Abstract
Objective and Design.
Inflammation is a key component of a number of diseases, including diabetic retinopathy. We investigated the cellular pathway by which protein kinase A (PKA) inhibited high mobility group box 1 (HMGB1).
Methods.
Primary human retinal endothelial cells (REC) were grown in normal glucose (5mM) or high glucose (25mM). Cells in high glucose were treated with exchange protein for cAMP 1 (Epac1) and IGFBP-3 siRNA. Additional cells in high glucose were treated with forskolin, a PKA agonist, and Epac1 siRNA. Some cells were treated with a plasmid for insulin-like growth factor binding protein 3 (IGFBP-3) that does not bind IGF-1. Finally, some REC received Ex527, a sirtuin 1 (SIRT1) antagonist, prior to forskolin treatment. Protein analyses were done for HMGB1, Epac1, IGFBP-3, SIRT1, and PKA.
Results.
PKA inhibited cytoplasmic HMGB1, independent of Epac1 actions. PKA activated IGFBP-3 and SIRT1 to inhibit cytoplasmic HMGB1. High glucose inhibited SIRT1 levels and increased cytoplasmic HMGB1 in REC.
Conclusions.
PKA requires active IGFBP-3 and SIRT1 to inhibit HMGB1 inflammatory actions in the retina vasculature. Activation of these pathways may offer new targets for therapy development.
Keywords: PKA, HMGB1, SIRT1, inflammation, retinal endothelial cells
1. Introduction
Inflammation is a key component of a number of diseases. We and others have shown that inflammatory mediators contribute to retinal damage in diabetic retinopathy both in vivo and in vitro [1–5]. One of the primary goals of therapy is to determine the regulation of these inflammatory mediators, so that downstream pathways can be inhibited. For these studies, we focused on the cellular regulation of high mobility group box 1 (HMGB1). We have previously reported that exchange protein for cAMP 1 (Epac1) can inhibit HMGB1 levels [6]. Both PKA and Epac1 pathways may become activated after β-adrenergic receptor stimulation, leading to initiation of distinct signaling cascades [7]. Therefore, in these studies we sought to better understand the cellular signaling by which protein kinase A may regulate HMGB1, as HMGB1 has been linked to inflammation [8, 9]. Focusing on the retina, work in 1-month diabetic rats showed that glycyrrhizin, a HMGB1 inhibitor, significantly reduced HMGB1, ERK1/2, caspase-3, and glutamate levels [10]. Additionally, work in receptor for advanced glycation end products (RAGE) knockout mice showed that ischemia/reperfusion (I/R) caused a significant increase in HMGB1 levels in the retina, which was attenuated using a HMGB1 neutralizing antibody [11]. Inhibition of HMGB1 also reduced neuronal cell loss in these mice. We found similar results using glycyrrhizin in a mouse model of retinal ischemia/reperfusion [12]. Additionally, co-cultures of pericytes and retinal endothelial cells (REC) showed that HMGB1 directly induced REC apoptosis [13]. The authors suggested that REC cell death may be due to HMGB1-induced cytotoxic activity in glial cells or a direct effect of HMGB1 on the endothelial cells [13]. Taken together, it is clear that HMGB1 is toxic to the retina.
Our goal to dissect the specific contribution of PKA to the regulation of HMGB1 stems from findings that PKA and Epac1 regulate macrovascular and microvascular endothelial actions differently [14]. Studies have shown that PKA can directly phosphorylate HMGB1 in the Box A segment of yeast to decrease its activity [15]. Furthermore, a mosquito form of HMGB1 (AaHMGB1) is phosphorylated by PKA and PKC [16]. Other than these studies, little has been reported for PKA regulation of HMGB1.
