Voltage-gated Na+ and K+ channels are known to underlie the temporal characteristics of action potentials. Corbin-Leftwich et al. establish reliable action potential recordings from Xenopus oocytes coexpressing these channels and show how different K+ channel subtypes can modulate excitability.
Abstract
Action potentials (APs) are the functional units of fast electrical signaling in excitable cells. The upstroke and downstroke of an AP is generated by the competing and asynchronous action of Na+- and K+-selective voltage-gated conductances. Although a mixture of voltage-gated channels has been long recognized to contribute to the generation and temporal characteristics of the AP, understanding how each of these proteins function and are regulated during electrical signaling remains the subject of intense research. AP properties vary among different cellular types because of the expression diversity, subcellular location, and modulation of ion channels. These complexities, in addition to the functional coupling of these proteins by membrane potential, make it challenging to understand the roles of different channels in initiating and “temporally shaping” the AP. Here, to address this problem, we focus our efforts on finding conditions that allow reliable AP recordings from Xenopus laevis oocytes coexpressing Na+ and K+ channels. As a proof of principle, we show how the expression of a variety of K+ channel subtypes can modulate excitability in this minimal model system. This approach raises the prospect of studies on the modulation of APs by pharmacological or biological means with a controlled background of Na+ and K+ channel expression.
Introduction
The action potential (AP) is a self-regenerating electrical signal and is the basis for long-distance signaling in the nervous system, skeletal muscles, and the heart. APs are also observed in plants, where they are thought to control several physiological processes (Choi et al., 2016). The plasma membrane of living cells is electrically polarized, with an intracellular negative voltage with respect to the extracellular environment. An AP consists of a transient change of the membrane potential toward canceling its electrical polarity (depolarization), followed by the establishment of positive intracellular potentials (polarity reversion or antipolarization) and, subsequently, a return to the initial negatively polarized state (repolarization). Based on the seminal work of Hodgkin and Huxley (1939, 1952) on the squid giant axon, we know that at least two competing ionic currents are essential for the generation of APs. In general, the first current consists of an influx of positive charges, usually carried by Na+ ions, driving a change toward positive membrane potentials. The second current is mainly carried by K+ ions and flows in the opposite direction through a K+-selective pathway that is also activated by depolarization. Today, we know that these pathways are typically embodied in Na+- and K+-selective voltage-gated (NaV and KV, respectively) ion channels (Hille, 2001).
Understanding how specific membrane transport pathways contribute to AP firing is the subject of considerable research effort (Bean, 2007). In excitable cells, a rich diversity of voltage-gated and non-voltage-gated channels and ion exchangers shape the AP, determining properties such as AP threshold, duration, amplitude, and firing frequency (Ballerini et al., 1997; Hille, 2001; Wang and Huang, 2006; Bean, 2007; Jan and Jan, 2012). The silencing of certain ion channel genes (Macica et al., 2003; Peters et al., 2005; Speca et al., 2014) or inhibition of specific channels by toxins or drugs (Bekkers and Delaney, 2001; Pathak et al., 2016; Hu and Bean, 2018) have been useful subtractive methods contributing to understanding the role of specific ion channel pathways in AP firing. Alternatively, an additive approach could be useful to understanding the role of different channels incorporated into a background of the minimal components necessary for APs. Computational models often use an additive design to study membrane excitability in silico (Rudy and Silva, 2006; Marder and Taylor, 2011). To advance this goal in living cells, we turned to the Xenopus laevis oocyte expression system (Gurdon et al., 1971) because it is a highly used tool for the study of membrane transport proteins foreign to the amphibian oocyte and allowed us to select for the simultaneous expression of specific proteins (Dascal, 1987; Sigel, 1990).
To date, there are more than 8,000 published works on the properties of ion channels expressed in Xenopus oocytes describing various experimental approaches, including techniques such as two-electrode voltage clamp (TEVC), patch clamp, or cut-open oocyte voltage clamp. However, investigation of the capacity of Xenopus oocytes to generate all-or-none APs is only sparsely addressed in the research literature. The present study explores a relatively simple and inexpensive adaptation of conventional TEVC recordings in Xenopus oocytes as an approach to the study the additive effects of K+ channel subtypes on the generation of APs. We build from prior demonstrations of regenerative APs in oocytes (Shapiro et al., 2012, 2013; Corbin-Leftwich et al., 2016) and test the sufficiency of Na+ channel expression alone, as well as the addition of non-voltage-gated and/or voltage-gated K+ conductances to document the reliability of AP generation and the impact of K+ channel diversity on APs. Furthermore, we demonstrate that APs recorded in Xenopus oocytes can induce repetitive firing. The results are important in their ability to replicate key features of APs and explore the minimal components needed for AP generation. This work has wide applications to the study of APs, including the role of specific ion channel subunits, the impact of ion channel mutations and modulation, pharmacologic screening of small molecules, and comparative physiology of ion channels from diverse species. The approach also has educational value for inquiry-based learning and helps clarify common misconceptions about APs.
Materials and methods
Preparation of oocytes and RNA injections
RNA preparation, Xenopus laevis oocyte isolation and preparation, and oocyte injection were performed using published methods (Heler et al., 2013; Corbin-Leftwich et al., 2016). Animal protocols were approved by Institutional Animal Care and Use Committees at the University of Richmond, Virginia Commonwealth University, and the University of the Pacific and conform to the requirements in the Guide for the Care and Use of Laboratory Animals from the National Academy of Sciences. Some oocytes were prepared from ovarian lobules purchased from Xenopus 1, and some oocytes were purchased from EcoCyte Bioscience. Results from many batches of oocytes were combined. Each oocyte was injected with 24–46 nl RNA and studied 2–4 d later. Oocytes were maintained at 14–17°C in a solution of (in mM) 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES, 5 sucrose, and 2.5 Na pyruvate, pH 7.4, with 50 U/ml penicillin G and 50 µg/ml streptomycin or 25 µg/ml gentamycin.
Electrophysiology
Ionic currents were recorded in two electrode voltage clamp (TEVC) from oocytes with Warner OC-725B or OC-725C amplifiers using maximum clamp speed and gain (Warner Instruments). Voltage and current recording pipettes were filled with a filtered solution containing 1 M KCl, 10 mM EGTA, 10 mM HEPES, pH 7.4.
To obtain AP recordings, we avoided voltage clamping the oocyte by decreasing the amplifier’s feedback gain (Corbin-Leftwich et al., 2016). As in many TEVC systems, our amplifiers consisted of two voltage-clamping circuits. One controls the intracellular potential, holding it at 0 mV with respect to a reference (“ground”). The second circuit (“bath clamp”) controls the extracellular potential, setting it to the negative of the command voltage (VCMD). Thus, an oocyte is voltage-clamped at −70 mV by setting the extracellular voltage to +70 mV. Our approach was, therefore, to make one of these two circuits as inefficient as possible, without hampering the ability to depolarize the cell. To achieve this, a simple modification was made. A 50-megaohm resistor was added between the current (feedback) electrode and the headstage to decrease the ability of the amplifier to voltage clamp the interior of the oocytes at 0 mV. To depolarize the membrane, we added a second resistor (10-megaohm) connected in series to a diode and placed in parallel to the first resistor. The diode was oriented with the cathode pointing toward the cell (Fig. 1, A and B). This arrangement allowed us to readily depolarize the membrane, but not efficiently repolarize it (Figs. S1 and S3). A switch was added to bypass this circuit, allowing us to toggle between the “loose” voltage clamp mode for AP recordings and the traditional TEVC mode for current recordings.
