Abstract
Introduction:
As Zika virus infection during pregnancy can cause a range of congenital anomalies, pregnant women may be a target population for vaccination in future outbreaks. Their inclusion in vaccine trials is critical to ensure safe and effective vaccines in pregnancy. Though many vaccine candidates are in development, pregnant women’s willingness to participate in Zika virus vaccine research is unknown. This study aims to describe pregnant women’s attitudes towards Zika virus vaccine research participation, as well as perceived barriers to and facilitators of enrollment.
Methods:
Pregnant and recently postpartum women (n=128) attending prenatal care at Massachusetts General Hospital completed surveys querying their willingness to participate in four hypothetical Zika virus vaccine trials and their motivations for participation. Demographics, information on prior Zika virus exposure, and vaccine acceptance were collected.
Results:
Most women (77%) accepted participation in at least one hypothetical Zika virus vaccine trial, and women were significantly more likely to accept prospective enrollment in an inactivated vaccine trial compared to a live-attenuated vaccine trial (p-value <0.0001) or a nucleic acid-based vaccine trial (p-value<0.0444). Important motivators for participation included evidence from research with pregnant and non-pregnant people, a desire to protect the baby from Zika, perceptions of vaccine safety, and provider recommendation.
Conclusions:
A majority of women in this cohort were willing to participate in a Zika virus vaccine trial while pregnant however differences in acceptance exist between vaccine platforms. The high value placed on evidence by participants highlights the importance of gathering and communicating pregnancy-specific data to potential research participants and their providers. Women’s motivations for accepting research participation during pregnancy are important to inform the Zika virus vaccine research agenda, candidate prioritization, and trial design.
Introduction:
Pregnant women and their offspring face the greatest risk of harm during Zika virus outbreaks, given the association between Zika virus infection and life altering congenital anomalies known as Congenital Zika Syndrome. Congenital Zika Syndrome is characterized by fetal anomalies including microcephaly, seizures, brainstem dysfunction and ocular abnormalities, and while the risk of harm from congenital infection is greatest in the first trimester, it is possible in the second and third trimesters as well [1,2]. Due to the asymptomatic nature of most infections, the potential for sexual transmission, the poor performance of screening tests, and the prediction of recurring outbreaks [2,3] a vaccine against Zika virus will likely be the primary public health defense for future outbreaks.
For Zika virus vaccine development, women of childbearing potential and those already pregnant are precisely the populations for whom the Zika virus vaccine may offer the most meaningful protection. Therefore, obtaining data about safety and efficacy of vaccines in pregnancy in crucial. Historically, pregnant women have been excluded from vaccine trials due to a range of legal and ethical concerns. In fact, for all vaccines recommended for use in pregnancy, there exists no pregnancy-specific indication and no pre-licensure clinical trials with pregnant women were conducted [4]. However, increasing efforts around maternal immunization are challenging this status quo, and orienting the research community toward considering earlier inclusion of pregnant women in the vaccine research and development agenda [5].
Responsible inclusion of pregnant women in Zika virus vaccine research will be necessary to ensure that marketed products are both safe and effective for this patient population. While a few studies have explored pregnant women’s willingness to participate in vaccine research and receive vaccines in clinical contexts [6–13], little is known about pregnant women’s willingness to participate in Zika virus vaccine trials. While all vaccines currently recommended for use in pregnancy are inactivated, several leading Zika virus vaccine candidates use platforms whose safety in pregnancy is not well established [14,15]. Thus, understanding women’s views about participating in trials utilizing a range of platforms is critical. In this study, we sought to describe pregnant women’s attitudes around participation in Zika virus vaccine trials involving a range of vaccine platforms, and to identify both predictive factors and barriers to their participation.
