater reduction. Supplemental Tables S1.1 and S1.2 summarize the statistics on CTT.
PAC-SYM, PAC-QoL, and BFI
The results for the PAC-SYM, PAC-QoL, and BFI, measured on Days 0 (baseline) and 28 (end of study), are presented in Table 2. The statistics for PAC-SYM are presented in Tables S2.1 and S2.2, Tables S3.1 and S3.2 for PAC-QoL, and Tables S4.1 and S4.2 for BFI.
In the ITT population, PAC-SYM scores were homogeneous between treatment groups, except for the Rectal Symptoms Score between the high-dose HN019 and placebo groups, wherein placebo changes after the intervention were higher (P value = 0.04). There were no significant changes in PAC-QoL or BFI scores between treatments.
In the ITT population, PAC-SYM, PAC-QoL and BFI scores differed significantly from baseline over time (P value < 0.01), indicating a placebo effect in all treatments.
In the ITT population, PAC-SYM scores differed significantly between sexes at baseline for the Abdominal Symptoms Score (P value < 0.01)—females had higher scores—as did overall scores (P value = 0.02).
In the BMF ≤ 3/week population, PAC-SYM, PAC-QoL, and BFI scores were homogeneous between treatment groups. In this subgroup, PAC-SYM, PAC-QoL, and BFI scores differed significantly from their baselines values in all treatments (P value ≤ 0.01), reflecting a strong placebo effect in all treatments.
In the BMF ≤ 3/week population, PAC-SYM scores differed significantly between sexes at baseline for the Abdominal Symptoms Score (P value ≤ 0.01)—females had higher scores. Rectal Symptoms Scores also differed by sex on Day 28 (P value = 0.01), for which males had higher scores.
Outcomes measured with participants' diaries
The results for BMF, BSS, degree of straining, self-assessment of complete bowel emptying, and sensations of abdominal pain and bloating, as measured by the participants' diaries, are presented in Table 3. The statistics for BMF are listed in Tables S5.1 and S5.2, and the RMANOVA values for BMF are shown in Tables S6.1 to S6.5. The statistics for BSS are shown in Tables S7.1 and S7.2, for degree of straining in Tables S8.1 and S8.2, for complete bowel emptying in Tables S9.1 and S9.2, for abdominal pain statistics in Tables S10.1 and S10.2, and for bloating in Tables S11.1 and S11.2.
No laxatives were taken by the participants during the intervention; therefore, all bowel movements were spontaneous.
In the ITT population, there were no significant differences in the parameters that were measured with the participants' diaries after the intervention. Over time, the diary-based parameters differed significantly from their baseline values (P value<0.01), indicating a placebo effect in all treatments.
Regarding differences by sex in the ITT population, females had higher bloating scores at Day 0 (baseline) (P value < 0.05) and Day 28 (end of study) (P value = 0.02).
In the BMF ≤ 3/week population, there were also no significant changes in the diary-based parameters. These parameters also differed significantly from baseline (P value < 0.01), suggesting a placebo effect for all treatments.
In the BMF ≤ 3/week population, by RMANOVA, there was a significant effect in BMF (differing on at least 1 BM per day). No other differences between product groups were observed by Tukey's adjustment, despite the global effect being significant (P value = 0.04). RMANOVA was also performed in the BMF ≤ 3/week population with the high- and low-dose HN019 groups pooled, wherein the values changed significantly for the active versus placebo groups (P value = 0.01). Fig. 3 shows the change in BMF under the 3 treatments during the 28-day intervention in the BMF ≤ 3/week population.
Figure 3.
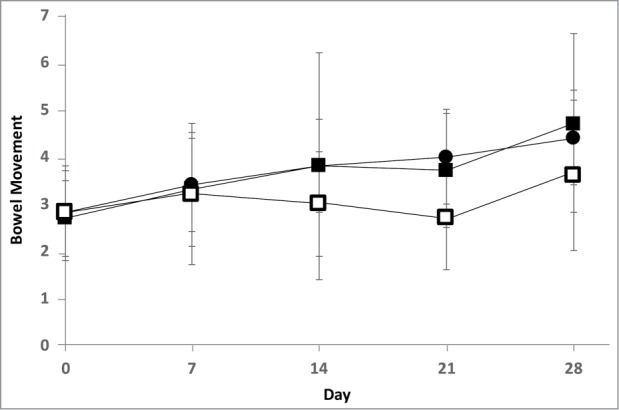
Bowel movement frequency during the 28-day intervention in the BMF≤3/week population—participants from the ITT population who had fewer than or equal to 3 bowel movements per week during the entire 2-week run-in period. ▪, high-dose group (1 × 1010 CFU of HN019/day, n = 19); •, low-dose group (1 × 109 CFU of HN019/day, n = 23); and □, placebo group (n = 23). Values are mean ± SD. By RMANOVA, on pooling the high- and low-dose groups—the active groups—there was significant improvement in bowel movement frequency compared with the placebo group (P value = 0.01).
