Abstract
Clinical and laboratory studies have implicated black raspberries (BRBs) and their associated phytochemicals in the modulation of several chronic diseases. Most research on the health benefits of BRBs is conducted using freeze-dried or otherwise minimally processed products, yet BRBs are typically consumed as thermally processed goods like jams and syrups. The objective of this work was to profile the chemical changes that result from thermal processing of BRB powder into a nectar beverage. Using an untargeted UHPLC-QTOF-MS metabolomics approach, key degradation products of anthocyanins were identified along with several other proposed phenolic degradants. The effects of processing on other key BRB compound groups, including ellagitannins, are also discussed. This work demonstrates the utility of an untargeted metabolomics approach in describing the chemistry of complex food systems and provides a foundation for future research on the impact of processing on BRB product bioactivity.
1. Introduction
Black raspberries (BRBs) are heavily researched for their anti-cancer properties. In vitro models suggest BRBs may be active against a variety of cancer types (Seeram et al., 2006), while animal studies and clinical trials provide compelling evidence for the potential role of BRBs, and their phytochemical components, in preventing aerodigestive cancers (Bishayee et al., 2015; Kresty et al., 2001). For example, a two week treatment with BRB-based troches reduced malignant tumor levels of hallmark biomarkers of cancer in oral cancer patients (Knobloch et al., 2016). Studies such as this support further research on BRBs as part of a food-based approach for cancer prevention.
BRBs contain a wide array of phytochemicals including anthocyanins, flavonols, ellagitannins, and hydroxycinnamic acids, among others (Paudel et al., 2013). Work to understand which of these components contribute to the bioactivity of BRBs has shown that singular phytochemicals cannot explain the complete bioactivity of the fruit (Paudel et al., 2014; Wang et al., 2009). Thus, the complete phytochemical profile of the fruit is critical when conducting research on the potential health effects of BRBs.
In assessing BRB bioactivity, laboratory and clinical studies have historically used lyophilized BRB powder in treatments, though consumers do not typically encounter these berries in their freeze-dried or even fresh forms. Instead, BRBs are more commonly found incorporated into thermally processed products, such as jams and syrups. Knowledge of how thermal processing affects the phytochemical profile of BRB products is limited to a few select compounds (Gu et al., 2014; Hager, Howard, Prior, & Brownmiller, 2008), despite the biological importance of the whole phytochemical profile. Untargeted metabolomics is an analytical technique that aims to provide a comprehensive chemical profile of as many small molecules in a system as possible, which allows for a more thorough and encompassing analysis of molecular composition than traditional methods.
The objective of this study is to understand how thermal processing impacts the phytochemical profile of BRBs using an untargeted metabolomics approach. The product used in this work is a BRB nectar beverage, which has been previously described (Gu et al., 2014). The nectar is an optimal product to study, as it contains the whole BRB fruit and components typically incorporated in other BRB products, such as sugar and pectin, and thus applicable to additional BRB products.
2. Materials and Methods
2.1. Chemicals
All chemicals used were obtained from Fisher Scientific (Pittsburgh, PA).
2.2. Nectar Production
Nectar was produced in 250 g batches (n = 3) according to the formulation described by Gu and colleagues, with slight modifications (J. Gu et al., 2014). The modified formula is as follows (% wet basis): water (89.9%), sucrose (3.0%), pectin (0.5%), corn syrup (1.0%), BRB powder (5.6%). The BRB (Rubus occidentalis cv. Jewel) powder used in this work was acquired from Stokes Berry Farm (Wilmington, OH) as freeze-dried product and was produced from a single lot of berries. To manufacture the nectar, all components, except the BRB powder, were combined and heated to approximately 30 °C with constant stirring. Once the pectin was dissolved, the BRB powder was added and the product was heated to 95 °C with constant stirring until a final soluble solids content of 9 °Brix was achieved (~20 min); these parameters were chosen to model the pasteurization procedure previously described for this product (Gu et al., 2014). Nectar was immediately flash frozen with liquid nitrogen, lyophilized, and stored at −20 °C until analysis. A process blank, which consisted of all nectar components without the BRB powder (i.e. water, sugar, corn syrup, pectin), was also produced in the same manner as the BRB nectars.
2.3. Determination of Total Monomeric Anthocyanins
BRB powder and lyophilized nectar were extracted according to Hager and colleagues with slight modification (Hager, Howard, Prior, & Brownmiller, 2008). Briefly, 100 mg was combined with 2 mL of 60:37:3 methanol:water:formic acid and vortexed for 15 sec. Following centrifugation at 4000 × g for 10 min, the supernatants were decanted and pellets extracted twice more. Pooled extracts were diluted to 10 mL with 0.01% aqueous HCl, and total monomeric anthocyanin content was determined as previously described (Giusti & Wrolstad, 2001). Results are expressed as mg cyanidin-3-glucoside equivalents/g powder (using ε = 26900 and MW = 449.2 amu), and levels in the lyophilized nectar were multiplied by a factor of 1.8 to account for additional dry ingredients in the nectar formulation (BRB powder constitutes 55.4% of the dried nectar solids).
2.4. Sample Preparation for Untargeted Metabolomics
Lyophilized nectar and BRB powder were extracted using identical protocols except 180 mg of nectar was extracted, compared to 100 mg of BRB powder, to account for other ingredients used in the nectar formulation. Briefly, 1 mL of 75% methanol in water, with 0.1% formic acid, was added to each sample. Samples were sonicated in an ice bath for 15 min, centrifuged at 21,130 x g for 2 min, and decanted into glass vials. The resulting pellets were extracted twice more with the use of a probe sonicator (8 sec, Branson Ultrasonics; Danbury, CT). Each nectar batch was extracted in triplicate (total n=9), and an equal number of BRB powder samples were extracted (n=9). The nectar process blank (no BRB added) was also extracted using this protocol. Extracts were centrifuged at 21,130 x g for 4 min and the supernatant immediately analyzed. A set of quality control (QC) samples was produced by pooling equal volume aliquots from all nectar and BRB powder extracts.
