Abstract
Purpose
Accumulation of iodide and other substrates for the human sodium/iodide symporter (hNIS) is fundamental to imaging and therapy of thyroid disease, hNIS reporter gene imaging and hNIS-mediated gene therapy. There is no readily available positron emission tomography (PET) tracer for hNIS. Our aim was to develop a colon carcinoma cell line stably expressing hNIS, and use it to evaluate a novel hNIS PET tracer, [18F]-tetrafluoroborate.
Methods
Colon carcinoma cell line HCT116 was stably transfected with hNIS, producing cell line HCT116-C19 with high hNIS expression. Rat thyroid cells FRTL5, which express rat sodium-iodide symporter (rNIS) when stimulated with thyroid stimulating hormone, were used for comparison. Accumulation of [188Re]-perrhenate, [99mTc]-pertechnetate and [18F]-tetrafluoroborate was evaluated with and without perchlorate inhibition using an automated radioimmune assay system, LigandTracer™. Affinity of [18F]-tetrafluoroborate for hNIS, and its IC50 for inhibition of [99mTc]-pertechnetate transport were determined from plateau accumulation of [18F]-tetrafluoroborate and [99mTc]-pertechnetate, respectively, as a function of tetrafluoroborate concentration.
Results
[18F]-tetrafluoroborate accumulated effectively in both FRTL5 and HCT116-C19 cells. Accumulation in HCT116-C19 cells (plateau accumulation 31%) was comparable to that of [188Re]-perrhenate (41%) and [99mTc]-pertechnetate (46%). Its affinity for hNIS and IC50 for inhibition of pertechnetate uptake were approximately micromolar.
Conclusions
We have produced a human colon cell line with stable constitutive expression of functional hNIS (HCT116-hNIS-C19). [18F]-tetrafluoroborate accumulates in cells expressing hNIS or rNIS and is a potential PET imaging agent in thyroid disease and hNIS reporter gene imaging.
Keywords: Tetrafluoroborate, sodium/iodide symporter, F-18, PET, Thyroid
Introduction
The sodium/iodide symporter (NIS) is a plasma membrane glycoprotein that mediates the active transport of iodide in the thyroid and other NIS-expressing cells or tissues. The ability of thyroid cells to accumulate radioiodine via NIS has been instrumental to the management of thyroid disease using nuclear medicine techniques for imaging and therapy. Since the cloning and characterisation of the human NIS gene (hNIS) in 1996 [1], there has been great interest in the potential of using hNIS as a reporter gene for trafficking implanted cells and monitoring cell differentiation and survival in vivo [2–4], and for imaging and therapy of tumours [5–7]. Radionuclide imaging and therapy via hNIS may be feasible since hNIS is expressed only in a small number of normal tissues (thyroid gland, salivary glands, gastric mucosa, thymus, lactating breast and kidney) [8–11].
Although iodide is the natural substrate for NIS alternative tracers with improved imaging characteristics and better therapeutic emissions have been investigated. [99mTc]-pertechnetate is commonly used as an alternative to [131/123I]-iodide for single-photon emission computed tomography (SPECT) imaging of the thyroid [12, 13]; the perchlorate ion (ClO4-) is a known specific inhibitor of hNIS [14–16]; and [188Re]-perrhenate [17, 18] and [211At]-astatide [19, 20] have been proposed for radionuclide therapy [17–19, 21, 22]. SPECT scans using [99mTc]-pertechnetate and [123I]-iodide produce images of similar quality [13]. However, [99mTc] results in a lower radiation dose and is more readily available. Despite the success of SPECT tracers for imaging thyroid disease, current SPECT tracers have limitations in detecting small metastases and low volume disease. This along with the increased interest in using hNIS as a reporter gene led to a need to develop a radiotracer with improved imaging characteristics to image small lesions and to track transgenic cells in vivo.
Positron emission tomography (PET) is generally regarded as a superior imaging technique as it offers improved resolution, sensitivity and quantification of radiotracer accumulation compared to SPECT. PET imaging of NIS-expressing tissues can be performed with [124I]-iodide. Several groups have used [124I]-iodide to image NIS transgene expression in vivo [23, 24]. Iodide accumulation measured by PET imaging correlated well with post-mortem gamma counting and with hNIS gene expression measured by the quantitative real-time polymerase chain reaction [23]. However, 124I undergoes a complex decay scheme with a low abundance of positrons (23 %). In addition it emits high energy gamma photons (Emax = 2.14 MeV) which may interfere with annihilation photons leading to increased background noise and poor image quality. In addition, the long half-life of 124I results in a high radiation dose to the patient. An ideal PET tracer for NIS would be based on fluorine-18 as it has good imaging characteristics: short half life (110 min), high positron yield (97 %), low positron energy (Emax = 0.634 MeV) and ready availability by production on a medical cyclotron. The tetrafluoroborate ion (BF4-) has been shown to be a substrate for NIS by electrochemical studies and accumulation in the thyroid of rats [25–27]. We recently described the synthesis of [18F]-tetrafluoroborate by isotopic exchange with BF4- and demonstrated its promise for PET imaging using pre-clinical PET/CT imaging in rodents [28].
The options for commercially available cell lines that express NIS are limited. The Fisher rat thyroid cell line FRTL5 has been used previously for in vitro studies of rNIS radiotracer accumulation [15, 28]. In this study the hNIS gene was stably transfected into the human colon colorectal cell line HCT116 to provide a human cell line with high expression of hNIS in order to evaluate and compare the accumulation of [18F]-tetrafluoroborate with other NIS tracers. Experimental cancer cell lines expressing reporter genes are useful tools in preclinical cancer research.
In this study the accumulation kinetics of [99mTc]-pertechnetate, [188Re]-perrhenate and [18F]-tetrafluoroborate were compared using LigandTracer™ [29]. This instrument is designed to measure radioligand-cell interactions using automated radioimmunoassay technology. It measures in vitro accumulation of radiotracers repeatedly in real time and is less labour-intensive compared to standard protocols in which cells are seeded into dishes or multi-well plates and radiotracer is added, followed by harvesting cells at least in triplicate over a range of time points and measurement of accumulation of radiotracer in each sample by gamma counting. Here we describe the use of LigandTracer to evaluate the accumulation of radiolabelled hNIS substrates into target cells for radiopharmaceutical development.