Methods:
Instrument development:
In order to explore women’s views about vaccine research participation during pregnancy, we developed a scenario-based survey assessing pregnant women’s attitudes regarding participation in Zika virus vaccine trials employing a range of vaccine platforms. We chose a vignette-based survey approach given its specific methodological strength in assessing decision-making in complex contexts [16,17]. The survey tool presented four hypothetical vaccine trials (Figure 1, Supplement 1) and asked participants to indicate whether or not they would be willing to enroll in each study in a context where they were at risk for Zika infection due to infectious mosquitos in their neighborhood. Each scenario was based on either a specific vaccine in the pipeline or represented aspects of several different vaccine candidates that used the same platform and were currently in development. More than one factor was changed in each scenario so that each best reflected realistic future trials; e.g. inactivated vaccines typically require more doses than live vaccines. Scenarios included: (1) a three dose trial of an inactivated Zika virus vaccine that enrolled pregnant women prospectively (Scenario 1 –inactivated); (2) a two dose trial of an inactivated Zika virus vaccine that had excluded pregnant women, but allowed women who became pregnant after the first dose to continue on the trial and receive the second dose (Scenario 2 – inactivated continuation)1; (3) A one dose trial of a live-attenuated Zika virus vaccine shown to be safe and effective in pregnant animal models and non-pregnant people that enrolled pregnant women prospectively (Scenario 3 – live); and (4) a two dose trial of a nucleic acid-based vaccine, described as a new vaccine technology shown to be safe and effective in pregnant animals and in non-pregnant people that enrolled pregnant people prospectively (Scenario 4 – nucleic acid). Scenarios were presented in the same order for each participant. Participants were also asked to rate how safe they perceived the vaccine would be for themselves and for their baby. Participant demographics, influenza and Tdap vaccine acceptance during pregnancy, and exposure to and attitudes about Zika virus during pregnancy were also collected.
Figure 1:
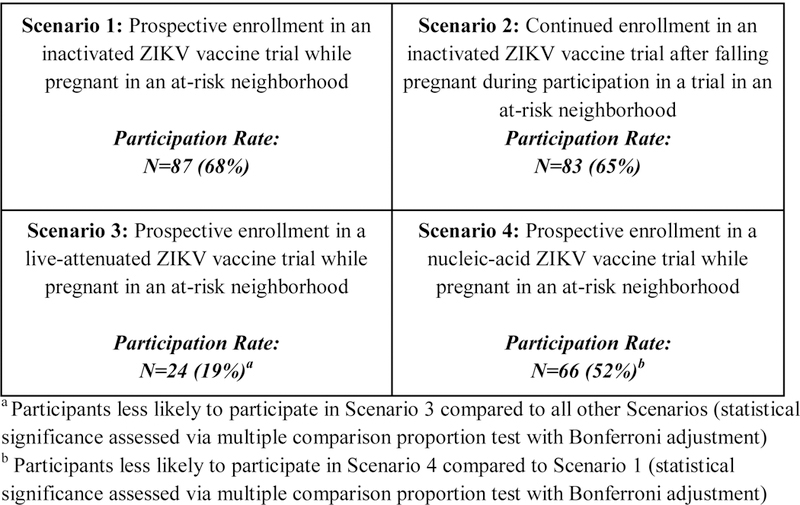
Hypothetical Zika virus vaccine trial participation rates amongst survey respondents (n=128)
Study setting:
The obstetrics department at the Massachusetts General Hospital is a large faculty practice made up of obstetricians, midwives, and perinatologists that provides prenatal care for a diverse patient population at multiple outpatient clinical sites. The approach to screening and testing for Zika virus at this medical center was standardized and includes the following: (1) All pregnant women were screened for travel or sexual exposure at intake to prenatal care and tested according to the evolving guidelines published by the Centers for Disease Control and Prevention and in collaboration with the Massachusetts Department of Public Health. (2) The patients were also universally counseled to avoid travel to areas of active Zika virus transmission and to use condoms when the partners’ travels were unavoidable. This level of patient engagement was hospital and department protocol, not related to this study. The study protocol did not provide any information about Zika virus, nor did we query knowledge about Zika virus.
Study methods:
Consent forms and English language surveys were approved by the IRB at Partners Healthcare (IRB# 2017P000489). English-speaking pregnant and postpartum (within one year) women presenting for care at the Massachusetts General Hospital prenatal clinic from May 2017 to August 2017 were eligible. Recruitment was designed around clinic flow and based on the limited availability of study staff and as such, a non-response rate was not calculated. Written informed consent was obtained by a trained research assistant prior to questionnaire administration. Surveys were then distributed at the time of the participant’s clinic visit and assessed for completeness. The survey took participants approximately 20 to 30 minutes to complete and $20 remuneration was provided (to offset the price of parking).