In order to determine potential intermediary pathways between PKA and HMGB1, we focused on insulin-like growth factor binding protein 3 (IGFBP-3), as we have previously reported that PKA activates IGFBP-3 to prevent REC apoptosis [17]. We also focused on sirtuin 1 (SIRT1) as a key regulator of HMGB1, since SIRT1 promotes the deacetylation of HMGB1 to reduce cytoplasmic translocation and downstream inflammatory pathways [18]. In that study, mice with endothelial cell specific knockout of SIRT1 and vascular cell culture studies showed that SIRT1 is key to the reduced HMGB1 translocation in kidney lysates, leading to decreased renal inflammation [18]. Work in A549 and mouse embryonic cells showed that PKA can activate SIRT1 by disrupting the interaction between SIRT1 and the deleted in breast cancer 1 (DBC1) gene [19]. Additional work in HepG2 showed that PKA activates SIRT1 to reduce ER stress [20]. However, work in HepG2 cells suggested that PKA, not SIRT1, was key to hepatic gluconeogenesis [21].
In the present study, we dissected potential cellular pathways by which PKA reduces cytoplasmic HMGB1. We used REC grown in normal (5mM) and high glucose (25mM) with a PKA agonist, SIRT1 antagonist, and IGFBP-3 and Epac1 siRNA. We hypothesized that PKA would increase IGFBP-3 and SIRT1 levels, leading to decreased cytoplasmic translocation of HMGB1 in REC grown in high glucose. These actions of PKA would be independent of Epac1.
2. Materials and Methods
Retinal Endothelial Cells (REC).
Primary human retinal endothelial cells (REC) were purchased from Cell Systems Corporation (CSC, Kirkland, Washington). Cells were grown in Cell Systems Medium, either basal (normal glucose, 5mM glucose) or high glucose medium (25mM glucose), augmented with microvascular growth factors (MVGS), 10ug/mL gentamycin, and 0.25ug/mL amphotericin B (Invitrogen, Carlsbad, CA) on attachment factor coated dishes. Only cells prior to passage 6 were used. Cells were quiesced by incubating in the appropriate medium without MVGS for 24 hours prior to use. Cells are kept in high glucose for a minimum of 3 days before experimentation.
Cell Treatments.
Some REC were transfected with Epac1 siRNA, IGFBP-3 siRNA, or scrambled siRNA (Dharmacon, Lafayette, CO) using RNAiMax based upon manufacturer’s instructions. The scrambled siRNA was used to insure that the transfection protocol does not alter REC responses. Following transfection, some dishes were treated with forskolin (20uM for 2 hours). Additional cells in normal and high glucose were transfected with an IGFBP-3 plasmid that cannot bind IGF-1 at 1ug/ul using lipofectamine [22]. Additional cells were treated with Ex527, a SIRT1 inhibitor (200nM, 1 hour), prior to forskolin treatment.
Nuclear vs. cytoplasmic extraction.
For assessment of HMGB1, protein lysates were processed to isolate the nuclear and cytoplasmic portions, using the NE-PER kit (Fisher Scientific, Pittsburgh, PA). Separation was done following the manufacturer’s instructions.
Western blotting.
Retinal cell lysates were collected into lysis buffer containing protease and phosphatase inhibitors. Equal amounts of protein were separated onto a pre-cast tris-glycine gel (Invitrogen, Carlsbad, CA), blotted onto nitrocellulose membrane and blocked with TBST (10mM Tris-HCl buffer, pH 8.0, 150 mM NaCl, 0.1% Tween 20) and 5% (w/v) BSA. Membranes were treated with HMGB1, IGFBP-3, Epac1(Abcam, Cambridge, MA) or beta actin (Santa Cruz Biotechnology, Santa Cruz, CA) primary antibodies followed by incubation with horseradish peroxidase labeled secondary antibodies. Antigen-antibody complexes were detected by chemilluminescence reagent kit (Thermo Scientific, Pittsburgh, PA) and data was acquired using an Azure C500 (Azure Biosystems, Dublin, CA). Western blot data were assessed using Image Studio Lite software.