Figure 1.
A reliable approach to recording APs in Xenopus oocytes. (A and B) Schematic of the loose-clamp electronics used to measure APs with a TEVC amplifier. The dashed red box shows the adapted circuitry, which is enlarged in B. Electrical recordings in oocytes injected 2–4 d prior with an RNA mixture for sodium and potassium channels: 10 ng Nav1.4, 2.5 ng Navβ1, 5 ng Kv7.2, 5 ng Kv7.3, and 0.6 ng ShakerΔ. (C) In the loose clamp, 1-ms voltage pulses were applied to depolarize the membrane. Top: Membrane current produced by the depolarizing stimulus. Bottom: Membrane depolarization. The inset in C shows a subthreshold depolarization (black trace), and the next pulse stimulated a voltage change that is an all-or-none AP (red trace). (D) For the same RNA mixture as in C, the APs in oocytes were examined with paired pulses to measure the refractory period. The 1-ms pulses (top) were 16 ms apart for the traces on the left. The black traces for the pulses were for stimuli of the same amplitude, whereas red traces signify that the second pulse was of greater amplitude than the first. The corresponding membrane voltage recordings are shown in the bottom left with the same color coding. On the right side, the 1-ms depolarizing pulses (top) were 9 ms apart. The black traces were for stimuli of the same amplitude, whereas blue traces signify that the second pulse was of greater amplitude. The corresponding membrane voltage recordings are shown in the bottom right. The second stimulus was increased four times without eliciting an AP.
In the loose-clamp mode, we were able to readily depolarize the membrane (Fig. S2). For AP triggering, brief depolarizing pulses (0.5–1 ms) were used to elicit single APs, and longer duration stimuli, sometimes in the form of ramps, were used to generate multiple APs, as noted in the figure legends.
Experiments were conducted at room temperature (20–23°C) in a recording chamber that was perfused continuously with a standard 2 Ko solution containing (in mM) 2 KCl, 98 NaCl, 2 MgCl2, and 5 HEPES, pH 7.4. A 10 Ko solution (10 KCl with 90 Na or N-methyl-d-glucamine Cl) was used to study inwardly rectifying K+ (Kir) currents in voltage clamp (Fig. 4). Alternative solutions are noted in figure legends. Data were recorded and analyzed on computers equipped with Digidata 1320A A/D hardware and Clampex and Clampfit software (Molecular Devices). Data were sampled at 10–50 kHz and filtered at 1–2 kHz. Some experiments used a USB-6251 multifunction acquisition board (National Instruments) controlled by an in-house program coded in LabVIEW (National Instruments; details available from C.A. Villalba-Galea upon request). For these, the electrical signals were filtered at 100 kHz, oversampled at 500 kHz to 2 MHz, and stored at 5–25 kHz for offline analysis. All data were transferred to Excel (Microsoft) and Origin (OriginLab Corp.) for additional analysis and the production of figures; some data were analyzed using custom Java-based software (details available from C.A. Villalba-Galea, upon request). For box-and-whisker plots (Figs. 3 and 4), the ends of the box are the upper and lower quartiles, and the median is marked by a horizontal line inside the box. The whiskers are the maximum and minimum values, and the mean is designated with a solid data point inside the box. Significant differences in resting membrane potential (VREST) measurements (Fig. 4) were determined by ANOVA followed by a Tukey–Kramer multiple comparisons test. P < 0.05 was considered significant.
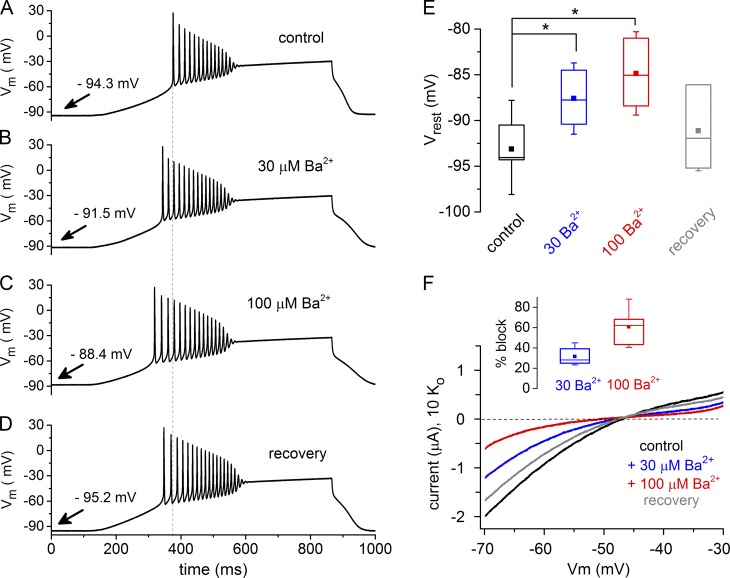
Figure 4.
Maintenance of VREST by inwardly rectifying K+ channels regulates AP generation in Xenopus oocytes. Xenopus oocytes were injected with an RNA mixture of 10 ng Nav1.4, 2.5 ng Navβ1, 2.5 ng ShakerΔ, and 10 or 20 ng Kir2.1 and incubated for 3 d before experiments. (A–E) Loose-clamp AP recordings were done using a ramp to depolarize the membrane in the absence of Ba2+ (2 Ko control; A), plus 30 µM Ba2+ (B), plus 100 µM Ba2+ (C), and upon recovery from Ba2+ block of Kir current by washout with 2 Ko (D). The dashed, vertical line in A–D designates the approximate time of onset of the first AP in a ramp under control conditions. Arrows in A–D designate the value of VREST for each recording condition. (E) Comparison of VREST measured before the current ramp that elicited APs, recorded as in A–D. The impact of 30 and 100 µM Ba2+ was measured (n = 6); significant differences from control values are noted by *. Box plots show 25–75% of the range (the box); the solid square inside the box is the mean VREST, and the whiskers depict the maximum and minimum values. The line within the box represents the median value. (F) For the same cells as in A–E, the expression of the Kir2.1 channel current was observed in TEVC recordings using a 10 Ko solution (black trace) and a voltage ramp to elicit the K+ currents. The current–voltage plot shows an example of block of the inward Kir current by 30 µM Ba2+ (blue trace) and 100 µM Ba2+ (red trace). The recovery is in 10 Ko (gray trace). The inset quantifies the percentage of the 10 Ko inward current at −70 mV that was blocked by 30 or 100 µM Ba2+ (n = 6).
Figure 3.
