Abstract
An obligate intracellular bacterium Chlamydia trachomatis requires host lipid ceramide for their replication within an intracellular membranous compartment, the inclusion. Chlamydial inclusion membrane protein D (IncD) composed of two closely linked long hydrophobic domains with their N- and C-termini exposed to the host cytosol, binds directly to the pleckstrin homology (PH) domain of the ceramide transport protein (CERT), likely redirecting ceramide to the inclusion. The precise regions of IncD required for this interaction have not been delineated. Using co-transfection studies together with phylogenetic studies, we demonstrate that both the IncD N- and C-terminal regions are required for binding to the CERT PH domain and define key interaction residues. Native gel electrophoresis analysis demonstrates that the transmembrane region of IncD forms SDS-resistant but dithiothreitol-sensitive homodimers, which in turn can assemble to form higher order oligomers through additional N- and C-terminal domain contacts. We propose that IncD oligomerization may facilitate high affinity binding to CERT, allowing C. trachomatis to efficiently redirect host ceramide to the inclusion.
Keywords: ceramide, Chlamydia trachomatis, contact sites, IncD, oligomerization, PH domain
1. Introduction
Chlamydia trachomatis is an obligate intracellular bacterium that is a major causative pathogen of a bacterial sexually transmitted disease in developed countries and also the leading cause of non-congenital blindness in developing countries [1]. After the entry into mammalian cells by receptor-mediated endocytosis Chlamydiae quickly dissociates from the canonical endo-lysosomal pathway and proliferates in a parasitophorous vacuole, the inclusion [2]. Chlamydiae selectively interact with multiple host cell compartments and trafficking pathways to acquire nutrients, in part by modifying the inclusion membrane through secretion and insertion of a unique set of effectors, the Inclusion membrane proteins (Incs). These proteins contain a bilobed hydrophobic domain composed of two closely spaced membrane-spanning regions that are separated by a short hairpin loop, with their N- and C-termini predicted to extend into the cytosplasm of the host cell [2]. The Chlamydial intracellular life cycle is unusual for a bacteria in that it requires host cell sphingolipids and cholesterol for replication and maintenance of inclusion membrane integrity [3–6]. The ceramide transport protein (CERT) is the major mediator of ceramide transport from the endoplasmic reticulum (ER) to the Golgi apparatus, where ceramide is converted to sphingomyelin [7]. Recent studies have revealed that the inclusion membrane protein IncD binds directly to and recruits CERT to the inclusion membrane [8–10]. Inhibition or depletion of CERT inhibits sphingolipid acquisition and intracellular replication of several Chlamydia species, including C. muridarum [6], C. trachomatis (9), and C. psittaci [11]. Together, these studies suggest that recruitment of CERT and subversion of the lipid synthesis pathway is required for the Chlamydial intracellular life cycle.
CERT contains three functional regions: an N-terminal pleckstrin homology (PH) domain that binds phosphatidylinositol-4-phosphate (PI4P) at the trans-Golgi; a two phenylalanines in an acidic tract (FFAT) motif that binds the ER integral membrane proteins VAP-A and VAP-B; and a C-terminal steroidogenic acute regulatory protein-related lipid transfer (START) domain that binds to and extracts ceramide from the ER membrane [7,12]. The N-terminal region of CERT including the PH domain, but not its FFAT motif, is necessary for recruitment to the inclusion and for binding to IncD [8,10]. However, the molecular details of how IncD binds to the CERT PH domain are unknown. In this work, we defined the structural features of IncD that are crucial for its binding to the CERT PH domain. Our studies suggest that both the N- and C-terminal regions of IncD coordinately bind to CERT PH domain while the IncD transmembrane (TM) domains facilitate formation of multimers of IncD dimers.
2. Materials and methods
This section is described in Supplementary Materials.
3. Results
Both the N- and C-terminal Cytosolic Regions of IncD Are Required for Binding to CERT PH Domain.