ELISA
A PKA ELISA (Enzo Lifesciences, Ann Arbor, MI) and SIRT1 ELISA (Abcam, Cambridge, MA) were done on protein lysates based upon manufacturer’s instructions, with the exception that the antibody reaction was left overnight at 4oC.
Statistics.
Non-parametric Kruskal-Wallis with Dunn’s post-hoc testing was done with P<0.05 considered statistically significant. We did non-parametric analyses due the N<5 in cell culture samples and with a potential for non-normal distribution. A representative Western blot is shown where appropriate.
3. Results
High glucose decreased SIRT1 and increased cytoplasmic HMGB1 in REC, which are regulated by IGFBP-3 or Epac1.
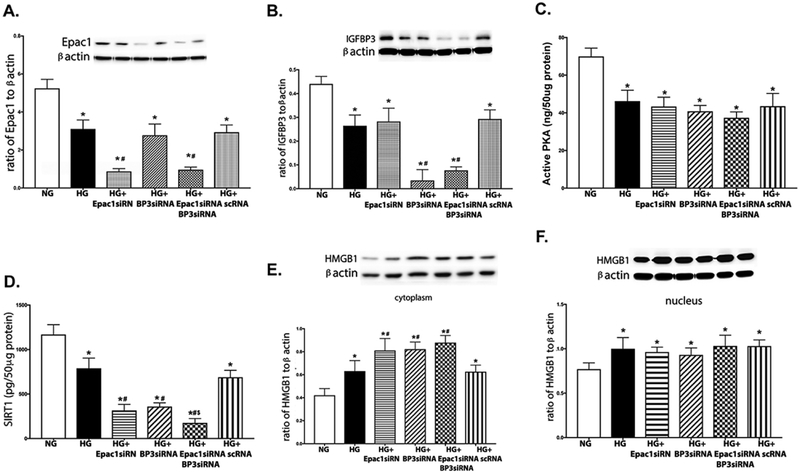
Figures 1A, B are controls to show successful knockdown of Epac1 and IGFBP-3, respectively. Figure 1C shows that high glucose decreased PKA activity, which agrees with our previous findings [5]. Figure 1D shows that high glucose significantly decreased SIRT1 levels, which was further reduced when IGFBP-3 and Epac1 were blocked by siRNA. Figure 1E shows that high glucose alone increased cytoplasmic HMGB1 levels, which was regulated by Epac1 and IGFBP-3 since high glucose+IGFBP-3 siRNA and high glucose+Epac1 siRNA were significantly higher than high glucose only. Figure 1F shows that high glucose also increased nuclear HMGB1, which was not altered by Epac1 or IGFBP-3. Taken together, these results suggest that high glucose altered HMGB1 levels through IGFBP-3 and SIRT1.
Figure 1.
High glucose decreases SIRT1 levels, while increasing cytoplasmic HMGB1 levels. REC were grown in normal glucose (5mM, NG), high glucose (25mM, HG), HG+Epac1 siRNA, HG+IGFBP-3 siRNA, HG+Epac1 and IGFBP-3 siRNA, or HG+scrambled siRNA (HG+Sc). Panel A shows Epac1 levels and Panel B shows IGFBP-3 levels demonstrating successsful knockdown by siRNA. Panel C shows high glucose decreased PKA activity, independent of Epac1 and IGFBP-3. Panel D is SIRT1 levels, Panel E is cytoplasmic HMGB1, and Panel F is nuclear HMGB1 levels. *P<0.05 vs. NG, #P<0.05 vs. HG, $P<0.05 vs. Epac1 or IGFBP-3 siRNA. Data are mean ± SEM. N=4 for all groups.
Forskolin can increase SIRT1, leading to decreased cytoplasmic HMGB1 independent of Epac1.
