ABSTRACT
The advent of industrial revolution caused a large inflow of synthetic chemicals for medical, agricultural, industrial and other purposes in the world. In general, these chemicals were subjected to toxicological risk assessment for human health and ecology before release for public use. But today we are witnessing a negative impact of some of these chemicals on human health and environment indicating an underestimation of toxic effects by current risk assessment protocol. Recent studies established gut microbiota as one of the key player in intercession of toxicity of drugs and synthetic chemicals. Hence, the need of the hour is to include the assessment for microbiota specifically gut microbiota in human toxicological risk assessment protocol. Herewith we are proposing a framework for assessment of gut microbiota upon exposure to drugs or chemicals.
KEYWORDS: drugs, environmental chemicals, gut microbiota, risk assessment, toxicology
Introduction
During past few centuries, humans had produced a wide variety of natural and synthetic chemicals by different processes that transformed the entire world. These chemicals initially led to industrial revolution followed by agricultural revolution in the form of synthetic fertilizers and pesticides, medical revolution as synthetic drugs and continuing today as electronic revolution. The synthetic chemicals produced for different purposes are normally subjected to human and ecological risk assessment and only the chemicals that pass through this assessment are released to public or environment.1 Toxicological risk assessment is defined as the process to estimate the nature and probability of adverse health effects in humans or other organisms who may be exposed to a particular chemical at present or in future.1,2 Based on this risk assessment, the chemicals are currently categorized based on their health effects that includes acute toxicity, skin irritation, eye damage, respiratory sensitizer, skin sensitizer, germ cell mutagenicity, carcinogenicity, toxic to reproduction, effect on or via lactation, teratogenicity, specific organ toxicity and so on.3
Recent studies indicate the impact of drugs and environmental chemicals on gut microbiota and also the impact of microbial metabolization of drugs.4-6 food7-8 and environmental chemicals9-11 and its subsequent effect on physiology and pathology of humans.4-11 Microbiota is today recognized as an organ of the human body that plays a vital role in human health and disease.12 Besides their role in body physiology, it plays a key role in digestion and metabolization of xenobiotic compounds. Microbial metabolism of chemicals by gut microbiota can be accompanied by microbial dysbiosis – a change in the microbial community structure, induction of specific bacterial genes, and microbial transformation of chemicals.12 In addition, chemicals can be absorbed and transported to the liver, where they are conjugated and excreted back into the gut through bile secretion for subsequent microbial metabolism.13,14 These studies indicate that human toxicological risk assessment of chemicals will not be complete without inclusion of gut microbiota as a parameter.
Proposed toxicological risk assessment protocol for gut microbiota
The outline of the proposed protocol for the risk assessment of microbiota for a known or new drug or chemical is provided in Fig. 1. It is essential that the microbiota is assessed at both acute and chronic dose of study chemical. In both cases, after exposure to the chemical, the faecal samples should be collected and processed for further experiments. The microbiota has to be assessed in two different ways: 1. What changes are induced by the chemical on the gut microbial community structure 2. What changes are incurred by gut microbes on the chemistry of the study compound? To address the former query, the whole genomic DNA can be isolated from faecal samples and 16S rRNA sequencing or qPCR can be performed using genera specific primers. Dysbiosis can be assessed by determining changes in abundance of members of microbial community. The observed changes in abundance or reduction of specific communities can be compared with previous reports relating to specific disease or other condition.13 In recent studies, bacterial species identified to be overexpressed during disease condition were proved to induce disease condition in rodent models like induction of colon tumoriogenesis by Fusobacterium nucleatum. 14 Similarly, Akkermansia muciniphila was noted to be decreased during obesity and diabetes and supplementation of A. muciniphila reduced the effect of these disorders.15 But it is important to note that it is unclear if a disease condition cause changes in the microbial community or vice-versa but hopefully in coming years this puzzle will be solved. Metagenomic DNA or metatranscriptomics analyses will provide the picture on the effect of the study chemical on functional effects of the gut microbiota.