In the BMF ≤ 3/week population, BSS was significantly elevated in the high-dose versus low-dose HN019 group (P value = 0.03) on Day 0 (baseline). Degree of straining improved in the high-dose HN019 compared with placebo group at Day 28 (end of study) (P value = 0.02).
In the BMF ≤ 3/week population, males had higher scores for complete bowel emptying at Day 0 (baseline) (P value < 0.01) wherein, and females had higher bloating scores at Day 0 (baseline) (P value < 0.05).
Adequate relief of constipation
The only significant difference in adequate relief of constipation was observed between the high-dose HN019 and placebo groups in the ITT population (P value = 0.05), the latter of which experienced a greater change from Day 0 to 28. The results on adequate relief of constipation are presented in Table 4, and the statistics are shown in Table S12.
Product satisfaction
There was no difference in overall product satisfaction between treatments in the ITT or BMF ≤ 3/week population at Day 28. The results and statistics on overall product satisfaction are presented in Table 5 and Table S13, respectively.
Table 5.
Overall product satisfaction at Day 28 (end of study).
| ITT |
BMF≤3/week |
||||||||
| |
Total |
High dose |
Low dose |
Placebo |
Total |
High dose |
Low dose |
Placebo |
|
| Not at all satisfied | 38 (16.7%) | 13 (17.1%) | 13 (17.1%) | 12 (15.8%) | 13 (20.0%) | 5 (26.3%) | 3 (13.0%) | 5 (21.7%) | |
| A little satisfied | 39 (17.1%) | 12 (15.8%) | 15 (19.7%) | 12 (15.8%) | 13 (20.0%) | 3 (15.8%) | 6 (26.1%) | 4 (17.4%) | |
| Moderately satisfied | 59 (25.9%) | 16 (21.1%) | 21 (27.6%) | 22 (28.9%) | 16 (24.6%) | 2 (10.5%) | 7 (30.4%) | 7 (30.4%) | |
| Quite satisfied | 60 (26.3%) | 24 (31.6%) | 14 (18.4%) | 22 (28.9%) | 19 (29.2%) | 8 (42.1%) | 4 (17.4%) | 7 (30.4%) | |
| Very satisfied | 28 (12.3%) | 9 (11.8%) | 11 (14.5%) | 8 (10.5%) | 4 (6.2%) | 1 (5.3%) | 3 (13.0%) | — | |
BMF≤3/week, participants from the ITT population who had fewer than or equal to 3 bowel movements per week during the entire 14-day run-in period; high-dose group, 1 × 1010 CFU of HN019/day; ITT, intention-to-treat population—all participants randomized at the second visit who consumed at least 1 dose of the study product; low-dose group 1 × 109 CFU of HN019/day. ‘-‘, no value. Values are number of participants (%).
Ancillary analysis
IPAQ scores were similar in the high- and low-dose HN019 groups compared with placebo (Tables S14.1. and S14.2). The only difference was observed at Day 0 between the high- and low-dose HN019 groups (P value = 0.05), the former of which had more participants with moderate physical activity.
No differences in components of the 24-h food recall index (in Table S15) or in body weight or BMI (Table S16) appeared between treatments.
Harms
There were no differences in harms between treatments. In total, 140 AEs were recorded for 90 subjects, 66 of which were TEAEs—24 in the high-dose HN019 group, 16 in the low-dose HN019 group, and 26 in the placebo group—and 74 were NTEAEs. Of the 66 TEAEs, 3 were judged to be unlikely related—nausea and vomiting in the same low-dose HN019 subject and abdominal pain in 1 placebo subject—and 63 were unrelated to the study products. Two unexpected emergent SAEs were reported during the intervention—1 in the high-dose HN019 group (appendicitis) and 1 in the low-dose HN019 group (pyelonephritis)—but they were deemed to be irrelevant to the study products.
