ABSTRACT
There is a paucity of information on diarrheagenic enterotoxigenic Escherichia coli (ETEC)’s interaction with innate immune cells, in part due to the lack of reliable models that recapitulate infection in human gut. In a recent publication, we described the development of an ex vivo enteroid-macrophage co-culture model using human primary cells. We reported that macrophages residing underneath the epithelial monolayer acquired “resident macrophage” phenotype characterized by lower production of inflammatory cytokines and strong phagocytic activity. These macrophages extended projections across the epithelium, which captured ETEC applied to the apical side of the epithelium and reduced luminal bacterial load. Additional evidence presented in this addendum confirms these findings and further demonstrates that macrophage adaptation occurs regardless of the stage of differentiation of epithelial cells, and that ETEC uptake arises rapidly after infection. The enteroid-macrophage co-culture represents a novel and relevant tool to study host-cell interactions and pathogenesis of enteric infections in humans.
KEYWORDS: Enterotoxigenic E. coli, macrophages, intestine, enteroid model
ETEC-induced intestinal epithelial cell dysfunction and innate immune activation
Enterotoxigenic Escherichia coli (ETEC) is highly prevalent in poor countries and in areas lacking adequate sanitary conditions. It is a leading cause of diarrhea in young children living in these endemic regions and the primary etiology of travelers’ diarrhea.1 ETEC infection manifests clinically by sudden watery diarrhea that lasts 3 to 4 days. Although generally self-limiting, ETEC diarrhea can lead to severe dehydration and death. Illness can resolve with rehydration and antibiotic treatment, although options have been limited due to increasing antibiotic-resistance.1 Since ETEC is a noninvasive bacterium, pathogenicity relies mainly on the secretion of enterotoxins: heat-labile toxin (LT), which has high homology with cholera toxin, and heat-stable toxin (ST), as well as a variety of colonization factors (CFs) which are fimbrial, fibrillar, or afimbrial structures on the bacterial surface.1, 2 Beyond this broad understanding, precise mechanisms and molecules involved in ETEC pathogenesis remain to be elucidated. Studies have traditionally focused on roles of the CFs and enterotoxins. CFs are known to facilitate ETEC attachment to the small bowel, allowing ETEC LT and/or ST to be produced in close proximity to the intestinal epithelium. Once internalized through specific cell ligands, the toxins induce intracellular accumulation of cyclic AMP and GMP, respectively, which causes loss of electrolytes and water.2 More recently, additional factors such as the secreted EtpA adhesin molecule, the EatA serine protease autotransporter (a member of the serine protease autotransporter of the Enterobacteriaceae [SPATE] protein family), and the secreted YghJ metalloprotease have been implicated in virulence, by enhancing colonization through mucus degradation and bacterial attachment.3-5 Despite the progress made in delineating ETEC adherence and subsequent damage to intestinal epithelial cells, its interaction with the intestinal immune system in the early stages of infection, a critical step in determining host resistance, remains unknown. The fact that the enterotoxins cause disease by themselves and that ETEC has typically been regarded as a non-inflammatory pathogen might have shifted attention away from anti-microbial innate immune defenses. Meanwhile, mounting evidence from human and animal studies support the notion that ETEC infection is associated with mild intestinal inflammation and increased expression of innate immune genes in the gut mucosa.6-8 Leukocytes and lactoferrin as well as elevated levels of IL-8, IL-6, IL-1β, IFN-γ, and IL-1RA pro-inflammatory cytokines were found in fecal samples from children and adult travelers with ETEC diarrhea.6, 9, 10 Likewise, calprotectin was detected in stool of ETEC-infected piglets, indicating active recruitment of neutrophils to the intestinal lumen.11 In a mouse pulmonary model of ETEC infection, lung histopathology revealed a moderate to severe infiltration of neutrophils and macrophages in the alveoli.12 In addition, cytokines with pro-inflammatory effects, including IL-2, IL-12p40, IL-1β, IL-6, IFN-γ, and TNF-α have been observed in serum of ETEC-infected pigs.7 A study performed by Loos and colleagues examined early immune responses to ETEC in orally infected piglets and showed rapid (4h) upregulation of intestinal genes involved in innate immune function, which included IL-8, IL-1 and IL-17 cytokines, and MMP1 and MMP3 matrix metalloproteinases.8 The TLR4 and NF-kB signaling pathways are rapidly activated following ETEC infection, which likely contributes to the upregulation of these innate immune mediators.8, 13, 14 Together, these results provide strong evidence of active recruitment of phagocytic cells to the site of infection and local production of inflammatory mediators, and support the notion that innate immune components are activated and deployed in the wake of ETEC infection.