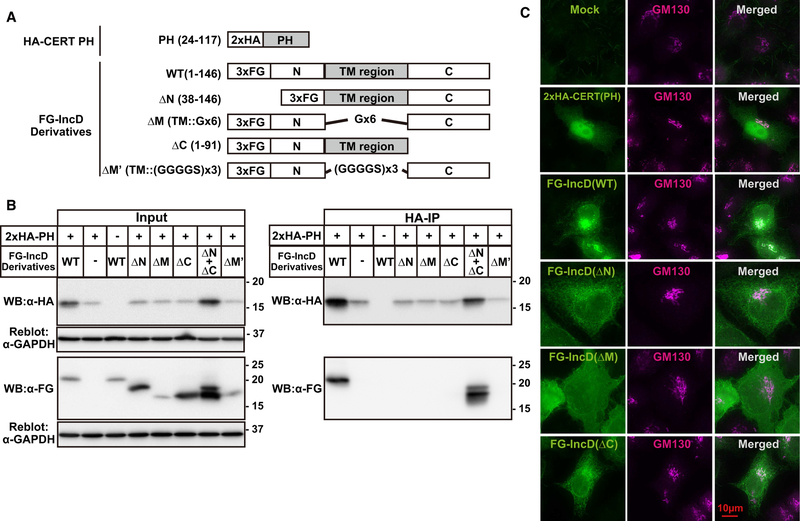
C. trachomatis IncD is a 146 amino acid protein comprised of two long TM domains (bilobed hydrophobic region) embedded in the inclusion membrane that are connected by a short cytosolic linker, with the N-terminal ~40 amino acids and C-terminal ~50 amino acids exposed to the host cytosol [9]. To determine the region(s) of IncD required for interaction with the CERT PH domain, we constructed N-terminal 3×FLAG-tagged expression plasmids that encoded full length IncD (FG-IncD WT (aa 1–146)) or deletion constructs in which either the N-terminal cytosolic exposed domain was deleted (FG-IncD ΔN (38–146)), the C-terminal cytosolic exposed domain was deleted (FG-IncD ΔC (aa 1–91)), or the TM domain was deleted (Fig. 1A). For the TM deletion constructs, we inserted two different linkers to connect the N and C-terminal regions (Fig. 1A): FG-IncD ΔM’, which contained a hexa-glycine linker or FG-IncD ΔM’, which utilized a longer and therefore more flexible linker (3×(Gly-Gly-Gly-Gly-Ser).
Figure 1. Molecular analysis of the interaction between IncD and CERT PH domain.
(A) Schematic diagram of relevant constructs. The TM region of IncD is highlighted by a grey box. The TM region is replaced with hexa-glycine linker (Gx6) in the construct of FG-IncD ΔM or with three tandem repeats of Gly-Gly-Gly-Gly-Ser (GGGGSx3) in the construct of FG-IncD ΔM’. (B) HeLa cells were co-transfected with pcDNA4/TO encoding the indicated derivatives of FG-IncD together with pcDNA3.1 neo (+) encoding HA-CERT PH. Total amounts of used plasmids for cells were adjusted to constant by adding mock plasmid. For the double transfection with FG-IncD ΔN and FG-IncD ΔC, the same amounts of each plasmid compared to single transfection was used. Cells were lysed with previously described lysis buffer containing 1% Triton X-100 [25]. Left panels: The cell extracts were analysed by Western blotting (Input). The amount loaded as “Input” is 1.0% of the amount used for immunoprecipitation when Western blotting with anti-HA antibody and 0.5% when Western blotting by anti-FLAG antibody. The membranes were reblotted with an anti-GAPDH antibody. Right panels: HA-CERT PH was immunoprecipitated with anti-HA antibody (HA-IP) from the cell extracts and analyzed by Western blotting with the indicated antibodies. Experiments were performed at least three times, and similar results were obtained. (C) HeLa cells were transiently expressed with various derivatives of FG-IncD or HA-CERT PH. The cells were double-labelled by indirect immunostaining with the anti-FLAG antibody (green) and anti-GM130 antibody (magenta) for the cells expressing derivative of FG-IncD or with the anti-HA antibody (green) and anti-GM130 antibody (magenta) for the cells expressing HA-CERT PH. The cells were observed by fluorescence microscopy. Experiments were performed at least three times, and similar results were obtained. Typical staining patterns are shown. The bar indicates 10 μm.
Lysates prepared from HeLa cells co-transfected with HA-CERT PH and the various FG-IncD constructs were immunoblotted with anti-FLAG or anti-HA antibody(input) or were immunoprecipitated with an anti-HA antibody followed by immunoblotting with anti-HA or anti-FLAG antibody (HA-IP) (Fig. 1B). All samples contained equivalent amounts of lysates as evidenced by immunoblotting with antibodies to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Despite the variable 3×FG-IncD levels, it is clear that co-immunoprecipitation of IncD with HA-CERT PH requires the TM region as well as the N- and C-terminal domains of IncD (lane 4–6 and 8 in right panel of Fig. 1B).