All AEs and SAEs were followed until full recovery by the participants. No deaths occurred during the study. The AEs that arose during the intervention period (TEAEs) are summarized in Table 6, and the AEs and SAEs are listed in Tables S17.1 and S17.2, respectively.
Table 6.
Treatment-emergent adverse events (TEAEs) registered in the study.
| Overall (n=228) |
High product dose (n=76) |
Low product dose (n=76) |
Low product dose (n=76) |
||||||||||
| |
N |
P |
% |
N |
P |
% |
N |
P |
% |
N |
P |
% |
|
| Relationship to study product | Unlikely | 3 | 2 | 0.9 | 0 | 0 | 0 | 2 | 1 | 1.3 | 1 | 1 | 1.3 |
| Unrelated | 63 | 49 | 21.5 | 24 | 19 | 25.0 | 14 | 12 | 15.8 | 25 | 18 | 23.7 | |
| Maximum intensity | Mild | 1 | 1 | 0.4 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1.3 |
| Moderate | 57 | 43 | 18.9 | 21 | 17 | 22.4 | 15 | 11 | 14.5 | 21 | 15 | 19.7 | |
| Severe | 8 | 7 | 3.1 | 3 | 2 | 2.6 | 1 | 1 | 1.3 | 4 | 4 | 5.3 | |
High-dose group, 1 × 1010 CFU of HN019/day; low-dose group 1 × 109 CFU of HN019/day; n; number of participants by product or overall; N, number of TEAEs; P, number of participants with at least 1 TEAE; %, incidence (P/n)*100.
Discussion
We have investigated the effects of a probiotic at 2 dosages of HN019—1 × 109 and 1 × 1010 CFU/day—in French adults who were diagnosed with functional constipation according to Rome III criteria. There were no differences in the primary or secondary outcomes after the intervention, based on the primary analysis. In a post hoc analysis, HN019 improved bowel movement frequency in adults with fewer than 3 stools per week.
We included middle-aged (41.7 years) and normal-weight and overweight (24.4 kg/m2) male (20%) and female (80%) participants; challenges in the recruitment prompted 2 protocol amendments to redefine the sex ratios. Per Rome III, more than 90% of the enrolled population was diagnosed with straining, lumpy, and hard stools, and a sensation of incomplete bowel movement existed for at least 25% of defecations; 1 in 5 presented with anorectal obstructions, and approximately half declared fewer than 3 bowel movements per week. Therefore, we concluded that our cohort represents the typical profile of adults with functional constipation, based on the Rome III criteria, in the French region of Auvergne-Rhône-Alpes and that our results are valid for this population.
We did not observe any significant differences in CTT, the primary outcome, in the ITT or BMF ≤ 3/week population. Overall, males had shorter CTTs than females, as expected.26 The CTT of the entire population at baseline (64 h) is consistent with previous studies that enrolled participants per Rome III criteria.27-29 Waller et al. reported significant reductions in CTT that were induced by HN019 dose-dependently (decreases of 28.1 h and 18.5 h with 1 × 1010 and 1 × 109 CFU of HN019/day, respectively) compared with placebo (a rise of 1.3 h) after 14 days—but their baseline CTT values were lower (49.2 h, 59.5 h, and 42.5 h for the high-dose HN019, low-dose HN019, and placebo groups, respectively) versus our study.15 The population in Waller et al. comprised middle-aged (∼44 years) and overweight and obese (∼31 kg/m2) adults—over 70% of them were African-Americas and Hispanics—from Houston, TX.15 We can not explain why we could not replicate these declines in CTT15 but highlight the differences in our protocol design with regard to length of treatment, baseline CTT values, and population characteristics.
With regard to the other parameters at Days 0 and 28—PAC-SYM, PAC-QoL, and BFI—all treatments, including placebo, relieved constipation to some degree according to these scores, improving most extensively in the BMF ≤ 3/week population; however, in all cases except for 1 score, the active groups differed from the placebo. Overall, baseline PAC-SYM scores were lower compared with other studies that enrolled participants according to Rome III criteria—ie, our population was less constipated.30-32 The only significant effect was a reduction in the Rectal Symptoms Score, which the placebo decreased significantly compared with high-dose HN019; however, this change might have been an artifact, because with high-dose HN019, the Rectal Symptoms Score did not change over time. The lower constipation in our population was confirmed by the finding that other studies that enrolled participants per Rome III criteria had higher baseline PAC-QoL scores32-35 and BFI,33 which might be why we did not observe any differences in these parameters.