Modeling gut microbial-host cell interaction in a human enteroid-macrophage co-culture
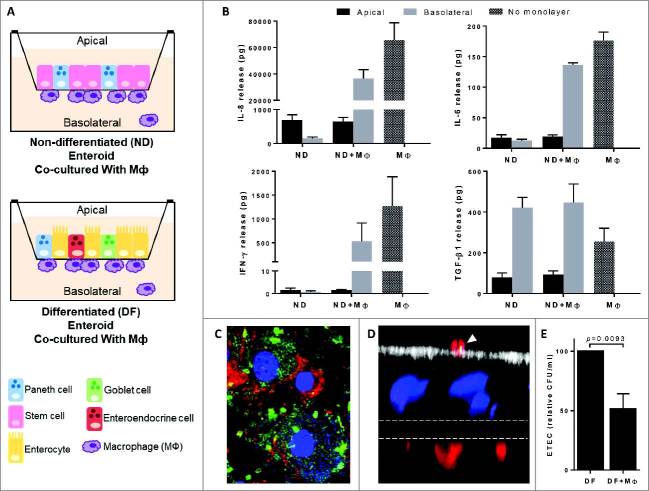
In a recent paper published by our group, we described the development of an in vitro model consisting of human primary intestinal epithelium and macrophages to study early innate immune activation at the mucosal interface upon pathogen exposure (Fig. 1A).15 The model is built on 2-dimensional (2D) enteroid monolayers established from human intestinal crypt-derived stem cells. Significantly, the model recreates the “crypt-like” (non-differentiated) and “villus-like” (differentiated) cellular diversity of the human intestinal epithelium that harbors the major intestinal epithelial cell lineages (i.e. Lgr5+ stem cells, Paneth cells, enterocytes, goblet cells, and enteroendocrine cells) and mucus layer, as schematically represented in Fig. 1A.15-18 Microbial-host cell interactions have traditionally been studied using transformed cell lines, yet their multiple genetic alterations and dysregulated function compromise the value and applicability of the results obtained. Further confounding factors are introduced by long-term cultures that fail to represent normal conditions. In preceding publications, our group described the successful establishment of enteroid and colonoid cultures from small intestinal and colonic tissue, respectively.15, 19 It is worth emphasizing the practical advantages of enteroid monolayers, which unlike the 3D culture configuration (i.e. spheroids) maintained in Matrigel, facilitate controlled access to the apical epithelial cell surface for treatment, as it occurs in vivo. In the enteroid-macrophage co-culture model we recently established, the monocyte-derived macrophages were purposefully seeded on the basolateral side of the enterocytes to mimic their location in vivo (Fig. 1A)15. This configuration allows for epithelial cell-macrophage interactions and because cells reside in separate compartments, it also allows the interrogation of the polarity (i.e. apical or basolateral) of cellular responses. The majority of macrophages remains firmly adhered to the filter, although a small fraction detach during culture.
Figure 1.