Statistical analyses were performed using Stata SE 14.0 (College Station, Texas). Categorical data are presented as frequency counts (percent) and compared using the chi-square or Fisher’s exact statistic as appropriate. Continuous variables are reported as means and compared using the Student’s t-test. A p-value of <0.05 was considered statistically significant, and Bonferroni corrected p-values were utilized in the context of multiple comparisons across scenarios. Generalized multivariable linear model analysis was performed to assess independent predictors of vaccine trial participation for individual scenarios. Factors included in the model were age, parity, education, race, prior vaccine acceptance, and Zika virus exposure during pregnancy. We considered women to be Zika exposed if they reported any of the following: personal or partner travel to a Zika virus outbreak area, being tested for Zika, or thinking they were infected at any time during pregnancy. These variables were decided a priori.
Results:
Between May and August 2017 a total of 128 women completed the study. Sociodemographic variables are presented in Table 1. The sample was relatively homogeneous: 72% were Caucasian and 80% were college educated. 92% of women demonstrated general vaccine acceptance which was defined as reported receipt of vaccination during pregnancy with influenza or Tdap as well as planned vaccination during pregnancy given varying gestational ages at time of survey completion. Zika virus related concerns of participants are summarized in Table 2. Twenty women (32%) traveled outside of the contiguous United States during their pregnancy, while 54 women (43%) changed their travel plans due to the Zika virus outbreak, and approximately two thirds reported feeling some level of concern about Zika virus during pregnancy. Eighteen women (15%) were tested for Zika virus infection during their pregnancy, and one woman tested positive. 43 women (33%) were considered “Zika exposed.”
Table 1:
Demographic characteristics of survey respondents to hypothetical Zika virus vaccine trials (n=128)
| Survey respondent frequency (n=128), n(%) | |
|---|---|
| Age (mean +− SD) | 32.3 (+/−4.11) |
| Education | |
| High school | 5 (3%) |
| Any college | 63 (49%) |
| Post graduate degree | 60 (46%) |
| Hispanic/Latina Ethnicity | 13 (10%) |
| Race | |
| African American | 8 (6%) |
| White | 94 (73%) |
| Asian | 15 (12%) |
| Other | 11 (9%) |
| Currently pregnant | 118 (93%) |
| Marital Status | |
| Partnered | 114 (89%) |
| Nulliparous | 53 (41%) |
| Participated in prior research | 48 (37%) |
| If yes, while pregnant? | 18 (40%) |
|
Received recommended vaccinations during this or any previous pregnancy |
92 (74%) |
| Tdap | 77 (60%) |
| Flu | 72 (56%) |
| Other (free text) * | 3 (3%) |
| Can’t remember | 5 (4%) |
| Not yet | 6 (5%) |
One participant wrote in “pneumovax” and two others wrote in “rhogam” which is an injectable medication but not a vaccine.
Table 2:
Zika virus-related characteristics of survey respondents to hypothetical Zika virus vaccine trials, n=128
| Variables | Survey respondent frequency (n=128), n (%) |
|---|---|
| Traveled during this pregnancy | 63 (49%) |
| Outside contiguous United States* | 20 (32%) |
| Changed travel plans due to ZIKV concerns | 54 (43%) |
| Tested for ZIKV in this pregnancy | 18 (15%) |
| Positive | 1 (6%) |
| Negative | 17 (94%) |
| Thought ZIKV virus infected this pregnancy | 11 (9%) |
| Worried about ZIKV during this pregnancy | 43 (33%) |
Travel outside the 48 adjoining U.S. states on the continent of North America
Participants indicated a high level of willingness to participate in trials of an inactivated virus vaccine (65% and 68% for Scenario 1 and 2, respectively) as well as a nucleic acid-based vaccine (52%, Scenario 3) as demonstrated in Figure 1. Willingness to participate in a live-attenuated virus vaccine trial (19%, Scenario 4) was considerably lower than that of the other scenarios. Acceptance of prospective enrollment in an inactivated Zika virus vaccine trial (Scenario 1) was significantly higher than acceptance of prospective enrollment in a live Zika virus vaccine trial (Scenario 3) [p-value<0.0001)] or a nucleic acid-based Zika virus vaccine trial (Scenario 4) [p-value<0.0444]. However, women were significantly more willing to participate in a trial of a nucleic acid based Zika virus vaccine (Scenario 4) than a trial of a live Zika virus vaccine, (Scenario 3), [p-value<0.0001].