All bowel movements that were recorded in our study were spontaneous—no laxatives were taken by the participants during the intervention. In addition, we determined whether the bowel movements were complete, but because there were no significant differences in completeness of BM between treatments, we chose to report the results as total BM to avoid excluding any data.
BMF and BSS were self-assessed by participants using diaries. In a recent meta-analysis by our group—after finalization of the present study—we found that the random effects of weekly BMF and BSS according to Rome III criteria for functional constipation were 2.7 [95% CI: 2.4-3.0] and 2.4 [95% CI: 2.1-2.6], respectively.25 In our study, the baseline weekly BMF (4.6) and BSS values (3.3) were above the confidence intervals in the meta-analysis, supporting that our population was less constipated than expected. We anticipated an improvement in BMF, because non-constipated healthy adults have an average of 7.7 bowel movements per week.36 However, we expected little improvement in BSS, given that non-constipated healthy adults report a weekly stool score of 3.6,36 close to the baseline BSS in our study.
Consequently, we conducted a post hoc analysis with a cutoff of 3 bowel movements per week, yielding a weekly baseline BMF of 2.8 in the BMF ≤ 3/week population; by RMANOVA, the pooled HN019 groups improved their weekly BMF significantly compared with placebo after the 28-day intervention. But, this subpopulation (BMF ≤ 3/week) was not sufficiently powered to detect changes in weekly BMF or other parameters, assessed according to the study protocol, with any HN019 dose against placebo. Nevertheless, considering the baseline BMF levels in the BMF ≤ 3/week subpopulation, the rise in weekly BMF in the high- (+2.0) and low-dose HN019 groups (+1.7) is meaningful and physiological relevant.2,37 As a reference, the use of laxatives and fiber can lead to an overall increase of approximately 1.4 bowel movements per week [95% CI: 1.1-1.8], which might be considered to be clinically significant.2
Further, an increase in stool frequency without causing diarrhea in the general population is physiologically relevant, according to the European Food Safety Authority (EFSA), within the scope of “maintaining normal defecation.”38 For example, a recent study indicated that inulin enhances stool frequency by 1 BMF/week,39 which might have prompted this health claim approval.
With regard to the other parameters that were recorded in the diaries, all treatments, including placebo, relieved constipation. Only degree of straining differed significantly, declining with high-dose HN019 versus placebo at Day 28 in the BMF ≤ 3/week subpopulation; but, there were no differences in the changes from baseline to the end of the study between these groups. There are conflicting data on the typical baseline straining values for participants who are enrolled per Rome III criteria for functional constipation: studies have reported higher,30,40,41 similar,42,43 and lower44 levels compared with us. However, our population was less constipated, according to the complete bowel emptying, abdominal pain, and bloating scores. Studies that included subjects according to Rome III criteria for functional constipation have had lower baseline values for complete bowel emptying34,41,42,45-47 and higher baseline scores for abdominal pain30,35,41,46,48 and bloating30,41,48 versus our trial.
All participants experienced the same placebo effect with regard to adequate relief from constipation after the intervention period; more participants improved versus worsened, although the placebo was the only group that improved this parameter significantly over baseline in the ITT population. Similarly, the overall results for product satisfaction at the end of the study showed that all treatments elicited similar subjective responses, including placebo, with which over 80% of participants were moderately, quite, and very satisfied.
Generally, all treatments, including placebo, were well tolerated and did not induce changes in physical activity, food intake, or body weight. The number of AEs that were recorded was typical of a well-controlled GCP study of these characteristics. The 3 unlikely related TEAEs—2 in the same low-dose HN019 same participant and 1 in the placebo group—and the 2 unrelated SAEs—1 each in the high- and low-dose HN019 groups—were monitored until a full recovery was made. Thus, our findings do not raise any concerns over the safety of HN019.
The Rome III classification system for functional constipation was issued with the expectation that it would become the standard for clinical practice and research.3 However, its accuracy as a clinical tool has been debated, due to its inadequacy in properly differentiating certain disorders—eg, between functional constipation and irritable bowel syndrome49—and its lack of sensitivity in detecting meaningful changes in response to treatment strategies over time.8 In our study, we noted that Rome III created several subgroups, based on the requirement that participants fulfill at least 2 of the 6 criteria. For example, a standard combination analysis—in which 6 criteria (n) choose 2 criteria (k)—shows that 15 subgroups (or subpopulations) are possible.50 Therefore, the result is a mixture of several subpopulations, collectively defined as ‘functional constipation’ per Rome III, creating a lack of homogeneity that could have significant disadvantages in clinical research.