Materials and methods
Radiotracers
Sodium [18F]-tetrafluoroborate was prepared from [18F]-fluoride, obtained direct from proton-irradiated [18O]-water (97 atom %, Isochem Ltd., Hook, UK; 11 MeV protons from a CTI RDS 112 cyclotron, beam current 30 μA, irradiation time 10 – 20 min). Sodium [99mTc]-pertechnetate was obtained by elution of a 99Mo/99mTc generator (Drytec, GE Healthcare, Little Chalfont, UK) with saline. Sodium [188Re]-perrhenate was eluted from a 188W/188Re generator in accordance with manufacturers’ instructions (Institute of Atomic Energy-POLATOM, Otwock-Świerk, Poland).
Radiochemistry
Synthesis of [18F]-tetrafluoroborate was performed using an automated labelling protocol developed using an Eckert and Ziegler ModularLab module (Imaging Equipment Ltd, Bristol, UK) as described previously [28]. Briefly, [18F]-fluoride (12 – 18 GBq) was trapped by passage of the irradiated water (4 mL) through a QMA cartridge (Waters SepPak Light QMA, conditioned with 1.0 M sodium hydrogen carbonate). The [18F]-fluoride was eluted from the cartridge with 1.2 mL of 1.5 M HCl into the reactor which contained sodium tetrafluoroborate (1 mg in 0.1 mL 1.5 M HCl). The reaction mixture (1.3 mL) was heated to 120 °C for 10 min, cooled to 25° C and passed through a silver ion-loaded cation exchange cartridge (OnGuard II AG, Dionex, Leeds, UK, conditioned with 10 mL water) to remove chloride and raise the pH, and through two alumina columns (Waters SepPak Light Alumina N, conditioned with 10 mL water and 5 mL air) to remove unreacted [18F]-fluoride and a sterile Millex-GS 0.22 μm filter unit (Millipore UK, Watford, UK), and washed through with a further 2 mL water for injections into a nitrogen-filled sterile vial. The purified [18F]-tetrafluoroborate stock thus produced contained about 10% of the starting activity, and had a specific activity of 1-10 x 108 MBq/mol and a maximum tetrafluoroborate concentration of 1 mM.
Cell culture
Media and reagents were obtained from PAA (Yeovil, UK) and hormones from Sigma-Aldrich (Poole, UK). The Fisher rat thyroid cell line FRTL5 was kindly donated by Prof. Jan Smit and Dr. Guido Hovens (Leiden University, The Netherlands) and grown in a humidified incubator at 37°C with 5% CO2 in Hams:F12 medium supplemented with 10% fetal bovine serum, penicillin/streptomycin, minimal essential medium amino acids and a hormone mixture containing insulin (10 μg/mL), hydrocortisone (3.6 ng/mL), glycyl-histidyl-lysine acetate (10 ng/mL), transferrin (5 μg/mL), somatostatin (10 ng/mL) and bovine thyroid stimulating hormone (TSH) (1 mU/mL). The human colon cancer cell line HCT116 was grown in a humidified incubator at 37°C with 5% CO2 in McCoys 5A medium supplemented with 10% fetal bovine serum, L-glutamine (2 mM) and penicillin/streptomycin.
Transfection with hNIS cDNA
Full-length hNIS cDNA cloned into the EcoRI site of pcDNA3 [30] was used to transfect HCT116 cells. HCT116 cells were grown to 90% confluency in 24 well plates. Stable transfection was performed using Lipofectamine 2000 in accordance with the manufacturers’ protocol (Invitrogen, Paisley, UK). Twenty-four hours after transfection cells were passaged into a 25 cm tissue culture flask and after a further 24 hours cells were cultured in medium containing 250 μg/mL geneticin (Invitrogen, Paisley, UK). After 14 days of antibiotic selection cells were counted and seeded into 96 well plates at a density of <1 cell per well. Single colonies were selected and bulked up to 24 well plates. Colonies with hNIS expression were selected on the basis of [99mTc]-pertechnetate accumulation.
Selection of clones with hNIS expression
HCT116 hNIS clones were seeded in 24 well plates at a density of 1.5 x 105 cells per well and incubated in a humidified incubator at 37°C with 5% CO2 for 24 hours. Cells were washed twice in Hanks balanced salt solution (HBSS) before incubating for 30 minutes with 500 μL HBSS and 0.1 MBq [99mTc]-pertechnetate in a volume not exceeding 5 % of the total volume. Then cells were washed twice with 500 μL cold HBSS and extracted with 500 μL 1M NaOH for 10 minutes. The cell extracts were counted in a gamma counter (Wallac 1282-001 Compugamma CS).