2.5. UHPLC-QTOF-MS Metabolomics Data Acquisition
BRB and nectar samples were randomized for run order, and QC samples were positioned after every sixth injection. The use of QC samples allows for monitoring of instrument stability over the sample set. Untargeted full-scan data was acquired using a 1290 Infinity II series UHPLC (Agilent, Santa Clara, CA) coupled to an Agilent iFunnel 6550 QTOFMS. Samples were injected (3 μL) onto a 100 × 2.1 mm Agilent SB-Aq column (1.8 μm particle size) maintained at 50 °C. The mobile phase consisted of (A) 0.1% formic acid in water and (B) 0.1% formic acid in methanol at a flow rate of 0.6 mL/min. The mobile phase composition was as follows: 0–2 min, 0% B; 2–3 min increase to 10% B; 3–8 min, increase to 40% B; 8–14 min increase to 100% B; 14–16 min, hold at 100% B; 16–18 min, immediate switch to 0% B for a total run time of 18 min. The UHPLC was interfaced with the QTOF-MS with an ESI source operated in negative ion mode. Relevant MS parameters were as follows: gas temp 150 °C, drying gas 18 L/min, nebulizer 30 psig, sheath gas temp 350 °C, sheath gas flow 12 L/min, VCap 4000 V, nozzle voltage 2000 V, acquisition mode was 2 GHz extended dynamic range with a mass range of 50–1700 m/z.
2.6. Data Pre-processing and Analysis
Full scan UHPLC-QTOF-MS data was processed using the batch recursive feature extraction algorithm in Agilent Profinder (B.06.00). This process bins mass spectral features according to expected isotope patterns, adducts, and charge states, and then aligns them across all samples. Feature groups that appeared in at least two samples in either the nectar or BRB powder were retained and re-extracted across all samples. The recursive nature of this workflow ensures high quality data for statistical analysis. Further filtering of the data was performed in Agilent Mass Profiler Professional. First, features unique to the processing blank, which consisted of all nectar components except BRB powder, were removed from analysis. The analysis was then restricted to features with retention times between 1–12.5 min and a calculated neutral mass <1200 amu. Finally, features that were present in at least 66.6% of nectar or BRB samples, and those with a CV<25% in either of these groups were retained for statistical analyses. All data was log2 transformed and median-centered prior to analysis. Differential analysis was performed using an unpaired t-test (P < 0.05) with the Benjamini-Hochberg false discovery rate multiple testing correction applied.
2.7. Compound Identification
Features that differed significantly between the BRB powder and nectar were considered for identification if their average ion abundance was > 1.0 × 105 in either sample group. Highly abundant features (abundance > 1 × 106) that differed < 2 times between BRB powder and nectar were also considered for identification. These thresholds were used to ensure high data quality and sufficient signal for further experimentation. Identification was achieved using a combination of MS/MS fragmentation spectra, accurate mass, isotope analysis, and database matches when available. For MS/MS fragmentation studies, extracts were injected on the previously mentioned UHPLC-QTOF-MS system operated in targeted or auto MS/MS mode using collision energies of 10, 20, and 40 eV. Fragmentation patterns were compared to literature or curated spectra in the FooDB (Wishart et al., 2013) and Metlin (metlin.scripps.edu) databases when available. CFM-ID (Allen, Pon, Wilson, Greiner, & Wishart, 2014) was used to rationalize proposed molecular structures as needed. Feature spectral data and retention times were compared to authentic standards as available, and metabolites were annotated according to the Metabolomics Standard Initiative guidelines (Sumner et al., 2007).
3. Results and Discussion
In the present work, we compared the metabolomic profiles of lyophilized BRB powder and a thermally processed nectar beverage made from that same powder. This allows us a more holistic view on both phytochemicals that change, and those that do not change, with thermal treatment. A metabolomic approach has been previously used to understand chemical changes during BRB ripening (Kim et al., 2011), as well as the potential bioactivity of BRB phytochemicals (Jo et al., 2015; Paudel et al., 2014). In an effort to provide context for the practical translation of clinical research that supports consumption of BRB phytochemicals, we used this approach to more comprehensively characterize the effects of producing a beverage that incorporates the entire BRB fruit.
3.1. Monomeric anthocyanin content
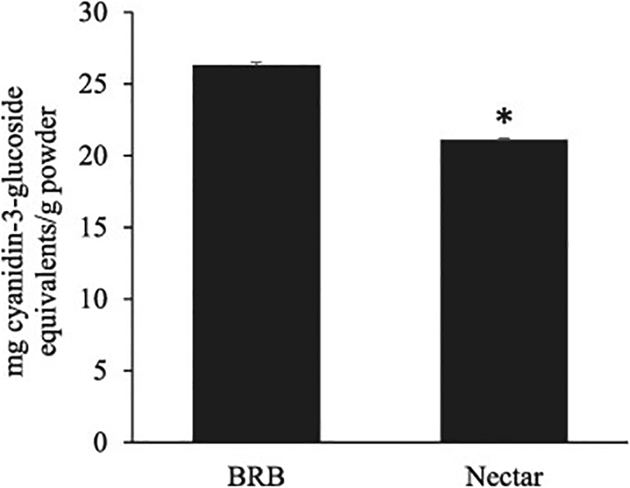
Because the UHPLC-QTOF-MS method used in this study was not optimized for anthocyanin analysis, a spectrophotometric approach was used to quantify these compounds. The total content of monomeric anthocyanins in nectar was approximately 80% of that in the BRB powder (Figure 1), indicating anthocyanin loss due to heat treatment. Similar decreases in anthocyanin content have been described in previous work on BRB nectars (Gu et al., 2014). This is a higher level of anthocyanin retention than observed in other processed BRB food products (Hager et al., 2008), however the fewer number of processing steps used here may account for the greater retention in this nectar product.
Figure 1.