To rule out the possibility that the loss of interaction of the IncD deletion mutants with the CERT PH domain was due to altered subcellular distribution of transfected IncD constructs, we compared the subcellular localization of each of the IncD constructs by indirect immunofluorescence. Transfected HA-CERT PH localized to cytosol, nucleus, and Golgi (Fig. 1C), consistent with our previously published localization data of a CERT construct comprised of amino acids 24–152 [13]. Full-length IncD (FG-IncD WT) as well as the FG-IncD ΔN and FG-IncD ΔC constructs exhibited an ER-like reticular staining pattern with some enrichment to a GM-130 positive (i.e., Golgi) structure (Fig. 1C). Not unexpectedly, FG-IncD ΔM displayed largely diffuse cytosolic staining, though there was some co-localization with the Golgi marker. Together, these data indicate that some populations of the FG-IncD deletion constructs localized to the same subcellular compartments as CERT-PH. These results suggest that the failure the FG-IncD ΔN, FG-IncD ΔC, and the FG-IncD ΔM mutants to interact with CERT-PH was not due to altered subcellular distribution.
To determine whether the N- or C-terminal regions of IncD can transcomplement each other for binding to CERT-PH, we performed HA immunoprecipitations of lysates of cells that were triply co-transfected with FG-IncD ΔN, FG-IncD ΔC, and HA-CERT PH. Under these conditions, both the IncD constructs co-immunoprecipitated with HA-CERT PH (lane 7 in right panel of Fig. 1B). These results eliminate the possibility that the inability of the FG-IncD ΔN or FG-IncD ΔC constructs to interact with HA-CERT PH is due to IncD protein misfolding. These findings are consistent with the formation of a trimolecular complex in which the two IncD variants heterodimerize through their TM domains to form a functional CERT-PH binding interface (Fig. 1B).
We noted that the expression level of HA-CERT PH was markedly enhanced when co-transfected with full-length IncD or with either FG-IncD ΔN or FG-IncD ΔC. This result suggests that the CERT PH domain is stabilized upon association with IncD.
Defining IncD residues required for the interaction with the CERT PH domain.
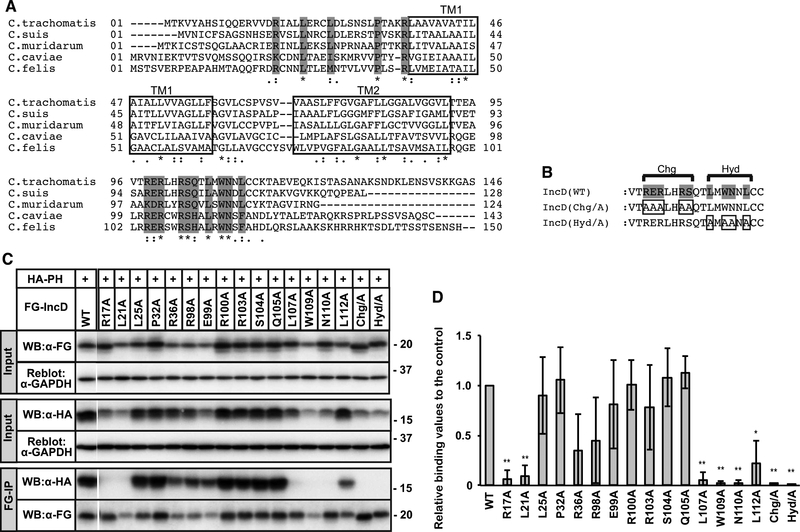
We next sought to define specific regions or residues in the IncD N- and C-terminal domains that are required for CERT-PH binding. As IncD from multiple Chlamydia species, including C. trachomatis [8], C. muridarum [6], and C. psittaci [11], has been shown to bind to CERT, we hypothesized that residues conserved in IncD N-and C-terminal host cell cytosolic-exposed domains would be required for CERT binding. Amino acid sequence alignment of IncD from C. trachomatis, C. suis, C. muridram, C. caviae and C. felis (Fig. 2A) reveals that the two predicted TM domains are highly conserved, while the N- and C-termini showed significant divergence; overall identity of these regions among the different species is less than 38% for the N-termini and 57% for the C-termini. Nonetheless, several amino acid residues are conserved in both the N- and C-terminal regions, notably a charged block (Chg) C-terminal to the second TM domain as well as an adjacent stretch of hydrophobic residues (Hyd). Highly conserved residues in N- and C-terminus of IncD were replaced with alanine either individually, or all of the Chg or Hyd region (Fig. 2B). Each of the constructs were transfected into HeLa cells together with HA-CERT PH, and FG immunoprecipitations were performed (Fig. 2C). Due to variations in the expression levels of the transfected proteins, we normalized the immunoprecipitated HA-CERT PH to the input levels of HA-CERT PH (Fig. 2D). Mutation of the conserved arginine 17 or leucine 21 in the N-terminal region abolished IncD interaction with HA-CERT PH (Fig. 2C, 2D). Replacement of all the conserved residues in the Chg or Hyd blocks with alanines also abrogated IncD interaction with HA-CERT PH (Fig. 2C, 2D). Finally, replacement of leucine 107, tryptophan 109, or asparagine 110 with alanine, conserved residues in the C-terminus, abrogated IncD binding to CERT-PH (Fig. 2C, 2D). Together, these results are consistent with a requirement for both the N- and C-terminal cytosolic regions of IncD for binding to CERT-PH and suggest that despite rapid evolution amongst Chlamydia species, the ability of IncD to bind to CERT-PH has been maintained.
