Abstract
Stockbridge highlights new work revealing an allosteric inactivation mechanism for the bestrophin channel.
The story of the bestrophin family of calcium-activated chloride channels began two decades ago with the discovery of the gene responsible for a form of juvenile onset macular degeneration called Best disease (Petrukhin et al., 1998; Sun et al., 2002). Fruitful years of whole cell electrophysiology experiments followed, outlining the basic mechanistic properties of this channel. In 2014, x-ray crystal structures of a pair of bestrophin homologues heralded the second chapter of the story (Kane Dickson et al., 2014; Yang et al., 2014). In this issue of the Journal of General Physiology, Vaisey and Long pursue this new chapter in a follow up to the Long laboratory’s 2014 structure of the chicken BEST1 homologue. The authors exploit their crystallography-pure protein preparations to perform planar lipid bilayer electrophysiology with reconstituted bestrophin channels (Vaisey and Long, 2018). They focus on understanding the molecular mechanism of bestrophin inactivation and on understanding the functional consequences of the crystallization chaperones that they used to obtain the structure in the first place.
Basics of channel architecture and ion permeation
After the discovery of the bestrophin family channels, Jeremy Nathans’ and Criss Hartzell’s laboratories led the initial investigative charge, performing a series of electrophysiological studies that revealed molecular and regulatory properties of the four human paralogues in the family (BEST1-BEST4). Though these four proteins differ in regulation and tissue distribution, major functional properties are shared, including anion selectivity and activation in response to cytosolic calcium (Hartzell et al., 2008). The Hartzell laboratory also observed a decrease in ionic current over the minutes after patch break-in, or “rundown.” In a 2008 JGP paper, they showed that both calcium and a C-terminal autoinhibitory motif contribute to rundown (Xiao et al., 2008). However, in whole cell experiments, it is never quite clear whether current rundown is an intrinsic molecular property or the result of an association with some unknown cellular factor.
The structural era began when Stephen Long and Wayne Hendrickson’s groups published simultaneous x-ray crystal structures of bestrophin: a chicken BEST1 homologue in Nature and a prokaryotic bestrophin homologue in Science, respectively (Kane Dickson et al., 2014; Yang et al., 2014). Although the overall architecture of both ion channels is strikingly similar, the chicken structure elucidated by Veronica Kane Dickson and Stephen Long is perhaps better suited to understanding the human homologues. The chicken channel is 74% identical to human BEST1 and conserves many of the molecular properties, including anion selectivity (the prokaryotic channel is cation selective) and activation by intracellular calcium (Kane Dickson et al., 2014).
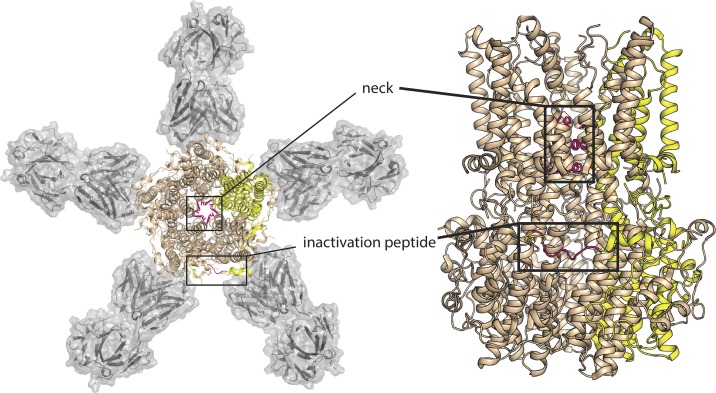
The structure revealed a pentameric assembly of subunits spanning the membrane and extending 55 Å into the cytosol (Fig. 1). The long, 95-Å pore narrows at two pinch points, the so-called “neck” midway through the membrane, and the “aperture” located at the farthest cytosolic reach, where selectivity among anions occurs (Vaisey et al., 2016). The constriction at the neck is lined by an isoleucine (I76) and a pair of phenylalanines (F80 and F84). Its diameter is just large enough for a dehydrated Cl− ion to pass, but the region is awfully hydrophobic, leaving the question of whether the structure represents an open channel unresolved. Subsequent functional experiments by the Long laboratory showed that the neck acts as the calcium-sensitive gate (Vaisey et al., 2016); opening when Ca2+ binds to its intracellular binding pockets. There are five such pockets, one in each subunit, identified unambiguously in the crystal structure by their anomalous difference electron density (Kane Dickson et al., 2014). By carving out space in the narrow neck with a triple-alanine mutation, robust anionic currents were observed in the absence of Ca2+. Further, a structure of this mutant showed that the diameter of the neck had increased as expected, although no other structural changes were registered (Vaisey et al., 2016).