In vitro radiotracer accumulation
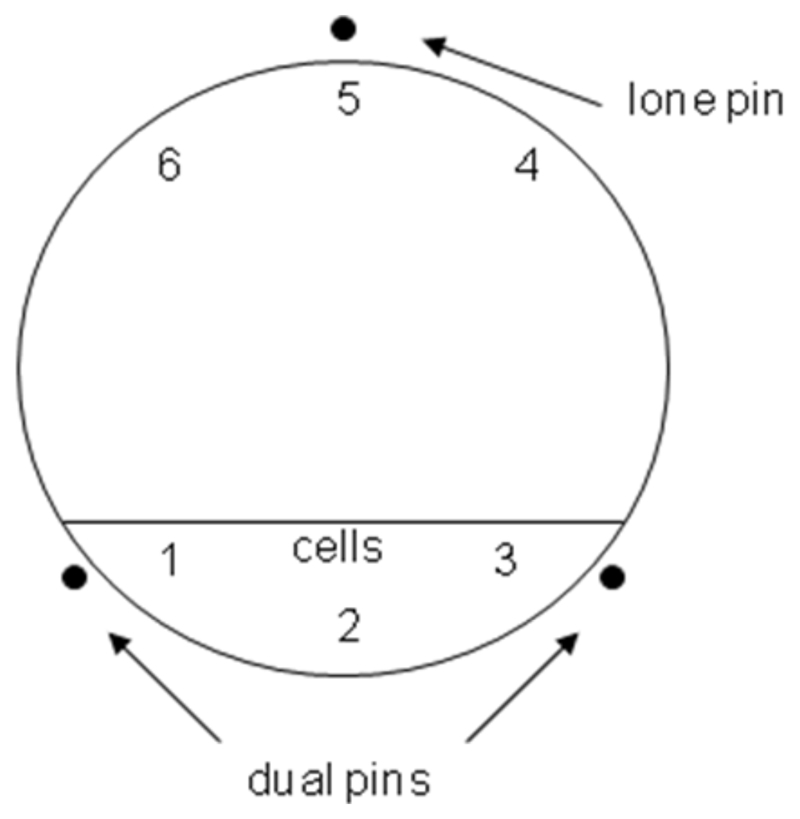
[18F]-tetrafluoroborate, [99mTc]-pertechnetate and [188Re]-perrhenate accumulation were evaluated in FRTL5, HCT116 Clone 19 (high hNIS expression) with or without sodium perchlorate to control for hNIS- or rNIS-specific accumulation, and HCT116 untransfected cells. Accumulation was measured in real-time using LigandTracer® Yellow in accordance with the manufacturer’s instructions (Ridgeview Instruments AB, Uppsala, Sweden). Briefly, 2 x 106 cells in 2 mL growth medium were seeded into 100 mm cell bind® Petri dishes (Corning, NY, USA) and incubated on an incline for 16-24 hours so that target cells adhered to small section of the plate (Figure 1). The medium was replaced with 10 mL fresh medium and cells were incubated for a further 18-24 hours. Cells were then washed twice in HBSS before incubating with 2 mL HBSS with or without 10 μM sodium perchlorate for 10 minutes. The LigandTracer was equilibrated to 37 °C before placing the Petri dish in the machine with the target cells opposite the “lone pin” (Figure 1). The LigandTracer was set up to measure 3 + 3 positions at opposite sides of the dish. Background counts were collected for 6 minutes prior to the addition of 0.5 MBq of the radiotracer in a volume not exceeding 5% of the total volume and accumulation data were collected for up to 60 minutes with sampling every 12 seconds. At the end of the run the medium was collected and cells were washed briefly in cold HBSS and the wash was combined with the medium. The cells were extracted in 2 mL 1 M NaOH for radioactivity counting. All liquids were counted on a gamma counter and accumulation in cells was calculated as a percentage of total activity.
Figure 1.
Ligandtracer measurement locations in 3 + 3 configuration. The Petri dish was placed on the rotating platform so that area in which cells were seeded was located opposite the lone pin. The detector then measured at three locations within the area populated by cells and at 3 locations in a cell free area of the dish. The accumulation was calculated at each measurement by subtracting minimum from maximum activity values
Inhibition of [18F]-tetrafluoroborate and [99mTc]-pertechnetate accumulation with cold sodium tetrafluoroborate
HCT116-hNISC19 cells were seeded in 24-well plates at a density of 1.5 x 105 cells per well and incubated in a humidified incubator at 37°C with 5% CO2 for 48 hours. Cells were washed twice in HBSS before incubating for 30 minutes at 37°C with 500 μL HBSS containing cold sodium tetrafluoroborate at concentrations ranging from 1 x 10-2 to 1 x 10-12 M. Next, 0.1 MBq [99mTc]-pertechnetate or [18F]-tetrafluoroborate in a volume not exceeding 5 % of the total volume was added and cells were incubated at 37°C for 30 minutes. Then cells were washed with 500 μL cold HBSS and extracted with 500 μL 1M NaOH for 10 minutes. The cell medium, washes and cell extracts were counted in a gamma counter (Wallac 1282-001 Compugamma CS). Percent accumulation was plotted versus log10[tetrafluoroborate]. The sigmoid inhibition curve was fitted using Graphpad Prism 5.0 software using a four parameter variable Hill slope equation and this was used to calculate IC50 values.