TREK (K2P) channel expression affects AP duration and VREST. (A) APs recorded in oocytes after expression of Nav channels (Nav1.4 α, 10 ng; Navβ1, 2.5 ng) plus 5 ng each of Kv7.2 and Kv7.3 or Nav channels plus TREK-1 (right, 3.0–6.5 ng E306A mutant; see Methods). The insets in A show the Kv7 or TREK-modified AP on different time scales. 3 d after RNA injection and incubation, the membrane potential recordings were done from a VREST of −60 mV using 1-ms depolarizing pulses. (B) APs from four different oocytes with low (blue trace) to high (orange trace) expression of TREK currents are shown. Inset: The half-width of each AP was measured and plotted as a function of the estimated magnitude of the outward TREK current at +35 mV. In the inset, the four symbols in color (orange, green, pink, and blue) correspond to the traces shown on the left for low to high TREK currents, respectively. (C and D) VREST was recorded after penetration of both recording pipettes into oocytes expressing Nav channels with the addition of the Kv7.2/7.3 subunits (red) or TREK (blue). Box plots show 25–75% of the range (the box); the solid square inside the box is the mean VREST, and the whiskers depict the maximum and minimum values. The line within the box represents the median value. Complete data for VREST measured in cells expressing Nav channels plus TREK (blue box plot) are expanded in D for individual cells with outward currents ranging from 3 to ∼20 μA at a command potential of +35 mV in TEVC.
Plasmid constructs
Ion channel plasmids used in this study included rat Nav1.4 (α subunit), rat Navβ1, human Kv7.1, human Kv7.2, human Kv7.3, rat Kir2.1, Shaker with fast inactivation-removed (Shaker Δ6–46, hereafter ShakerΔ), rat Kv4.2Δ2-40 (slows inactivation and enhances channel expression), rat Kv2.1, and mouse TREK-1 E306A. Mutations or deletions were introduced and confirmed by published methods (Heler et al., 2013). The mass of each RNA injected is noted in each figure legend. The Navβ1 subunit was always coinjected with the Navα subunit to enhance channel expression and produce fast Na+ channel gating (Makita et al., 1994; Isom et al., 1995).
Online supplemental material
Figs. S1 and S2 show the performance of the loose TEVC clamp. Fig. S3 shows how the kinetics of membrane repolarization in loose TEVC is impacted by an outwardly rectifying, voltage-gated K+ conductance. Fig. S4 shows evidence for a contribution from native oocyte Cl− conductance to the repolarization of APs. Fig. S5 shows that Kir channels impact VREST but not VTHR in regulating AP excitability.
Results
We expressed Na+ and K+ channels in Xenopus oocytes to determine the minimal requirements for inducing all-or-none regenerative electrical activity in the membrane of a large, nonexcitable cell. Fig. 1 shows the loose-clamp electronics used to measure the APs in oocytes (Fig. 1, A and B) and demonstrates APs recorded in oocytes with high expression of Nav1.4 (α and β subunits) and Kv currents (Fig. 1 C).
The resistor-diode device described in Methods allowed depolarization of the membrane, but not its repolarization (Fig. S1, A and B; and Fig. S2). Also, the expression of a KV channel resulted in a rapid repolarization of the membrane after depolarization (Fig. S1, D and E) with a repolarization rate that was a function of the depolarizing pulse amplitude (Fig. S3 C). In oocytes expressing NaV and KV channels, we were able to elicit APs once a threshold potential was surpassed by the application of 1-ms depolarizing pulses. We attributed the fast upstroke of this electrical response and the subsequent rapid repolarization to the activity of NaV and KV channels, respectively. These APs produced in Xenopus oocytes expressing NaV and KV channels had a quick rising phase, were all or none (see inset to Fig. 1 C), and reached a depolarized potential of approximately +20 mV under the conditions tested. The repolarizing or decaying phase of the AP was mainly driven by the large outward current from the ShakerΔ channels. We also observed that repolarization of Xenopus oocyte APs was often followed by an afterhyperpolarization (Fig. 1 C) as long as Kv channels were used in the RNA injection mixture. The same type of recordings in uninjected oocytes produced electrotonic responses, displaying only slow, nonundershooting membrane potential changes (Figs. S1 and S3).
APs in Xenopus oocytes also demonstrate refractory periods (Fig. 1 D). We observed a relative refractory period when a second AP could be initiated only after applying a stimulus stronger than that used to elicit the first AP (Fig. 1 D, left). An absolute refractory period was determined as the shortest time interval during which a second AP could not be elicited, despite increases in stimulus magnitude (Fig. 1 D, right). Thus, the APs in Xenopus oocytes demonstrate the hallmark features of APs that are critical to setting the firing rate of excitable cells.
Are Nav channels alone sufficient to produce an AP?
For the generation of an AP, both a depolarizing conductance and a repolarizing voltage-dependent conductance are thought to be required. Membrane repolarization was slow but steady in uninjected oocytes that were depolarized by a step pulse (Fig. S1 B). This result suggests that the expression of Nav channels in oocytes combined with endogenous conductances in oocytes should produce a transient response, resembling an AP. To test this hypothesis, we injected oocytes with only cRNAs encoding NaV α and β subunits. Fig. 2 shows that long-lasting, all-or-none depolarizing membrane potential changes could be generated with sufficiently large Nav channel expression (even in the absence of K+ channel RNA in the injection mixture). Similar to uninjected cells, an oocyte expressing only a small inward Na+ current (∼1 µA at the peak inward current in TEVC; Fig. 2 A, inset) generated only passive membrane depolarizations (electrotonic potential) in loose clamp (Fig. 2 A), and cells with low levels of Na+ current expression failed to trigger an AP (Fig. 2 B). However, for oocytes with inward Na+ currents greater than ∼20 μA, APs were often elicited (Fig. 2, C and D). With the highest expression of Na+ currents measured (∼40 μA or more), all-or-none responses reached a peak depolarization near the Nernst potential for Na+ (approximately +40 mV; Fig. 2 D). We speculate that, given the large size of the oocytes, a high density of current was required to overcome the background repolarizing conductances in the oocyte and depolarize the entire cell.
Figure 2.
Testing for AP generation in oocytes after expression of sodium channel RNA only. Oocytes were injected with 10 ng Nav1.4 and 2.5 ng Navβ1 subunits only and recorded 2–4 d postinjection. K+ channel RNA was not included. (A–D) Representative membrane potentials recorded from oocytes maintained at a membrane voltage of approximately −50 mV, in response to stepwise increases in depolarizing pulses (1 ms) in the loose clamp recording mode. The insets in each panel show TEVC current recordings (not leak-subtracted) of the estimated maximum inward Na+ current recorded for each oocyte: 1 µA (A), 17 µA (B), 25 µA (C), and 43 µA (D). Voltage and current recordings in A–D are all on the same axis scales, for comparison of amplitudes and kinetics. The upward current deflections in the insets (A–D) are unsubtracted capacitive artifacts (truncated in B–D). These are representative examples from among a larger population of oocytes showing variable levels of Na+ current expression. (E and F) The probability of firing an all-or-none AP was determined for all cells injected with Na+ channel α and β RNAs only and expressing <20 µA (E) or ≥20 µA (F) of peak inward current. AP genesis was determined in response to variable-amplitude 1-ms depolarizing pulses in the loose-clamp mode for VREST values between −42.5 and −72.5 mV (grouped in increments of 5 mV). The number on each bar represents the number of recordings at each range of VREST. The dashed lines in E and F designate the maximum probability of firing an AP for the conditions of low (E) and high (F) Na+ current expression.