Further, a diagnosis per Rome III without the support of additional measures to narrow the symptoms during enrollment can lead to substantial deviations in the diagnosis—eg, without information from the participants' diaries. For example, at baseline, 55.7% (127 participants) of the ITT population was diagnosed with fewer than 3 bowel movements per week, whereas based on the participants' journals, this subgroup (BMF ≤ 3/week) represented 28.5% (65 participants); this subgroup conflicted with its Rome III diagnosis for this criterion, which resulted in 64.6% of participants instead of the expected 100% rate per the diaries.
Similar conclusions can be drawn by comparing other items from these diaries with the parameters that are assessed with Rome III, such as degree of straining, number of hard stools, and the sensation of incomplete bowel movements. We noted these inconsistencies after the end of the study, when the diaries were analyzed. For this reason, we included a post hoc analysis of the BMF ≤ 3/week population that was more homogeneous and sensitive to HN019 with regard to BMF. Based on the results of this study, we hypothesize that the effects of HN019 on BMF are greater in those with low BMF. The hypothesis that meaningful improvements in BMF occur in those with fewer than 3 BMs/week remains to be confirmed in well-powered interventions.
In conclusion, there were no differences in the primary or secondary outcomes after the intervention, according to the primary analysis. A post hoc analysis demonstrated that restricting the population to participants who reported fewer than 3 bowel movements per week during the run-in period allowed us to detect physiologically meaningful improvements in weekly BMF after the intervention period, on pooling the HN019 groups versus the placebo group. All treatments, including the placebo, were well tolerated. Future studies on the effects of HN019 should target improvements in BMF in participants with low stool frequency (eg, the BMF ≤ 3/week population).
Supplementary Material
Abbreviations
- AEs
adverse events
- BFI
Bowel Function Index
- BMF
Bowel Movement Frequency
- BMF ≤ 3/week
participants from the ITT population who had less than or equal to 3 bowel movements per week during the entire 14-day run-in period
- BSS
Bristol Stool Scale
- CTT
Colonic Transit Time
- GI
gastrointestinal
- EFSA
European Food Safety Authority
- HN019
Bifidobacterium animalis subsp. lactis HN019
- IPAQ
International Physical Activity Questionnaire
- NTEAEs
non-treatment-emergent AEs
- PAC-QoL
Patient Assessment of Constipation Quality of Life
- PAC-SYM
Patient Assessment of Constipation Symptoms
- RMANOVA
repeated measures ANOVA
- TEAEs
treatment-emergent AEs.
Conflicts of interest
Alvin Ibarra and Arthur C. Ouwehand were employees of E.I. DuPont de Nemours & Co., the manufacturer of Bifidobacterium animalis subsp. lactis HN019, during the study. Mathilde Latreille-Barbier, Yves Donazzolo, and Xavier Pelletier were employees of Eurofins Optimed SAS, a company that was hired by E.I. DuPont de Nemours & Co. to conduct the study. The authors declare no other conflicts of interest.
Acknowledgments
The authors participated in the preparation and revision of the manuscript and gratefully acknowledge help from the personnel of E.I. DuPont de Nemours & Co. and Eurofins Optimed SAS. Special thanks to Tad Stuart, Angela Paulsen, Myriam Mouhib, and Laure Anne Giannone. The study team acknowledges the high adherence of the participants to the study protocol.
Sources of support
This study was fully sponsored by E.I. DuPont de Nemours & Co.
Ethical approval
The protocol and informed consent forms were approved by Comité de Protection des Personnes Sud-Est III (Lyon, FR).
Clinical trial registry
ClinicalTrials.gov, NCT02189707; French Health Authorities, ID-RCB 2014-A00166-41.
