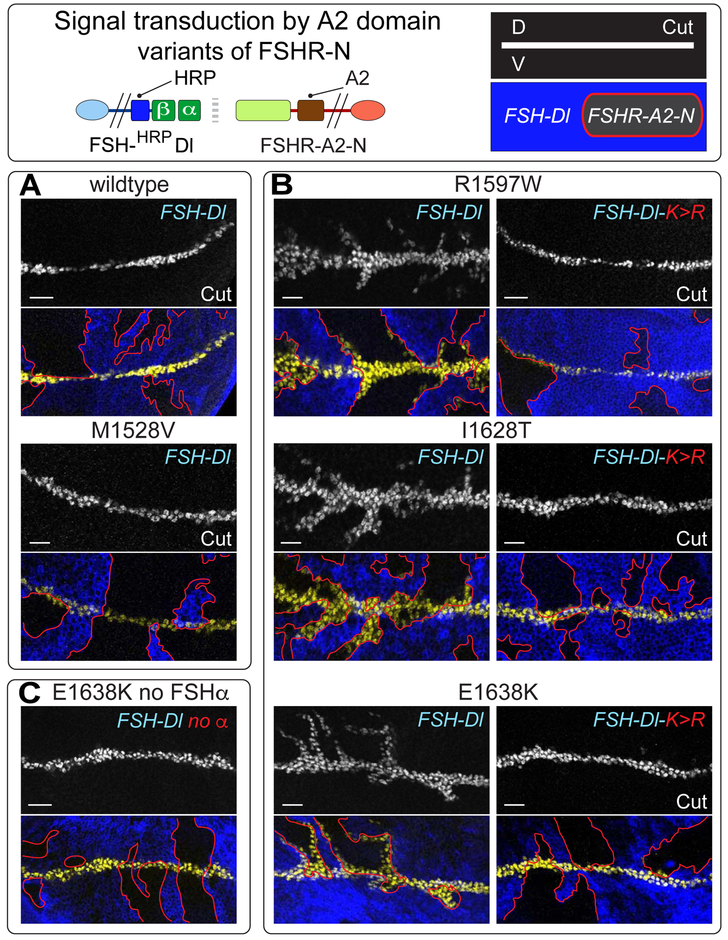
Figure 6. Signal transduction by FSHR-N receptors containing the force sensing A2 domain of von Willibrand Factor in place of the NRR.
A) UAS>FSH-Dl sending cells (blue) fail to induce UAS>FSHR-A2-N receiving cells (black, outlined in red) to ectopically express Cut (white in upper panels, yellow in the lower panels) when the A2 domain is wildtype or carries the disease associated M1528V mutation, which modestly elevates its potential to be cleaved by mechanical stress in blood. B,C) FSHR-A2-N receptors that contain any one of three other disease-associated mutant A2 domains that are more readily cleaved in blood are activated by FSH-Dl, as indicated by ectopic Cut expression (B, left); the response is limited to 5-10 cell diameters of the D/V boundary indicating that it is weaker than canonical FSH-Dl/FSHR-N signaling. Activation of all three receptors requires Epsin-mediated ligand endocytosis, as indicated by their failure to respond to FSH-Dl-K>R (B, right), and by the requirement for FSHα (C). Scale bar: 10μm.