Figure 2. Identification of IncD residues necessary for binding to the CERT PH domain.
(A) Alignment of amino acid sequence of IncD from the indicated Chlamydia species. The predicted TM ™ regions are indicated as white rectangle. Invariant amino acids are shaded grey. (B) The C-terminal amino acids that form the conserved Charged (Chg) and Hydrophobic (Hyd) clusters are indicated. Invariant residues are shaded grey and the residues that were mutated are boxed. (C) HeLa cells were co-transfected with pcDNA4/TO encoding the indicated derivatives of FG-IncD and with pcDNA3.1 neo (+) encoding HA-CERT PH. Samples were prepared as described in Fig. 1. The cell extracts were analysed by Western blotting (Input). The amount loaded as “Input” is 0.6% of the amount used for immunoprecipitation when Western blotting with HA antibody and 2.0% when Western blotting by FLAG antibody. Immunoprecipitated fractions with anti-FLAG antibody (FG-IP) from the cell extracts were analyzed by Western blotting with anti-HA antibody. The membranes were reblotted with an anti-GAPDH antibody. A portion of the gel (between WT and R17A) was spliced out. (D) For the Input fraction and the FG-IP fraction, each band in the image of Western blotting with anti-HA antibody was quantified. The Quantitative values of FG-IP fractions were divided by that of input fractions to correct expression levels and the values are shown as relative values to the control fraction (FG-IncD WT). See Supplementary Material for detail. The data shown are the means ± S.D. from three experiments. *p<0.05 **p<0.01
The Bilobed Hydrophobic Region is Required for IncD oligomerization
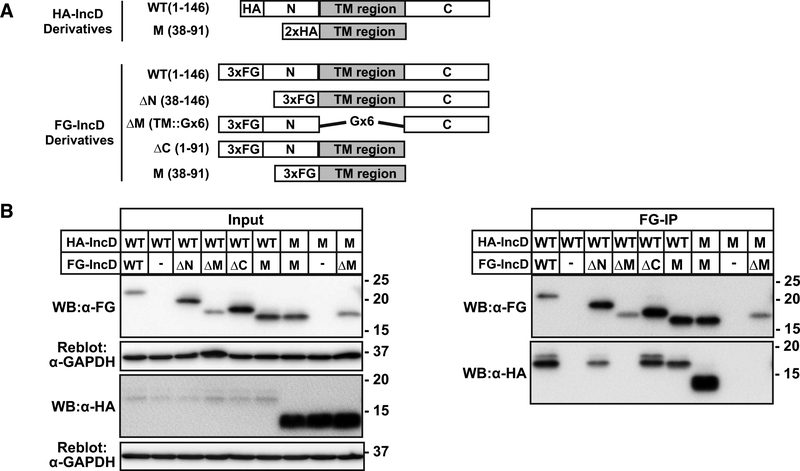
As previous studies have demonstrated that IncD can form homo-oligomers [14,15], we defined the domains of IncD that are necessary and/or sufficient for homodimerization. For these studies, HA-tagged full-length IncD (HA-IncD WT) or TM domain (HA-IncD M) together with FG-tagged constructs as described in Fig. 1A were co-transfected into HeLa cells and lysates immunoprecipitated with anti-FG (Fig. 3A, 3B). The TM domain of IncD was necessary and sufficient for homotypic interactions (Fig. 3B). We conclude that the IncD TM region is involved in homo-oligomerization, whereas the N- and C-terminal domains are required for binding to the CERT-PH domain.