Sub-epithelial macrophages exhibited lower production of pro-inflammatory cytokines and high phagocytic activity. A) Schematic representation of enteroid-macrophages co-cultures established with non-differentiated (ND) and differentiated (DF) enteroid monolayers. B) IL-8, IL-6, IFN-γ, and TGF-β1 produced by ND enteroid-macrophage co-culture. Lower levels of IL-8, IL-6, and IFN-γ, but not TGF-β1, were produced by the ND enteroid-macrophage co-culture as compared to macrophages alone. Data correspond to mean ± SEM of multiple independent experiments; n = 6 for IL-8, n = 4 for TGF-β1, and n = 2 for IL-6 and IFN-γ. C) ETEC are phagocytosed by human macrophages following overnight infection. DNA (bacterium and macrophage DNA), blue; ETEC, green; CD14 (macrophages), red. D) Apical ETEC (yellow) phagocytosed by sub-epithelial macrophages extending dendrites across the epithelial cell monolayer. Actin (microvilli), white; nuclei, blue; ETEC, green; CD14 (macrophages), red; filter, dashed lines. E) ETEC CFU recovered from the apical compartment 30 min after infection was greatly reduced in the macrophage-enteroid co-culture as opposed to enteroid alone. Data represent mean ± SEM of N = 9 from 4 independent experiments. Methods and experimental conditions were the same as described in our previous publication.15
Different from traditional tissue culture systems, our enteroid-macrophage co-culture avoids macrophages being directly exposed to a high bacterial load, which may lead to events that bear little relevance to pathophysiological conditions.20 Instead, the sub-epithelial seeded macrophages more faithfully represent the shielding of non-activated phagocytic cells by the intestinal epithelial barrier in the human intestine.
In our recent publication, we demonstrated that macrophages could be engrafted underneath both non-differentiated and differentiated enteroid monolayers.15 We also showed that chemokines and cytokines known to play key roles in immune activation and maintenance of homeostasis in the gut mucosa, including TGF-β1, IL-8, IL-6, and IFN-γ, were secreted by the “villus-like” enteroid-macrophage co-culture. Both macrophages and differentiated epithelial cells produced these cytokines when cultured alone and are therefore believed to contribute to the levels measured in the combined culture. It was clear, however, that macrophages were responsible for the higher basolateral levels of IL-8, IL-6, and IFN-γ produced by the co-culture, while TGF-β1 was produced by both enterocytes and macrophages.15 New data presented in this addendum confirm these results and further reveal a similar pattern of cytokine and chemokine production by macrophages and the enterocytes bearing “crypt-like” non-differentiated format (Fig. 1B). In both differentiated and non-differentiated epithelium cultures, the IL-8, IL-6, and IFN-γ produced were released primarily to the apical side, as depicted by the higher amounts contained in the apical culture supernatant (Fig. 1B).15 The fact that cytokines produced by intestinal epithelial cells are mainly secreted towards the lumen as opposed to the basolateral side where immune cells reside in vivo, was intriguing. The apical production of immune mediators has been also observed in epithelium from other organs such as lung and uterus and may hint to the migration of immune cells beyond the intestinal barrier, which would depend on cytokine signals for stimulation.21-23 We have also shown in our previous paper that macrophages enhanced the epithelial barrier function, evidenced by increased electrical resistance and increased enterocyte height suggesting a novel paracrine role for macrophages in intestinal epithelial differentiation.15
Macrophages in the intestinal enteroid co-culture model have reduced pro-inflammatory function
Macrophages comprise a heterogeneous population. Various distinct phenotypes have been defined through in vivo and in vitro monocyte differentiation studies.24, 25 Mature macrophages originate from circulating monocytes and can be activated by discrete stimuli to become M1, which are pro-inflammatory and drive T helper (Th) 1 type responses, or M2, which are anti-inflammatory and promote tissue repair and drive Th2 responses. The gut lamina propria harbors a phenotypically and functionally distinct population known as “resident” macrophages. Although derived from blood monocytes, unlike M1 macrophages, human intestinal resident macrophages display profound inflammatory anergy and maintain homeostasis.26 Despite the downregulation of pro-inflammatory cytokine production, resident macrophages have high phagocytic capacity (for luminal bacteria, non-harmful foreign molecules and cellular debris) as well as bactericidal activity.26-29 During the development and optimization of the enteroid-macrophage co-culture model, mature (not activated) macrophages, also known as M0, were added to the enteroid monolayer. This non-activated phenotype was selected to ascertain discernible changes (i.e. activation towards a resident, M1, or M2 phenotype) prompted by molecules expressed, released, and/or induced by the intestinal epithelium. In our recent publication, we showed that macrophages engrafted into the differentiated “villus-like” enteroid monolayers (for as little as 24h) exhibited a marked reduction in their capacity to produce chemokines and pro-inflammatory cytokines IL-8, IL-6, and IFN-γ.15 In contrast, the levels of TGF-β1, a strong promoter of homeostasis,30 remained unchanged. The same was observed in the additional experiments described in this addendum using co-cultures containing non-differentiated “crypt-like” enteroids (Fig. 1B). Unlike the other cytokines, which were released apically by the epithelial cells (regardless of differentiation), TGF-β1 was mainly secreted to the basolateral side. The direct exposure of macrophages to the enterocyte-released TGF-β1 in the basolateral compartment could explain their acquisition of a resident phenotype.26, 28 It would be interesting to test whether enteroid-derived TGF-β1 induces macrophage differentiation and function. In this addendum, we confirm our observations suggesting that monocyte-derived macrophages acquire a resident “tolerogenic” function when cultured with differentiated epithelial cell monolayers, this time demonstrating that the same cell phenotypic change occurs when M0 macrophages are cultured in the presence of “crypt-like” intestinal epithelial cells (Fig. 1B). Further analysis of cell surface marker expression and cytokine expression in both conditions would allow establishing the identity and exact phenotype of these enteroid-interacting macrophages.