Total monomeric anthocyanins (expressed as mg cyanidin-3-glucoside equivalents) in BRB powder and lyophilized nectar. A numeric correction was applied to the nectar values to account for other solids present. (*)Decrease in anthocyanin content was statistically significant (P<0.01).
3.2. Untargeted metabolomics
Following pre-processing of the data, a total of 4411 molecular features were evaluated in subsequent analyses. As part of the pre-processing procedure, features present in a “processing blank,” which consisted of all nectar ingredients, minus the BRB powder, were removed from analysis. Thus, this final list of 4411 features does not include those native to non-BRB nectar ingredients, but it does contain features that correspond to BRB phytochemicals and any potential products of interactions between BRB phytochemicals and other nectar components.
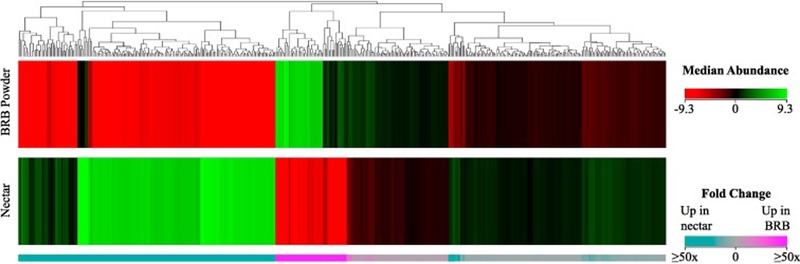
To focus on features that differed significantly between the BRB powder and nectar, an unpaired t-test and fold change analysis was performed on each molecular feature. Following manual review for peak quality, a total of 547 features were found to differ by at least two-fold (P<0.05) between the BRB powder and nectar. Unsupervised hierarchical clustering analysis was performed on these features according to their normalized abundances. The median centered abundances and average fold changes of these features are summarized in Figure 2. Large differences in abundance were evident between the two sample groups, as evidenced by the clusters with large fold change values denoted at the bottom of Figure 2. A majority of features were present at higher levels in the nectar than the BRB powder. Interestingly, 170 features were unique to the nectar while 40 were unique to the BRB powder (Figure 2). We hypothesize that the features unique to the BRB powder were present in the fruit but completely degraded over thermal processing, while those unique to the nectar represent degradation or reaction products. It is also possible that thermal processing enhanced the extractability of some compounds, which may additionally account for some of the unique features.
Figure 2.
Hierarchical clustering analysis on features that differ ≥2 fold between lyophilized BRB powder and nectar made from that powder (P < 0.05). Features were clustered using Euclidian distance metrics and Ward’s linkage rule.
3.3. Identification of Features Elevated in Thermally Processed BRBs
Given the large number of features elevated in the nectar, special attention was paid to annotating those that were most abundant with a fold change >3. A total of nine potential degradation or reaction products were annotated on the basis of their accurate mass, relative retention time and MS/MS fragmentation spectra (Table 1). Of these, three were assigned tentative identifications as anthocyanin-derived degradation products including cyanidin chalcone rutinoside, protocatechuic acid, and phloroglucinaldehyde. Cyanidin chalcone rutinoside is a thermal degradation product of cyanidin-3-O-rutinoside, the major anthocyanin present in BRBs. This product results from the oxidative opening of the flavilium C-ring (Markakis & Jurd, 1974). Similar chalcone glycosides have been observed in thermally processed strawberry and elderberry concentrates (Sadilova, Carle, & Stintzing, 2007). Protocatechuic acid and phloroglucinaldehyde are well-characterized thermal degradation products of cyanidin that result from the liberation of the catecholic B-ring and trihydroxyphenolic A-ring, respectively (Sadilova, Stintzing, & Carle, 2006; Sadilova, Carle, & Stintzing, 2007). Protocatechuic acid can also originate from flavonoids such as quercetin (Buchner, Krumbein, Rohn, & Kroh, 2006), which is also present in BRBs. Comparison of retention times of authentic standards confirmed the identities of protocatechuic acid and phloroglucinaldehyde. The rise in all three of these compounds is well explained by the simultaneous decrease in total monomeric anthocyanin content, and demonstrates the potential of metabolomics in monitoring phytochemical degradation in complex food mixtures.
Table 1.