Figure 3. Molecular analysis of the homotypic interaction of IncD.
(A) Schematic diagram relevant derivatives of FG-IncD or HA-IncD. (B) HeLa cells were co-transfected with pcDNA4/TO encoding the indicated derivatives of FG-IncD or an empty vector and with pcDNA3.1 neo (+) encoding the indicated derivatives of HA-IncD. Samples were prepared as described in Fig. 1. Left panels: The cell extracts were analysed by Western blotting. The amount loaded as “Input” is 0.4% of the amount used for immunoprecipitation when Western blotting with HA antibody and 1.5% when Western blotting by FLAG antibody. The membranes were reblotted with an anti-GAPDH antibody. Right panels: FLAG-tagged IncD derivatives were immunoprecipitated with anti-FLAG antibody (FG-IP) from the cell extracts and analyzed by Western blotting with the indicated antibodies. Experiments were performed at least three times, and similar results were obtained.
IncD is capable of forming higher order oligomers of dimers
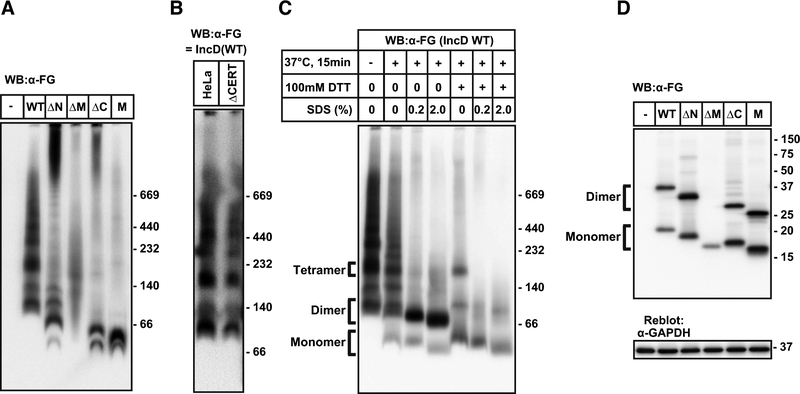
We used non-denaturing gel electrophoresis to investigate whether transfected IncD was capable of forming higher order oligomers. Lysates from HeLa cells transfected with FG-IncD constructs were subjected to Blue-Native PAGE and immunoblotted with anti-FLAG antibody. FG-IncD WT demonstrated multiple oligomeric complexes with masses ranging from ~80 kDa to ~1000 kDa (Fig. 4A). Because hydrophobic regions of proteins bind more dye molecules in Blue-Native PAGE than the non-hydrophobic regions, the apparent molecular mass values of membrane proteins on Blue-Native PAGE were corrected by a factor of 1.8 to predict the actual mass value [16], yielding a corrected mass of 44 kDa for IncD on Blue-Native PAGE, a value close to the predicted mass of (38.8 kD). In contrast to the laddering pattern of FG-IncD WT, FG-IncD M exhibited only the two lower molecular weight bands (Fig. 4A). FG-IncD ΔN and FG-IncD ΔC exhibited more complex patterns: two bands with apparent low molecular mass values, a partial ladder pattern, and a smear of bands with very high molecular mass, likely representing aggregates (Fig. 4A). These results suggest that the IncD TM domains facilitate homo-dimerization, whereas the N- and C-terminal regions contribute to higher order oligomerization. IncD oligomerization was not dependent upon its association with CERT, as we observed similar oligomeric states in HeLa cells in which CERT was inactivated by gene-editing technology (Fig. 4B)[17].
Figure 4. IncD forms higher order oligomers by dimers.