ETEC is captured by sub-epithelial resident macrophages
Human monocyte-derived macrophages phagocytose ETEC in vitro (Fig 1C). How human intestinal macrophages interact with ETEC is not known. It has been shown in mice that intestinal resident macrophages can sample and process luminal antigens and organisms, and stimulate an adaptive immune response in collaboration with dendritic cells.27, 31, 32 In our recent paper, we showed that human macrophages engrafted with enteroid monolayers develop projections (i.e. transepithelial dendrites), which extended across the intestinal epithelium without disturbing the barrier integrity.15 This was the first demonstration of macrophage luminal sampling in a model of human primary cells. Interestingly, when ETEC was applied to the apical surface of the enteroid monolayer, the number of macrophage extensions moving through the monolayer toward the bacteria markedly increased. Macrophage transepithelial dendrites in contact with luminal bacteria were visualized by immunofluorescent confocal microscopy (Fig. 1D). The number of apical colony-forming units (CFU) in ETEC-exposed co-culture was significantly reduced following overnight infection, which reflects successful macrophage sampling and phagocytic activity. The strong phagocytic activity of the macrophages cultured with epithelial cells, along with the downregulation of pro-inflammatory cytokines, such as IL-8, IL-6, and IFN-γ, further support their resident functional profile.
In this addendum, we confirm the phenotypic adaptation and ETEC phagocytic activity of the co-cultured macrophages and further characterize this process by showing that it occurs very quickly, as revealed by a remarkable reduction of CFU as early as 30 min post-infection (Fig. 1E). The luminal ETEC sampling by sub-epithelial human macrophages in the co-culture model is in agreement with finding of intracellular bacilli in murine lung macrophages following pulmonary ETEC challenge.12 The molecular factors that either directly or indirectly (through epithelial cells) triggered and enhanced macrophage transepithelial projections are not known. These findings are intriguing and open an entire new avenue of investigation. Recent studies have shown that Pet toxin, a SPATE produced by enteroaggregative E. coli (EAEC), stimulates motility and cytokine expression by macrophages.33 SPATEs form a large family of many related proteins among the pathogenic E. coli strains; it would be worth investigating whether similar factors are produced by ETEC to trigger an intestinal macrophage response.