Women chose from a list of factors that influenced their decision to participate (Figure 2) or not participate (Figure 3). Among women who accepted participation in any scenario, 65% cited their desire to protect themselves and almost all (98%) cited a desire to protect their babies as motivating factors, while obtaining extra medical care (25%) or contributing to science (20%) played a smaller role. Evidence in non-pregnant human trials was of importance to the majority of women who accepted (59%) and women who declined (68%) vaccine trial participation. Among women who believed the vaccine was “definitely or likely safe” for their baby (70, 70, 34, and 58 women for scenarios 1–4, respectively), a majority accepted trial participation (87%, 90%, 62%, and 79% for scenarios 1–4, respectively). Conversely, among women who were unsure about vaccine safety for their baby or believed the vaccine to be “likely or definitely not safe” for the baby (55, 54, 93, and 63 women for scenarios 1–4, respectively), a majority (56%, 69%, 97%, and 71% for scenarios 1–4, respectively) declined trial participation.
Figure 2:
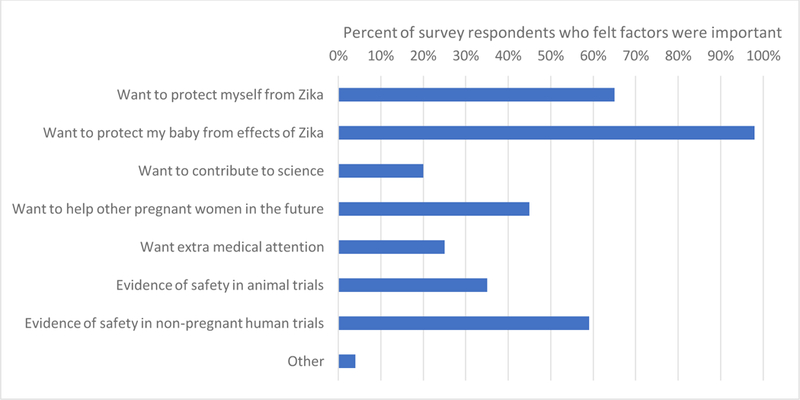
Important factors for survey respondents that accepted hypothetical Zika virus vaccine trial participation
Figure 3:
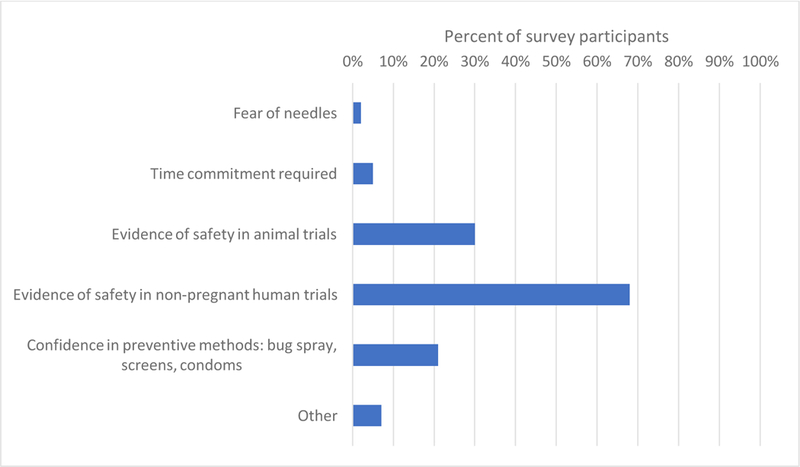
Important factors for survey respondents that declined hypothetical Zika virus vaccine trial participation.
Women who indicated they would decline participation in vaccine trials were also asked what factors would change their decision (Figure 4). Regardless of vaccine type, evidence of safety from women who had fallen pregnant on vaccine trials (over 90% across all scenarios) and healthcare provider recommendations (over 60% across all scenarios) were most important.