Figure 1.
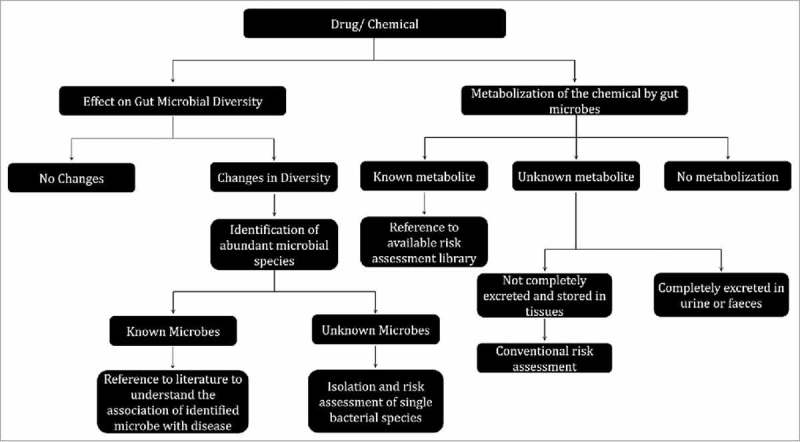
Proposed work flow for toxicological risk assessment of gut microbiota for a known or new drug and chemical.
The metabolizing potential of gut microbiota is vast and several studies proven that the drugs and environmental chemicals were metabolized by the gut microbiota. The metabolites produced by gut microbial process were proven to alter the host physiology and pathology.13,16 Hence it is essential to assess whether a specific chemical can be metabolized or not by the gut microbiota. After the exposure period, changes in the metabolite profile of faecal samples can be studied by mass spectrometry techniques. This will allow identification of the metabolites resulting from biotransformation of the chemical of interest by the gut microbiota. In case of known compounds, the toxicity profile can be assessed from the database while in case of unknown compound, all the protocols in conventional toxicological risk assessment must be performed.
Models to assess gut microbiota
Small animals specifically mice and rats are largely used as experimental animals for toxicological studies due to their similarity with anatomy and physiology of humans. Mice are widely used as a model for gut microbiota studies as they have quite similar structure of gastrointestinal tract with a few prominent differences and availability of germ-free and genetically modified mouse models.17 The gut microbiota of human and mice are dominated by two major phyla, Bacteriocides and Firmicutes but there are differences at the species level. Despite similarities, multiple factors including host-microbe response are different between humans and mice.17 To overcome this pitfall, human microbiota-associated (HMA) mice were developed by inoculating a human microbiota in germ-free mice.18 This HMA mice are regarded as the best model to study gut microbiota. For toxicological risk assessment, the effect of study chemicals need to be assessed at both acute and chronic level. In case of drugs, the recommended daily dosage and in case of environmental chemicals, theoretical maximum daily intake (TMDI) dose can be employed for chronic exposure studies. Based on mode of exposure in practice, the study chemical can be given to the model animal by oral gavage, drinking water or feed or by nasal route or skin exposure and the subsequent effect on gut microbiota can be assessed.
The in vitro human/ animal cell lines are used as a model for toxicological risk assessment primarily to reduce time and to avoid sacrificing of large number of experimental animals. Similarly, to study gut microbiota, an in vitro system was developed and christened as “The stimulator of intestinal microbial ecosystem (SHIME)”.19 SHIME mimics the entire gastrointestinal tract including stomach, small intestine and colon. In parallel, bile and pancreatic juice are also fed to the stomach and small intestine compartments to mimic the natural system by maintaining specific feed, pH, temperature and nutrients. The faecal microbiota is used as the inoculum to this system and suitable period is provided for the microbiome to adapt to each segment. The complete details of the SHIME system, build-up, operation time, stability and reproducibility are discussed elsewhere.19 The effect of different dietary compounds20 and chlorpyrifos pesticide21 were studied using SHIME system.