(A) HeLa cells were transfected with pcDNA4/TO encoding FG-IncD WT, FG-IncD ΔN, FG-IncD ΔM, FG-IncD ΔC, FG-IncD M, or an empty vector. Cell extracts were prepared from the cells with 1% dodecylmaltoside-containing lysis buffer. The cell extracts were separated by Blue-Native-PAGE as manufacturer’s instruction and analysed by Western blotting with anti-FLAG antibody. (B) HeLa cells or CERT-deficient HeLa cells were transfected with plasmid encoding FG-IncD WT and cell extracts were prepared as described above. The cell extracts were separated by Blue-Native-PAGE and analysed by Western blotting with anti-FLAG antibody. (C) HeLa cells were transfected with pcDNA4/TO encoding FG-IncD WT and cell extracts were prepared as described. For samples of Blue-Native-PAGE, cell extracts were incubated at 37 °C for 15 min in the presence or absence of the indicated reagents. (D) The cells described in Fig. 4A were lysed by LDS-containing nonreducing loading buffer. The lysate was subjected to SDS-PAGE under non-reducing conditions and, after electrophoresis, IncD constructs were detected by Western blotting. The membranes were reblotted with an anti-GAPDH antibody.
We noted that lysates of FG-IncD WT-transfected cells that were prepared in buffer containing lithium dodecyl sulfate (LDS) and DTT migrated as an apparent monomer when electrophoresed on SDS-PAGE (Figs. 1–3), suggesting that detergents or reducing reagents could disrupt IncD oligomerization. To examine the stability of oligomers in more detail, cell extracts from the FG-IncD WT-expressing cells were pre-treated with SDS or DTT for 15 min at 37 °C and analyzed by Blue-Native PAGE (Fig. 4C). Heat treatment failed to affect oligomerization, but FG-IncD WT exhibited reduced oligomeric laddering pattern when the lysates were pretreated with 0.2% SDS or 100 mM DTT at 37° for 15 minutes. No oligomers were observed when the extracts were exposed to both SDS and DTT (Fig. 4C), suggesting that the fastest migrating bands observed in SDS/DTT-treated FG-IncD WT samples represent monomeric FG-IncD WT and that the second fastest migrating band represents an IncD dimer. Dimeric IncD was further dissociated upon treatment with DTT, suggesting that disulfide bond formation is required for stable formation of IncD homodimers.
To define the region(s) involved in the DTT-sensitive dimerization, IncD deletion constructs were analyzed by SDS-PAGE under non-reducing conditions. FG-IncD WT, FG-IncD ΔN, FG-IncD ΔC, and FG-IncD M migrated as a monomer and dimer, whereas IncD ΔM migrated only as a monomer (Fig. 4D). Thus, the TM region is necessary to form the DTT-sensitive dimeric unit. Collectively, these data indicated that IncD formed multiple oligomers composed of dimers linked by disulfide bond formation in the bilobed hydrophobic region.
4. Discussion
We have used co-transfection approaches to delineate regions of IncD that are required for its interaction with the CERT-PH domain. Our studies reveal that both the N- and C-terminal domains of transfected IncD are required for its interaction with the CERT-PH domain. The IncD N- and C-terminal regions can function in trans, suggesting that they both contribute to formation of the CERT-PH binding interface and that the requirement for both domains does not reflect allosteric effects. Guided by phylogenetic comparisons, we identified highly conserved residues in both the N- and C-terminal regions of IncD that are required for binding to the CERT PH domain. Even though the predicted N-terminal cytosolic-exposed region is short−−37 amino acids--and likely also encodes the type III secretion signal as well as a potential chaperone binding site [18], we identified at least two highly conserved residues that are required for binding to the CERT-PH domain. We believe that this is the first example of an N-terminal Inc region required for binding to the host interaction partner(s). For other Incs for which this has been examined, including IncE [19,20], IncA [21,22], and CT850 [23], the C-terminal regions have been shown to be sufficient for binding to the known host partners. We recognize that our conclusions are based upon interactions determined by co-transfection and that they await final validation by creating relevant mutations in the C. trachomatis IncD gene and testing them in the context of infection.
Our studies provide additional insights into the ability of IncD to form oligomeric structures [14,15]. The TM regions are required for homodimerization and for the ability of the individual N- and C-terminal regions of IncD to transcomplement each other for binding to the CERT-PH domain. IncD homodimerization was disrupted upon exposure to DTT, suggesting disulfide bonding within the TM or linker region. Our results differ slightly from those reported by Derre et al in which they overexpressed in Chlamydia chimeras of IncD and an unrelated Inc protein, IncE, to assess homodimerization (15). In their studies, the IncD TM region was not sufficient for homodimierization [15]. The somewhat discrepant results can be reconciled by our observation that the N- and C-terminal regions of IncD may contribute to formation of higher order oligomers.