Potential role of macrophages during human ETEC infection
ETEC is an extracellular organism that (in normal conditions) attaches but is not thought to translocate across the gut mucosal barrier. However, results reported in our previous publication and in this addendum demonstrate that the bacterium is sensed and taken up by human macrophages attached to the opposite side of the intestinal epithelium through transepithelial projections (Fig. 1D and 1E).15 These macrophages underwent adaptation and functional phenotypic changes triggered by epithelial cells. This unique ETEC-macrophage interaction is important on several levels: 1) upon microbial sensing, macrophages become activated and produce mediators that will attract other phagocytic cells, 2) being professional antigen presenting cells, together with dendritic cells, macrophages can present antigens to stimulate (or re-stimulate) T cells; antigen-loaded apoptotic macrophages (as well as released antigens) allow for cross-presentation by professional phagocytic cells and stimulation of B and T cells, and 3) macrophages could provide a means of microbial or antigenic translocation. Although the fate of the organisms within the macrophages was not evaluated in our study, one possible outcome is active intracellular killing, which is consistent with the prompt CFU reduction observed in our study and the heightened bactericidal activity ascribed to resident macrophages. The resulting ETEC antigens could be presented or cross-presented by macrophages and dendritic cells, resulting in T and B cell activation and induction of adaptive immunity. Alternatively, ETEC may simply translocate across the monolayer via macrophages; extracellular bacterial pathogens such as E. coli K1 and Enterococcus faecalis have been shown not only to survive but also replicate in the harsh intracellular macrophage environment.34 35 Unlike ETEC, however, these strains can produce a systemic infection believed to stem from bacteria that replicate within phagocytes and spread to distal sites. The fact that ETEC does not become systemic means that some sort of mechanism allows for its clearance in the intestine. Whether this is effectively and uniquely performed by macrophages or additional cells needs to be determined.
In addition to queries about the pathogen, there are many unanswered questions about the fate of the infected macrophages. It has been suggested that ETEC infection can kill murine macrophage cell lines; however, in contrast to other diarrheagenic E. coli, ETEC failed to induce markers of apoptosis (i.e. DNA fragmentation and TUNEL staining), leaving unknown the mechanism of death involved.20 While we did not observe significant death of ETEC-exposed macrophages in co-culture, a more comprehensive analysis of macrophage response to ETEC is warranted.
Conceivably, apoptotic macrophages could be captured by other macrophages or dendritic cells and microbial antigens cross-presented for activation of T cells. Apoptotic bodies could also prime B cells. This mechanism would explain the induction of systemic (even protective) immunity by a non-invasive pathogen. Indeed, ETEC-exposed individuals (also animals) develop strong adaptive immunity consisting of serum antibodies, CD4+ T cells expressing gut-homing markers, and IgA B memory cells.36 8 37 LT and CF antibodies are believed to contribute to protection according to human challenge studies.38 36 Furthermore, increased levels of IL-4 and IL-8 but not TNF-α, IL-6, and IFN-γ have been associated with reduced severity of disease, suggesting a protective role of Th2 type cytokines.9 The involvement of mucosal Th2 type cells (e.g. M2 macrophages, type 2 innate lymphoid cells, B cells) and molecules (e.g. IL-4, IL-5, IgA) during ETEC infection could be easily interrogated in a “crypt-like” or “villus-like” enteroid co-culture model as the one described herein.
Interrogating enteric infection and immunity ex vivo in a enteroid-immune model
Human enteroids or colonoids have been used successfully to study interactions of enteric pathogens with the human epithelial cell barrier; the complexity of the system is such that it allows us to dissect steps and cells involved in pathogenesis, including effects on enterocyte microvilli, tight junctional regulation, and ion/nutrient transporter activity that may be correlated to disease severity.39
The macrophage-enteroid co-culture model developed by our group provides a new tool to study host cell and pathogen interactions with innate immune cells. Although our work thus far has been focused on macrophages, we are currently expanding the system to include other innate phagocytic cell types (i.e. polymorphonuclear neutrophils and dendritic cells). Systems of higher complexity possibly containing B and T lymphocytes will be forthcoming. Distinct immune cell phenotypes can be incorporated one at a time or together to address specific questions in a reductionist system easy to manipulate and reproduce. Such models will generate mechanistic insight into how immune cells interact and respond to enteric pathogens. In addition, the co-culture systems offer a model to interrogate the mutual dependency of the intestinal epithelium and immune cells for survival, differentiation, and function using primary human cells arranged in a configuration that resembles the human gut in vivo. In our studies, the intestinal epithelial monolayer finely modulated macrophage phenotype and function, and in turn, these “intestine-adapted” macrophages supported the epithelial cell barrier.15 Saha and colleagues demonstrated that murine macrophages secrete Wnt ligands necessary to regenerate the intestinal epithelium following radiation injury.40 The fascinating inter cell coordination that takes place in the human gut has yet to be tapped.