Results
[99mTc]-pertechnetate accumulation following stable transfection with hNIS cDNA
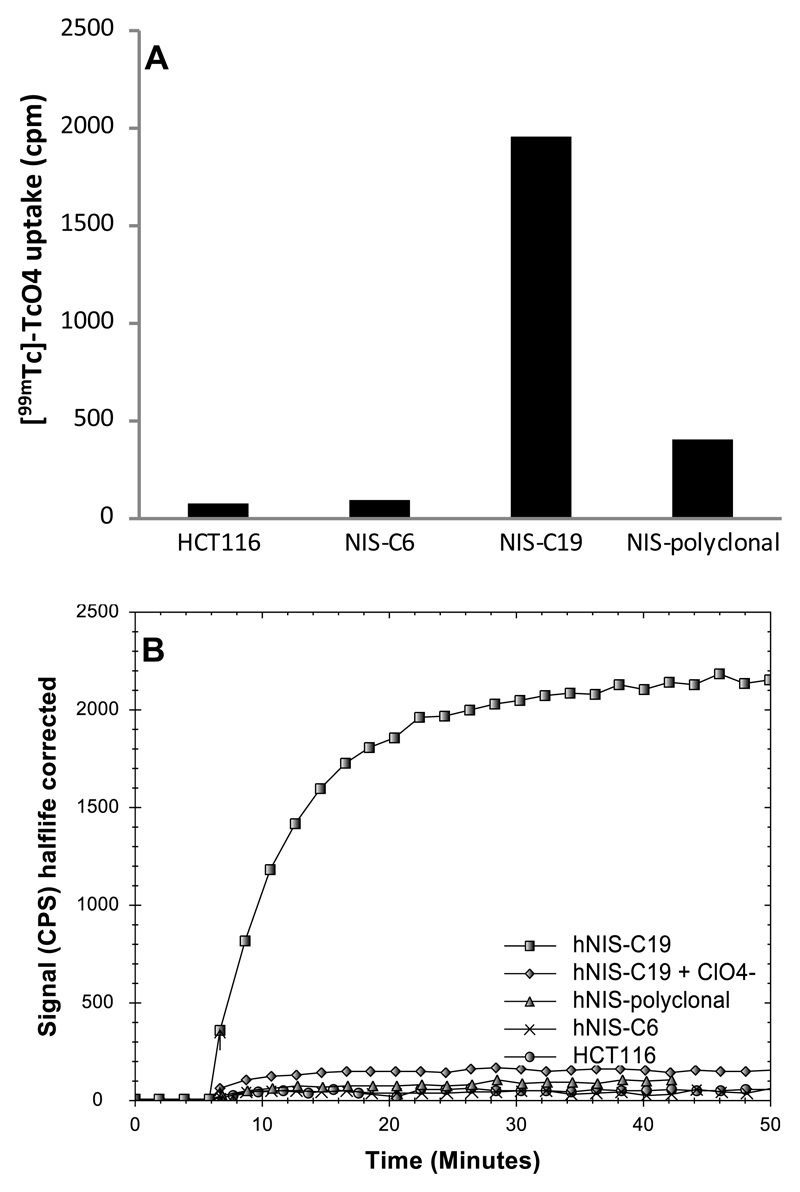
Twenty–one colonies of HCT116 cells transfected with hNIS were identified and selected for the [99mTc]-pertechnetate accumulation assay. The concentration of pertechnetate in the wells was estimated to be approximately 5 x 10-11 M. The highest accumulation was observed in HCT116-hNIS-clone 19 (C19). Cell associated [99mTc]-pertechnetate activity was significantly (approximately 20-fold) higher in hNIS-expressing C19 cells compared to the untransfected HCT116 control cell line (Figure 2A). C19 accumulated 1954 cpm, compared to 408 cpm for the polyclonal cell population and <100 cpm for HCT116 untransfected cells and HCT116-hNIS-C6. The accumulation of [99mTc]-pertechnetate in C19 cells was shown to be hNIS-dependent by assessing the accumulation in the presence of perchlorate, a known inhibitor of NIS. Sodium perchlorate reduced the accumulation of [99mTc]-pertechnetate in C19 cells by approximately 95%. Accumulation of [99mTc]-pertechnetate in C19 cells was rapid, reaching half maximal levels within 5 minutes after addition and approaching a plateau within 15-20 minutes (Figure 2B).
Figure 2.
(A) [99mTc]-pertechnetate accumulation was measured after 30 min in stably transfected HCT116 cells (polyclonal and 22 clones) and in untransfected control cells (n=1). HCT116-hNIS-polyclonal cells concentrated [99mTc]-pertechnetate 5-fold compared to the HCT116 untransfected control. HCT116-hNIS-clone 6 showed very low accumulation and HCT116-hNIS-clone 19 showed the highest accumulation of all 22 clones and concentrated [99mTc]-pertechnetate 24-fold more effectively than untransfected cells. (B) [99mTc]-pertechnetate accumulation in HCT116 control cells, hNIS-polyclonal, hNIS-clone 6 and hNIS-clone 19 (with and without sodium perchlorate) was followed in real time for 44 min (after the addition of radiotracer at 6 min) using LigandTracer. hNIS-clone 6, hNIS polyclonal and the parent cell line HCT116 all showed low accumulation of [99mTc]-pertechnetate whilst HCT116-hNIS-C19 concentrated the tracer about 20-fold more strongly and this accumulation was effectively blocked by sodium perchlorate.
Comparison of accumulation kinetics of [18F]-tetrafluoroborate, [99mTc]-pertechnetate and [188Re]-perrhenate in hNIS- and rNIS-expressing cells
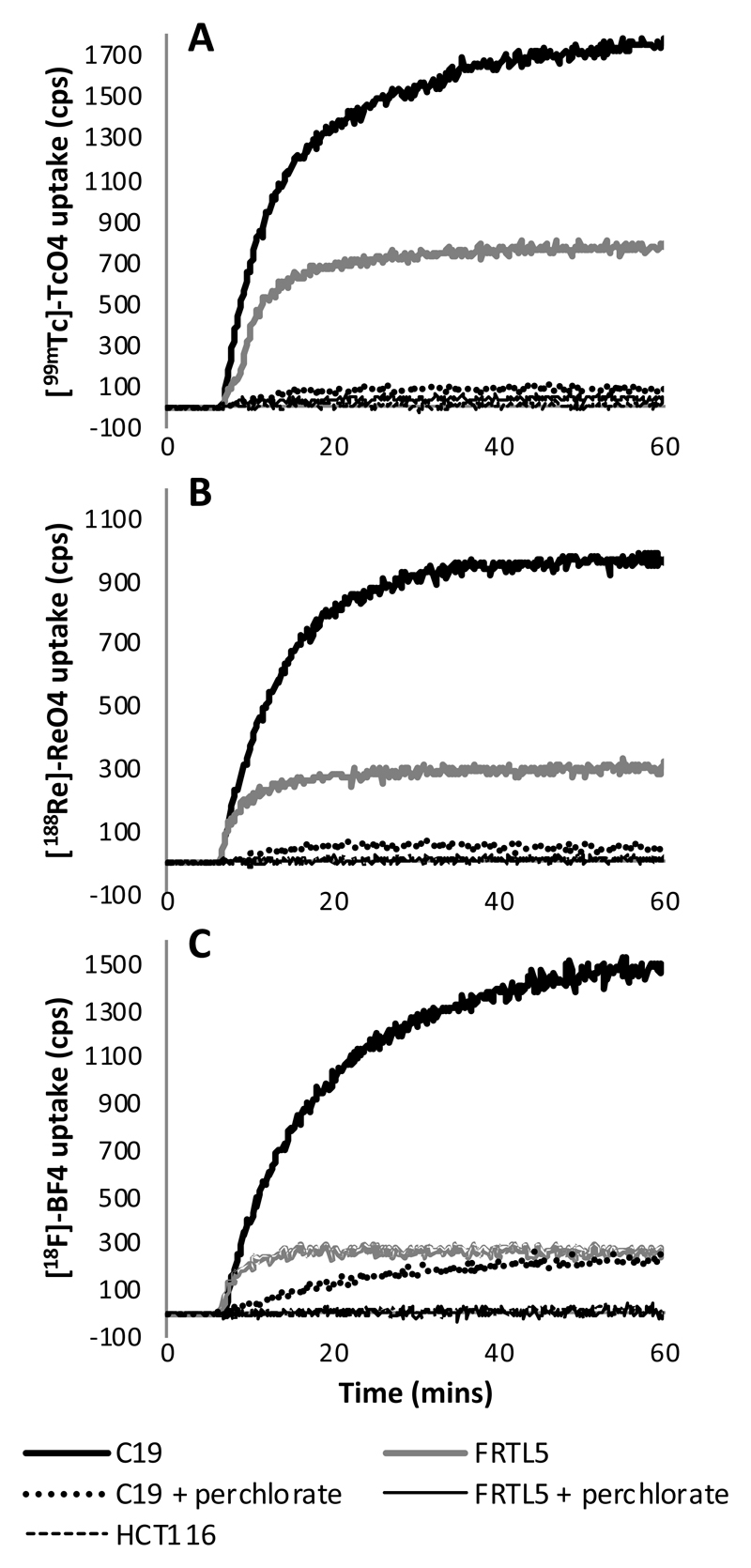
The kinetics of [18F]-tetrafluoroborate, [99mTc]-pertechnetate and [188Re]-perrhenate accumulation were studied in FRTL5, HCT116-hNIS-C19 and HCT116 cells (Figure 3). FRTL5 is a rat thyroid cell line which is known to express rNIS and was used as positive control to show that the radiotracers are taken up by NIS. The concentrations of [18F]-tetrafluoroborate, [99mTc]-pertechnetate and [188Re]-perrhenate added to each dish were estimated to be less than 1 x 10-7, 5 x 10 -11 and 4 x 10 -11 