Based on our results, we propose a model of the interaction between the CERT PH domain and IncD: The bilobed hydrophobic region of IncD forms a stable homotypic dimer via intermolecular disulphide-bonding, while additional interactions through the N- and/or C-terminal cytosolic exposed regions are required for higher order oligomerization to form a multivalent CERT PH domain binding interface, enhancing the efficiency of CERT recruitment to the inclusion. In this context, it is noteworthy that CERT behaves as oligomer (presumably trimer) in cells [24]. Given the rapid divergence of Incs, the conservation of these IncD residues suggests that binding to CERT-PH reflects evolutionary pressure to redirect and acquire host cell ceramide.
Supplementary Material
Highlights.
Both the N- and C-termini of IncD are required for the CERT-IncD interaction.
IncD residues essential for the binding to CERT are conserved among Chlamydiaces.
IncD forms multiple oligomers composed of a dimeric unit.
Acknowledgements
This work was supported by MEXT of Japan for Scientific Research on Innovative Areas (17H06417 to K.H.), Japan AMED for AMED-CREST (JP17gm0910005j0003 to K.H.), JSPS for Grant-in-Aid for Scientific Research (C) (18K06649 to K.K.), NIH R01 AI073770 (JE), NIH R01 AI122747 (JE), and NIH R21 AI105561 (JE).
Glossary
The Abbreviations used are:
- CERT
ceramide transport protein
- TM
transmembrane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Mandell GL, Bennett JE, Dolin R, Mandell, Douglas, and Bennett’s Principles and practice of infectious diseases. 7th edition, in: Churchill Livingstone/Elsevier, Philadelphia, PA, 2010. [Google Scholar]
- [2].Elwell C, Mirrashidi K, Engel J, Chlamydia cell biology and pathogenesis., Nat. Rev. Microbiol 14 (2016) 385–400. doi: 10.1038/nrmicro.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hackstadt T, Rockey DD, Heinzen RA, Scidmore MA, Chlamydia trachomatis interrupts an exocytic pathway to acquire endogenously synthesized sphingomyelin in transit from the Golgi apparatus to the plasma membrane., Embo J. 15 (1996) 964–977. [PMC free article] [PubMed] [Google Scholar]
- [4].Kesley Robertson D, Gu L, Rowe RK, Beatty WL, Inclusion biogenesis and reactivation of persistent Chlamydia trachomatis requires host cell sphingolipid biosynthesis, PLoS Pathog. 5 (2009). doi: 10.1371/journal.ppat.1000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Van Ooij C, Kalman L, Van Ijzendoorn S, Nishijima M, Hanada K, Mostov K, Engel JN, Host cell-derived sphingolipids are required for the intracellular growth of Chlamydia trachomatis, Cell. Microbiol 2 (2000) 627–637. doi: 10.1046/j.1462-5822.2000.00077.x. [DOI] [PubMed] [Google Scholar]
- [6].Elwell CA, Jiang S, Kim JH, Lee A, Wittmann T, Hanada K, Melancon P, Engel JN, Chlamydia trachomatis co-opts gbf1 and cert to acquire host sphingomyelin for distinct roles during intracellular development, PLoS Pathog. 7 (2011). doi: 10.1371/journal.ppat.1002198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M, Molecular machinery for non-vesicular trafficking of ceramide, Nature. 426 (2003) 803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- [8].Derré I, Swiss R, Agaisse H, The lipid transfer protein CERT interacts with the Chlamydia inclusion protein IncD and participates to ER-Chlamydia inclusion membrane contact sites, PLoS Pathog. 7 (2011). doi: 10.1371/journal.ppat.1002092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bauler LD, Hackstadt T, Expression and targeting of secreted proteins from Chlamydia trachomatis., J. Bacteriol 196 (2014) 1325–34. doi: 10.1128/JB.01290-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Agaisse H, Derré I, Expression of the effector protein IncD in Chlamydia trachomatis mediates recruitment of the lipid transfer protein CERT and the endoplasmic reticulum-resident protein VAPB to the inclusion membrane, Infect. Immun 82 (2014) 2037–2047. doi: 10.1128/IAI.01530-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Koch-Edelmann S, Banhart S, Saied EM, Rose L, Aeberhard L, Laue M, Doellinger J, Arenz C, Heuer D, The cellular ceramide transport protein CERT promotes Chlamydia psittaci infection and controls bacterial sphingolipid uptake, Cell. Microbiol 19 (2017) e12752. doi: 10.1111/cmi.12752. [DOI] [PubMed] [Google Scholar]