Another important factor to be considered was the VREST. In the absence of exogenous K+ channels, we were able to manipulate VREST by setting VCMD to more negative potentials. Thus, we used this procedure to study the role of NaV channel modulation by VREST in AP triggering because NaV channels are known to undergo closed-state inactivation, represented as h∞ in the equations of Hodgkin and Huxley (1952); see also Armstrong (2006). As a consequence, the number of NaV channels that can be activated increases as the membrane potential becomes more negative. On the other hand, hyperpolarization also decreases the rate of activation of channels, as described by Cole and Moore (1960). Consistent with this idea, we observed that AP triggering had a bell-shaped dependence on VREST (Fig. 2, E and F). In oocytes showing less than 20 µA of maximum Na+ current, AP triggering was more effective when VREST was between −52.6 and −62.5 mV (Fig. 2 E). In oocytes displaying more than 20 µA, triggering an AP was more effective when VREST was between −62.6 and −67.5 mV (Fig. 2 F). This small change in the optimal VREST for AP triggering is consistent with a role for NaV channel closed-state inactivation in cellular excitability.
Note that we routinely recorded ionic currents in the TEVC mode as a way to estimate the relative magnitude of currents resulting from the mixtures of ion channel RNAs injected. However, we are under no illusions that the voltage control in TEVC was adequate at all test potentials (Baumgartner et al., 1999). To achieve AP generation and test for the minimal requirements by continually increasing Na+ currents, we conceded this limitation of TEVC. In sum, to reliably elicit an all-or-none regenerative change in membrane potential in Xenopus oocytes, the critical issue is that the estimated peak, inward, voltage-gated Na+ current must be quite large (≥20 μA) and VREST must be optimized for Nav channel availability.
Are non-voltage-gated K+ conductances sufficient to support AP firing?
The ability to generate APs in oocytes in the absence of exogenous K+ channel expression indicates that native resting conductances in oocytes are sufficient to repolarize the NaV-only AP. Furthermore, some of the resting conductances must hyperpolarize the membrane sufficiently to reset the voltage sensors of the Nav channels and increase their availability to produce the all-or-none AP. The hyperpolarizing actions of the Na+/K+ ATPase pump would be of insufficient magnitude to perform these functions; ouabain inhibition of the pumps in cardiac muscle induces a membrane depolarization of only 5 mV (Miura and Rosen, 1978). An alternative explanation is that the oocyte plasma membrane possesses a sufficient number of its own K+ or Cl− channels, although their contributions cannot be distinguished when the expression of foreign channels is very high. We observed evidence that endogenous Cl− conductances, activated by the Nav-dependent depolarizations (Sanguinetti et al., 1996; Weber, 1999), contribute to the repolarization of the AP generated from Nav channels alone (Fig. S4). In oocytes expressing Na+ currents greater than ∼30 μA, we observed (in five of eight cells) APs with repolarizing phases that lasted from 390 to 2,970 ms, and we observed that reduction of external Cl− lengthened the repolarization phase (Fig. S4). In addition, “leak” K+ channels of the K2P type are present in Xenopus oocytes (Weber, 1999) and may also contribute to repolarization.
We tested the impact of experimentally induced K2P channel expression on Na+-generated APs in Xenopus oocytes in the absence of exogenous voltage-gated K+ channel expression (Fig. 3). Along with the Nav channels, we coexpressed TREK-1 channels possessing an E306A mutation so that these K2P channels would be constitutively active in the absence of thermal, chemical, or mechanical stimuli (Honoré et al., 2002), which might simultaneously impact Nav or endogenous channels. Notably, oocytes injected with RNA for Nav channels and only TREK-1 K+ channels demonstrated reliable APs (Fig. 3). Compared with oocytes expressing the slowly activating Kv7.2/7.3 channels, the expression of constitutively active TREK channels resulted in faster APs, although they lacked an undershoot after membrane repolarization (Fig. 3 A). TREK-1 conductances influenced the duration of the AP depolarization; as K+ current amplitude increased, the AP half-width decreased (Fig. 3 B). As described previously for large Na+ currents recorded in TEVC, large TREK-1 K+ currents introduced error in the voltage clamp recording, and so the values reported for TREK-1 current amplitudes (Fig. 3 B, inset; and Fig. 3 D) are estimates, with the largest values possessing the greatest error.
Increasing expression of K2P channels also increased the magnitude of the polarization of the VREST (Fig. 3 C). Of course, the oocyte VREST measured in TEVC is expected to be more depolarized than the true VREST because two micropipettes are penetrating and thus damaging the membrane. Despite this drawback, under the same conditions of measurement, the impact of K2P channels on VREST in Xenopus oocytes is similar to that of voltage-gated channels, with Kv7.2/7.3 channels used for comparison (Fig. 3 C). Fig. 3 D plots all of the VREST data for oocytes expressing TREK-1 (from the box plot in Fig. 3 C) so that the relationship between the variable expression of TREK-1 currents and VREST may be determined. Based on the impact of TREK-1 expression on AP duration and VREST, K2P channels support AP firing by facilitating Nav channel deactivation and minimizing inactivation, even in the absence of exogenous Kv channels.
Kir channel expression enhances excitability in Xenopus oocytes
We also studied the impact of Kir channels on AP generation in oocytes (Fig. 4). We coexpressed Nav channels with ShakerΔ plus Kir2.1 and used the loose-clamp approach to deliver depolarizing current ramps, which elicited a series of APs (Fig. 4 A). The APs eventually ceased because of the cumulative inactivation of Na+ channels. Block of Kir channels by external Ba2+ (30 or 100 µM) reduced the latency to fire APs and, sometimes, increased the number of APs fired during the current ramp (Fig. 4, B–D). This occurred with a reversible Ba2+-dependent depolarization of the VREST (Fig. 4 E). Using TEVC, we confirmed the presence of a Ba2+-sensitive Kir current in these cells by elevating the external K+ concentration from 2 mM to 10 mM Ko (Fig. 4 F). We tested if the enhanced excitability following Ba2+ block of Kir channels could be explained by a change in threshold (VTHR), but we found no evidence for this (Fig. S5). These results highlight the exquisite sensitivity of AP generation to the difference between VREST and threshold for the all-or-none response.