M, respectively. In FRTL5 and HCT116-hNIS-C19 cells rapid accumulation was observed with all three radiotracers and a plateau was reached within 20 minutes of addition of tracer. In HCT116 cells no measurable accumulation was observed. The addition of sodium perchlorate to the cell medium prevented the accumulation of all three tracers almost completely in C19 and FRTL5 cells indicating that accumulation was specific and mediated by hNIS or rNIS. The 10 μM perchlorate block was observed to be less effective for [18F]-tetrafluoroborate in C19 cells (83%) compared to [99mTc]-pertechnetate (93%) and [188Re]-perrhenate (96%).
Figure 3.
Time course of radiotracer accumulation in HCT116-hNIS-C19 and FRTL5 cells (with or without a sodium perchlorate block) and HCT116 cells. Figures show the accumulation of (A) [99mTc]-pertechnetate, (B) [188Re]-perrhenate and (C) [18F]-tetrafluoroborate in real time using the LigandTracer. Data represent the mean of two independent experiments for each tracer.
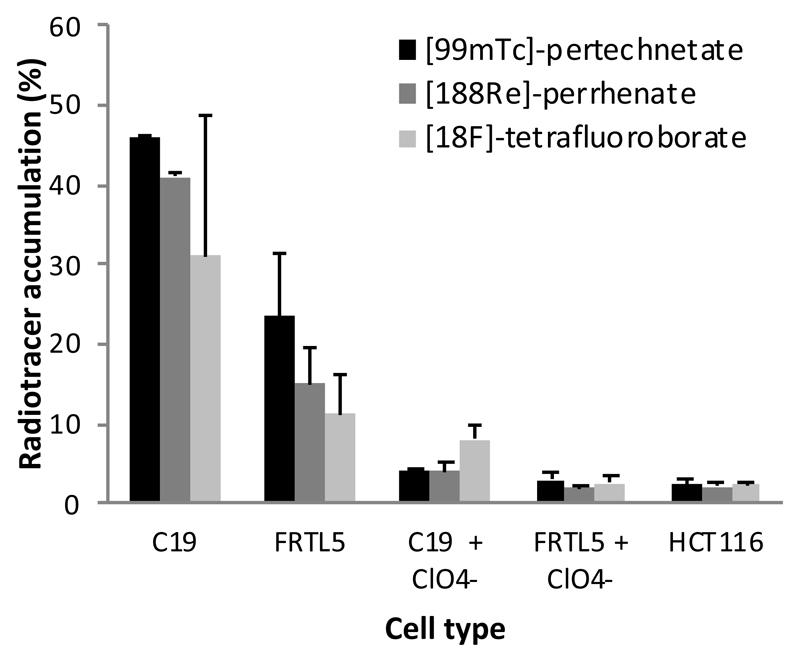
Comparison of accumulation of [18F]-tetrafluoroborate, [99mTc]-pertechnetate and [188Re]-perrhenate as a percentage of total radioactivity in hNIS expressing cells
The measurements of the cell-bound radioactivity do not provide a direct comparison of the extent of accumulation of tracers, since the detector sensitivity to the three radiotracers were different. Therefore, at the end of each run using LigandTracer the activity associated with cells and medium was counted and the percentage of total radioactivity accumulated in cells after accumulation plateau was determined (Figure 4). The accumulation as a percentage of total activity for [99mTc]-pertechnetate (46%) was higher than for [188Re]-perrhenate (41%) which in turn was higher than for [18F]-tetrafluoroborate (31%) in C19 cells; similarly in FRTL5 cells percentage accumulation of [99mTc]-pertechnetate, [188Re]-perrhenate and [18F]-tetrafluoroborate were 23%, 15% and 11%, respectively. The percentage accumulation of [18F]-tetrafluoroborate in C19 cells had higher variation between independent experiments than the other tracers. This may be due to variations in the specific activity of [18F]-tetrafluoroborate between two independent experiments. The specific activity was in the range 1-10 x 108 MBq/mol and would vary between experiments as the isotope decayed. At this level the concentration of tetrafluoroborate in the incubation medium would have been in the range 1-5 x 10 -7 M, which is relatively close to the IC50 (see below and Figure 5) whereas the concentration of pertechnetate and perrhenate would have been less than 4 x 10 -11 M. The relatively low specific activity [18F]-tetrafluoroborate could also account for its lower percent accumulation compared to pertechnetate and perrhenate, and is also consistent with the smaller fractional inhibition by perchlorate compared to the other tracers.