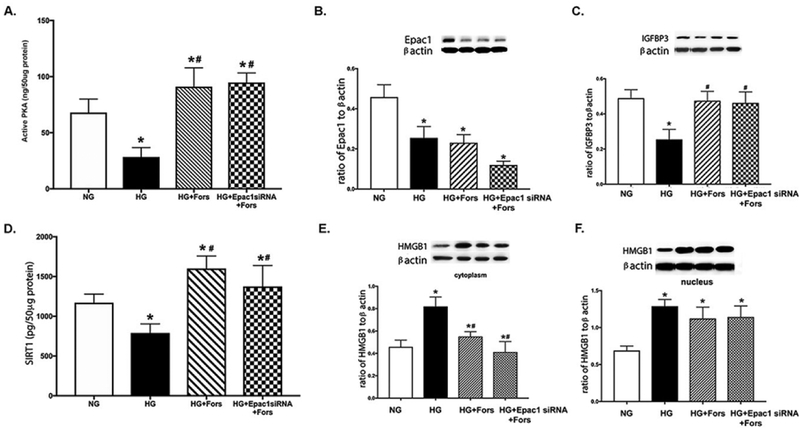
We have reported that Epac1 significantly reduced HMGB1 levels in REC [6]. However, both PKA and Epac1 may regulate this pathway independently. To test this, REC grown in high glucose were treated with forskolin following transfection with Epac1 siRNA. We used the Epac1 siRNA to insure that actions of PKA are independent of Epac1. Figure 2A confirmed that forskolin significantly increased PKA activity independent of Epac1. Figure 2B was a control to confirm successful knockdown of Epac1 by the siRNA. Figure 2C confirmed that high glucose decreased IGFBP-3 levels, which we have previously reported [5]. PKA significantly increased IGFBP-3 levels, independent of Epac1 (Figure 2C). High glucose decreased SIRT1 levels, which were increased by the PKA agonist (Figure 2D). Cytoplasmic HMGB1 were increased by the high glucose culturing conditions (Figure 2E). This response was reduced by forskolin, independent of Epac1. As shown in Figure 1F, high glucose increased nuclear HMGB1, which was not altered by forskolin. These data suggest that PKA reduced cytoplasmic HMGB1 levels, independent of Epac1. This regulation likely involved IGFBP-3 and SIRT1.
Figure 2.
Forskolin regulates HMGB1, independent of Epac1. REC were grown in normal glucose (5mM, NG), high glucose (25mM, HG), HG+forskolin (HG+Fors), or HG+forskolin+Epac1 siRNA (HG+Epac1 siRNA+Fors). Panel A shows PKA activity is increased by forskolin, and Panel B shows effective knockdown by Epac1 siRNA. Panel C shows IGFBP-3 levels, Panel D shows SIRT1 levels, Panel E shows cytoplasmic HMGB1 levels, and Panel F is nuclear HMGB1 levels. *P<0.05 vs. NG, #P<0.05 vs. HG. Data are mean ± SEM. N=4 for all groups.
IGFBP-3 increases SIRT1 and decreases HMGB1 in REC.