Identified features elevated in BRB nectar
| RT (min) | Tentative ID | Observed [M-H]- | Major fragments | Chemical formula | Δ ppm | Fold changea |
|---|---|---|---|---|---|---|
| 2.25 | 2-oxo-2-(2,4,6-trihydroxyphenyl) acetaldehydec | 181.0144 | 153.0186 125.0237 |
C8H6O5 | −2.7 | Nectar Only |
| 2.32 | Protocatechuic acidb | 153.0192 | 109.0296 | C7H6O4 | −0.65 | 4.0 |
| 4.86 | Phloroglucinaldehydeb | 153.0192 | 151.0034 125.0238 107.0137 |
C7H6O4 | −0.65 | 6.0 |
| 4.88 | Unknown phenolicd | 275.0552 | 165.0186 109.0294 |
C14H12O6 | −3.3 | 11.7 |
| 5.39 | Cyanidin chalcone rutinosidec | 611.1600 | 303.0497 | C27H32O16 | −2.9 | 9.2 |
| 6.33 | Unknown phenolicd | 319.0451 | 183.0287 153.0184 139.0390 109.0313 |
C15H11O8 | −2.2 | 8.4 |
| 8.01 | Unknown quercetin derivatived | 565.1547 | 499.0492 323.0176 301.0340 257.0451 151.0401 |
C26H30O14 | −1.8 | 7.4 |
| 8.45 | Unknown phenolicd | 301.0340 165.0187e |
165.0190 137.0240 137.0240 109.0290 |
C15H10O7 | −2.7 | 5.4 |
| 8.85 | Unknown phenolicd | 331.0441 | 221.0086 195.0294 193.0136 151.0181 109.0290 |
C16H12O8 | −3.9 | 6.2 |
| 9.53 | Unknown phenolicd | 313.0341 | 285.0403 109.0300 |
C16H10O7 | −2.2 | 10.7 |
aMean abundance in nectar vs BRB powder, Identified level Ib, IIc, IIId, according to the Metabolomics Standards Initiative, eIn source fragment
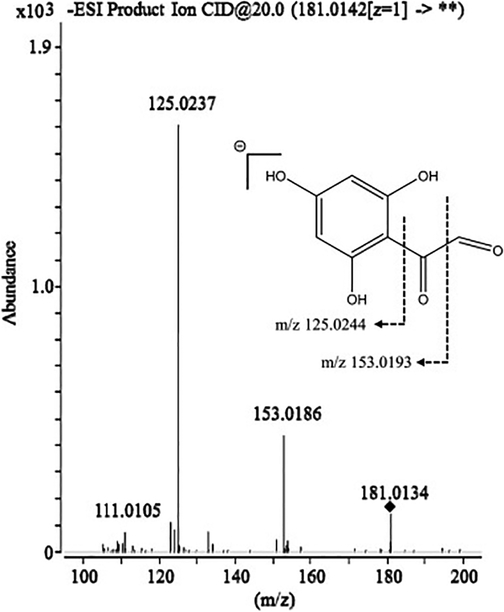
We were able to propose a structure for one additional feature, 2-oxo-2-(2,4,6-trihydroxyphenyl) acetaldehyde (Figure 3), level II identification. The MS/MS spectra of this compound contained successive neutral losses of 27.995 amu, which is associated with the loss of CO and characteristic of aldehyde functional groups (Levsen et al., 2007). The product ion at m/z 125.0237 suggests this is a degradation product that originates from the A ring of a flavonoid parent structure, similar to phloroglucinaldehyde. An analogous carboxylic acid product with an identical fragmentation pattern was observed in a study on the thermal degradation of quercetin (Barnes, Foss, & Schug, 2013), further supporting the structure we have proposed here. Based on the proposed structure, this compound may be a degradation product of a flavonol-type molecule.
Figure 3.
Fragmentation pattern of proposed degradation product, 2-oxo-2-(2,4,6-trihydroxyphenyl) acetaldehyde.
Additional potential degradation products were annotated based on characteristic fragments observed in MS/MS spectra. One was described as an unknown quercetin derivative because of the presence of the m/z 301.034 fragment and abundance of quercetin in BRBs. An additional five features were annotated as unknown phenolic compounds due to the presence of a m/z 109.029 fragment, which corresponds to a [catechol-H]− ion. This same ion has been noted in the fragmentation of quercetin degradation products where a carboxyl group is directly attached to the catecholic B ring of the parent structure (Barnes et al., 2013). Thus, we hypothesize that these features are potential phenolic degradation products, or potential reaction products that result from an interaction between phenolic degradants.
The identification of chemical features in metabolomics experiments remains challenging despite advances in the tools available to aid in this task (Dunn et al., 2013). Spectral libraries are integral parts of the compound identification process, and efforts are underway to develop databases specific to foods, such as FooDB, a subset of the Human Metabolite Database (Wishart et al., 2013). While these databases can contain information about thousands of phytochemicals, they do not tend to include information on their chemical degradation products, or interactions thereof. The curation of these types of compounds presents inherent difficulties, but represents a potential area for future growth. This is important because in some cases, bioactive phytochemicals may degrade to other bioactive molecules. For example, cyanidin-based anthocyanins degrade to protocatechuic acid, as described here, which has demonstrated chemopreventative activity against several different types of cancers in animal and cell studies (Tanaka, Tanaka, & Tanaka, 2011; Teegarden, Knobloch, Weghorst, Cooperstone, & Peterson, 2018). In fact, it has been proposed that degradation products of anthocyanins likely mediate their associated bioactivity (Kay, Kroon, & Cassidy, 2009). This may be partly explained by the ortho-dihydroxyphenyl chemical moiety shared between cyanidin and protocatechuic acid, which has been found to be critical for the biological properties of anthocyanins (Hou et al., 2003; Hou, Yanagita, Uto, Masuzaki, & Fujii, 2005). Thus, a greater understanding of compounds present in foods in the forms in which they are eaten (i.e. processed, stored) is relevant when considering health outcomes.
3.4. Identification of Features Stable to Thermal Processing
Because of the interest in the potential bioactive properties of BRB phytochemicals, we chose to complete a survey of abundant features that were minimally influenced by processing BRB powder into nectar. Significant losses of key phenolic compounds in processed berry products can occur depending on the techniques that are employed (Howard, Prior, Liyanage, & Lay, 2012). An auto-MS/MS approach, in which the most abundant ions at any given time are fragmented, was used to acquire additional spectral information for highly abundant compounds. Tentative identifications of features were assigned based off of characteristic fragmentation data reported in literature (Gu et al., 2014; Gu et al., 2003; Määttä-Riihinen, Kamal-Eldin, & Törrönen, 2004; Paudel et al., 2013), and authentic standards were used to confirm some identities, as noted in Table 2. Many of the key non-anthocyanin compounds associated with BRBs were identified via this untargeted approach.
Table 2.