Figure 1.
Structure of chicken BEST1 with neck and inactivation peptide highlighted. Two views of chicken BEST1 (PDB ID 4RDQ): at left, perpendicular to the membrane, and at right, parallel to the membrane. In each cartoon, four BEST1 subunits are colored in wheat, with one colored yellow for emphasis. The sidechains that define the neck (I76, I80, I84) and the inactivation peptide (356RPSFLGS362) from the yellow subunit are highlighted in hot pink. In the top-down view, Fab fragments 10D10 are shown with gray surface rendering.
Mechanism of inactivation
Having previously identified the neck as the Ca2+ responsive gate in the activation process (Vaisey et al., 2016), Vaisey and Long (2018) turn their attention in the present work to another calcium-dependent phenomenon, rundown. Although nanomolar concentrations of Ca2+ are required for channel activation, micromolar Ca2+ causes the currents to decrease substantially over time, and faster rundown kinetics occur with increasing concentrations of Ca2+. Working in their minimalist bilayer system, Vaisey and Long (2018) unambiguously identified rundown as an intrinsic property of the channel, and thus a molecular process—inactivation—ripe to be understood with additional mechanistic experiments.
Tipped off by the experiments performed in the Hartzell laboratory a decade prior (Xiao et al., 2008), the authors focused their attention on the C-terminal tail that wraps around the body of the channel, binding at a receptor site in an adjacent subunit (Fig. 1). Using the crystal structure to guide mutagenesis, the authors show that by altering important contacts between the tail and the receptor site in the main channel body (or by chopping the tail off altogether), inactivation can be mitigated without altering ion selectivity or Ca2+-dependent activation.
Binding of the tail is dynamic; it is protected from proteolysis in the presence of high Ca2+ (conditions that correspond to inactivation), but easily cleaved by proteases when Ca2+ is chelated. The tail is similarly dislodged and made susceptible to proteolysis by a point mutation in the tail, S358E. The present experiments reveal that this mutation to a negatively charged sidechain prevents inactivation (Vaisey and Long, 2018), reminiscent of an electrostatically homologous phosphorylation event at that same position that prevents current rundown of human BEST1 currents in cells (Xiao et al., 2009). From these experiments, Vaisey and Long (2018) propose that inactivation occurs when this C-terminal peptide binds to its receptor site. When mutation, phosphorylation, or low Ca2+ concentrations prevent binding and free the tail, the channels are able to open and conduct anions (Vaisey and Long, 2018).