Anaerobic culture methods can also be employed to assess the effect of drug or chemical on gut microbiota.4,9 In this culture method, human or animal faeces can be cultured in presence/ absence of the specific chemical and after the culture period, the changes can be assessed. In our anaerobic culture with faeces and with organophosphate insecticides, we observed the increase in the activity of xenobiotic degrading enzymes, expression of specific genes and degradation of insecticides into glucogenic substrates.9 The major advantages of these in vitro systems (SHIME and culture method) are short time span and employment of human feces directly as the inoculum. On the other hand, the negative features of in vitro models are lack of ability of the absorption of digested compounds that occurs naturally in intestine and absence of influence of host cell factors on the physiology of microbial system.19 Hence, these in vitro models could be a suitable system to study the effect of chemicals on microbial dysbiosis and not on the effect of the compound or its metabolites on different organs of host. The final culture from SHIME or anaerobic culture can be transplanted to rodents by oral gavage to understand its effect on host system.8,9
A new microfluidic technology termed as “Gut-on-a-Chip” can be exploited to understand the effect of chemicals on gut microbiota and its subsequent impact on host intestinal cells.22 In this system, epithelial cell line such as Caco2 cells are subjected to peristalsis-like motions and liquid flow leading to different types of differentiated epithelial cells. Single bacterium or whole microbiota can be co-cultured on this chip system with intestinal cells and their effect on host-microbe interaction can be studied.23 For toxicological assessment, the candidate drug or chemical can be mixed with flow liquid at desired concentration and its effect on both microbial cells and host cells can be studied simultaneously. It is also mandatory to develop modeling/ simulations for scaling up of the gut microbial changes from mice to human system and in vitro to in vivo systems.
Conclusion
Though our proposed assessment is focused only on gut microbiota, they can be extended to oral, skin, vaginal and other body microbiota for human health risk assessment and soil, water and air microbiota for ecological risk assessment. Our proposed assessment protocol is preliminary and it is essential that concerned bodies and toxicological societies constitute committees to formulate regulations for inclusion of microbiota in the conventional risk assessment for drugs and synthetic chemicals.
Acknowledgments
The author acknowledges Indian Institute of Technology Madras for providing Institute Post-doctoral fellowship.
References
- 1.Nielsen E, Ostergaard G, Larsen JC. Toxicological risk assessment of chemicals: a practical guide. Florida: CRC Press; 2008. [Google Scholar]
- 2.Torres J, Bobst S. Toxicological risk assessment for beginners. Switerland: Springer; 2015. [Google Scholar]
- 3.Hazard classification guidance for manufacturers, importers and employers 2016. Occupational safety and health administration, Department of Labour, USA. OSHA Publication 3844 (02/2016). https://www.osha.gov/Publications/OSHA3844.pdf [Google Scholar]
- 4.Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39–50. doi: 10.1016/j.cell.2012.10.052. PMID:23332745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haiser HJ, Gootenberg DB, Chatman K, Sirasani G, Balskus EP, Turnbaugh PJ. Predicting and manipulating cardiac drug inactivation by the human gut bacterium Eggerthella lenta. Science. 2013;341:295–8. doi: 10.1126/science.1235872. PMID:23869020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geller LT, Barzily-Rokni M, Danino T, Honas OH, Shental N, Nejman D, Gavert N, Zwang Y, Cooper ZA, Shee K, et al.. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357:1156–60. doi: 10.1126/science.aah5043. PMID:28912244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al.. Diet flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011. 472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A, et al.. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181–6. doi: 10.1038/nature13793. PMID:25231862. [DOI] [PubMed] [Google Scholar]