Figure 4.
Percent accumulation of [99mTc]-pertechnetate, [188Re]-perrhenate and [18F]-tetrafluoroborate. The accumulation of radiotracers in HCT116-hNIS-C19 cells and FRTL5 cells was calculated as a percentage of total activity (with and without a 10 μM sodium perchlorate block), and HCT116 cells. Results represent the mean ± 1 sd of two independent experiments for each tracer.
Figure 5.
Inhibition of [18F]-tetrafluoroborate (A) and [99mTc]-pertechnetate (B) accumulation in C19 cells by cold sodium tetrafluoroborate. Tetrafluoroborate concentration was adjusted to correct for the concentration of tetrafluoroborate added in the radiotracer. Sigmoid curves were fitted by non-linear regression using GraphPad Prism 5.0 software using the four parameter variable Hill slope equation. The IC50s were calculated to be 1.6 x 10-6 and 7.4 x 10-7 M for inhibition of tetrafluoroborate and pertechnetate accumulation, respectively.
Inhibition of [18F]-tetrafluoroborate and [99mTc]-pertechnetate accumulation with cold sodium tetrafluoroborate
The inhibition of [18F]-tetrafluoroborate and [99mTc]-pertechnetate accumulation in C19 cells using cold sodium tetrafluoroborate was investigated in order to estimate the ability of tetrafluoroborate to compete with pertechnetate as a substrate and the effect of specific activity on the accumulation of [18F]-tetrafluoroborate (Figure 5). In the experiment where [18F]-tetrafluoroborate was inhibited by itself, the tetrafluoroborate concentration was adjusted to take into account the concentration added as a radiotracer. The IC50 values of tetrafluoroborate as an inhibitor of [18F]-tetrafluoroborate and [99mTc]-pertechnetate accumulation were 1.6 x 10-6 and 7.4 x 10-7 M, respectively.
Discussion
The colon carcinoma cell line HCT116 was stably transfected with hNIS to provide a cell model with high hNIS expression. This cell line (HCT116-hNIS-C19) has proved to be a useful model for the investigation of novel NIS substrates. The full-length hNIS cDNA pcDNA3 plasmid was originally constructed by Smanik et al. [30]. This group transfected the COS-7 monkey kidney cell line with hNIS and observed a 9-fold increase in radioiodine accumulation compared to control cells [30]. Our hNIS-transfected HCT116 cell line C19 has proved to be a convenient cell model for studying the accumulation kinetics of radiotracers that are substrates for hNIS. The cell line grows quickly compared to FRTL5 cells and high accumulation of pertechnetate has been demonstrated in C19 cells that have been cryogenically stored and subsequently cultured for in excess of 30 passages, under the selection of 250 μg/ml Geneticin. This cell line has also been beneficial in our laboratory for use as a positive control for determination of hNIS expression in novel hNIS transgenic cells (results not shown). Since the parent cell line HCT116 is capable of forming human xenografts in the nude mouse [31], C19 cells may be a useful tool for evaluating hNIS targeting of tracers in tumour models in vivo using microPET/SPECT imaging.
We have previously demonstrated accumulation of [18F]-tetrafluoroborate in the rat thyroid cell line FRTL5 and in the thyroid gland of normal mice and TRβPV/PV transgenic mouse with a thyroid tumour [28]. The in vitro studies presented herein show that in addition to being a substrate for rNIS in rodent models, [18F]-tetrafluoroborate is taken up by a human colon carcinoma cell line with hNIS expression. The accumulation of [18F]-tetrafluoroborate in HCT116 cells transfected with hNIS was high and comparable with known substrates [99mTc]-pertechnetate and [188Re]-perrhenate, indicating that [18F]-tetrafluoroborate is a good substrate for the human sodium iodide symporter in addition to rNIS in rodent species. Like [99mTc]-pertechnetate and [188Re]-perrhenate, [18F]-tetrafluoroborate was taken up by both FRTL5 and HCT116-hNIS-C19 cells but not by the untransfected parent HCT116 cells. Since the accumulation is blocked by perchlorate, a known specific inhibitor of hNIS and rNIS [14, 16], it is clear that [99mTc]-pertechnetate accumulation is specific and mediated by hNIS. The data in Figure 5 show that tetrafluoroborate is able to inhibit accumulation of pertechnetate by hNIS with an IC50 of 7.4 x 10-7 M. This value is similar to the affinity of [18F]-tetrafluoroborate, based on measurement of inhibition of accumulation of [18F]-tetrafluoroborate by cold tetrafluoroborate. This is consistent with the assumption that both pertechnetate and tetrafluoroborate act similarly as substrates of hNIS and will therefore each competitively inhibit accumulation of the other.