References
- 1.Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: Systematic review and meta-analysis. American journal of gastroenterology. 2011;106:1582–91. doi: 10.1038/ajg.2011.164. [DOI] [PubMed] [Google Scholar]
- 2.Tramonte SM, Brand MB, Mulrow CD, Amato MG, O'Keefe ME, Ramirez G. The treatment of chronic constipation in adults. Journal of General Internal Medicine. 1997;12:15–24. doi: 10.1007/s11606-006-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. Journal of Gastrointestinal and Liver Diseases. 2006;15:237. [PubMed] [Google Scholar]
- 4.Drossman DA. The functional gastrointestinal disorders and the Rome III process. gastroenterology. 2006;130:1377–90. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Bouchoucha M, Devroede G, Faye A, Le Toumelin P Arhan P, Arsac M. Colonic response to food in constipation. International journal of colorectal disease. 2006;21:826–33. doi: 10.1007/s00384-005-0787-5. [DOI] [PubMed] [Google Scholar]
- 6.Riegler G, Esposito I. Bristol scale stool form. A still valid help in medical practice and clinical research. Techniques in coloproctology. 2001;5:163–4. doi: 10.1007/s101510100019. [DOI] [PubMed] [Google Scholar]
- 7.Frank L, Kleinman L, Farup C, Taylor L, Miner P. Psychometric validation of a constipation symptom assessment questionnaire. Scandinavian journal of gastroenterology. 1999;34:870–7. doi: 10.1080/003655299750025327. [DOI] [PubMed] [Google Scholar]
- 8.Marquis P, De La Loge C Dubois D, McDermott A, Chassany O. Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scandinavian journal of gastroenterology. 2005;40:540–51. doi: 10.1080/00365520510012208. [DOI] [PubMed] [Google Scholar]
- 9.Rentz A, Van Hanswijck De Jonge P Leyendecker P, Hopp M. Observational, nonintervention, multicenter study for validation of the Bowel Function Index for constipation in European countries. Current medical research and opinion. 2011;27:35–44. doi: 10.1185/03007995.2010.535270. [DOI] [PubMed] [Google Scholar]
- 10.Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, Soffer EE, Spiegel BM, Moayyedi P. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: Systematic review and meta-analysis. American journal of gastroenterology. 2014;109:1547–61. doi: 10.1038/ajg.2014.202. [DOI] [PubMed] [Google Scholar]
- 11.Miller LE, Zimmermann AK, Ouwehand AC. Contemporary meta-analysis of short-term probiotic consumption on gastrointestinal transit. World journal of gastroenterology. 2016;22:5122. doi: 10.3748/wjg.v22.i21.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goossens D, Jonkers D, Stobberingh E, Avd Bogaard, Russel M, Stockbrugger R. Probiotics in gastroenterology: Indications and future perspectives. Scandinavian Journal of Gastroenterology-Supplements. 2003;38:15–6. doi: 10.1080/00855920310002645. [DOI] [PubMed] [Google Scholar]
- 13.Ouwehand AC, Lagström H, Suomalainen T, Salminen S. Effect of probiotics on constipation, fecal azoreductase activity and fecal mucin content in the elderly. Annals of nutrition and metabolism. 2002;46:159–62. doi: 10.1159/000063075. [DOI] [PubMed] [Google Scholar]
- 14.Sanders ME. Summary of probiotic activities of Bifidobacterium lactis HN019. Journal of clinical gastroenterology. 2006;40:776–83. doi: 10.1097/01.mcg.0000225576.73385.f0. [DOI] [PubMed] [Google Scholar]
- 15.Waller PA, Gopal PK, Leyer GJ, Ouwehand AC, Reifer C, Stewart ME, Miller LE. Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scandinavian journal of gastroenterology. 2011;46:1057–64. doi: 10.3109/00365521.2011.584895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalziel JE, Anderson RC, Peters JS, Lynch AT, Spencer NJ, Dekker J, Roy NC. Promotility Action of the Probiotic Bifidobacterium lactis HN019 Extract Compared with Prucalopride in Isolated Rat Large Intestine. Frontiers in neuroscience. 2017;11. doi: 10.3389/fnins.2017.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Association WM World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. Jama. 2013;310:2191. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 18.International Conference on Harmonisation Working Group ICH harmonised tripartite guideline: Guideline for good clinical practice E6 (R1). International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. 1996;10:1–53. [Google Scholar]
- 19.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC medicine. 2010;8:1. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ioannidis JP, Evans SJ, Gøtzsche PC, O'neill RT, Altman DG, Schulz K, Moher D. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of internal medicine. 2004;141:781–8. doi: 10.7326/0003-4819-141-10-200411160-00009. [DOI] [PubMed] [Google Scholar]
- 21.Ibarra A, Olli K, Ouwehand AC. Correcting for non-compliance when determining colonic transit time with radio-opaque markers. World Journal of Gastroenterology. 2017;23:740–2. doi: 10.3748/wjg.v23.i4.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagströmer M, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public health nutrition. 2006;9:755–62. doi: 10.1079/PHN2005898. [DOI] [PubMed] [Google Scholar]
- 23.Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Safety. 1999;20:109–17. doi: 10.2165/00002018-199920020-00002. [DOI] [PubMed] [Google Scholar]
- 24.WHO World Health Organization (WHO) Anatomical Therapeutic Chemical (ATC) Classification System. Guidelines for ATC Classification. 1993:23–185. [Google Scholar]
- 25.Miller LE, Ibarra A, Ouwehand AC, Zimmermann AK. Normative values for stool frequency and form using Rome III diagnostic criteria for functional constipation in adults: systematic review with meta-analysis. Annals of Gastroenterology. 2017;30:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meier R, Beglinger C, Dederding J, MEYER‐WYSS B, Fumagalli M, Rowedder A, Turberg Y, Brignoli R. Influence of age, gender, hormonal status and smoking habits on colonic transit time. Neurogastroenterology & Motility. 1995;7:235–8. doi: 10.1111/j.1365-2982.1995.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 27.Camilleri M, Thorne NK, Ringel Y, Hasler WL, Kuo B, Esfandyari T, Gupta A, Scott SM, McCallum RW, Parkman HP, et al.. Wireless pH‐motility capsule for colonic transit: prospective comparison with radiopaque markers in chronic constipation. Neurogastroenterology & Motility. 2010;22:874–e233. doi: 10.1111/j.1365-2982.2010.01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park SY, Park HB, Lee JM, Lee HJ, Park CH, Kim HS, Choi SK, Rew JS. Relevance of colonic gas analysis and transit study in patients with chronic constipation. Journal of neurogastroenterology and motility. 2015;21:433–9. doi: 10.5056/jnm14109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polymeros D, Beintaris I, Gaglia A, Karamanolis G, Papanikolaou IS, Dimitriadis G, Triantafyllou K. Partially hydrolyzed guar gum accelerates colonic transit time and improves symptoms in adults with chronic constipation. Digestive diseases and sciences. 2014;59:2207–14. doi: 10.1007/s10620-014-3135-1. [DOI] [PubMed] [Google Scholar]
- 30.Fateh R, Iravani S, Frootan M, Rasouli M, Saadat S. Synbiotic preparation in men suffering from functional constipation: a randomised controlled trial. Swiss medical weekly. 2010;141:w13239–w. [DOI] [PubMed] [Google Scholar]
- 31.Neri L, Conway PM, Basilisco G. Confirmatory factor analysis of the Patient Assessment of Constipation-Symptoms (PAC-SYM) among patients with chronic constipation. Quality of Life Research. 2015;24:1597–605. doi: 10.1007/s11136-014-0886-2. [DOI] [PubMed] [Google Scholar]
- 32.Yiannakou Y, Piessevaux H, Bouchoucha M, Schiefke I, Filip R, Gabalec L, Dina I, Stephenson D, Kerstens R, Etherson K, et al.. A randomized, double-blind, placebo-controlled, phase 3 trial to evaluate the efficacy, safety, and tolerability of prucalopride in men with chronic constipation. American journal of gastroenterology. 2015;110:741–8. doi: 10.1038/ajg.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbott R, Ayres I, Hui E, Hui K-K. Effect of perineal self-acupressure on constipation: A randomized controlled trial. Journal of general internal medicine. 2015;30:434–9. doi: 10.1007/s11606-014-3084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Da N, Wang X, Liu H, Xu X, Jin X, Chen C, Zhu D, Bai J, Zhang X, Zou Y, et al.. The Effectiveness of Electroacupuncture for Functional Constipation: A Randomized, Controlled, Clinical Trial. Evidence-Based Complementary and Alternative Medicine. 2015;2015:1–5. doi: 10.1155/2015/670963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dupont C, Campagne A, Constant F. Efficacy and safety of a magnesium sulfate–rich natural mineral water for patients with functional constipation. Clinical Gastroenterology and Hepatology. 2014;12:1280–7. doi: 10.1016/j.cgh.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Saad RJ, Rao SS, Koch KL, Kuo B, Parkman HP, McCallum RW. Do Stool Form and Frequency Correlate With Whole-Gut and Colonic Transit? Results From a Multicenter Study in Constipated Individuals and Healthy Controls. American journal of gastroenterology. 2010;105:403–11. doi: 10.1038/ajg.2009.612. [DOI] [PubMed] [Google Scholar]
- 37.Heaton K, Radvan J, Cripps H, Mountford R, Braddon F, Hughes A. Defecation frequency and timing, and stool form in the general population: A prospective study. Gut. 1992;33:818–24. doi: 10.1136/gut.33.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.EFSA Panel on Dietetic Products Nutrition and Allergies (NDA) Scientific Opinion on the substantiation of a health claim related to “native chicory inulin” and maintenance of normal defecation by increasing stool frequency pursuant to Article 13.5 of Regulation (EC) No 1924/2006. EFSA Journal. 2015;13(1):3951–63. doi: 10.2903/j.efsa.2015.3951 2015. [DOI] [Google Scholar]
- 39.Micka A, Siepelmeyer A, Holz A, Theis S, Schön C. Effect of consumption of chicory inulin on bowel function in healthy subjects with constipation: A randomized, double-blind, placebo-controlled trial. International journal of food sciences and nutrition. 2017;68:82–9. doi: 10.1080/09637486.2016.1212819. [DOI] [PubMed] [Google Scholar]
- 40.de Gouveia Santos VLC, de Cássia Domansky R, Hanate C, Matos DS, Benvenuto CVC, Jorge JMN. Self-reported fecal incontinence in a community-dwelling, urban population in Southern Brazil. Journal of Wound Ostomy & Continence Nursing. 2014;41:77–83. doi: 10.1097/01.WON.0000438018.83110.88. [DOI] [PubMed] [Google Scholar]
- 41.Cheng CW, Bian ZX, Zhu LX, Wu JC, Sung JJ. Efficacy of a Chinese herbal proprietary medicine (Hemp Seed Pill) for functional constipation. American journal of gastroenterology. 2011;106:120–9. doi: 10.1038/ajg.2010.305. [DOI] [PubMed] [Google Scholar]
- 42.Kamm MA, Mueller–Lissner S, Wald A, Richter E, Swallow R, Gessner U. Oral bisacodyl is effective and well-tolerated in patients with chronic constipation. Clinical Gastroenterology and Hepatology. 2011;9:577–83. doi: 10.1016/j.cgh.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 43.Kang D-W, DiBaise JK, Ilhan ZE, Crowell MD, Rideout JR, Caporaso JG, Rittmann BE, Krajmalnik-Brown R. Gut microbial and short-chain fatty acid profiles in adults with chronic constipation before and after treatment with lubiprostone. Anaerobe. 2015;33:33–41. doi: 10.1016/j.anaerobe.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Yeun Y, Lee J. Effect of a double-coated probiotic formulation on functional constipation in the elderly: A randomized, double blind, controlled study. Archives of pharmacal research. 2015;38:1345–50. doi: 10.1007/s12272-014-0522-2. [DOI] [PubMed] [Google Scholar]
- 45.Bian Z, Cheng C, Zhu L. Chinese herbal medicine for functional constipation: A randomised controlled trial. Hong Kong medical journal = Xianggang yi xue za zhi/Hong Kong Academy of Medicine. 2013;19:44–6. [PubMed] [Google Scholar]
- 46.Bothe G, Coh A, Auinger A. Efficacy and safety of a natural mineral water rich in magnesium and sulphate for bowel function: A double-blind, randomized, placebo-controlled study. European Journal of Nutrition. 2015:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mueller-Lissner S, Kamm MA, Wald A, Hinkel U, Koehler U, Richter E, Bubeck J. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of sodium picosulfate in patients with chronic constipation. American journal of gastroenterology. 2010;105:897–903. doi: 10.1038/ajg.2010.41. [DOI] [PubMed] [Google Scholar]
- 48.Waitzberg DL, Logullo LC, Bittencourt AF, Torrinhas RS, Shiroma GM, Paulino NP, Teixeira-da-Silva ML. Effect of synbiotic in constipated adult women–a randomized, double-blind, placebo-controlled study of clinical response. Clinical nutrition. 2013;32:27–33. doi: 10.1016/j.clnu.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 49.Wong RK, Palsson OS, Turner MJ, Levy RL, Feld AD, Von Korff M Whitehead WE. Inability of the Rome III criteria to distinguish functional constipation from constipation-subtype irritable bowel syndrome. American journal of gastroenterology. 2010;105:2228–34. doi: 10.1038/ajg.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benjamin AT, Quinn JJ. Proofs that really count: The art of combinatorial proof. The Mathematical Association of America. 2003; 1:1–169. ISBN: 0-88385-333-7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