- 9.Velmurugan G, Ramprasath T, Swaminathan K, Mithieux G, Rajendhran J, Dhivakar M, Parthasarathy A, Babu DD, Thumburaj LJ, Freddy AJ, et al.. Gut microbial degradation of organophosphate insecticides induces glucose intolerance via gluconeogenesis. Genome Biol. 2017;18:8. doi: 10.1186/s13059-016-1134-6. PMID:28115022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Velmurugan G, Ramprasath T, Gilles M, Swaminathan K, Ramasamy S. Gut microbiota, endocrine-disrupting chemicals and the diabetes epidemic. Trends Endocrinol Metab. 2017;28:612–25. doi: 10.1016/j.tem.2017.05.001. PMID:28571659. [DOI] [PubMed] [Google Scholar]
- 11.Claus SP, Guillou H, Ellero-Simator S. The gut microbiota: a major player in the toxicity of environmental pollutants? Biofilms and Microbiomes. 2016;2:16003. doi: 10.1038/npjbiofilms.2016.3. PMID:28721242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13:260–70. doi: 10.1038/nrg3182. PMID:22411464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koppel N, Rekdal VM, Balskus EP. Chemical transformation of xenobiotics by the human gut microbiota. Science. 2017;356:eaag2770. doi: 10.1126/science.aag2770. PMID:28642381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y, Weng W, Penj J, Hong L, Toiyama Y, Gao R, Liu M, Yin M, Pan C, Li H, et al.. Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-κB, and Up-regulating Expression of MicroRNA-21. Gastroentrol. 2017;152:851–66. doi: 10.1053/j.gastro.2016.11.018.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roopchand DE, Carmody RN, Kuhn P, Moskal K, Rojas-Silva P, Turnbaugh PJ and Raskin I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes. 2015;64:2847–58. doi: 10.2337/db14-1916. PMID:25845659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haiser HJ, Turnbaugh PJ. Developing a metagenomic view of xenobiotic metabolism. Pharmacol Res. 2013;69:21–31. doi: 10.1016/j.phrs.2012.07.009. PMID:22902524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen TLA, Vieira-Silva S, Liston A, Raes J. How informative is the mouse for the human gut microbiota research? Dis Model Mech. 2015;8:1–16. doi: 10.1242/dmm.017400. PMID:25561744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arrieta M, Walter J, Finlay BB. Human microbiota-associated mice: a model with challenges. Cell Host Microb. 2016;19:575–8 doi: 10.1016/j.chom.2016.04.014.. [DOI] [PubMed] [Google Scholar]
- 19.de Wiele TV, Abbeele PV, Ossieur W, Possemiers S, Marzorati M. The stimulator of the human intestinal microbial ecosystem (SHIME) In: Verhoeckx K, et a (eds). The impact of food bioactives on health. Springer, Cham; 2015:305–317. [Google Scholar]
- 20.Duenas M, Munoz-Gonzalez I, Cueva C. Jiménez-Girón A, Sánchez-Patán F, Santos-Buelga C, Moreno-Arribas MV, Bartolomé B, A survey of modulation of gut microbiota by dietary polyphenols. 2015. Biomed. Res. Int. 2015:850902. PMID:25793210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joy C, Gay-Queheillard J, Leke A, Chardon K, Delanaud S, Bach V, Khorsi-Cauet H. Impact of chronic exposure to low doses of chlorpyrifos on the intestinal microbiota in the simulator of human intestinal microbial ecosystem (SHIME) and in the rat. Environ. Sci. Poll. Res. 2012;20:2276–734. [DOI] [PubMed] [Google Scholar]
- 22.Kim HJ, Ingber DE. Gut-on-a-chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr. Biol. (Camb). 2013;5:1130–40 doi: 10.1039/c3ib40126j. PMID:23817533. [DOI] [PubMed] [Google Scholar]
- 23.Kim HJ, Li H, Collins JJ, Ingber DE. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc. Natl. Acad. Sci. USA. 2016;113:E7–E15. doi: 10.1073/pnas.1522193112. [DOI] [PMC free article] [PubMed] [Google Scholar]