An additive approach to studying the impact of Kv channel diversity on APs
A diversity of AP waveforms could be achieved using the expression of different channel types (Fig. 5). When Nav channel RNA was coinjected with only a Kv4-type of K+ channel RNA, the oocyte APs were long and lacked a detectable undershoot after membrane repolarization (Fig. 5 A). The rapidly activating and inactivating Kv4 channels did not confer a rapid phase of repolarization, and the K+ conductance was not sustained long enough to contribute to an undershoot. However, when Nav channel RNA was coinjected with only a Kv7-type of K+ channel RNA, the oocyte APs were shorter in duration and demonstrated a prominent undershoot after membrane repolarization (Fig. 5, B and E). By comparison to Kv4, the more sustained activity of the Kv7 channels would be expected to contribute more to the repolarization and afterhyperpolarization. The combined expression of Kv4 and Kv7 types of channels gave rise to an intermediate-duration AP with a prominent undershoot. The kinetics of the Kv7 channel seem to dominate over the Kv4 channel, perhaps because the Kv4 currents are more transient. Neither Kv4 nor Kv7 K+ currents, alone nor combined, were sufficient to generate a fast AP with kinetics similar to neuronal APs. However, coexpression of Nav channels with ShakerΔ resulted in fast APs (Fig. 5 D). When combined with the Kv7 type of channels, the impact of ShakerΔ predominates and the APs are fast (Fig. 5 F). Importantly, when studying the additive effects of different K+ conductances on AP properties, the K+ channel currents were also observed in TEVC so that the presence of each current could be confirmed for each unique RNA. In sum, our results demonstrate the ability to induce diverse APs in oocytes, and future studies could use this additive approach to study in greater detail the contributions of different channel types to the AP waveform.
Figure 5.
Diversity of Kv channel expression modifies the oocyte AP waveforms. (A–F) Representative APs evoked in oocytes 3 d after injection with RNA mixtures for Nav1.4 α (10 ng) and Navβ1 (2.5 ng) plus Kv channels. The RNAs were mixed to achieve an injection of 20 ng Kv4.2Δ2-40 (A); 2.5 ng Kv7.2 and 2.5 ng Kv7.3 (B); 20 ng Kv4.2Δ2-40 plus 2.5 ng Kv7.2 and 2.5 ng Kv7.3 (C); 0.65 ng ShakerΔ (D); 5 ng Kv7.2 and 5 ng Kv7.3 (E); and 0.65 ng ShakerΔ plus 5 ng Kv7.2 and 5 ng Kv7.3 (F). In A–F, each set of three membrane voltage recordings shows one response which was just below threshold (blue trace), the first suprathreshold response (red trace), and a suprathreshold response for which increasingly larger pulses of 1-ms duration failed to change the membrane potential recording (black trace). A–F use the same voltage and time axes for ease of comparison; the insets in D and F show the faster APs on a different time scale.
Can oocytes generate repetitive AP firing?
Oocytes had the capacity to generate multiple APs in response to sustained stimuli, multiple stimuli, or in some cases, no direct stimulus. For example, very high expression of Nav channels along with very high expression of K+ channels could result in spontaneous APs, as the VREST hovered near the membrane’s threshold for firing (Fig. 6 A). Combinations of Kir2.1 plus ShakerΔ (Fig. 6 A) supported spontaneous APs. High expression of Kv7 channels could also support spontaneous APs, but with a different firing rate (Fig. 6 B). In addition, delivery of a sustained, suprathreshold depolarizing pulse from a loose clamp could excite a train of APs in oocytes expressing Kir2.1 plus ShakerΔ (Fig. 6 C). In sum, AP patterns induced in Xenopus oocytes represent an emergent property of the expression and interactions of a variety of ion channel types.
Figure 6.
Oocyte recordings may demonstrate multiple APs. 3 d before AP recordings, oocytes were injected with a mixture of 10 ng Nav1.4 α and 2.5 ng Nav1.4 β plus coexpressing K+ channel RNAs, as noted. (A) Spontaneous APs in oocytes coexpressing ShakerΔ (0.65 ng RNA) and Kir 2.1 (10 ng RNA). This is representative of four similar recordings from oocytes injected with the same mixture of RNAs. (B) Oscillatory APs were also spontaneously evoked in oocytes coexpressing Kv7.1 (2.5 ng RNA). This is representative of five similar recordings from oocytes injected with the same mixture of RNAs. (C) High-frequency AP firing in oocytes coexpressing ShakerΔ (0.65 ng RNA injected) and Kir 2.1 (10 ng) generated by a 1-s suprathreshold depolarizing stimulus applied from a loose clamp. Representative of four cells.
Discussion
The determinant of AP formation is the high expression of voltage-gated Na+ and K+ channels in a spatially compact and restricted area such as in the axon initial segment (Kole and Stuart, 2012). The high expression of Na+ and K+ channels in Xenopus oocytes affirmed these minimal channel requirements for inducing a rapid, all-or-none, regenerative electrical event. The findings in this paper extend the work of Shapiro et al. (2012), who demonstrated the ability of infrared radiation to depolarize a cell, and of Corbin-Leftwich et al. (2016), who studied the impact of a Kv7 modulator on cellular excitability. Unique to the present study is the assessment of membrane potentials generated in loose clamp by high expression of Nav channels (Fig. 2), examination of the role of non-voltage-gated K+ conductances in the genesis of APs (Figs. 3, 4, and S5), demonstration of the diversity of AP waveforms that can be generated in oocytes (Figs. 5 and 6), and documentation of reliable methods necessary to achieve the AP recordings (Figs. 1, S1, S2, and S3). Collectively, these findings provide valuable information about the parameters of APs induced in oocytes that can be used in diverse research and educational settings.
Notably, our study used skeletal muscle Nav1.4 α subunits, because plasmid DNA constructs for neuronal Nav channels are remarkably unstable in bacterial hosts. However, if methods are successfully used to prepare neuronal Nav plasmid DNA without rearrangements or uncontrolled mutations (Feldman and Lossin, 2014), it is reasonable to expect that neuronal Nav channels (e.g., Nav1.1, 1.2, and 1.6) could be used to generate APs in oocytes as well. This remains to be tested in future studies.
In neurons, the axon hillock (Fuortes et al., 1957) and the axon initial segment (Araki and Otani, 1955) are sites of AP initiation (Colbert and Johnston, 1996), although this functional polarization may be a vertebrate specialization (Kole and Stuart, 2012). The unique role of these structures is governed by the very high expression of voltage-gated Na+ and K+ channels in a spatially restricted area. Although there are differences in the channel density and geometry of the neuronal structures and the Xenopus oocyte, a requirement for very high expression of Na+ channels is a cornerstone of the present study. The genesis of all-or-none events that resemble APs in excitable cells required the coexpression of at least one type of voltage-gated or non-voltage-gated K+ conductance. Indeed, coexpression of the TREK-1 type of K2P channels with Nav channels was sufficient to allow rapid APs (Fig. 3). These results compare favorably with the conclusions from a mixed computer simulation/biological experiment in which virtual Na+ currents were introduced to HEK cell patch-clamp recordings with biologically expressed K2P channels to assess the ability to fire APs without the introduction of voltage-gated K+ channels (MacKenzie et al., 2015). Although background Kv channels may have been present in the HEK cells, and the study examined in silico but not biological Na+ channels, K2P channels generated the hyperpolarized VREST needed for APs, and they contributed to repolarization of the AP (MacKenzie et al., 2015). Likewise, in cerebellar Purkinje cells, high expression of K2P channels supports rapid AP firing (Brickley et al., 2007). The results of the present study support the hypothesis that the minimal requirement for an AP includes a sufficiently large K+ conductance, although it need not be a voltage-activated conductance. Mechanistically, our results suggest that a K2P conductance is sufficient to promote the deactivation of Na+ channels, at least for the capability of firing single APs.