Figure 4:
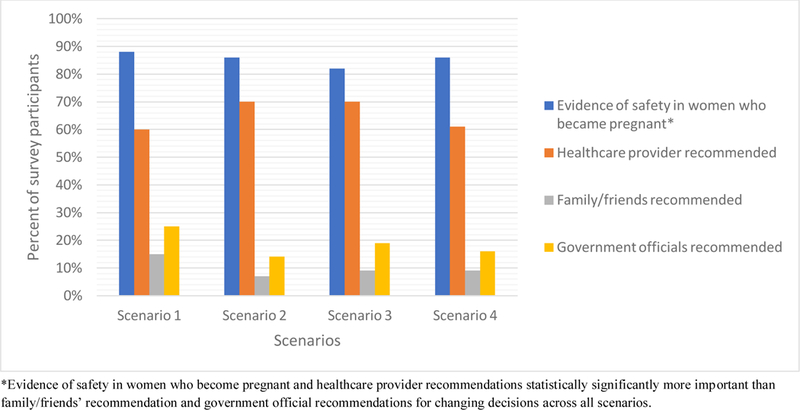
Important factors that would change participation decisions among survey respondents who declined participation in Scenarios 1–4
To identify possible predictors of trial participation, a multivariable generalized linear model was generated to evaluate the association between age, race, parity, education, prior vaccine acceptance, Zika virus exposure and scenario participation. In the case of all trials offering prospective enrollment (the inactivated, nucleic acid, and live platforms), no independent predictor variables were identified. Of interest, however, a history of Zika virus exposure was identified as an independent negative predictor of participation in Scenario 2 (aRR .67, 95% CI .48–.96) (Supplemental Table). Women with a Zika virus exposure during pregnancy were 23% less likely to continue with participation in a Zika virus vaccine trial after pregnancy was discovered than non-Zika exposed women after controlling for other potential confounders.
Discussion:
Development of Zika virus vaccines continues to be a priority of the global research agenda, with multiple vaccines under development and in early stage trials [14,15]. Because women of reproductive potential are a population that will be recruited for Zika virus trials – and may be pregnant or may become pregnant during studies – understanding their attitudes toward Zika virus vaccine research participation while pregnant is critical. In our study aimed to elicit pregnant women’s views about participation in Zika virus vaccine research, more than half of pregnant women indicated that they would participate in a trial of an inactivated or nucleic acid vaccine, where approximately one in five would participate in a live vaccine trial. Regardless of vaccine platform, safety data in pregnant women and provider recommendations were the factors that most affected women’s willingness to join a study.
Among the reasons that have historically been given to justify pregnant women’s exclusion from clinical trials has been that recruitment challenges would serve as a barrier. Yet consistent with other studies eliciting women’s willingness to participate in studies during pregnancy [6,7,9] we found instead a broad-based willingness to participate in Zika virus vaccine studies, even where risks of the intervention were unknown. Of note, women were most willing to participate in studies of inactivated vaccines, which have a long history of use in pregnant populations. Unexpectedly, more than half of women indicated they would participate in a trial of a nucleic acid vaccine, which does not have such history. Also, around one in five women indicated they would participate in a trial of a live vaccine, a platform generally recommended against in pregnancy due to theoretical risk. Together these findings are an indication that recruitment may not in fact be a significant barrier to the conduct of Zika virus vaccine trials in pregnant women, even those using platforms not widely accepted in pregnancy. Still, the higher willingness to participate in trials of inactivated vaccines compared to other platforms should be considered when designating research priorities, investments, and activities.
We also interrogated women’s reasoning about research participation, and found that protecting the baby was the strongest motivator for both research participation and non-participation. Fewer women accepted participation in the trials in which they expressed lower confidence in the safety of the vaccine for the baby and which were described as having a higher level of risk or uncertainty. Similarly, among women who expressed confidence that the vaccine was safe for the baby, a majority agreed to participate; among those who were unsure or viewed the vaccine as unsafe, a majority declined trial participation across scenarios. These findings highlight that for some women the background risk of Zika is more salient, and for others, the risk of a medical intervention is most motivating. As the risk profile associated with the vaccine shifted throughout the scenarios, some women’s decisions changed as well, demonstrating that there is range of values, priorities, risk perceptions and tolerances among individuals evaluating potential trial participation.