As observed for several other enteric pathogens, the host secretor status plays a role in susceptibility to ETEC infection and illness.41,42 We are currently addressing this question using enteroid-immune co-cultures which, unlike in vitro models using immortalized cell lines, allow for an in-depth analysis of individual biological diversity (secretors vs. non-secretors).
In conclusion, we have demonstrated that human monocyte-derived macrophages co-cultured with primary epithelial cell monolayers acquire a resident functional phenotype. These macrophages sense ETEC on apical side of the epithelial cells, develop transepithelial projections and quickly phagocytose the organism, greatly reducing the luminal bacterial load. The model is novel and relevant to the study of pathogenesis and host-cell interactions, and will advance our understanding of enteric infections in humans.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding Statement
This work was funded by Grand Challenge Exploration award OPP1118529 and National Institutes of Health awards P30-DK089502 and P01-AI125181.
Contributions
G.N. and M.D. performed experiments; NC.Z. obtained microscopy images; G.N. and MF.P. wrote the manuscript; all authors contributed ideas, reviewed, and edited the manuscript.
References
- 1.Qadri F, Svennerholm A-M, Faruque ASG, Sack RB. Enterotoxigenic Escherichia coli in Developing Countries: Epidemiology, Microbiology, Clinical Features, Treatment, and Prevention. Clinical Microbiology Reviews 2005; 18:465–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB. Recent Advances in Understanding Enteric Pathogenic Escherichia coli. Clinical Microbiology Reviews 2013; 26:822–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo Q, Kumar P, Vickers TJ, Sheikh A, Lewis WG, Rasko DA, Sistrunk J, Fleckenstein JM. Enterotoxigenic Escherichia coli Secretes a Highly Conserved Mucin-Degrading Metalloprotease To Effectively Engage Intestinal Epithelial Cells. Infection and Immunity 2014; 82:509–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roy K, Hilliard GM, Hamilton DJ, Luo J, Ostmann MM, Fleckenstein JM. Enterotoxigenic Escherichia coli EtpA mediates adhesion between flagella and host cells. Nature 2009; 457:594–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy K, Kansal R, Bartels SR, Hamilton DJ, Shaaban S, Fleckenstein JM. Adhesin Degradation Accelerates Delivery of Heat-labile Toxin by Enterotoxigenic Escherichia coli. The Journal of Biological Chemistry 2011; 286:29771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg DE, Jiang Z-D, Steffen R, Verenker MP, DuPont HL. Markers of Inflammation in Bacterial Diarrhea among Travelers, with a Focus on Enteroaggregative Escherichia coli Pathogenicity. The Journal of Infectious Diseases 2002; 185:944–9. [DOI] [PubMed] [Google Scholar]
- 7.Xu C, Wang Y, Sun R, Qiao X, Shang X, Niu W. Modulatory Effects of Vasoactive Intestinal Peptide on Intestinal Mucosal Immunity and Microbial Community of Weaned Piglets Challenged by an Enterotoxigenic Escherichia coli (K88). PLoS ONE 2014; 9:e104183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loos M, Geens M, Schauvliege S, Gasthuys F, van der Meulen J, Dubreuil JD, Goddeeris BM, Niewold T, Cox E. Role of Heat-Stable Enterotoxins in the Induction of Early Immune Responses in Piglets after Infection with Enterotoxigenic Escherichia coli. PLoS ONE 2012; 7:e41041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long KZ, Rosado JL, Santos JI, Haas M, Al Mamun A, DuPont HL, Nanthakumar NN, Estrada-Garcia T. Associations between Mucosal Innate and Adaptive Immune Responses and Resolution of Diarrheal Pathogen Infections. Infection and Immunity 2009; 78:1221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mercado EH, Ochoa TJ, Ecker L, Cabello M, Durand D, Barletta F, Molina M, Gil AI, Huicho L, Lanata CF, et al. Fecal Leukocytes in Children Infected with Diarrheagenic Escherichia coli. Journal of Clinical Microbiology 2011; 49:1376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao D, Wang Y, Liu G, He J, Qiu W, Hu X, Feng