Identified BRB phytochemicals with P > 0.05 or fold change < 2
| Ret. time (min) |
Tentative ID | Observed [M-H]- | Major fragments | Chemical formula | Δ ppm | Fold changea |
|---|---|---|---|---|---|---|
| Flavonols | ||||||
| 7.36 | Quercetin petosyl-rutinosidec | 741.1864 | 301.0322 | C32H38O20 | −2.7 | - |
| 7.97 | Quercetin hexuronidec | 477.0668 | 301.0354 | C21H18O13 | −1.3 | −1.09 |
| 8.10 | Quercetin rutinosideb | 609.1447 | 301.0345 | C27H30O16 | −2.3 | −1.08 |
| 8.22 | Quercetin glucosideb | 463.0867 | 301.0351 | C21H20O12 | −3.2 | −1.13 |
| 8.63 | Myricetin glucosidec | 479.0813 | 317.0299 | C21H20O13 | −3.8 | −1.12 |
| 9.60 | Quercetinb | 301.0345 | 151.0032 | C15H10O7 | −3.0 | 1.15 |
| Procyanidins | ||||||
| 4.60 | B-type procyanidinc | 577.134 | 289.0714 | C30H26O12 | −2.1 | - |
| 4.63 | Catechinb | 289.0714 | 245.0815 | C15H14O6 | −1.4 | - |
| 5.03 | B-type procyanidinc | 577.134 | 289.0714 | C30H26O12 | −2.1 | −1.23 |
| 5.28 | Epicatechinb | 289.0715 | 245.0817 | C15H14O6 | −1.0 | 1.17 |
| Phenolic acids | ||||||
| 1.40 | Gallic acidb | 169.0146 | 125.0243 | C7H6O5 | 2.4 | 1.92 |
| 3.06 | Salicylic acid hexosidec | 299.0773 | 137.0246 | C13H16O8 | 0.33 | - |
| 4.23 | Caffeic acid hexosidec | 341.087 | 179.0352 | C15H18O9 | −2.3 | −1.14 |
| 5.00 | Coumaric acid hexosidec | 325.0923 | 163.0399 | C15H18O8 | −1.8 | −1.24 |
| 5.11 | Caffeic acidb | 179.0361 | 135.0452 | C9H8O4 | 6.1 | 1.71 |
| 5.28 | Salicylic acidb | 137.0247 | C7H6O3 | 2.2 | 1.69 | |
aMean abundance in nectar vs BRB powder, fold change shown for compounds with P<0.05, Identified level Ib, IIc, IIId, according to the Metabolomics Standards Initiative
Quercetin is a flavonol ubiquitously found in edible berries (Häkkinen, Kärenlampi, Heinonen, Mykkänen, & Törronen, 1999). It is typically found conjugated to various glycosyl groups, which have been previously described in BRBs (Gu et al., 2014; Paudel et al., 2013). The potential biological significance of quercetin in several diseases has been previously reviewed (Erlund, 2004). Decreases in levels of quercetin glycosides were observed with a modest, concurrent increase in the aglycone quercetin. The thermal stability of quercetin and its rutinoside are enhanced in low pH systems (Buchner et al., 2006), like that of the nectar beverage (pH = 3.7). Changes in the abundance of quercetin and its glycosides may be due to hydrolysis of the sugar moieties and/or oxidative processes (Buchner et al., 2006; White, Howard, & Prior, 2011). Similar to quercetin-based compounds, the glucoside of myricetin was minimally degraded by processing.
Gallic acid and salicylic acid (and its glucoside) are hydroxybenzoic acids, which have been previously reported in BRBs (Kula, Majdan, Głód, & Krauze-Baranowska, 2016; Paudel et al., 2013). Coumaric acid, caffeic acid, and their glycosides are hydroxycinnamic acids that have also been previously reported in BRBs (Paudel et al., 2013). Phenolic acids have been extensively studied for their potential biological activities including antimicrobial and antitumorigenic effects (Heleno, Martins, Queiroz, & Ferreira, 2014).
B-type procyanidins are oligomers of catechin and/or epicatechin, and have been previously documented in BRBs (Kula et al., 2016). The potential bioactive properties of these compounds are thought to be primarily mediated through products of microbial catabolism (Monagas et al., 2010). Similar to our findings, Brownmiller and colleagues noted the stability of monomeric and dimeric procyanidins, relative to higher oligomers, in a variety of processed blueberry products (Brownmiller, Howard, & Prior, 2009). Increased loss of procyanidins has also been associated with increased number and complexity of steps involved in processing (Howard et al., 2012). Thus, like the relatively high retention of total anthocyanins, the simplistic processing design likely contributed to retention of procyanidins in the final product.
3.5. Ellagitannins
Ellagitannins are polymers that consist primarily of hexahydroxydiphenic acid units that, when hydrolyzed, lactonize to form ellagic acid (Quideau & Feldman, 1996). Ellagic acid and its related derivatives were tentiatively identified (level II) in BRB powder and nectar based on previous reports (Gu et al., 2014; Paudel et al., 2013). Similar to other compounds identified here, small decreases in ellagic acid and putative methylellagic acid glycosides were observed, concurrent with an increase in tentatively annotated aglycone forms likely due to hydrolysis over thermal processing. Similar changes were observed in the ellagic acid profile of red raspberries when processed into jam (Zafrilla, Ferreres, & Tomás-Barberán, 2001).
Collision-induced dissociation of ellagitannins produces characteristic product ions corresponding to ellagic acid (Mullen, Yokota, Lean, & Crozier, 2003). Several features produced this fragment (m/z 300.99) throughout the course of data analysis. Features consistent with ellagitannins were found to increase, decrease and remain constant with processing, as summarized in Table 3. The ellagitannins that decreased with processing may have depolymerized to smaller ellagitannin products, and thus contributed to the slight elevation of ellagic acid in the nectar. As ellagitannins are primarily concentrated in the seeds of BRBs (Howard et al., 2012), the increased levels of some ellagitannins in the nectar could have resulted from enhanced extraction during processing from thermal and shear induced destruction of cell walls. This increase could also be the result of hydrolysis of even larger ellagitannins. Structures as large as 3740 amu have been reported in Rubus fruits (Vrhovsek et al., 2006). In the current study, a mass cutoff of 1200 amu was applied, thus larger ellagitannins previously observed in BRBs are not included in this analysis. Interestingly, at least one putatively annotated ellagitannin, sanguiin-H4 (level II), was stable over processing. Hager and colleagues observed minimal changes in total ellagitannin content when blackberries were processed into various products containing the whole fruit (Hager, Howard, & Prior, 2010). Together with the modest increase in ellagic acid, our data suggests that the ellagitannin profile of BRBs may shift over processing into nectar without a large net decrease in total content.