Our data are consistent with findings from other recent studies exploring pregnant women’s willingness to receive a hypothetical Zika virus vaccine in different clinical contexts. The acceptance rates of hypothetical Zika vaccination among pregnant women were 48% and 72% among two cohorts in the United States [18,19] and 94% among a cohort of pregnant women in Malaysia, a country with a history and risk of local Zika transmission [20]. Of note, neither Alholm et al. nor Wong et al. reportedly described to participants the details around vaccine platform or prior research [18,20]. Fraiz et al. queried women about a “safe and effective Zika vaccine” but did not describe this in further detail [19]. While important to gauge women’s general willingness to receive a Zika virus vaccine in the clinical context, our study addresses different questions and is specific to the research setting – a setting that has not been previously explored. For example, we found there is variation in acceptability of vaccine platform among pregnant women. Our findings also highlight the relevance of evidence to women’s decisions about whether or not to participate in studies. In the recent study by Fraiz et al. 72% of pregnant women said they would agree to receive a “safe and effective Zika vaccine,” in the clinical context, raising important questions about how women understand different interventions to be “safe and effective” [19]. Our data suggest that pregnant women’s judgements about the safety of vaccines in pregnancy rely heavily on whether there are available data from studies in pregnant women, and to lesser extents non-pregnant women and pregnant animals. These results are consistent with previous findings in the context of vaccine trials for Group B streptococcus that women’s acceptance of vaccine research participation is associated with the amount of pregnancy-specific evidence [7]. The high value placed on evidence by participants also calls attention to the importance of accurately communicating prior research results in studies that include pregnant women – in the processes of community engagement, recruitment, and informed consent. Even when pregnant women are not prospectively enrolled in vaccine trials, pregnancies will occur during studies directed at women of reproductive potential. Responsibly communicating vaccine safety in these circumstances is important to avoiding unnecessary terminations and anxiety.
It is worth noting that acceptance rates for participation in Zika vaccine trials exceeded acceptance of currently recommended vaccines [21]. While our data do not indicate reasons for this interesting comparative finding, further investigation of some possible explanations may warrant pursuit. For instance, a qualitative study asking pregnant women about their reasoning around participation in an H1N1 vaccine trial indicated that many felt safer receiving a vaccine as a part of a research study than in the clinical setting [6]. Other possibilities include that the hypothetical nature of the trial, or greater concern about the health effects of Zika (e.g., birth defects) than of influenza or neonatal pertussis drove the higher acceptance rates. Future studies that explore women’s reasoning on these issues may help inform approaches both to recruitment for trials and acceptance of recommended vaccines.
Women’s decisions were also strongly shaped by provider recommendation. This result is consistent with other literature around vaccine acceptance in pregnancy [10–13,19]. That over half of women who declined participation in any of the Zika virus vaccine trial scenarios would reconsider their decisions if a provider recommended they enroll reflects the strength of this influence on pregnant women’s decisions on vaccine acceptance. Notably, recommendations from government and family and friends were significantly less likely to change or affect women’s trial participation decisions. These findings highlight the importance of efforts to engage and educate providers around vaccine research and use in pregnancy.
Our study had several limitations, including the demographic homogeneity and narrow geographic representation of our sample, limiting the generalizability of the findings. Though at least one Zika virus vaccine candidate is currently undergoing trials in Boston, Zika vaccine candidates will undergo larger trials in endemic areas, which have predominantly been low and middle income countries. 2 Our findings suggest trends that may be observed at other high income setting urban academic medical centers where vaccine trials are being conducted, but future research should explore women’s opinions in areas where outbreaks are projected to occur and where differences in culture and knowledge about Zika as well as research might inform willingness to participate in studies. Future evaluations of attitudes should include women in endemic areas, particularly given our unexpected finding that Zika exposure was identified as an independent negative predictor for certain vaccine trials compared to the findings from Wong et al. of a 92% Zika vaccine acceptability among pregnant women in a Zika affected area [20]. Additionally, our study population was not racially diverse enough to identify a statistically significant difference between attitudes across racial and ethnic groups. Future studies should aim to enroll a more racially and ethnically diverse population to better define potentially significant differences in motivation and participation willingness.