- [12].Kawano M, Kumagai K, Nishijima M, Hanada K, Efficient trafficking of ceramide from the endoplasmic reticulum to the golgi apparatus requires a VAMP-associated protein-interacting FFAT motif of CERT, J. Biol. Chem 281 (2006) 30279–30288. doi: 10.1074/jbc.M605032200. [DOI] [PubMed] [Google Scholar]
- [13].Sugiki T, Egawa D, Kumagai K, Kojima C, Fujiwara T, Takeuchi K, Shimada I, Hanada K, Takahashi H, Phosphoinositide binding by the PH domain in ceramide transfer protein (CERT) is inhibited by hyperphosphorylation of an adjacent serine-repeat motif., J. Biol. Chem (2018) jbc.RA118.002465. doi: 10.1074/jbc.RA118.002465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gauliard E, Ouellette SP, Rueden KJ, Ladant D, Characterization of interactions between inclusion membrane proteins from Chlamydia trachomatis, Front. Cell. Infect. Microbiol 5 (2015) 1–11. doi: 10.3389/fcimb.2015.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Han Y, Derré I, A Co-infection Model System and the Use of Chimeric Proteins to Study Chlamydia Inclusion Proteins Interaction, Front. Cell. Infect. Microbiol 7 (2017) 1–9. doi: 10.3389/fcimb.2017.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Heuberger EHML, Veenhoff LM, Duurkens RH, Friesen RHE, Poolman B, Oligomeric state of membrane transport proteins analyzed with blue native electrophoresis and analytical ultracentrifugation, J. Mol. Biol 317 (2002) 591–600. doi: 10.1006/jmbi.2002.5416. [DOI] [PubMed] [Google Scholar]
- [17].Yamaji T, Hanada K, Establishment of HeLa cell mutants deficient in sphingolipid-related genes using TALENs, PLoS One. 9 (2014). doi: 10.1371/journal.pone.0088124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mueller KE, Plano GV, Fields KA, New frontiers in type III secretion biology: The Chlamydia perspective, Infect. Immun 82 (2014) 2–9. doi: 10.1128/IAI.00917-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Elwell CA, Czudnochowski N, von Dollen J, Johnson JR, Nakagawa R, Mirrashidi K, Krogan NJ, Engel JN, Rosenberg OS, Chlamydia interfere with an interaction between the mannose-6-phosphate receptor and sorting nexins to counteract host restriction, Elife. 6 (2017) 1–17. doi: 10.7554/eLife.22709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Paul B, Kim HS, Kerr MC, Huston WM, Teasdale RD, Collins BM, Structural basis for the hijacking of endosomal sorting nexin proteins by Chlamydia trachomatis, Elife. 6 (2017) 1–23. doi: 10.7554/eLife.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Weber MM, Noriea NF, Bauler LD, Lam JL, Sager J, Wesolowski J, Paumet F, Hackstadt T, A Functional Core of IncA Is Required for Chlamydia trachomatis Inclusion Fusion, J. Bacteriol 198 (2016) 1347–1355. doi: 10.1128/JB.00933-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ronzone E, Wesolowski J, Bauler LD, Bhardwaj A, Hackstadt T, Paumet F, An α-helical core encodes the dual functions of the chlamydial protein IncA, J. Biol. Chem 289 (2014) 33469–33480. doi: 10.1074/jbc.M114.592063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mital J, Lutter EI, Barger AC, Dooley CA, Hackstadt T, Chlamydia trachomatis inclusion membrane protein CT850 interacts with the dynein light chain DYNLT1 (Tctex1), Biochem. Biophys. Res. Commun 462 (2015) 165–170. doi: 10.1016/j.bbrc.2015.04.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Charruyer A, Bell SM, Kawano M, Douangpanya S, Yen TY, Macher BA, Kumagai K, Hanada K, Holleran WM, Uchida Y, Decreased ceramide transport protein (CERT) function alters sphingomyelin production following UVB irradiation, J. Biol. Chem 283 (2008) 16682–16692. doi: 10.1074/jbc.M800799200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kumagai K, Kawano-Kawada M, Hanada K, Phosphoregulation of the ceramide transport protein CERT at serine 315 in the interaction with VAMP-associated protein (VAP) for inter-organelle trafficking of ceramide in mammalian cells, J. Biol. Chem 289 (2014) 10748–10760. doi: 10.1074/jbc.M113.528380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.