Kir channels have a complex relationship with cellular excitability. Neurons with naturally large Kir currents tend to have strongly hyperpolarized VREST values (Hibino et al., 2010), which may suppress excitability (Leao et al., 2012; Li et al., 2013), and genetic overexpression of Kir channels can be used to silence neuronal electrical activity (Nitabach et al., 2002). Correspondingly, genetic inhibition of Kir2.1 reduces membrane hyperpolarization and promotes rhythmic firing in ventricular myocytes (Miake et al., 2002). The dampening of excitability by Kir channels may seem paradoxical, because membrane hyperpolarization promotes recovery from inactivation of Nav channels. However, a membrane that is strongly hyperpolarized at rest may not generate a large enough depolarization in a spatially restricted area to engage the regenerative cycle of depolarization and Na+ channel activation, even when Na+ channels are available. Kir channels thus influence cellular excitability not simply by maintaining a hyperpolarized VREST or altering VTHR, but by regulating the relationship between VREST and Nav channel availability (Figs. 4 and S5).
Kir channel expression promoted spontaneous firing (Fig. 6 A) and high-frequency firing in response to long current injections (Fig. 6 C), probably by finely regulating the Nav channel availability. Indeed, other mechanisms that allowed the membrane potential to hover near threshold while also maintaining sufficient Nav channel availability for AP firing could reveal excitability in the oocyte membrane; even Kv7 channels could support spontaneous firing (Fig. 6 B). Correspondingly, we observed that in oocytes with a high expression of Nav channels and the capability of generating APs, we could prevent AP generation by artificially hyperpolarizing the membrane in the loose-clamp recordings. Together, these experiments in oocytes help us understand how Kir channels influence the dynamic relationship between Nav channel availability and VREST, with significant impact on cellular excitability.
We have shown that AP generation in Xenopus oocytes provides a way to additively change an AP waveform in living cells and study the role of different channels on AP diversity (Yue and Yaari, 2004; Bean, 2007; Larsson, 2013). Such an approach could be particularly useful in reconstituting APs from ion channels cloned from invertebrates in which recording from neural tissue is challenging or using channels from unicellular organisms such as diatoms or algal cells. Other specific applications include investigating the role of accessory subunits for many of the channels (Patton et al., 1994), RNA editing (Patton et al., 1997; Garrett and Rosenthal, 2012), glycosylation (Johnson and Bennett, 2008), or disease-causing, gain-of-function mutations in ion channels (Lossin et al., 2012; Cannon, 2015; Dell’Orco et al., 2017). Especially useful would be the expression of mutations in accessory subunits whose influences are difficult to predict. An example is the C121W mutation in a neuronal Nav β1 subunit, which is associated with a form of epilepsy (Wallace et al., 1998). Additional applications of this approach are to test hypotheses about the impact of drugs and modulators on AP waveforms (Corbin-Leftwich et al., 2016) using controlled combinations of channel RNAs. Perhaps researchers may also be able to induce propagated APs in gap junction–associated oocytes (Swenson et al., 1989) or stimulate APs in oocytes using channelrhodopsins or ligand-gated channels.
When adding different ionic conductances to the model system, the ease of repeatedly switching between voltage clamp and AP recording makes it possible to assess relative magnitudes of ionic currents and membrane potential changes. However, precise measurements of the kinetics and amplitudes of ionic current recordings are compromised by space clamp artifacts (Baumgartner et al., 1999). The key adaptation of the TEVC method that is needed for AP recordings in oocytes is to weaken the voltage clamp so that the membrane potential changes as a function of the ionic currents. Thus, the approach described here is best considered as an AP recording method; requirements for simultaneous voltage clamp recordings of time- and voltage-dependent currents should be used only to confirm that certain channel types are functional and to estimate expression levels. A second limitation is in studying the role of calcium-dependent conductances on AP firing, due to the presence of endogenous Ca2+-activated Cl− conductances in the oocyte (Weber, 1999). Additional limitations of the oocyte expression system or any other in vitro expression system are not restricted to the loose-clamp method of AP recording. Despite these limitations, the model has identified research applications and remains open to the possibility of future, creative adaptations as well.
An unexpected benefit that we experienced in exploring this AP model is the facilitation of student learning about the properties of APs. Undergraduate and graduate students alike benefit from the hands-on recording and analysis of APs, an experience that is not tractable for many students when using cultured cells or brain slices and patch-clamp methods. The approach offers the enjoyment of interacting with the data in real time and in a living cell; the ability to use the model for hypothesis-testing promotes inquiry-based learning, which deepens understanding and increases knowledge retention (Kober, 2015; Waldrop, 2015). The minimal AP model can certainly help to clarify common misconceptions about APs. For example, students, faculty, and researchers alike often believe that the value of −50 or −55 mV (as shown in textbooks) is the value of “threshold” in all cells. This minimal AP model, therefore, could certainly help investigators recognize the impact of ion channel composition and resting potential on threshold and appreciate that it is neither a set value for every excitable cell nor invariable in an individual cell. Academics also often believe that all neurons fire APs. The present approach helps students formulate their own explanation for why certain neurons do not show spiking behavior in native systems (Bufler et al., 1992; Baden et al., 2013) and what is distinctive about an excitable membrane. This helps encourage discussion and understanding about how channel localization and clustering may affect AP firing and facilitates appreciation for the structure and function of the axon hillock, initial segments, and nodes of Ranvier.
In conclusion, this work demonstrates reliable, useful, and simple approaches to induce APs in Xenopus oocytes with many applications for research and teaching.
Supplementary Material
Acknowledgments
Special thanks to undergraduate students Julian Butler, Bridgette Heine, Trevor Larry, and Shriraj Patel for discussion and feedback about experiments, and Sayeed M. Mossadeq for preliminary work on the methods. Thanks to Scott O’Grady and Kelly Lambert for feedback on the manuscript.
This research was supported by National Institutes of Health (NIH) grants 2R15-GM096142 (to L.M. Boland) and R01HL059949 (subcontract to C.A. Villalba-Galea), undergraduate research grants from the Howard Hughes Medical Institute Undergraduate Science Education Program and Beckman Scholars Foundation to the University of Richmond, summer undergraduate research fellowships from the University of Richmond School of Arts and Sciences, and the Nutting-Davidson Sinai Research Award (to C.A. Villalba-Galea). This paper is subject to the NIH Public Access Policy.
The authors declare no competing financial interests.
Author contributions: All authors performed experiments and analyzed data. A. Corbin-Leftwich, H.E. Small, and H.H. Robinson contributed to preliminary drafts of the manuscript. L.M. Boland and C.A. Villalba-Galea designed the concept of the research and wrote and edited the paper. All authors approved the final manuscript.
Kenton J. Swartz served as editor.