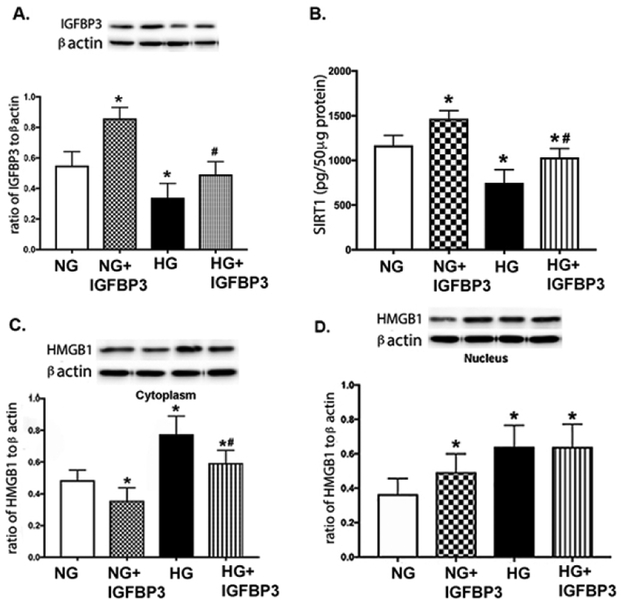
We have previously reported that PKA can regulate IGFBP-3 actions in REC to prevent apoptosis [17]. In these studies, we used the same IGFBP-3 plasmid that does bind IGF-1 to determine whether IGFBP-3 regulated SIRT1 and HMGB1 levels. Figure 3A shows that the plasmid effectively increased IGFBP-3 levels in REC grown in normal or high glucose as a control. Figure 3B shows that high glucose decreased SIRT1 levels. The IGFBP-3 plasmid increased SIRT1 levels in both normal and high glucose. Cytoplasmic HMGB1 was increased in high glucose, but significantly decreased when the IGFBP-3 plasmid was added (Figure 3C). The IGFBP-3 plasmid decreased cytoplasmic HMGB1 in normal glucose as well (Figure 3C). In both normal and high glucose, the IGFBP-3 plasmid increased nuclear HMGB1 levels, but the plasmid did not significantly increase nuclear HMGB1 levels in REC grown in high glucose compared to cells grown in high glucose alone (Figure 3D). These data suggest that IGFBP-3 can regulate SIRT1 and HMGB1 levels in REC.
Figure 3.
IGFBP-3 regulates SIRT1 and HMGB1. REC were grown in normal glucose (5mM, NG), high glucose (25mM, HG), NG+IGFBP-3 plasmid (does not bind IGF-1), and HG+IGFBP-3 plasmid. Panel A is a control to show that the plasmid increased IGFBP-3 levels. Panel B shows SIRT1 levels, Panel C is cytoplasmic HMGB1, and Panel D is nuclear HMGB1 levels. *P<0.05 vs. NG, #P<0.05 vs. HG. Data are mean ± SEM. N=4 for all groups
Forskolin cannot inhibit HMGB1 when SIRT1 is blocked with Ex527.
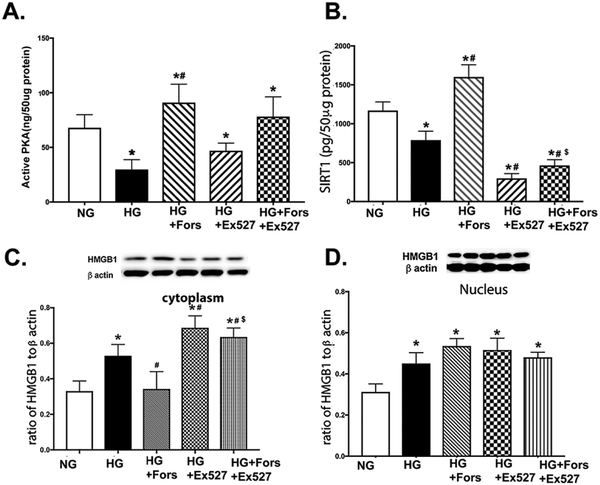
To determine the role of SIRT1 in the pathway, we used Ex527 to inhibit SIRT1 levels prior to forskolin treatment. Figure 4A shows that forskolin increased PKA actions, which were not altered by SIRT1 inhibition. Figure 4B shows that forskolin overcame the high glucose-induced decrease in SIRT1. Ex527 was effective in reducing SIRT1 levels, and forskolin was able to significantly (although slightly) increase SIRT1 levels after Ex527 treatment. Panels 4C confirmed that PKA can overcome the high glucose-induced increase in cytoplasmic HMGB1. Inhibition of SIRT1 significantly increased cytoplasmic HMGB1 levels, which was reduced by forskolin treatment. Forskolin did not reduce nuclear HMGB1 levels, nor did the inhibition of SIRT1. Taken together, these data suggest that PKA requires SIRT1 to regulate cytoplasmic HMGB1 levels.
Figure 4.