Z, Ran M, Nyachoti CM, Kim SW, et al. Effects of Chitosan on Intestinal Inflammation in Weaned Pigs Challenged by Enterotoxigenic Escherichia coli. PLoS ONE 2014; 9:e104192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byrd W, Mog SR, Cassels FJ. Pathogenicity and Immune Response Measured in Mice following Intranasal Challenge with Enterotoxigenic Escherichia coli Strains H10407 and B7A. Infection and Immunity 2003; 71:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finamore A, Roselli M, Imbinto A, Seeboth J, Oswald IP, Mengheri E. Lactobacillus amylovorus Inhibits the TLR4 Inflammatory Signaling Triggered by Enterotoxigenic Escherichia coli via Modulation of the Negative Regulators and Involvement of TLR2 in Intestinal Caco-2 Cells and Pig Explants. PLoS ONE 2014; 9:e94891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Zhang L, Chen L, Zhu Q, Wang W, Qiao J. Lactobacillus acidophilus alleviates the inflammatory response to enterotoxigenic Escherichia coli K88 via inhibition of the NF-kB and p38 mitogen-activated protein kinase signaling pathways in piglets. BMC Microbiology 2016; 16:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noel G, Baetz NW, Staab JF, Donowitz M, Kovbasnjuk O, Pasetti MF, Zachos NC. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Scientific Reports 2017; 7:45270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato T, Stange DE, Ferrante M, Vries RGJ, van Es JH, van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, et al. Long-term Expansion of Epithelial Organoids From Human Colon, Adenoma, Adenocarcinoma, and Barrett's Epithelium. Gastroenterology 2011; 141:1762–72. [DOI] [PubMed] [Google Scholar]
- 17.Stelzner M, Helmrath M, Dunn JCY, Henning SJ, Houchen CW, Kuo C, Lynch J, Li L, Magness ST, Martin MG, et al. A nomenclature for intestinal in vitro cultures. American Journal of Physiology – Gastrointestinal and Liver Physiology 2012; 302:G1359–G63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zachos NC, Kovbasnjuk O, Foulke-Abel J, In J, Blutt SE, de Jonge HR, Estes MK, Donowitz M. Human Enteroids/Colonoids and Intestinal Organoids Functionally Recapitulate Normal Intestinal Physiology and Pathophysiology. Journal of Biological Chemistry 2016; 291:3759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.In J, Foulke-Abel J, Zachos NC, Hansen A-M, Kaper JB, Bernstein HD, Halushka M, Blutt S, Estes MK, Donowitz M, et al. Enterohemorrhagic Escherichia coli Reduces Mucus and Intermicrovillar Bridges in Human Stem Cell-Derived Colonoids. Cellular and Molecular Gastroenterology and Hepatology 2016; 2:48–62.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai X-H, Xu J-G, Melgar S, Uhlin BE. An apoptotic response by J774 macrophage cells is common upon infection with diarrheagenic Escherichia coli. FEMS Microbiology Letters 1999; 172:29–34. [DOI] [PubMed] [Google Scholar]
- 21.Fahey JV, Schaefer TM, Channon JY, Wira CR. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Human Reproduction 2005; 20:1439–46. [DOI] [PubMed] [Google Scholar]
- 22.Chow AW, Liang JF, Wong JS, Fu Y, Tang NL, Ko WH. Polarized Secretion of Interleukin (IL)-6 and IL-8 by Human Airway Epithelia 16HBE14o- Cells in Response to Cationic Polypeptide Challenge. PLoS ONE 2010; 5:e12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szabady RL, McCormick BA. Control of Neutrophil Inflammation at Mucosal Surfaces by Secreted Epithelial Products. Frontiers in Immunology 2013; 4:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bain CC, Mowat AM. Macrophages in intestinal homeostasis and inflammation. Immunological Reviews 2014; 260:102–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mills CD, Ley K. M1 and M2 Macrophages: The Chicken and the Egg of Immunity. Journal of innate immunity 2015; 6:716–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. Journal of Clinical Investigation 2005; 115:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazzini E, Massimiliano L, Penna G, Rescigno M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1(+) macrophages to CD103(+) dendritic cells. Immunity 2014; 40:248–61. [DOI] [PubMed] [Google Scholar]