Conclusion:
Product development of a range of Zika virus vaccines is currently underway, and researchers, vaccine developers, regulators, and other relevant agents face challenging questions about when, whether, and how to include pregnant women and their specific interests in future Zika virus vaccine trials [5]. Decisions around participating in clinical research for experimental biomedical interventions during pregnancy can be complex. In the context of an urgent epidemic threat, the complexity of these decisions is compounded. Our findings establish willingness of pregnant women to participate in Zika virus vaccine trials and offer insight about how different vaccine platforms may affect decisions about trial participation. Given recent developments in the Zika virus vaccine pipeline, such as the discontinued study of an inactivated vaccine candidate that had shown success in Phase 1 trials and the announcement of a new trial of a live-attenuated Zika virus vaccine, these findings may be useful to those working to develop a Zika vaccine that can be safely used in pregnancy [22–24]. Further, these findings may be useful to those considering enrollment of pregnant women in trials testing vaccines against other pathogens, especially as novel platforms emerge. Our findings also highlight the importance of both fetal safety and provider recommendations in the vaccine research context. As Zika virus vaccine development moves forward, considering the interests of pregnant women in designing and conducting research will help ensure that pregnant women are well-served as both participants in and beneficiaries of this critical scientific and public health effort.
Supplementary Material
Highlights:
Most pregnant women demonstrated willingness to participate in ZIKV vaccine trials
There was higher acceptance of non-replication competent vaccine platforms trials.
A desire to protect the baby was the most important decisional factor.
Most women who declined would reconsider given evidence of safety in pregnancy.
Acknowledgements:
We are grateful to all of the women who participated in our study, as well as the providers and staff at Massachusetts General Hospital Department of Obstetrics and Gynecology.
Funding: This work was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI108368. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This scenario was designed based on a concern heard from vaccine investigators about women who became pregnant in between vaccine doses. For this incident pregnancy scenario, we chose the vaccine prototype most prioritized in the Target Product Profile document released by the World Health Organization and the vaccine platform most likely to be considered for continued use after incident pregnancy given its record in pregnancy.
A Phase 1 trial of an inactivated Zika virus vaccine is ongoing in Boston, Massachusetts, at the Center for Virology and Vaccine Research Clinical Trials Unit, Beth Israel Deaconess Medical Center; ClinicalTrials.gov Identifier: NCT02937233. Several other vaccine trials are ongoing at similar academic medical centers in the US, see ClinicalTrials.gov.
Conflict of Interest: None
References:
- [1].Brasil P, Pereira JP, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N Engl J Med 2016;375:2321–34. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fitzgerald B, Boyle C, Honein MA. Birth defects potentially related to zika virus infection during pregnancy in the United States. JAMA 2018;319:1195–6. doi: 10.1001/jama.2018.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Siedner MJ, Ryan ET, Bogoch II. Gone or forgotten? The rise and fall of Zika virus. Lancet Public Health 2018;3:e109–10. doi: 10.1016/S2468-2667(18)30029-X. [DOI] [PubMed] [Google Scholar]
- [4].Beigi RH, Fortner KB, Munoz FM, Roberts J, Gordon JL, Han HH, et al. Maternal Immunization: Opportunities for Scientific Advancement. Clin Infect Dis 2014;59:S408–14. doi: 10.1093/cid/ciu708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ethics, pregnancy, and ZIKV vaccine research & development. Vaccine 2017;35:6819– 22. doi: 10.1016/j.vaccine.2017.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lyerly AD, Namey EE, Gray B, Swamy G, Faden RR. Women’s views about participating in research while pregnant. IRB Ethics Hum Res 2012;34. [PubMed] [Google Scholar]