The accumulation of [18F]-tetrafluoroborate in NIS expressing C19 cells was comparable in terms of percent accumulation to that of [188Re]-perrhenate and [99mTc]-pertechnetate with accumulation of 31%, 41% and 46%, respectively. The concentration ratio (Cin:Cout) in C19 cells were estimated as 295:1, 243:1 and 157:1 for [99mTc]-pertechnetate, [188Re]-perrhenate and [18F]-tetrafluoroborate, respectively. The relatively low specific activity of [18F]-tetrafluoroborate, and the variation in specific activity between different batches of [18F]-tetrafluoroborate may account for the lower percent accumulation and the greater variability in accumulation in C19 cells compared to the other tracers. The specific activity was estimated to be in the range 1-10 x 108 MBq/mol and would vary between experiments as the isotope decayed. At this specific activity the concentration of tetrafluoroborate in the incubation medium would have been in the range 1-5 x 10 -7 M, which is relatively close to the IC50 which was estimated at 1.6 x 10-6 M, hence accumulation is likely to depend quite strongly on concentration. Conversely, the concentration of pertechnetate and perrhenate would have been less than 4 x 10-11 M, well below the reported IC50 values of 1 - 2 x 10-6 M [15] for inhibition of sodium perrhenate on the accumulation of [125I]-iodide and [99mTc]-pertechnetate, and hence is not likely to adversely affect accumulation. The extracellular concentration of tetrafluoroborate in patients after being injected with a suitable imaging dose of [18F]-tetrafluoroborate will be significantly lower than the lowest concentrations in our in vitro experiments (< 0.1 micromolar). Therefore the low specific activity will not adversely affect accumulation in vivo in humans. Although the accumulation of [18F]-tetrafluoroborate is lower than that of [99mTc]-pertechnetate, PET scanning with this agent may be advantageous because of better spatial resolution and attenuation properties compared to SPECT with 99mTc.
To our knowledge this study is the first to compare accumulation of [18F]-tetrafluoroborate with existing radiotracers for accumulation by NIS. Comparisons of accumulation of NIS radiotracers between different experiments are difficult due to differences in cell types used, methodologies and presentation of data. Van Sande et al. [15] studied the accumulation of pertechnetate and perrhenate in FRTL5 and hNIS transfected COS cells. Both tracers showed similar accumulation kinetics to our work reaching a plateau within 20 minutes, but in contrast to this study Van Sande et al showed higher accumulation of perrhenate compared to pertechnetate. Kang et al. also showed slightly higher accumulation of perrhenate compared to pertechnetate in an hepatocellular carcinoma cell line transfected with hNIS [32].
As NIS can facilitate the accumulation of a range of different isotopes there has been a great deal of interest in its use in cancer gene therapy with particle-emitting radionuclides. In addition to [131I]-iodide, NIS is also capable of transporting the beta-emitter [188Re]-perrhenate and the alpha emitter [211At]-astatide. In this study perrhenate accumulation and pertechnetate accumulation were comparable at 41 and 46%, respectively, in C19 cells which would suggest that [188Re]-perrhenate may be able to accumulate in sufficient concentrations to deliver a therapeutic dose to tumour tissues expressing NIS. Several studies have shown decreased clonogenic survival in tumour cells targeted with radioiodine via NIS using tissue-specific promoters, for example the prostate-specific antigen (PSA) [22] and the carcinoembryonic antigen (CEA) [33]. Willhauck et al. showed that [131I]-iodide and [188Re]-perrhenate were equally effective at treating smaller tumours in a mouse model and that [188Re]-perrhenate had a superior therapeutic effect in tumours with a relatively large volume (> 200 mm3) [22].
LigandTracer proved to be an effective method for the evaluation of radiotracer accumulation in target cells, giving reproducible data from much higher sampling rates than feasible by conventional manual methods. The automated system reduces operator errors that occur due to harvesting cells from multi-well plates and errors due to variability in cell numbers and distribution in wells. In addition, the reduction in liquid handling steps reduces radiation exposure to the operator. However, the disadvantage of the LigandTracer system is that only one experiment can be run at a time, so controls cannot be run simultaneously with the experimental sample. This may be an issue particularly when using short half-life isotopes, as after decay correction the radiotracer added to experiments performed later in the day will have diminishing specific activity, and thus controls will use radiotracers with different specific activity to test runs.
Conclusion
[18F]-Tetrafluoroborate accumulates in cells expressing hNIS and has potential for use as a PET agent for imaging thyroid disease and hNIS reporter gene imaging. As tetrafluoroborate is labelled with 18F it has ideal imaging characteristics that may deliver the spatial resolution and sensitivity required for imaging small lesions, reporter gene imaging and cell migration studies. The new colon cancer cell line HCT116-hNIS-C19 stably and constitutively expresses hNIS and promises to be a useful research tool in preclinical cancer research. The LigandTracer instrument is useful for kinetic measurements of tracer accumulation in cells, offering high sampling rate and reproducibility and low operator error and radiation absorbed dose.
Acknowledgments
AJW and MJO were supported by Guy’s & St Thomas’ Charity (grant to PJB), the Centre of Excellence in Medical Engineering at King’s College London funded by the Wellcome Trust and EPSRC (Grant WT 088641/Z/09/Z) (studentship to JEB), the EPSRC (postdoctoral fellowship to MJO) and the KCL-UCL Comprehensive Cancer Imaging Centre, Cancer Research UK & EPSRC, in association with the MRC and DoH (England). The authors would like to thank Ridgeview instruments for the loan of the LigandTracer instrument and Cancer Research UK for a grant to purchase the rhenium-188 generator. We thank the staff of the Guy’s & St Thomas’ NHS Foundation Trust Radiopharmacy for supplying technetium-99m.
Financial support: AJW and MJO were supported by Guy’s & St Thomas’ Charity (grant to PJB), the Centre of Excellence in Medical Engineering at King’s College London funded by the Wellcome Trust and EPSRC (Grant WT 088641/Z/09/Z) (studentship to JEB), EPSRC (postdoctoral fellowship to MJO) and the KCL-UCL Comprehensive Cancer Imaging Centre, funded by Cancer Research UK & EPSRC, in association with the MRC and DoH (England). The authors thank Ridgeview instruments for the loan of the LigandTracer™ instrument and Cancer Research UK for a grant to purchase the rhenium-188 generator.