Table 3.
Identified ellagic acids (EA) and ellagitannins (ET)
| Ret. time (min) |
Tentative ID | Observed [M-H]- | Major fragments | Chemical formula | Δ ppm | Fold changea |
|---|---|---|---|---|---|---|
| Ellagic acid derivatives | ||||||
| 8.57 | EA pentoside | 433.0388 | 300.9983 | C19H14O12 | −5.8 | −1.07 |
| 8.90 | Ellagic acidb | 300.9978 | 229.0134 | C14H6O8 | −3.9 | 1.43 |
| 9.87 | Methyl EA | 300.9978 | 300.9940 | C15H8O8 | 0.95 | 1.39 |
| 10.12 | Acylated methyl EA pentoside | 489.0667 | 315.0136 | C22H18O13 | −1.6 | −1.1 |
| 10.97 | Malonyl methyl EA pentoside | 531.0773 | 315.0136 | C24H20O14 | −1.3 | −1.13 |
| Ellagitannins | ||||||
| 3.92 | Unknown ET | 484.0263 (−2H) | 300.9995 | C44H26O26 | 4.1 | −17.1 |
| 3.92 | Unknown ET | 473.0354 (−2H) | 300.9991 | C42H28O26 | 1.8 | −8.3 |
| 4.45 | Unknown ET | 480.0432 (−2H) | 300.9970 | C43H30O26 | −0.74 | −4.9 |
| 4.68 | Sanguiin H4 | 633.0738 | 300.9985 | C27H22O18 | 0.79 | - |
| 5.54 | Unknown ET | 587.0564 (−2H) | 300.9989 | C55H36O30 | −1.5 | 9.5 |
| 6.63 | Unknown ET | 587.0568 (−2H) | 300.9994 | C55H36O30 | −0.85 | 5.2 |
| 7.34 | Unknown ET | 587.0575 (−2H) | 300.9984 | C55H36O30 | 0.34 | 5.1 |
aMean abundance in nectar vs BRB powder, fold change shown for compounds with P<0.05, Identified level Ib, IIc, IIId, according to the Metabolomics Standards Initiative
While in vitro studies suggest ellagic acid and ellagitannins may be bioactive in various assays, they are poorly bioavailable. Urolithins are bioavailable microbial catabolites of ellagic acid and ellagitannins and are hypothesized to be at least partially responsible for their associated bioactivity in vivo (Cerdá, Periago, Espín, & Tomás-Barberán, 2005; Landete, 2011). Truchado and colleagues recently compared urinary excretion of urolithins after subjects were fed fresh and processed strawberries. Despite differences in the ellagitannin/ellagic acid profiles of the treatments, no change in urolithin excretion was observed (Truchado et al., 2012). We speculate similar results would be observed after feeding processed BRB nectar, indicating no impact on ellagitannin-associated bioactivity after thermal processing of BRBs.
4. Conclusions
Here we have described the use of an untargeted metabolomics approach to extract >4000 chemical features in BRB powder and nectar. Using this technique, we profiled the global phytochemical changes that occur when producing a thermally-processed nectar product from lyophilized BRB powder by broadly describing the differences and similarities between the two products. For instance, key BRB components such as quercetin, phenolic acids, and ellagic acid were relatively stable to processing, while a decrease in total anthocyanin content was observed concurrent with large increases in degradation products. Since phytochemical profile is thought to be a driver of bioactivity, the implications of thermal processing of BRB food products should be considered in future studies using BRB-based functional foods. This work demonstrates the utility of an untargeted metabolomics approach in analyzing and understanding complex food systems.
Metabolomics is useful for broadly describing the chemistry of berry foods
Metabolomics uncovered flavonoid/phenolic thermal degradants in black raspberries
Modulation of ellagitannin profile was observed
Key phytochemicals were surveyed and demonstrated stability over thermal processing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- Allen F, Pon A, Wilson M, Greiner R, & Wishart D (2014). CFM-ID: A web server for annotation, spectrum prediction and metabolite identification from tandem mass spectra. Nucleic Acids Research, 42(W1), 94–99. 10.1093/nar/gku436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JS, Foss FW, & Schug KA (2013). Thermally accelerated oxidative degradation of quercetin using continuous flow kinetic electrospray-ion trap-time of flight mass spectrometry. Journal of the American Society for Mass Spectrometry, 24(10), 1513–1522. 10.1007/s13361-013-0698-6 [DOI] [PubMed] [Google Scholar]
- Bishayee A, Haskell Y, Do C, Siveen KS, Mohandas N, Sethi G, & Stoner GD (2015). Potential Benefits of Edible Berries in the Management of Aerodigestive and Gastrointestinal Tract Cancers: Preclinical and Clinical Evidence. Critical Reviews in Food Science and Nutrition, 8398(June 2016), 0 10.1080/10408398.2014.982243 [DOI] [PubMed] [Google Scholar]
- Brownmiller C, Howard LR, & Prior RL (2009). Processing and Storage Effects on Procyanidin Composition and Concentration of Processed Blueberry Products. Journal of Agricultural and Food Chemistry, 57, 1896–1902. 10.1021/jf102964b [DOI] [PubMed] [Google Scholar]
- Buchner N, Krumbein A, Rohn S, & Kroh LW (2006). Effect of thermal processing on the flavonols rutin and quercetin. Rapid Communications in Mass Spectrometry, 20, 3229–3235. 10.1002/rcm [DOI] [PubMed] [Google Scholar]
- Cerdá B, Periago P, Espín JC, & Tomás-Barberán FA (2005). Identification of urolithin A as a metabolite produced by human colon microflora from ellagic acid and related compounds. Journal of Agricultural and Food Chemistry, 53(14), 5571–5576. 10.1021/jf050384i [DOI] [PubMed] [Google Scholar]