PKA requires SIRT1 to decrease cytoplasmic HMGB1. REC were grown in normal glucose (5mM, NG), high glucose (25mM, HG), HG+forskolin (HG+Fors), HG+Ex527 (Sirt1 inhibitor), or HG+Ex527+Forskolin (HG+Fors+Ex527). Panel A shows that forskolin increased PKA activity. Panel B shows that Ex527 inhibited SIRT1, Panel C is cytoplasmic HMGB1, and Panel D is nuclear HMGB1 levels. *P<0.05 vs. NG, #P<0.05 vs. HG, $P<0.05 vs. HG+Fors. Data are mean ± SEM. N=4 for all groups.
4. Discussion
Diabetic retinopathy, particularly type 2 diabetes, is increasingly linked to inflammation. We have previously reported that Compound 49b, a novel β-adrenergic receptor agonist, protected the retina against diabetes-induced damage [5]. We sought to determine key factors downstream of β-adrenergic receptors that may mediate these responses in the retinal vasculature. We focused on the regulation of HMGB1, as others have reported that HMGB1 mediated retinal neuropathy in rats [10]. Members of the same group also showed that HMGB1 and reactive oxygen species cause apoptosis in REC and diabetic human and rat retinas [23]. Additionally, work in RAW264.7 cells showed that isoproterenol, a β-adrenergic receptor agonist, decreased HMGB1 levels and increased survival in septic mice [24]. We found that high glucose alone significantly increased cytoplasmic HMGB1 levels, with decreased SIRT1 levels. These high glucose mediated responses were exacerbated when Epac1 or IGFBP-3 were blocked with siRNA.
We have recently showed that Epac1 reduced inflammatory mediators, including HMGB1 [6, 25]. However, there is also literature that PKA may regulate HMGB1 [15]. Additionally, PKA and Epac1 can regulate vascular changes independently [14]. To test the actions of PKA independent of Epac1, Figure 2 shows that forskolin increased both SIRT1 and IGFBP-3 levels, while decreasing cytoplasmic HMGB1 levels when Epac1 was blocked by siRNA. There is not a great deal of literature on PKA regulation of HMGB1, particularly in the retina. However, work in the mosquito showed that PKA inhibited HMGB1 [16]. Similarly, studies in monocytes showed that histamine can inhibit HMGB1 actions through PKA activity [26]. In contrast, work in lung cancer cells showed that HMGB1 regulates PKA activity to suppress metastasis and cell motility [27]. Nonetheless, our findings do agree with studies showing that PKA inhibits HMGB1 actions.
In an attempt to determine the cellular mechanisms by which PKA blocks HMGB1 actions, we started with IGFBP-3, as we have previously showed that PKA increases IGFBP-3 actions to reduce REC apoptosis [17]. Others have reported that IGFBP-3 is neuroprotective in the hypothalamus in type 1 diabetic mice [28]. In a subsequent study, these authors also found that the decrease in hypothalamic activity of type 1 diabetic mice was correlated with cytoplasmic translocation of HMGB1 [29]. We focused on nuclear versus cytoplasmic levels of HMGB1 to address the cytoplasmic translocation of HMGB1, which leads to inflammatory responses. Our findings in REC agree with the work in the hypothalamus, showing that the non-IGF binding IGFBP-3 plasmid decreased cytoplasmic HMGB1, which was associated with increased SIRT1 levels. The interaction of IGFBP-3 and SIRT1 was also observed in diabetic keratopathy models [30]. In addition to IGFBP-3, our findings also showed that forskolin requires active SIRT1 to decrease cytoplasmic HMGB1 levels, as forskolin was ineffective on cytoplasmic HMGB1 when Ex527 was used. The exact mechanisms by which IGFBP-3 can regulate HMGB1 will be focus of future studies.