References
- Araki T., and Otani T.. 1955. Response of single motoneurons to direct stimulation in toad’s spinal cord. J. Neurophysiol. 18:472–485. 10.1152/jn.1955.18.5.472 [DOI] [PubMed] [Google Scholar]
- Armstrong C.M. 2006. Na channel inactivation from open and closed states. Proc. Natl. Acad. Sci. USA. 103:17991–17996. 10.1073/pnas.0607603103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden T., Euler T., Weckström M., and Lagnado L.. 2013. Spikes and ribbon synapses in early vision. Trends Neurosci. 36:480–488. 10.1016/j.tins.2013.04.006 [DOI] [PubMed] [Google Scholar]
- Ballerini L., Bracci E., and Nistri A.. 1997. Pharmacological block of the electrogenic sodium pump disrupts rhythmic bursting induced by strychnine and bicuculline in the neonatal rat spinal cord. J. Neurophysiol. 77:17–23. 10.1152/jn.1997.77.1.17 [DOI] [PubMed] [Google Scholar]
- Baumgartner W., Islas L., and Sigworth F.J.. 1999. Two-microelectrode voltage clamp of Xenopus oocytes: voltage errors and compensation for local current flow. Biophys. J. 77:1980–1991. 10.1016/S0006-3495(99)77039-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B.P. 2007. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 8:451–465. 10.1038/nrn2148 [DOI] [PubMed] [Google Scholar]
- Bekkers J.M., and Delaney A.J.. 2001. Modulation of excitability by α-dendrotoxin-sensitive potassium channels in neocortical pyramidal neurons. J. Neurosci. 21:6553–6560. 10.1523/JNEUROSCI.21-17-06553.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley S.G., Aller M.I., Sandu C., Veale E.L., Alder F.G., Sambi H., Mathie A., and Wisden W.. 2007. TASK-3 two-pore domain potassium channels enable sustained high-frequency firing in cerebellar granule neurons. J. Neurosci. 27:9329–9340. 10.1523/JNEUROSCI.1427-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufler J., Zufall F., Franke C., and Hatt H.. 1992. Patch-clamp recordings of spiking and nonspiking interneurons from rabbit olfactory bulb slices: membrane properties and ionic currents. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 170:145–152. [DOI] [PubMed] [Google Scholar]
- Cannon S.C. 2015. Channelopathies of skeletal muscle excitability. Compr. Physiol. 5:761–790. 10.1002/cphy.c140062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W.-G., Hilleary R., Swanson S.J., Kim S.-H., and Gilroy S.. 2016. Rapid, long-distance electrical and calcium signaling in plants. Annu. Rev. Plant Biol. 67:287–307. 10.1146/annurev-arplant-043015-112130 [DOI] [PubMed] [Google Scholar]
- Colbert C.M., and Johnston D.. 1996. Axonal action-potential initiation and Na+ channel densities in the soma and axon initial segment of subicular pyramidal neurons. J. Neurosci. 16:6676–6686. 10.1523/JNEUROSCI.16-21-06676.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole K.S., and Moore J.W.. 1960. Potassium ion current in the squid giant axon: dynamic characteristic. Biophys. J. 1:1–14. 10.1016/S0006-3495(60)86871-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin-Leftwich A., Mossadeq S.M., Ha J., Ruchala I., Le A.H., and Villalba-Galea C.A.. 2016. Retigabine holds KV7 channels open and stabilizes the resting potential. J. Gen. Physiol. 147:229–241. 10.1085/jgp.201511517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N. 1987. The use of Xenopus oocytes for the study of ion channels. CRC Crit. Rev. Biochem. 22:317–387. 10.3109/10409238709086960 [DOI] [PubMed] [Google Scholar]
- Dell’Orco J.M., Pulst S.M., and Shakkottai V.G.. 2017. Potassium channel dysfunction underlies Purkinje neuron spiking abnormalities in spinocerebellar ataxia type 2. Hum. Mol. Genet. 26:3935–3945. 10.1093/hmg/ddx281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D.H., and Lossin C.. 2014. The Nav channel bench series: Plasmid preparation. MethodsX. 1:6–11. 10.1016/j.mex.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuortes M.G., Frank K., and Becker M.C.. 1957. Steps in the production of motoneuron spikes. J. Gen. Physiol. 40:735–752. 10.1085/jgp.40.5.735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett S., and Rosenthal J.J.C.. 2012. RNA editing underlies temperature adaptation in K+ channels from polar octopuses. Science. 335:848–851. 10.1126/science.1212795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J.B., Lane C.D., Woodland H.R., and Marbaix G.. 1971. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature. 233:177–182. 10.1038/233177a0 [DOI] [PubMed] [Google Scholar]
- Heler R., Bell J.K., and Boland L.M.. 2013. Homology model and targeted mutagenesis identify critical residues for arachidonic acid inhibition of Kv4 channels. Channels (Austin). 7:74–84. 10.4161/chan.23453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino H., Inanobe A., Furutani K., Murakami S., Findlay I., and Kurachi Y.. 2010. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol. Rev. 90:291–366. 10.1152/physrev.00021.2009 [DOI] [PubMed] [Google Scholar]
- Hille B. 2001. Ion Channels of Excitable Membranes. Sinauer, Sunderland, MA. [Google Scholar]
- Hodgkin A.L., and Huxley A.F.. 1939. Action potentials recorded from inside a nerve fibre. Nature. 144:710–711. 10.1038/144710a0 [DOI] [Google Scholar]
- Hodgkin A.L., and Huxley A.F.. 1952. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J. Physiol. 116:449–472. 10.1113/jphysiol.1952.sp004717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E., Maingret F., Lazdunski M., and Patel A.J.. 2002. An intracellular proton sensor commands lipid- and mechano-gating of the K+ channel TREK-1. EMBO J. 21:2968–2976. 10.1093/emboj/cdf288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., and Bean B.P.. 2018. Differential control of axonal and somatic resting potential by voltage-dependent conductances in cortical layer 5 pyramidal neurons. Neuron. 97:1315–1326.e3. 10.1016/j.neuron.2018.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom L.L., Scheuer T., Brownstein A.B., Ragsdale D.S., Murphy B.J., and Catterall W.A.. 1995. Functional co-expression of the beta 1 and type IIA alpha subunits of sodium channels in a mammalian cell line. J. Biol. Chem. 270:3306–3312. 10.1074/jbc.270.7.3306 [DOI] [PubMed] [Google Scholar]
- Jan L.Y., and Jan Y.N.. 2012. Voltage-gated potassium channels and the diversity of electrical signalling. J. Physiol. 590:2591–2599. 10.1113/jphysiol.2011.224212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D., and Bennett E.S.. 2008. Gating of the shaker potassium channel is modulated differentially by N-glycosylation and sialic acids. Pflugers Arch. 456:393–405. 10.1007/s00424-007-0378-0 [DOI] [PubMed] [Google Scholar]