- [7].McQuaid F, Jones C, Stevens Z, Plumb J, Hughes R, Bedford H, et al. Factors influencing women’s attitudes towards antenatal vaccines, group B Streptococcus and clinical trial participation in pregnancy: an online survey. BMJ Open 2016;6:e010790. doi: 10.1136/bmjopen-2015-010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Palmer S, Pudwell J, Smith GN, Reid RL. Optimizing Participation of Pregnant Women in Clinical Trials: Factors Influencing Decisions About Participation in Medication and Vaccine Trials. J Obstet Gynaecol Can n.d;38:945–54. doi: 10.1016/j.jogc.2016.04.100. [DOI] [PubMed] [Google Scholar]
- [9].Rodger MA, Makropoulos D, Walker M, Keely E, Karovitch A, Wells PS. Participation of Pregnant Women in Clinical Trials: Will They Participate and Why? Am J Perinatol 2003;20:069–76. [DOI] [PubMed] [Google Scholar]
- [10].Goldfarb I, Panda B, Wylie B, Riley L. Uptake of influenza vaccine in pregnant women during the 2009 H1N1 influenza pandemic. Am J Obstet Gynecol 2011;204:S112–5. doi: 10.1016/j.ajog.2011.01.007. [DOI] [PubMed] [Google Scholar]
- [11].Shavell VI, Moniz MH, Gonik B, Beigi RH. Influenza immunization in pregnancy: overcoming patient and health care provider barriers. Am J Obstet Gynecol 2012;207:S67–74. doi: 10.1016/j.ajog.2012.06.077. [DOI] [PubMed] [Google Scholar]
- [12].Wilson RJ, Paterson P, Jarrett C, Larson HJ. Understanding factors influencing vaccination acceptance during pregnancy globally: A literature review. Vaccine 2015;33:6420– 9. doi: 10.1016/j.vaccine.2015.08.046. [DOI] [PubMed] [Google Scholar]
- [13].Wiley KE, Cooper SC, Wood N, Leask J. Understanding Pregnant Women’s Attitudes and Behavior Toward Influenza and Pertussis Vaccination. Qual Health Res 2014;25:360–70. doi: 10.1177/1049732314551061. [DOI] [PubMed] [Google Scholar]
- [14].Hombach J Update on Zika vaccines and related WHO activities 2017.
- [15].Wilder-Smith A, Vannice K, Durbin A, Hombach J, Thomas SJ, Thevarjan I, et al. Zika vaccines and therapeutics: landscape analysis and challenges ahead. BMC Med 2018;16:84. doi: 10.1186/s12916-018-1067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Evans SC, Roberts MC, Keeley JW, Blossom JB, Amaro CM, Garcia AM, et al. Vignette methodologies for studying clinicians’ decision-making: Validity, utility, and application in ICD-11 field studies. Int J Clin Health Psychol 2015;15:160–70. doi: 10.1016/j.ijchp.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ramirez R, Mukherjee M, Vezzoli S, Kramer AM. Scenarios as a scholarly methodology to produce “interesting research.” Futures 2015;71:70–87. doi: 10.1016/j.futures.2015.06.006. [DOI] [Google Scholar]
- [18].Alholm Z, Ault K, Zwick R, Fitzgerald S, Satterwhite C. Pregnant Women’s Acceptance of Hypothetical Zika Vaccine. Open Forum Infect Dis 2017;4:S458–S458. doi: 10.1093/ofid/ofx163.1167. [DOI] [Google Scholar]
- [19].Fraiz LD, de Roche A, Mauro C, Catallozzi M, Zimet GD, Shapiro GK, et al. U.S. pregnant women’s knowledge and attitudes about behavioral strategies and vaccines to prevent Zika acquisition. Vaccine 2018;36:165–9. doi: 10.1016/j.vaccine.2017.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wong LP, Alias H, Hassan J, AbuBakar S. Attitudes towards Zika screening and vaccination acceptability among pregnant women in Malaysia. Vaccine 2017;35:5912–7. doi: 10.1016/j.vaccine.2017.08.074. [DOI] [PubMed] [Google Scholar]
- [21].Ding H, Black C, Fink R, Williams W, Fiebelkorn A, Lu P, et al. Influenza Vaccination Coverage Among Pregnant Women — United States, 2016–17 Influenza Season. MMWR Morb Mortal Wkly Rep 2017;66:1016–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sanofi Statement on Zika Vaccine License 2017.
- [23].Modjarrad K, Lin L, George SL, Stephenson KE, Eckels KH, De La Barrera RA, et al. Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: phase 1, randomised, double-blind, placebo-controlled clinical trials. The Lancet 2018;391:563–71. doi: 10.1016/S0140-6736(17)33106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Evaluation of the Safety and Immunogenicity of the Live Attenuated Zika Vaccine rZIKV/D4Δ30–713 in Flavivirus-naïve Adults. ClinicalTrials.gov n.d. https://clinicaltrials.gov/ct2/show/NCT03611946.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