In conclusion, our findings expand work in other systems to show that high glucose decreases SIRT1 and increases cytoplasmic HMGB1 levels in REC through IGFBP-3 actions. PKA mediates the reduction in cytoplasmic HMGB1 through increased IGFBP-3 and SIRT1 actions, as forskolin was unable to reduce cytoplasmic HMGB1 when IGFBP-3 siRNA or SIRT1 inhibitors were used. PKA can mediate the actions on cytoplasmic HMGB1 through IGFBP-3 and SIRT1 independent of Epac1, since the actions of forskolin occurred when Epac1 siRNA was used. These studies provide novel targets to reduce HMGB1-induced retinal inflammation.
Acknowledgement of Support:
R01EY028442 (JJS), P30EY04068 (Hazlett), and an Unrestricted Grant to the Department of Ophthalmology from Research to Prevent Blindness (Kresge Eye Institute). The funders did not influence these design or execution of these studies.
References.
- 1.Joussen AM, Poulaki V, Le ML, et al. , A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004; 18 1450–2. [DOI] [PubMed] [Google Scholar]
- 2.Tang J and Kern TS, Inflammation in diabetic retinopathy. Progress in retinal and eye research. 2011; 30 343–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abcouwer SF, Lin CM, Shanmugam S, et al. , Minocycline prevents retinal inflammation and vascular permeability following ischemia-reperfusion injury. Journal of neuroinflammation. 2013; 10 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Q and Steinle JJ, IGFBP-3 inhibits TNF-alpha production and TNFR-2 signaling to protect against Retinal Endothelial Cell Apoptosis. Microvascular research. 2014; 95 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Q, Guy K, Pagadala J, et al. , Compound 49b Prevents Diabetes-Induced Apoptosis through Increased IGFBP-3 Levels. Investigative ophthalmology & visual science. 2012; 53 3004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang Y, Liu L, Curtiss E, and Steinle JJ, Epac1 Blocks NLRP3 Inflammasome to Reduce IL-1beta in Retinal Endothelial Cells and Mouse Retinal Vasculature. Mediators Inflamm. 2017; 2017 2860956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C, Du J, Feng W, et al. , beta-Adrenergic receptors stimulate interleukin-6 production through Epac-dependent activation of PKCdelta/p38 MAPK signalling in neonatal mouse cardiac fibroblasts. British journal of pharmacology. 2012; 166 676–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu H, Chen Z, Xie J, et al. , High Mobility Group Box-1: A Missing Link between Diabetes and Its Complications. Mediators Inflamm. 2016; 2016 3896147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun X, Zeng H, Wang Q, et al. , Glycyrrhizin ameliorates inflammatory pain by inhibiting microglial activation-mediated inflammatory response via blockage of the HMGB1-TLR4-NF-kB pathway. Exp Cell Res. 2018. [DOI] [PubMed] [Google Scholar]
- 10.Abu El-Asrar AM, Siddiquei MM, Nawaz MI, Geboes K, and Mohammad G, The proinflammatory cytokine high-mobility group box-1 mediates retinal neuropathy induced by diabetes. Mediators Inflamm. 2014; 2014 746415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dvoriantchikova G, Hernandez E, Grant J, et al. , The high-mobility group box-1 nuclear factor mediates retinal injury after ischemia reperfusion. Investigative ophthalmology & visual science. 2011; 52 7187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Jiang Y, and Steinle JJ, Inhibition of HMGB1 protects the retina from ischemia-reperfusion, as well as reduces insulin resistance proteins. PloS one. 2017; 12 e0178236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos AR, Dvoriantchikova G, Li Y, et al. , Cellular mechanisms of high mobility group 1 (HMGB-1) protein action in the diabetic retinopathy. PloS one. 2014; 9 e87574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Netherton SJ, Sutton JA, Wilson LS, Carter RL, and Maurice DH, Both protein kinase A and exchange protein activated by cAMP coordinate adhesion of human vascular endothelial cells. Circulation research. 2007; 101 768–76. [DOI] [PubMed] [Google Scholar]