- 28.Smythies LE, Shen R, Bimczok D, Novak L, Clements RH, Eckhoff DE, Bouchard P, George MD, Hu WK, Dandekar S, et al. Inflammation anergy in human intestinal macrophages is due to Smad-induced IkappaBalpha expression and NF-kappaB inactivation. The Journal of Biological Chemistry 2010; 285:19593–19604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith PD, Smythies LE, Shen R, Greenwell-Wild T, Gliozzi M, Wahl SM. Intestinal Macrophages and Response to Microbial Encroachment. Mucosal immunology 2011; 4:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li MO, Sanjabi S, Flavell Richard A. Transforming Growth Factor-b Controls Development, Homeostasis, and Tolerance of T Cells by Regulatory T Cell-Dependent and -Independent Mechanisms. Immunity 2006; 25:455–71. [DOI] [PubMed] [Google Scholar]
- 31.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, et al. CX3CR1-Mediated Dendritic Cell Access to the Intestinal Lumen and Bacterial Clearance. Science 2005; 307:254. [DOI] [PubMed] [Google Scholar]
- 32.Gross M, Salame T-M, Jung S. Guardians of the Gut – Murine Intestinal Macrophages and Dendritic Cells. Frontiers in Immunology 2015; 6:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rocha-Ramirez LM, Hernandez-Chinas U, Banos-Rojas D, Xicohtencatl-Cortes J, Chavez-Berrocal ME, Rico-Rosillo G, Kretschmer R, Eslava CA. Pet serine protease from enteroaggregative Escherichia coli stimulates the inflammatory response activating human macrophages. BMC Microbiology 2016; 16:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sukumaran SK, Shimada H, Prasadarao NV. Entry and Intracellular Replication of Escherichia coli K1 in Macrophages Require Expression of Outer Membrane Protein A. Infection and Immunity 2003; 71:5951–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baldassarri L, Bertuccini L, Creti R, Filippini P, Ammendolia MG, Koch S, Huebner J, Orefici G. Glycosaminoglycans Mediate Invasion and Survival of Enterococcus faecalis into Macrophages. The Journal of Infectious Diseases 2005; 191:1253–62. [DOI] [PubMed] [Google Scholar]
- 36.McArthur MA, Chen WH, Magder L, Levine MM, Sztein MB. Impact of CD4+ T Cell Responses on Clinical Outcome following Oral Administration of Wild-Type Enterotoxigenic Escherichia coli in Humans. PLOS Neglected Tropical Diseases 2017; 11:e0005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byrd W, Cassels FJ. Intranasal immunization of BALB/c mice with enterotoxigenic Escherichia coli colonization factor CS6 encapsulated in biodegradable poly(dl-lactide-co-glycolide) microspheres. Vaccine 2006; 24:1359–66. [DOI] [PubMed] [Google Scholar]
- 38.Svennerholm A-M. From cholera to enterotoxigenic Escherichia coli (ETEC) vaccine development. The Indian Journal of Medical Research 2011; 133:188–94. [PMC free article] [PubMed] [Google Scholar]
- 39.In JG, Foulke-Abel J, Estes MK, Zachos NC, Kovbasnjuk O, Donowitz M. Human mini-guts: new insights into intestinal physiology and host-pathogen interactions. Nature reviews Gastroenterology & hepatology 2016; 13:633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saha S, Aranda E, Hayakawa Y, Bhanja P, Atay S, Brodin NP, Li J, Asfaha S, Liu L, Tailor Y, et al. Macrophage-derived extracellular vesicle-packaged WNTs rescue intestinal stem cells and enhance survival after radiation injury. Nat Commun 2016; 7:13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jansson L, Tobias J, Lebens M, Svennerholm A-M, Teneberg S. The Major Subunit, CfaB, of Colonization Factor Antigen I from Enterotoxigenic Escherichia coli Is a Glycosphingolipid Binding Protein. Infection and Immunity 2006; 74:3488–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed T, Lundgren A, Arifuzzaman M, Qadri F, Teneberg S, Svennerholm AM. Children with the Le(a+b-) Blood Group Have Increased Susceptibility to Diarrhea Caused by Enterotoxigenic Escherichia coli Expressing Colonization Factor I Group Fimbriae. Infection and Immunity 2009; 77:2059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]