- Dunn WB, Erban A, Weber RJM, Creek DJ, Brown M, Breitling R, … Viant MR (2013). Mass appeal: Metabolite identification in mass spectrometry-focused untargeted metabolomics. Metabolomics, 9(SUPPL.1), 44–66. 10.1007/s11306-012-0434-4 [DOI] [Google Scholar]
- Erlund I (2004). Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutrition Research, 24 (10), 851–874. 10.1016/j.nutres.2004.07.005 [DOI] [Google Scholar]
- Giusti M, & Wrolstad RE (2001). Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy In Wrolstad RE (Ed.), Current Protocols in Food Analytical Chemistry. New York: John Wiley; 10.1002/0471142913.faf0102s00 [DOI] [Google Scholar]
- Gu J, Ahn-Jarvis J, Riedl KM, Schwartz SJ, Clinton SK, & Vodovotz Y (2014). Characterization of black raspberry functional food products for cancer prevention human clinical trials. J Agric Food Chem, 62, 3997–4006. 10.1021/jf404566p [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, & Prior RL (2003). Screening of Foods Containing Proanthocyanidins and Their Structural Characterization Using LC-MS/MS and Thiolytic Degradation. Journal of Agricultural and Food Chemistry, 51(25), 7513–7521. 10.1021/jf034815d [DOI] [PubMed] [Google Scholar]
- Hager A, Howard LR, Prior RL, & Brownmiller C (2008). Processing and Storage Effects on Monomeric Anthocyanins, Percent Polymeric Color, and Antioxidant Capacity of Processed Black Raspberry Products. Journal of Food Science, 73(6), H134–H140. 10.1111/j.1750-3841.2008.00855.x [DOI] [PubMed] [Google Scholar]
- Hager TJ, Howard LR, & Prior RL (2010). Processing and storage effects on the ellagitannin composition of processed blackberry products. Journal of Agricultural and Food Chemistry, 58, 11749–11754. 10.1021/jf102964b [DOI] [PubMed] [Google Scholar]
- Häkkinen SH, Kärenlampi SO, Heinonen IM, Mykkänen HM, & Törronen AR (1999). Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. Journal of Agricultural and Food Chemistry, 47(6), 2274–2279. 10.1021/jf9811065 [DOI] [PubMed] [Google Scholar]
- Heleno SA, Martins A, Queiroz MJRP, & Ferreira ICFR (2014). Bioactivity of phenolic acids: metabolites versus parent compounds: a review Centro de Investigação de Montanha, Escola Superior Agrária, Campus de Santa. Food Chemistry, 173, 501–513. 10.1016/j.foodchem.2014.10.057 [DOI] [PubMed] [Google Scholar]
- Hou D-X, Kai K, Li J-J, Lin S, Terahara N, Wakamatsu M, … Colburn N (2003). Anthocyanidins inhibit activator protein 1 activity and cell transformation: structure-activity relationship and molecular mechanisms. Carcinogenesis, 25(1), 29–36. 10.1093/carcin/bgg184 [DOI] [PubMed] [Google Scholar]
- Hou DX, Yanagita T, Uto T, Masuzaki S, & Fujii M (2005). Anthocyanidins inhibit cyclooxygenase-2 expression in LPS-evoked macrophages: Structure-activity relationship and molecular mechanisms involved. Biochemical Pharmacology, 70(3), 417–425. 10.1016/j.bcp.2005.05.003 [DOI] [PubMed] [Google Scholar]
- Howard LR, Prior RL, Liyanage R, & Lay JO (2012). Processing and storage Effect on berry polyphenols: Challenges and implications for bioactive properties. Journal of Agricultural and Food Chemistry, 60(27), 6678–6693. 10.1021/jf2046575 [DOI] [PubMed] [Google Scholar]
- Jo Y-H, Park H-C, Choi S, Kim S, Bao C, Kim HW, … Auh J-H (2015). Metabolomic Analysis Reveals Cyanidins in Black Raspberry as Candidates for Suppression of Lipopolysaccharide-Induced Inflammation in Murine Macrophages. Journal of Agricultural and Food Chemistry, 63(22), 5449–5458. 10.1021/acs.jafc.5b00560 [DOI] [PubMed] [Google Scholar]
- Kay CD, Kroon PA, & Cassidy A (2009). The bioactivity of dietary anthocyanins is likely to be mediated by their degradation products. Molecular Nutrition and Food Research, 53(SUPPL. 1), 92–101. 10.1002/mnfr.200800461 [DOI] [PubMed] [Google Scholar]
- Kim HS, Park SJ, Hyun SH, Yang SO, Lee J, Auh JH, … Choi HK (2011). Biochemical monitoring of black raspberry (Rubus coreanus Miquel) fruits according to maturation stage by 1H NMR using multiple solvent systems. Food Research International, 44(7), 1977–1987. 10.1016/j.foodres.2011.01.023 [DOI] [Google Scholar]
- Knobloch TJ, Uhrig LK, Pearl DK, Casto BC, Warner BM, Clinton SK, … Weghorst CM (2016). Suppression of Proinflammatory and Prosurvival Biomarkers in Oral Cancer Patients Consuming a Black Raspberry Phytochemical-Rich Troche. Cancer Prevention Research, 9(2), 159–171. 10.1158/1940-6207.CAPR-15-0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresty LA, Morse MA, Morgan C, Carlton PS, Lu J, Gupta A, … Stoner GD (2001). Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Research, 61(16), 6112–6119. [PubMed] [Google Scholar]
- Kula M, Majdan M, Głód D, & Krauze-Baranowska M (2016). Phenolic composition of fruits from different cultivars of red and black raspberries grown in Poland. Journal of Food Composition and Analysis, 52, 74–82. 10.1016/j.jfca.2016.08.003 [DOI] [Google Scholar]
- Landete JM (2011). Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Research International, 44(5), 1150–1160. 10.1016/j.foodres.2011.04.027 [DOI] [Google Scholar]