- 15.Cho JH, Lee YK, and Chae CB, The modulation of the biological activities of mitochondrial histone Abf2p by yeast PKA and its possible role in the regulation of mitochondrial DNA content during glucose repression. Biochimica et biophysica acta. 2001; 1522 175–86. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro FS, de Abreu da Silva IC, Carneiro VC, et al. , The dengue vector Aedes aegypti contains a functional high mobility group box 1 (HMGB1) protein with a unique regulatory C-terminus. PloS one. 2012; 7 e40192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Q and Steinle JJ, DNA-PK phosphorylation of IGFBP-3 is required to Prevent Apoptosis in Retinal Endothelial cells cultured in High Glucose. Investigative ophthalmology & visual science. 2013; 54 3052–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabadi MM, Xavier S, Vasko R, et al. , High-mobility group box 1 is a novel deacetylation target of Sirtuin1. Kidney Int. 2015; 87 95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nin V, Escande C, Chini CC, et al. , Role of deleted in breast cancer 1 (DBC1) protein in SIRT1 deacetylase activation induced by protein kinase A and AMP-activated protein kinase. The Journal of biological chemistry. 2012; 287 23489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Dou X, Li S, et al. , Nicotinamide ameliorates palmitate-induced ER stress in hepatocytes via cAMP/PKA/CREB pathway-dependent Sirt1 upregulation. Biochimica et biophysica acta. 2015; 1853 2929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi YJ, Choi SE, Ha ES, et al. , Extracellular visfatin activates gluconeogenesis in HepG2 cells through the classical PKA/CREB-dependent pathway. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2014; 46 233–9. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, Jiang Y, Miller MJ, et al. , IGFBP-3 and TNF-alpha Regulate Retinal Endothelial Cell Apoptosis. Investigative ophthalmology & visual science. 2013; 54 5376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohammad G, Alam K, Nawaz MI, et al. , Mutual enhancement between high-mobility group box-1 and NADPH oxidase-derived reactive oxygen species mediates diabetes-induced upregulation of retinal apoptotic markers. J Physiol Biochem. 2015; 71 359–72. [DOI] [PubMed] [Google Scholar]
- 24.Ha YM, Ham SA, Kim YM, et al. , beta(1)-adrenergic receptor-mediated HO-1 induction, via PI3K and p38 MAPK, by isoproterenol in RAW 264.7 cells leads to inhibition of HMGB1 release in LPS-activated RAW 264.7 cells and increases in survival rate of CLP-induced septic mice. Biochem Pharmacol. 2011; 82 769–77. [DOI] [PubMed] [Google Scholar]
- 25.Liu L, Jiang Y, Chahine A, Curtiss E, and Steinle JJ, Epac1 agonist decreased inflammatory proteins in retinal endothelial cells, and loss of Epac1 increased inflammatory proteins in the retinal vasculature of mice. Molecular vision. 2017; 23 1–7. [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi H, Sadamori H, Teshigawara K, et al. , Histamine inhibits high mobility group box 1-induced adhesion molecule expression on human monocytes. Eur J Pharmacol. 2013; 718 305–13. [DOI] [PubMed] [Google Scholar]
- 27.Zuo Z, Che X, Wang Y, et al. , High mobility group Box-1 inhibits cancer cell motility and metastasis by suppressing activation of transcription factor CREB and nWASP expression. Oncotarget. 2014; 5 7458–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu P, Thinschmidt JS, Caballero S, et al. , Loss of survival factors and activation of inflammatory cascades in brain sympathetic centers in type 1 diabetic mice. Am J Physiol Endocrinol Metab. 2015; 308 E688–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thinschmidt JS, Colon-Perez LM, Febo M, et al. , Depressed basal hypothalamic neuronal activity in type-1 diabetic mice is correlated with proinflammatory secretion of HMBG1. Neuroscience letters. 2016; 615 21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Zhao X, Shi D, et al. , Overexpression of SIRT1 promotes high glucose-attenuated corneal epithelial wound healing via p53 regulation of the IGFBP3/IGF-1 R/AKT pathway. Investigative ophthalmology & visual science. 2013; 54 3806–14. [DOI] [PubMed] [Google Scholar]