References
- 1.Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379:458–460. doi: 10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
- 2.Kang JH, Lee DS, Paeng JC, Lee JS, Kim YH, Lee YJ, et al. Development of a sodium/iodide symporter (NIS)-transgenic mouse for imaging of cardiomyocyte-specific reporter gene expression. J Nucl Med. 2005;46:479–483. [PubMed] [Google Scholar]
- 3.Miyagawa M, Beyer M, Wagner B, Anton M, Spitzweg C, Gansbacher B, et al. Cardiac reporter gene imaging using the human sodium/iodide symporter gene. Cardiovasc Res. 2005;65:195–202. doi: 10.1016/j.cardiores.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Terrovitis J, Kwok KF, Lautamaki R, Engles JM, Barth AS, Kizana E, et al. Ectopic Expression of the Sodium-Iodide Symporter Enables Imaging of Transplanted Cardiac Stem Cells In Vivo by Single-Photon Emission Computed Tomography or Positron Emission Tomography. J Am Coll Cardiol. 2008;52:1652–1660. doi: 10.1016/j.jacc.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Altmann A, Mier W, Eskerski H, Leotta K, Guo L, et al. Radioiodine therapy of hepatoma using targeted transfer of the human sodium/iodide symporter gene. J Nucl Med. 2006;47:854–862. [PubMed] [Google Scholar]
- 6.Chen LB, Altman A, Mier W, Lu HK, Zhu RS, Haberkorn U. Tc-99m-pertechnetate uptake in hepatoma cells due to tissue-specific human sodium iodide symporter gene expression. Nucl Med Biol. 2006;33:575–580. doi: 10.1016/j.nucmedbio.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Marsee DK, Shen DH, MacDonald LR, Vadysirisack DD, Lin XQ, Hinkle G, et al. Imaging of metastatic pulmonary tumors following NIS gene transfer using single photon emission computed tomography. Cancer Gene Ther. 2004;11:121–127. doi: 10.1038/sj.cgt.7700661. [DOI] [PubMed] [Google Scholar]
- 8.Dohan O, de la Vieja A, Paroder V, Riedel C, Artani M, Reed M, et al. The sodium/Iodide symporter (NIS): characterization, regulation, and medical significance. Endocrine Rev. 2003;24:48–77. doi: 10.1210/er.2001-0029. [DOI] [PubMed] [Google Scholar]
- 9.Vayre L, Sabourin JC, Caillou B, Ducreux M, Schlumberger M, Bidart JM. Immunohistochemical analysis of Na+/I- symporter distribution in human extrathyroidal tissues. Eur J Endocrinol. 1999;141:382–386. doi: 10.1530/eje.0.1410382. [DOI] [PubMed] [Google Scholar]
- 10.Spitzweg C, Joba W, Eisenmenger W, Heufelder AE. Analysis of human sodium iodide symporter gene expression in extrathyroidal tissues and cloning of its complementary deoxyribonucleic acids from salivary gland, mammary gland, and gastric mucosa. J Clin Endocrinol Metab. 1998;83:1746–1751. doi: 10.1210/jcem.83.5.4839. [DOI] [PubMed] [Google Scholar]
- 11.Spitzweg C, Dutton CM, Castro MR, Bergert ER, Goellner JR, Heufelder AE, et al. Expression of the sodium iodide symporter in human kidney. Kidney Int. 2001;59:1013–1023. doi: 10.1046/j.1523-1755.2001.0590031013.x. [DOI] [PubMed] [Google Scholar]
- 12.Sparagana M, Little A, Kaplan E. Rapid Evaluation of Thyroid Nodules Using 99mTc-Pertechnetate Scanning. J Nucl Med. 1970;11:224–225. [PubMed] [Google Scholar]
- 13.Ryo UY, Vaidya PV, Schneider AB, Bekerman C, Pinsky SM. Thyroid imaging agents: a comparison of I-123 and Tc-99m pertechnetate. Radiology. 1983;148:819–822. doi: 10.1148/radiology.148.3.6308711. [DOI] [PubMed] [Google Scholar]
- 14.Carrasco N. Iodide Transport in the Thyroid-Gland. Biochimica Et Biophysica Acta. 1993;1154:65–82. doi: 10.1016/0304-4157(93)90017-i. [DOI] [PubMed] [Google Scholar]
- 15.Van Sande J, Massart C, Beauwens R, Schoutens A, Costagliola S, Dumont JE, et al. Anion selectivity by the sodium iodide symporter. Endocrinol. 2003;144:247–252. doi: 10.1210/en.2002-220744. [DOI] [PubMed] [Google Scholar]
- 16.Saito K, Yamamoto K, Takai T, Yoshida S. Inhibition of iodide accumulation by perchlorate and thiocyanate in a model of the thyroid iodide transport-system. Acta Endocrinol. 1983;104:456–461. doi: 10.1530/acta.0.1040456. [DOI] [PubMed] [Google Scholar]
- 17.Dadachova E, Nguyen A, Lin EY, Gnatovskiy L, Lu P, Pollard JW. Treatment with rhenium-188-perrhenate and iodine-131 of NIS-expressing mammary cancer in a mouse model remarkably inhibited tumor growth. Nucl Med Biol. 2005;32:695–700. doi: 10.1016/j.nucmedbio.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Zuckier LS, Dohan O, Li Y, Chang CJ, Carrasco N, Dadachova E. Kinetics of perrhenate uptake and comparative biodistribution of perrhenate, pertechnetate, and iodide by NaI symporter-expressing tissues in vivo. J Nucl Med. 2004;45:500–507. [PubMed] [Google Scholar]
- 19.Carlin S, Mairs RJ, Welsh P, Zalutsky MR. Sodium-iodide symporter (NIS)-mediated accumulation of [211At]astatide in NIS-transfected human cancer cells. Nucl Med Biol. 2002;29:729–739. doi: 10.1016/s0969-8051(02)00332-3. [DOI] [PubMed] [Google Scholar]
- 20.Petrich T, Helmeke HJ, Meyer GJ, