Abstract
Preclinical studies indicate that lorcaserin (Belviq®), an FDA-approved serotonin 2C receptor agonist, may be a promising medication for the treatment of stimulant addiction. However, few studies have investigated its effects on self-administration of drugs of abuse from other pharmacological classes, including opioids. Here, adult male rhesus macaques (N=3) responded under a fixed ratio 30: time-out 60-s schedule of food (1-g banana-flavored pellets) and IV heroin delivery. Following stable self-administration of a range of heroin doses (0.32 – 32 μg/kg/inj, IV), the effects of acute pretreatment with lorcaserin (0.32 – 1.0 mg/kg, IM) on heroin- and food-maintained responding was determined. The ability of repeated treatment with lorcaserin (1.0 mg/kg) to produce sustained decreases in heroin and/or food intake and reinforcing strength were then analyzed using behavioral economic demand procedures. Results show that self-administration of intravenous heroin was dose-dependent, with peak responding maintained, on average, by the unit dose of 3.2 μg/kg/inj. Lorcaserin pretreatment produced a dose-dependent flattening of the dose-response function for heroin self-administration in each subject. On the other hand, lorcaserin did not decrease food-maintained responding. Repeated lorcaserin treatment reduced heroin intake by selectively decreasing its reinforcing strength, as evidenced by a leftward shift in the demand curves for heroin and the absence of comparable changes in the reinforcing strength of food. These results indicate that serotonin 2C receptor agonists, such as lorcaserin, have behaviorally selective effects on IV self-administration behavior, and deserve further consideration as candidates for the management of opioid use disorder.
Keywords: lorcaserin, opioid, heroin, serotonin, 5-hydroxytryptamine (5-HT), self-administration
1. Introduction
Epidemic levels of opioid use and abuse over the past two decades have resulted in a dramatic increase in overdose deaths and addiction in the United States (Volkow, 2017). While some opioid-based medications (e.g., methadone, buprenorphine) are available for managing opioid use disorder (OUD), concerns regarding their diversion and safety, and/or the stigma and complexity of treating OUD with other opioids has resulted in their underutilization by healthcare providers (Connery, 2015). Consequently, outcomes have been poor regarding treatment adoption of currently available medication options, and there is an urgent need for safe and effective non-opioid medication alternatives.
The serotonin 2C receptor subtype has emerged as a promising target in medication development efforts for drug addiction because of its well-documented role in modulating dopamine release in the mesolimbic system (Bubar et al, 2008; Howell and Cunningham, 2015). Studies in both rodents and nonhuman primates have demonstrated that cocaine- or amphetamine-induced elevations in extracellular dopamine levels in striatum can be decreased or further increased by, respectively, serotonin 2C receptor agonists (Manvich et al, 2012; Berro et al, 2017) and antagonists (Manvich et al, 2012; Zayara et al, 2011; Navailles et al, 2007; Filip and Cunningham, 2002). As well, we and others have reported that lorcaserin (Belviq®), an FDA-approved weight loss medication, like other serotonin 2C receptor agonists (e.g., Ro 60–0175), markedly reduces self-administration of stimulant drugs such as cocaine or amphetamines (Harvey-Lewis et al, 2016; Collins et al, 2016; Gerak et al, 2016; Gannon et al, 2018; though see Banks et al, 2017) and nicotine (Levin et al, 2011; Fletcher et al, 2012; Jacobs et al, 2017). Further, these effects occur at doses that do not appreciably alter other ongoing behaviors, suggesting that serotonin 2C receptor agonists, and in particular, lorcaserin, may be viable candidates as medication for the management of stimulant use disorder.
While the influence of activity at 5-HT2 receptor subtypes on stimulant-like behaviors has been well established, the role of 5-HT2 receptor subtype modulation of the abuse-related effects of opioids have received less attention. However, several published reports indicate that serotonin 2A and 2C receptors may modulate many of the behavioral effects of opioids (Zhang et al., 2016; Wu et al., 2015; Fletcher et al., 2006; Li et al, 2011; Li et al, 2013) including opioid self-administration (Maguire et al., 2012) and opioid-induced increases in extracellular dopamine levels (Willins and Meltzer, 1998). More recently, lorcaserin, which has agonist activity at both serotonin 2C and 2A receptor subtypes (7.5-times higher affinity for serotonin 2C vs. serotonin 2A; Thomsen et al, 2008) has been studied for its ability to alter oxycodone self-administration and reinstatement in rats. This study demonstrated that lorcaserin blunted the ability of a drug-paired cue light to elicit extinguished drug-seeking behavior and suppressed self-administration of a high dose of oxycodone (Neelakantan et al, 2017). Taken together, these data suggest that the serotonin 2C receptor system may be an important target for medications designed to lessen the abuse-related behavioral effects of opioid drugs of abuse. The present study was conducted to further examine the potential utility of lorcaserin as a candidate medication for substance abuse by determining how lorcaserin may alter heroin- and food-maintained responding in rhesus monkeys.
2. Materials and Methods
2.1. Animals
Three adult male rhesus macaques (Macacca mulatta) with histories of drug self-administration and prepared with intravenous (IV) catheters for drug delivery (surgical procedures described in Kohut et al, 2013) served as subjects in this study. Monkeys had continuous access to water and received sufficient High Protein Monkey Diet (Purina Mills International, Brentwood, MO) chow with fresh fruit and vegetables to maintain body weight in addition to 1-g banana-flavored food pellets (Formula 5TUR, Purina Mills Test Diet, Richmond, IN) during experimental sessions. A 12-h light-dark cycle was in effect (lights on 7:00 AM – 7:00 PM) except during experimental sessions (see below). Subjects lived in stainless-steel cages with side and front visual access to the colony room. A food cup attached outside the bottom left of the front side allowed access to response-contingent food pellets that could be delivered during experimental sessions from a pellet dispenser (Gerbrands Model G5210, Arlington, MA). The pellet dispenser was positioned above the cage next to a syringe pump that could be activated by programming software for IV injections (Model 981210, Harvard Apparatus, Inc., South Natick, MA). A custom-designed operant response panel containing three square translucent response keys arranged horizontally was attached to the front of the cage (Kohut et al, 2013). Each key could be transilluminated with stimulus lights differing in color (SuperBright LEDs; Fairchild Semiconductor, San Jose, CA). All experimental events were controlled by a desktop PC running Med Associates (Georgia, VT) software.
Animal care and research were conducted according to guidelines provided by the Institute of Laboratory Animal Resources and the National Institutes of Health (NIH) Office of Laboratory Animal Welfare. The facility is licensed by the U.S. Department of Agriculture and all experimental protocols were approved by the Institutional Animal Care and Use Committee at McLean Hospital. Enrichment was provided through access to mirrors and toys in the home-cage, music during non-experimental periods, and interactions with technical staff. Veterinary staff monitored the health of the colony on an ongoing basis.
2.2. Self-Administration Procedures
Each daily 2-h behavioral session consisted of three response components separated by 5-min time-out (TO) periods. During the first and last components (Food 1 and Food 2), red lights illuminating the center response key signaled the availability of 1-g banana-flavored food pellets for 5-min under a fixed ratio 30 (FR30): TO 10-s schedule of reinforcement. Animals could earn a maximum of 50 food pellets during the two food components of the daily experimental session. During the heroin self-administration component, green lights illuminating the center response key signaled the availability of intravenous (IV) injections of heroin or saline for 100-min under an FR30: TO 60-s schedule. This component was immediately preceded by illumination of a yellow light for 10-s and the non-contingent delivery of a single injection of saline or heroin at the dose that was subsequently available for IV self-administration. The number of drug injections per daily session was only limited by session duration and schedule parameters. During the TO following each reinforcer delivery (food or heroin), the center response key was illuminated with yellow stimulus lights. During inter-component TO periods, all lights were off and responding had no scheduled consequences.
During control sessions, saline (S) or a unit dose of heroin (H) was available for IV self-administration under a double alternation schedule (e.g, SS HH, SS, HH, etc.). Once responding under control conditions was stable, the heroin self-administration dose–effect function was determined over a dose range of 0.32 – 32 μg/kg/inj heroin. Responding was considered stable if the number of injections was within 15% of the mean of control sessions for that unit dose of IV heroin.
2.2.1. Effects of Lorcaserin on Heroin- and Food-maintained Responding
The effects of acute pretreatment with lorcaserin was studied in test sessions that were routinely conducted on the second day of the double alternation schedule, if control rates and patterns of self-administration behavior were evident in the previous session. Lorcaserin was administered 15-min prior to the onset of experimental sessions; doses were selected on the basis of previous studies to evaluate those that produced moderate (0.32 mg/kg, IM) and marked (1.0 mg/kg, IM) decreases in nicotine self-administration in rhesus monkeys (Jacobs et al, 2017). The effects of each dose of lorcaserin during sessions in which saline and a range of heroin doses (0.32 – 32 μg/kg/inj, IV) were available for IV self-administration were studied in an irregular order across subjects. The effects of each dose of lorcaserin across the range of heroin doses was determined once in each subject.
2.2.2. Behavioral Economic Analysis of Lorcaserin’s Effects on Heroin and Food Intake
Changes in the reinforcing strength of heroin and food during repeated treatment with lorcaserin were determined using behavioral economic demand analysis (Hursh and Silberberg, 2008). These studies were identical to acute pretreatment studies except that saline or 1.0 mg/kg, IM lorcaserin was administered daily 15-min prior to the start of each experimental session. The effects of repeated saline or lorcaserin administration was studied for at least 3 days under maintenance schedule requirements (FR30:TO60 s) to ensure stable levels of heroin- and food-maintained responding prior to the initiation of demand curves. Once stable, a behavioral economic demand protocol was utilized in which the response requirement for either food or drug was increased across successive sessions (e.g., FR 10, 32, 56, 100, … etc.) until subjects failed to earn a single reinforcer. Heroin availability remained unlimited during this procedure, but food-maintained responding components were limited to a 10-min response period and a maximum of 50 earned food pellets to limit the role of satiety on the assessment of reinforcing strength (Kohut and Bergman, 2016).
2.3. Data Analysis
The primary dependent variables were the total number of heroin or saline injections and food pellets earned in each session. Data for acute effects of lorcaserin at each dose of heroin was evaluated using two-way repeated measures analysis of variance (ANOVA). A significant ANOVA was followed by Dunnett’s multiple comparison test to determine whether a dose of lorcaserin significantly decreased heroin self-administration relative to control. Repeated measures one-way ANOVA with Dunnett’s multiple comparison tests were used to determine the significance of the effects of lorcaserin (0.32 and 1.0 mg/kg) on saline self-administration and food-maintained responding during Food 1 and Food 2 components of the session.
The demand analysis employed the exponential model of demand (Hursh and Silberberg, 2008; Hursh, 2014) and curves were generated according to the equation: logQ = logQ0 = k(e-αP− 1). Q0 (demand intensity) and α (demand elasticity) were free parameters and experimentally derived. Values used for the scaling parameter, k, were determined as the best fit for each dataset and were 2.6, 5.7, and 2.7 for, respectively, 3.2 and 10 μg/kg heroin, and banana-flavored food pellets. Comparisons of demand parameters between treatment (lorcaserin and saline) conditions were performed using an extra-sum-of-squares F test as has been used previously to compare medication effects on demand for drug reinforcers (c.f., McClure et al, 2012). All statistical analyses and figures were carried out using GraphPad Prism 6.0 for Macintosh (GraphPad Software Inc.).
2.4. Drugs
Lorcaserin hydrochloride ((R)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine) was obtained from Cayman Chemical Company (Ann Arbor, MI). Heroin hydrochloride was provided by the NIDA Drug Supply program. All drugs were dissolved in saline vehicle and doses are expressed in terms of base weight. Intravenous heroin was filtered to sterility with a syringe-driven 0.22-micron filter (Millipore Corporation, Billerica, MA).
3. Results
3.1. Baseline heroin- and food-maintained responding
Initially, control values were established for the self-administration of IV saline and several doses of IV heroin. When saline was available for self-administration, subjects generally responded for <20 injections/session. When heroin was available for self-administration, the heroin dose-effect curve described an inverted-U shaped function in all monkeys: the number of injections per session increased in a dose-dependent manner, with 3.2 μg/kg/inj heroin, on average, producing peak levels of responding (~46.5 inj/session) that declined as the unit dose increased further. Food-maintained responding was relatively consistent between the Food 1 and Food 2 session components under baseline conditions with subjects earning 14.8 (+/−5.43) and 16 (+/− 4.09) pellets in the respective components.
3.2. Effects of acute pretreatment with lorcaserin on heroin- and food-maintained responding
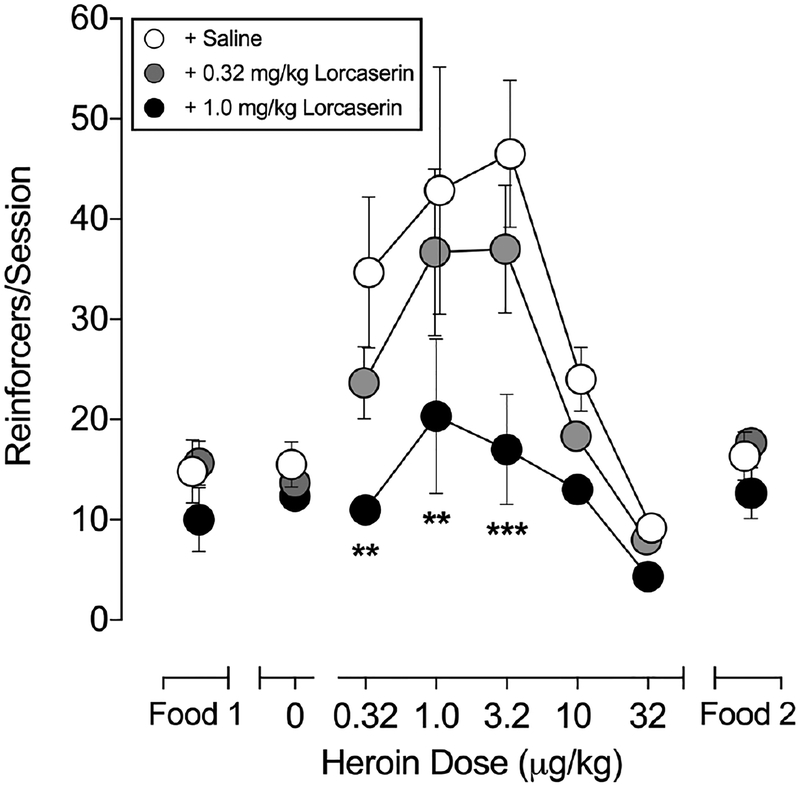
Doses of lorcaserin were then studied for their ability to alter IV self-administration across a 100-fold range of self-administered unit doses of heroin. Fig. 1 shows the effects of lorcaserin (0.32 and 1.0 mg/kg, IM) on responding maintained by a range of IV unit doses of heroin and food delivery during the Food 1 and Food 2 components. When averaged for the group, the lowest dose of lorcaserin (0.32 mg/kg, IM) produced a slight (~20% decrease from baseline), but non-significant, decrease in self-administration across the range of heroin doses tested (all P’s > 0.05). A higher dose of lorcaserin (1.0 mg/kg, IM) flattened the dose-response function for self-administered heroin (F(2,4)=7.369; P=0.046), reducing responding maintained by unit doses of 0.32–3.2 μg/kg to levels observed during saline availability (all P’s <0.004). Lorcaserin did not significantly decrease food-maintained responding during either the Food 1 or Food 2 session components (both F’s <0.9; P’s > 0.05).
Fig. 1.
Effects of acute treatment with lorcaserin (1.0 mg/kg) on heroin (0.32 – 32 μg/kg/inj)- and food (1 g banana-flavored pellet)-maintained responding. Abscissa, heroin dose in μg/kg/injection (log scale). Ordinate, total heroin injections earned per session or 1-g banana flavored food pellet deliveries per component. All data points represent mean ± S.E.M. of three subjects.
3.3. Effects of lorcaserin on the reinforcing strength of heroin and food
Demand curves in Fig. 2 show intake of 3.2 or 10 μg/kg heroin and 1-g banana flavored food pellets during repeated treatment with saline or 1.0 mg/kg lorcaserin averaged for the three subjects that were studied. Under the lowest response requirement (i.e., FR10), approximately 43 (+/− 16) and 22 (+/− 6) inj/session of, respectively, 3.2 and 10 μg/kg heroin were earned following saline pretreatment. As following acute pretreatment, repeated lorcaserin treatment decreased the number of heroin injections earned to 13.67 (+/− 5.24) at 3.2 μg/kg and 9.67 (+/− 2.96) at 10 μg/kg. Food-maintained responding was not similarly decreased; subjects earned 47 (+/− 2) pellets/session following saline pretreatment and 45 (+/− 3) pellets/session following 1.0 mg/kg lorcaserin treatment. In demand procedures, intake of both doses of heroin and food pellets decreased as price increased under both saline and lorcaserin treatment conditions. The exponential model of demand provided a good fit for all data sets (all R2s > 0.94): comparison of demand for heroin alone or in the presence of lorcaserin (see Table 1; Fig. 2) indicates that lorcaserin treatment significantly decreased the reinforcing strength of heroin as defined by increases in demand elasticity (i.e., α) of both heroin dose conditions. Further, as in acute pretreatment experiments, repeated lorcaserin treatment decreased demand intensity (Q0) when 3.2, but not 10, μg/kg heroin was the dose available for self-administration (Table 1). In contrast, lorcaserin treatment did not significantly alter the reinforcing strength of banana-flavored food pellets in the presence of repeated lorcaserin administration (see Table 1; Fig. 2).
Fig. 2.
Demand curves for IV heroin (3.2 μg/kg, left panel; 10 μg/kg, middle panel) and 1-g banana-flavored food pellets (right panel) alone (grey symbols) and during repeated administration of 1.0 mg/kg lorcaserin (open symbols). Each data point represents the mean number of heroin injections and food pellet reinforcers earned as a function of price. Lines represent the best fit for each data set determined using the exponential model of demand (Hursh and Silberberg, 2008).
Table 1.
Comparison of behavioral economic indices of demand intensity (Q0) and elasticity (α) for heroin or food pellets during treatment with 1.0 mg/kg, IM lorcaserin (LOR) or saline vehicle.
| Reinforcer | Q0 | α | R2 | ||
|---|---|---|---|---|---|
| 3.2 μg/kg/inj Heroin | 48 | 1.3e-005 | 0.98 | ||
|
3.2 μg/kg/inj Heroin +1.0 mg/kg LOR 10 μg/kg/inj Heroin |
14* | F(1,8) = 20; P = 0.002 |
6.7e-005* | F(1,8) = 92; P < 0.0001 |
0.94 |
| 25 | 3.5e-005 | 0.98 | |||
|
10 μg/kg/inj Heroin +1.0 mg/kg LOR 1-g Banana-flavored pellets |
17 | F(1,9) = 0.86; P = 0.379 |
0.00015* | F(1,9) = 35; P = 0.0002 |
0.94 |
| 38 | 2.1e-005 | 0.98 | |||
|
1-g Banana-flavored pellets +1.0 mg/kg LOR |
41 | F(1,11) = 0.068; P = 0.798 |
2.8e-005 | F(1,11) = 3.6; P = 0.085 |
0.98 |
4. Discussion
Previous research has implicated the 5-HT2-family of receptor subtypes as key neural substrates that modulate dopamine release in the mesolimbic system (Howell and Cunningham, 2015). In this regard, we and others have shown that serotonin 2C agonists, including lorcaserin, can markedly reduce IV self-administration of several classes of abused drugs. The present study was designed to further explore serotonin 2C modulation of the behavioral effects of drugs of abuse by evaluating the ability of lorcaserin to decrease heroin self-administration and food-maintained responding in adult rhesus monkeys using acute and repeated dosing protocols. Further, behavioral economic demand analysis, in which the response requirement for drug (i.e., heroin) or food reinforcer delivery is increased across multiple sessions (i.e., 10, 32, 56, 100, etc), was utilized to provide important insight into the behavioral mechanisms responsible for reduced drug intake. Taken together, the present results indicate that lorcaserin decreases overall intake and reinforcing effectiveness of IV heroin in a sustained and behaviorally selective manner, adding further support to the view that 5-HT2 receptor subtypes are rational targets for the development of medications to manage opioid use disorder.
The present study shows that acute pretreatment with lorcaserin produces dose-related and nearly complete attenuation of self-administration across a 10–30-fold range of IV heroin doses, with little effect on food-maintained responding. These data are consistent with previous studies showing flattening of dose-response curves for stimulants such as nicotine (Jacobs et al, 2017), cocaine (Collins et al 2016), and methampetamine (Gerak et al, 2016) by lorcaserin in nonhuman primates. The present study extends this previous work to the abused opioid, heroin. Recently, lorcaserin pretreatment also has been shown to dose-dependently decrease self-administration of IV oxycodone in rats (Neelakanten et al, 2017). In that study, 1.0 mg/kg subcutaneous lorcaserin decreased self-administration of 0.1 mg/kg oxycodone by about 40%, a decrease similar in magnitude to that observed for doses of heroin on the descending limb of the dose-response function in the present study. Taken together, these data suggest that lorcaserin may be effective to decrease self-administration across a number of opioid drugs. However, additional studies with higher efficacy ligands such as fentanyl will be necessary for a full evaluation of lorcaserin as a candidate medication for opioid use disorder.
The selective serotonin 2C receptor antagonist, SB242,084, previously has been shown to dose-dependently block lorcaserin-induced decreases in drug taking, suggesting that its effects on drug intake are primarily mediated through serotonin 2C rather than serotonin 2A receptor mechanisms (Gannon et al, 2018; Neelakantan et al,. 2017; Fletcher et al, 2003; Levin et al, 2011). Although the role of selective serotonin 2A versus serotonin 2C receptor activation on the abuse-related effects of opioids has not yet received widespread attention, Maguire et al (2013) previously have shown that the selective serotonin 2A receptor agonist DOM could reduce rates of heroin-maintained responding in rhesus monkeys. However, in contrast to the present results with lorcaserin, DOM did not decrease overall intake. Moreover, DOM pretreatment appeared to increase self-administration of the lowest dose of heroin in 3 of 4 subjects, suggesting that serotonin 2A activation might enhance the reinforcing effects of low unit doses of heroin. Such differences in behavioral effects of serotonin 2A or serotonin 2C agonists, though they remain to be further explored, are consistent with their well-recognized pharmacological modulation of DA activity. For example, studies in rodents have found that activation of mesolimbic serotonin 2A receptors both increases DA release in a serotonin 2A antagonist-sensitive manner (Bortolozzi et al, 2005) and potentiates amphetamine-induced DA release in nucleus accumbens (Kuroki et al, 2003). The effects of serotonin 2C ligands generally are opposite to those of serotonin 2A-selective drugs, with agonists decreasing, and antagonists increasing, DA activity (Navailles et al, 2007; Filip and Cunningham, 2002). Thus, cocaine-induced increases in DA levels in striatum can be decreased or further increased by, respectively, the selective serotonin 2C receptor agonist Ro 60–0175 (Manvich et al, 2012a) and antagonist SB242,084 (Manvich et al, 2012b; Zayara et al, 2011). Such attenuation of dopamine release in reward-related brain regions may help explain lorcaserin’s effects on the abuse-related effects of drugs across pharmacological class.
Simple FR schedules of reinforcement, such as that used in the present study, have consistently provided a great deal of information regarding the ability of candidate medications to selectively reduce drug self-administration but are not designed to identify treatment-induced changes in the reinforcing effectiveness of a self-administered drug. In this regard, behavioral economic procedures provide powerful quantitative metrics for scaling the strength of reinforcers across type and after treatment regimens but have not yet been widely applied to the evaluation of medications for substance use disorder. Consequently, a behavioral economic demand analysis was employed during the regimen of daily lorcaserin treatment to quantitatively assess treatment-related changes in the reinforcing strength of heroin, and, for comparison, the palatable food that also maintained fixed-ratio responding (i.e., banana-flavored food pellets). The results of these studies offer two important insights. First, repeated dosing with IM lorcaserin was well-tolerated, with effects on heroin self-administration that were comparable to those observed when lorcaserin was given acutely and that persisted throughout the treatment regimen. Second, as evidenced by leftward shifts in the heroin demand curves indicative of increased elasticity, the demand analysis suggests that lorcaserin reduced drug intake by decreasing the reinforcing strength of heroin without comparable changes in the reinforcing strength of food. This type of selective action on the reinforcing strength of self-administered drugs is highly encouraging and suggests that lorcaserin’s ability to decrease drug intake is not due to decreases in general motivational processes. Additionally, increased demand elasticity for heroin (α) in the present studies was found even for 10 μg/kg, a self-administered unit dose of heroin for which demand intensity (Q0), or total intake, was not suppressed by lorcaserin. The ability to dissociate these behavioral economic indices—elasticity and intensity—further highlights the utility of behavioral economic procedures as a framework for evaluating candidate medications for substance abuse. Overall, the results of the present study extend previous findings, indicating that lorcaserin decreases heroin intake with behavioral selectivity and support the view that serotonin 2C receptor agonism is a promising target mechanism for the development of medications for opioid use disorder.
Acknowledgments
This work was supported by the National Institutes of Health grants DA002519 and DA039306. The authors thank Olga Smirnova, Nathaniel Gillis, and Kevin Costa for technical assistance with conducting these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bortolozzi A, Diaz-Mataix L, Scorza MC, Celada P, Artigas F (2005) The activation of 5-HT receptors in prefrontal cortex enhances dopaminergic activity. J Neurochem. 95, 1597–1607. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA (2008) Prospects for serotonin 5-HT 2R pharmacotherapy in psychostimulant abuse. Prog Brain Res. 172, 319–346. [DOI] [PubMed] [Google Scholar]
- Collins GT, Gerak LR, Javors MA, France CP (2016) Lorcaserin Reduces the Discriminative Stimulus and Reinforcing Effects of Cocaine in Rhesus Monkeys. Journal of Pharmacology and Experimental Therapeutics. 356, 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connery HS (2015) Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry. 23(2), 63–75. [DOI] [PubMed] [Google Scholar]
- Filip M, Cunningham KA (2002) Serotonin 5-HT(2C) receptors in nucleus accumbens regulate expression of the hyperlocomotive and discriminative stimulus effects of cocaine. Pharmacology, Biochemistry and Behavior. 71, 745–756. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Sinyard J, Higgins GA (2006) The effects of the 5-HT(2C) receptor antagonist SB242084 on locomotor activity induced by selective, or mixed, indirect serotonergic and dopaminergic agonists. Psychopharmacology (Berl). 187, 515–525. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Noble K, Soko AD, Silenieks LB, Lê AD, Higgins GA (2012) Effects of the serotonin 2C receptor agonist Ro60–0175 and the serotonin 2A receptor antagonist M100907 on nicotine self-administration and reinstatement. Neuropharmacology. 62, 2288–2298. [DOI] [PubMed] [Google Scholar]
- Gannon BM, Sulima A, Rice KC, Collins GT (2018) Inhibition of Cocaine and 3,4-Methylenedioxypyrovalerone (MDPV) self-administration by lorcaserin is mediated by serotonin 2C receptors in rats. J Pharmacol Exp Ther. 364, 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, Collins GT, France CP (2016) Effects of Lorcaserin on Cocaine and Methamphetamine Self-Administration and Reinstatement of Responding Previously Maintained by Cocaine in Rhesus Monkeys. J Pharmacol Exp Ther. 359, 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey-Lewis C, Li Z, Higgins GA, Fletcher PJ (2016) The 5-HT(2C) receptor agonist lorcaserin reduces cocaine self-administration, reinstatement of cocaine-seeking and cocaine induced locomotor activity. Neuropharmacology. 101, 237–245. [DOI] [PubMed] [Google Scholar]
- Howell LL and Cunningham KA (2015) Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev 67, 176–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR (2014) Chapter 12 Behavioral Economics and the Analysis of Consumption and Choice The Wiley Blackwell Handbook of Operant and Classical Conditioning. [Google Scholar]
- Hursh SR, Silberberg A (2008) Economic demand and essential value. Psychological Review. 115, 186–198. [DOI] [PubMed] [Google Scholar]
- Jacobs DS, Barkin CE, Kohut MR, Bergman J, Kohut SJ (2017) Effects of lorcaserin (Belviq®) on nicotine- and food-maintained responding in non-human primates. Drug and Alcohol Depend 181, 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohut SJ, Bergman J (2016) Reinforcing effectiveness of nicotine in nonhuman primates: effects of nicotine dose and history of nicotine self-administration. Psychopharmacology. 233, 2451–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohut SJ, Fivel PA, Mello NK, Mello NK (2013) Differential effects of acute and chronic treatment with the α2-adrenergic agonist, lofexidine, on cocaine self-administration in rhesus monkeys. Drug Alcohol Depend. 133, 593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki T, Meltzer HY, Ichikawa J (2003) serotonin 2A receptor stimulation by DOI, a serotonin 2A/2C receptor agonist, potentiates amphetamine-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Brain Research. 972, 216–221. [DOI] [PubMed] [Google Scholar]
- Levin ED, Johnson JE, Slade S, Wells C, Cauley M, Petro A, Rose JE (2011) Lorcaserin, a serotonin 2C agonist, decreases nicotine self-administration in female rats. J Pharmacol Exp Ther 338, 890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Shah AP, Patel SK, Rice KC, France CP (2013) Modification of the behavioral effects of morphine in rats by serotonin 5-HT1A and 5-HT2A receptor agonists: antinociception, drug discrimination, and locomotor activity. Psychopharmacology (Berl). 225(4), 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Koek W, Rice KC, France CP (2011) Effects of direct- and indirect-acting serotonin receptor agonists on the antinociceptive and discriminative stimulus effects of morphine in rhesus monkeys. Neuropsychopharmacology 36, 940–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Li JX, Koek W, France CP (2013) Effects of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM) and quipazine on heroin self-administration in rhesus monkeys. Psychopharmacology (Berl) 225(1), 173–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manvich DF, Kimmel HL, Cooper DA, Howell LL (2012a) The serotonin 2C receptor antagonist SB 242084 exhibits abuse-related effects typical of stimulants in squirrel monkeys. J Pharmacol Exp Ther. 342, 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manvich DF, Kimmel HL, Howell LL (2012b) Effects of serotonin 2C receptor agonists on the behavioral and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 341, 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Vandrey RG, Johnson MW, Stitzer ML (2013) Effects of varenicline on abstinence and smoking reward following a programmed lapse. Nicotine Tob Res. 15(1), 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navailles S, Moison D, Cunningham KA, Spampinato U (2007) Differential Regulation of the Mesoaccumbens Dopamine Circuit by Serotonin2C Receptors in the Ventral Tegmental Area and the Nucleus Accumbens: An In Vivo Microdialysis Study with Cocaine. Neuropsychopharmacology. 33, 237–246. [DOI] [PubMed] [Google Scholar]
- Neelakantan H, Holliday ED, Fox RG, Stutz SJ, Comer SD, Haney M, et al. (2017) Lorcaserin Suppresses Oxycodone Self-Administration and Relapse Vulnerability in Rats. ACS Chem Neurosci. 8(5), 1065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, et al. (2008) Lorcaserin, a Novel Selective Human 5-Hydroxytryptamine2C Agonist: in Vitro and in Vivo Pharmacological Characterization. J Pharmacol Exp Ther. 325, 577–587. [DOI] [PubMed] [Google Scholar]
- Volkow ND (2017) Medications for opioid use disorder: bridging the gap in care. Lancet 391, 285–287. [DOI] [PubMed] [Google Scholar]
- Willins DL,Meltzer HY (1998) Serotonin serotonin 2C agonists selectively inhibit morphine-induced dopamine efflux in the nucleus accumbens. Brain Research 781(1–2), 291–9. [DOI] [PubMed] [Google Scholar]
- Wu X, Pang G, Zhang YM, Li G, Xu S, Dong L, Stackman RW Jr, Zhang G (2015) Activation of serotonin 5-HT(2C) receptor suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in heroin-treated mice. Neurosci Lett 607, 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayara AE, McIver G, Valdivia PN, Lominac KD, Mccreary AC, Szumlinski KK (2011) Blockade of nucleus accumbens serotonin 2A and serotonin 2C receptors prevents the expression of cocaine-induced behavioral and neurochemical sensitization in rats. Psychopharmacology. 213, 321–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Wu X, Zhang YM, Liu H, Jiang Q, Pang G, Tao X, Dong L, Stackman RW Jr (2016) Activation of serotonin 5-HT(2C) receptor suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in morphine-dependent mice. Neuropharmacology 101, 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]