- Levsen K, Schiebel H, Terlouw JK, Jobst KJ, Elend M, Preiß A, … Ingendoh A (2007). Even-electron ions: a systematic study of the neutral species lost in the dissociation of quasi-molecular ions. Journal of Mass Spectrometry, 42, 1024–1044. 10.1002/jms [DOI] [PubMed] [Google Scholar]
- Määttä-Riihinen KR, Kamal-Eldin A, & Törrönen AR (2004). Identification and quantification of phenolic compounds in berries of Fragaria and Rubus species (family rosaceae). Journal of Agricultural and Food Chemistry, 52(20), 6178–6187. 10.1021/jf049450r [DOI] [PubMed] [Google Scholar]
- Markakis P, & Jurd L (1974). Anthocyanins and their stability in foods. Critical Reviews in Food Technology, 4(4), 437–456. 10.1080/10408397409527165 [DOI] [Google Scholar]
- Monagas M, Urpi-Sarda M, Sánchez-Patán F, Llorach R, Garrido I, Gómez-Cordovés C, … Bartolomé B (2010). Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food & Function, 1(3), 233–253. 10.1039/c0fo00132e [DOI] [PubMed] [Google Scholar]
- Mullen W, Yokota T, Lean MEJ, & Crozier A (2003). Analysis of ellagitannins and conjugates of ellagic acid and quercetin in raspberry fruits by LC–MSn. Phytochemistry, 64(2), 617–624. 10.1016/S0031-9422(03)00281-4 [DOI] [PubMed] [Google Scholar]
- Paudel L, Wyzgoski FJ, Giusti MM, Johnson JL, Rinaldi PL, Scheerens JC, … Reese RN (2014). NMR-based metabolomic investigation of bioactivity of chemical constituents in black raspberry (Rubus occidentalis L.) fruit extracts. Journal of Agricultural and Food Chemistry, 62(8), 1989–1998. 10.1021/jf404998k [DOI] [PubMed] [Google Scholar]
- Paudel L, Wyzgoski FJ, Scheerens JC, Chanon AM, Reese RN, Smiljanic D, … Rinaldi PL (2013). Nonanthocyanin secondary metabolites of black raspberry (Rubus occidentalis L.) Fruits: Identification by HPLC-DAD, NMR, HPLC-ESI-MS, and ESIMS/MS Analyses. Journal of Agricultural and Food Chemistry, 61(49), 12032–12043. 10.1021/jf4039953 [DOI] [PubMed] [Google Scholar]
- Quideau S, & Feldman KS (1996). Ellagitannin Chemistry. Chemical Reviews, 96(1), 475–504. 10.1021/cr940716a [DOI] [PubMed] [Google Scholar]
- Sadilova E, Carle R, & Stintzing FC (2007). Thermal degradation of anthocyanins and its impact on color and in vitro antioxidant capacity. Molecular Nutrition and Food Research, 51(12), 1461–1471. 10.1002/mnfr.200700179 [DOI] [PubMed] [Google Scholar]
- Sadilova E, Stintzing FC, & Carle R (2006). Thermal degradation of acylated and nonacylated anthocyanins. Journal of Food Science, 71(8). 10.1111/j.1750-3841.2006.00148.x [DOI] [Google Scholar]
- Seeram NP, Adams LS, Zhang Y, Lee R, Sand D, Scheuller HS, & Heber D (2006). Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. Journal of Agricultural and Food Chemistry, 54(25), 9329–9339. 10.1021/jf061750g [DOI] [PubMed] [Google Scholar]
- Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, … Viant MR (2007). Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Inititative (MSI). Metabolomics, 3(3), 211–221. 10.1007/s11306-007-0082-2.Proposed [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Tanaka T, & Tanaka M (2011). Potential Cancer Chemopreventive Activity of Protocatechuic Acid. Journal of Experimental and Clinical Medicine, 3(1), 27–33. 10.1016/j.jecm.2010.12.005 [DOI] [Google Scholar]
- Teegarden MD, Knobloch TJ, Weghorst CM, Cooperstone JL, & Peterson DG (2018). Storage conditions modulate the metabolomic profile of a black raspberry nectar with minimal impact on bioactivity. Food & Function. 10.1039/C8FO00639C [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truchado P, Larrosa M, García-Conesa MT, Cerdá B, Vidal-Guevara ML, Tomás-Barberán FA, & Espín JC (2012). Strawberry processing does not affect the production and urinary excretion of urolithins, ellagic acid metabolites, in humans. Journal of Agricultural and Food Chemistry, 60(23), 5749–5754. 10.1021/jf203641r [DOI] [PubMed] [Google Scholar]
- Vrhovsek U, Palchetti A, Reniero F, Guillou C, Masuero D, & Mattivi F (2006). Concentration and mean degree of polymerization of Rubus ellagitannins evaluated by optimized acid methanolysis. Journal of Agricultural and Food Chemistry, 54(12), 4469–4475. 10.1021/jf060404w [DOI] [PubMed] [Google Scholar]
- Wang LS, Hecht SS, Carmella SG, Yu N, Larue B, Henry C, … Stoner GD (2009). Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prevention Research, 2(1), 84–93. 10.1158/1940-6207.CAPR-08-0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BL, Howard LR, & Prior RL (2011). Impact of different stages of juice processing on the anthocyanin, flavonol, and procyanidin contents of cranberries. Journal of Agricultural and Food Chemistry, 59(9), 4692–4698. 10.1021/jf200149a [DOI] [PubMed] [Google Scholar]
- Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, … Scalbert A (2013). HMDB 3.0-The Human Metabolome Database in 2013. Nucleic Acids Research, 41(D1), 801–807. 10.1093/nar/gks1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafrilla P, Ferreres F, & Tomás-Barberán FA (2001). Effect of processing and storage on the antioxidant ellagic acid derivatives and flavonoids of red raspberry (Rubus idaeus) jams. Journal of Agricultural and Food Chemistry, 49(8), 3651–3655. 10.1021/jf010192x [DOI] [PubMed] [Google Scholar]