- Kober N. 2015. Reaching Students: What Research Says about Effective Instruction in Undergraduate Science and Engineering. National Academies Press, Washington, D.C., 10.17226/18687 [DOI] [Google Scholar]
- Kole M.H.P., and Stuart G.J.. 2012. Signal processing in the axon initial segment. Neuron. 73:235–247. 10.1016/j.neuron.2012.01.007 [DOI] [PubMed] [Google Scholar]
- Larsson H.P. 2013. What determines the kinetics of the slow afterhyperpolarization (sAHP) in neurons? Biophys. J. 104:281–283. 10.1016/j.bpj.2012.11.3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao R.M., Li S., Doiron B., and Tzounopoulos T.. 2012. Diverse levels of an inwardly rectifying potassium conductance generate heterogeneous neuronal behavior in a population of dorsal cochlear nucleus pyramidal neurons. J. Neurophysiol. 107:3008–3019. 10.1152/jn.00660.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Blankenship M.L., and Baccei M.L.. 2013. Inward-rectifying potassium (Kir) channels regulate pacemaker activity in spinal nociceptive circuits during early life. J. Neurosci. 33:3352–3362. 10.1523/JNEUROSCI.4365-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossin C., Nam T.S., Shahangian S., Rogawski M.A., Choi S.Y., Kim M.K., and Sunwoo I.N.. 2012. Altered fast and slow inactivation of the N440K Nav1.4 mutant in a periodic paralysis syndrome. Neurology. 79:1033–1040. 10.1212/WNL.0b013e3182684683 [DOI] [PubMed] [Google Scholar]
- Macica C.M., von Hehn C.A.A., Wang L.-Y., Ho C.-S., Yokoyama S., Joho R.H., and Kaczmarek L.K.. 2003. Modulation of the kv3.1b potassium channel isoform adjusts the fidelity of the firing pattern of auditory neurons. J. Neurosci. 23:1133–1141. 10.1523/JNEUROSCI.23-04-01133.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie G., Franks N.P., and Brickley S.G.. 2015. Two-pore domain potassium channels enable action potential generation in the absence of voltage-gated potassium channels. Pflugers Arch. 467:989–999. 10.1007/s00424-014-1660-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita N., Bennett P.B. Jr., and George A.L. Jr. 1994. Voltage-gated Na+ channel β 1 subunit mRNA expressed in adult human skeletal muscle, heart, and brain is encoded by a single gene. J. Biol. Chem. 269:7571–7578. [PubMed] [Google Scholar]
- Marder E., and Taylor A.L.. 2011. Multiple models to capture the variability in biological neurons and networks. Nat. Neurosci. 14:133–138. 10.1038/nn.2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miake J., Marbán E., and Nuss H.B.. 2002. Biological pacemaker created by gene transfer. Nature. 419:132–133. 10.1038/419132b [DOI] [PubMed] [Google Scholar]
- Miura D.S., and Rosen M.R.. 1978. The effects of ouabain on the transmembrane potentials and intracellular potassium activity of canine cardiac Purkinje fibers. Circ. Res. 42:333–338. 10.1161/01.RES.42.3.333 [DOI] [PubMed] [Google Scholar]
- Nitabach M.N., Blau J., and Holmes T.C.. 2002. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 109:485–495. 10.1016/S0092-8674(02)00737-7 [DOI] [PubMed] [Google Scholar]
- Pathak D., Guan D., and Foehring R.C.. 2016. Roles of specific Kv channel types in repolarization of the action potential in genetically identified subclasses of pyramidal neurons in mouse neocortex. J. Neurophysiol. 115:2317–2329. 10.1152/jn.01028.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton D.E.L.L., Isom L.L., Catterall W.A., and Goldin A.L.. 1994. The adult rat brain beta 1 subunit modifies activation and inactivation gating of multiple sodium channel alpha subunits. J. Biol. Chem. 269:17649–17655. [PubMed] [Google Scholar]
- Patton D.E., Silva T., and Bezanilla F.. 1997. RNA editing generates a diverse array of transcripts encoding squid Kv2 K+ channels with altered functional properties. Neuron. 19:711–722. 10.1016/S0896-6273(00)80383-9 [DOI] [PubMed] [Google Scholar]
- Peters H.C., Hu H., Pongs O., Storm J.F., and Isbrandt D.. 2005. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat. Neurosci. 8:51–60. 10.1038/nn1375 [DOI] [PubMed] [Google Scholar]
- Rudy Y., and Silva J.R.. 2006. Computational biology in the study of cardiac ion channels and cell electrophysiology. Q. Rev. Biophys. 39:57–116. 10.1017/S0033583506004227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti M.C., Curran M.E., Zou A., Shen J., Spector P.S., Atkinson D.L., and Keating M.T.. 1996. Coassembly of KVLQT1 and minK (IsK) proteins to form cardiac IKS potassium channel. Nature. 384:80–83. 10.1038/384080a0 [DOI] [PubMed] [Google Scholar]
- Shapiro M.G., Homma K., Villarreal S., Richter C.P., and Bezanilla F.. 2012. Infrared light excites cells by changing their electrical capacitance. Nat. Commun. 3:736 10.1038/ncomms1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M.G., Priest M.F., Siegel P.H., and Bezanilla F.. 2013. Thermal mechanisms of millimeter wave stimulation of excitable cells. Biophys. J. 104:2622–2628. 10.1016/j.bpj.2013.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E. 1990. Use of Xenopus oocytes for the functional expression of plasma membrane proteins. J. Membr. Biol. 117:201–221. 10.1007/BF01868451 [DOI] [PubMed] [Google Scholar]
- Speca D.J., Ogata G., Mandikian D., Bishop H.I., Wiler S.W., Eum K., Wenzel H.J., Doisy E.T., Matt L., Campi K.L., et al. 2014. Deletion of the Kv2.1 delayed rectifier potassium channel leads to neuronal and behavioral hyperexcitability. Genes Brain Behav. 13:394–408. 10.1111/gbb.12120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson K.I., Jordan J.R., Beyer E.C., and Paul D.L.. 1989. Formation of gap junctions by expression of connexins in Xenopus oocyte pairs. Cell. 57:145–155. 10.1016/0092-8674(89)90180-3 [DOI] [PubMed] [Google Scholar]
- Waldrop M.W. 2015. Why we are teaching science wrong, and how to make it right. Nature. 523:272–274. 10.1038/523272a [DOI] [PubMed] [Google Scholar]
- Wallace R.H., Wang D.W., Singh R., Scheffer I.E., George A.L. Jr., Phillips H.A., Saar K., Reis A., Johnson E.W., Sutherland G.R., et al. 1998. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel β1 subunit gene SCN1B. Nat. Genet. 19:366–370. 10.1038/1252 [DOI] [PubMed] [Google Scholar]
- Wang Y.-C., and Huang R.-C.. 2006. Effects of sodium pump activity on spontaneous firing in neurons of the rat suprachiasmatic nucleus. J. Neurophysiol. 96:109–118. 10.1152/jn.01369.2005 [DOI] [PubMed] [Google Scholar]
- Weber W. 1999. Ion currents of Xenopus laevis oocytes: state of the art. Biochim. Biophys. Acta. 1421:213–233. 10.1016/S0005-2736(99)00135-2 [DOI] [PubMed] [Google Scholar]
- Yue C., and Yaari Y.. 2004. KCNQ/M channels control spike afterdepolarization and burst generation in hippocampal neurons. J. Neurosci. 24:4614–4624. 10.1523/JNEUROSCI.0765-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.