Channel inactivation by a terminal peptide is a familiar phenomenon in ion channel circles. Classical “ball-and-chain” inactivation of voltage-gated Na+ and K+ channels was first proposed in the 1970s by Clay Armstrong (Armstrong and Bezanilla, 1977), and over the years, the molecular mechanisms of inactivation have been elucidated in atomic detail. For the voltage-gated potassium channels, the cumulative work by some of the luminaries of ion channel structure and function has shown that an N-terminal appendage enters a binding site within the inner vestibule, physically occluding the pore (Hoshi et al., 1990; Zagotta et al., 1990; Choi et al., 1991; Zhou et al., 2001; Gonzalez et al., 2011). In the case of voltage-gated Na+ channels, a brand new structure of Nav1.4 from Nieng Yan’s laboratory suggests a different mechanism of inactivation. In that case, the N-terminal inactivation peptide is bound in a pocket peripheral to the pore, suggesting that it exerts an allosteric influence on the intracellular gate (Pan et al., 2018). In a similar manner, Vaisey and Long argue that, in bestrophin, C-terminal peptide binding and inactivation are allosterically linked but that this allosteric signal is transmitted over an even longer distance. They show that the hydrophobic constriction at the neck, previously implicated in Ca2+-dependent channel activation (Vaisey et al., 2016), is also responsible for inactivation, because the same triple alanine mutation abolishes both processes. The tail binds at a site that is a whopping 30 Å from the neck (Fig. 1), demanding long-range allosteric communication between these two regions of the channel. Though activation and inactivation share a gate and a dependence on Ca2+, Vaisey and Long (2018) propose that the Ca2+ binding sites differ between the activation and inactivation processes.
Probing the functional consequences of crystallization chaperone binding
Perhaps what I appreciated most about this work was the repurposing of structural antibodies, selected to act as crystallization chaperones, as mechanistic probes. Whether a nanobody, monobody, or Fab fragment, structural crystallization chaperones are selected against the predominant, low-energy conformation (or multiple low-energy conformations) present in solution, stabilizing that conformation and increasing the probability of seeing it in the crystal structure. But which of a functioning protein’s many conformational states does that crystal structure correspond to? In the case of the chicken BEST1 structure, the Fab plucked from the immunogenic mileau preferentially recognized the calcium-bound channel (Kane Dickson et al., 2014), and thus the Long group proposed that their crystal structure represented an open (or near-open) conformation, despite the rather narrow, hydrophobic constriction at the neck (Kane Dickson et al., 2014).
But in the mechanistic experiments described in this work, Vaisey and Long realize that the early inference wasn’t quite right. In fact, the crystallization Fab, dubbed 10D10, hastens inactivation, leading Vaisey and Long to revise that original hypothesis; the Ca2+-bound form in the structure may not represent an open state, but might in fact represent that other conformation that becomes populated as Ca2+ levels increase: the inactivated state. Analyzing the structure, which features the C-terminal tail bound to its receptor (Fig. 1), it appears that 10D10, through additional contacts to the C tail, might stabilize the inactivation-associated binding between the inactivation peptide and channel body (Vaisey and Long, 2018).
In addition to examining the functional effects of their crystallization chaperone, Vaisey and Long also comb through their collection of rejected Fab fragments, and show that these, though not the golden ticket for bestrophin crystallization, can be quite useful for mechanistic analysis. A second structural Fab, 8G5, rapidly restores currents of Ca2+-inactivated channels, and the limited proteolysis assay demonstrates that this Fab, like the phosphorylation-mimicking S358E mutation, dislodges the C-terminal tail, effectively outcompeting the inactivation peptide for binding to the receptor (Vaisey and Long, 2018).
Through long years toiling to crystallize this protein, the Long group has been rewarded with a stable, biochemically tractable homologue that can be purified and reconstituted, allowing the group to dispense with all of the complicating factors found in cells and cell membranes that might influence channel function in some unknown way. Not only that—they’ve also got a stable of Fab fragments that bind and stabilize different conformations of chicken BEST1 and induce different behaviors in functional experiments. By using these tools in their continued investigation of the bestrophin mechanism, long after the channel’s star-making structural turn in the pages of Nature, Vaisey and Long have revealed important information about the channel’s regulatory mechanisms and have helped us to better read the structure itself. With any luck, a structure of the open state will be solved someday soon, so that we can see how the hydrophobic constriction at the neck opens to allow ion permeation.
Acknowledgments
The author declares no competing financial interests.
Kenton J. Swartz served as editor.
References
- Armstrong C.M., and Bezanilla F.. 1977. Inactivation of the sodium channel. II. Gating current experiments. J. Gen. Physiol. 70:567–590. 10.1085/jgp.70.5.567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.L., Aldrich R.W., and Yellen G.. 1991. Tetraethylammonium blockade distinguishes two inactivation mechanisms in voltage-activated K+ channels. Proc. Natl. Acad. Sci. USA. 88:5092–5095. 10.1073/pnas.88.12.5092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C., Lopez-Rodriguez A., Srikumar D., Rosenthal J.J., and Holmgren M.. 2011. Editing of human K(V)1.1 channel mRNAs disrupts binding of the N-terminus tip at the intracellular cavity. Nat. Commun. 2:436 10.1038/ncomms1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H.C., Qu Z., Yu K., Xiao Q., and Chien L.T.. 2008. Molecular physiology of bestrophins: multifunctional membrane proteins linked to best disease and other retinopathies. Physiol. Rev. 88:639–672. 10.1152/physrev.00022.2007 [DOI] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W.N., and Aldrich R.W.. 1990. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 250:533–538. 10.1126/science.2122519 [DOI] [PubMed] [Google Scholar]
- Kane Dickson V., Pedi L., and Long S.B.. 2014. Structure and insights into the function of a Ca(2+)-activated Cl(-) channel. Nature. 516:213–218. 10.1038/nature13913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Li Z., Zhou Q., Shen H., Wu K., Huang X., Chen J., Zhang J., Zhu X., Lei J., et al. . 2018. Structure of the human voltage-gated sodium channel Nav1.4 in complex with beta1. Science. 10.1126/science.aau2486 [DOI] [PubMed] [Google Scholar]
- Petrukhin K., Koisti M.J., Bakall B., Li W., Xie G., Marknell T., Sandgren O., Forsman K., Holmgren G., Andreasson S., et al. . 1998. Identification of the gene responsible for Best macular dystrophy. Nat. Genet. 19:241–247. 10.1038/915 [DOI] [PubMed] [Google Scholar]
- Sun H., Tsunenari T., Yau K.W., and Nathans J.. 2002. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc. Natl. Acad. Sci. USA. 99:4008–4013. 10.1073/pnas.052692999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisey G., and Long S.B.. 2018. An allosteric mechanism of inactivation in the calcium-dependent chloride channel BEST1. J. Gen. Physiol. 10.1085/jgp.201812190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisey G., Miller A.N., and Long S.B.. 2016. Distinct regions that control ion selectivity and calcium-dependent activation in the bestrophin ion channel. Proc. Natl. Acad. Sci. USA. 113:E7399–E7408. 10.1073/pnas.1614688113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q., Prussia A., Yu K., Cui Y.Y., and Hartzell H.C.. 2008. Regulation of bestrophin Cl channels by calcium: role of the C terminus. J. Gen. Physiol. 132:681–692. 10.1085/jgp.200810056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q., Yu K., Cui Y.Y., and Hartzell H.C.. 2009. Dysregulation of human bestrophin-1 by ceramide-induced dephosphorylation. J. Physiol. 587:4379–4391. 10.1113/jphysiol.2009.176800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Liu Q., Kloss B., Bruni R., Kalathur R.C., Guo Y., Kloppmann E., Rost B., Colecraft H.M., and Hendrickson W.A.. 2014. Structure and selectivity in bestrophin ion channels. Science. 346:355–359. 10.1126/science.1259723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta W.N., Hoshi T., and Aldrich R.W.. 1990. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 250:568–571. 10.1126/science.2122520 [DOI] [PubMed] [Google Scholar]
- Zhou M., Morais-Cabral J.H., Mann S., and MacKinnon R.. 2001. Potassium channel receptor site for the inactivation gate and quaternary amine inhibitors. Nature. 411:657–661. 10.1038/35079500 [DOI] [PubMed] [Google Scholar]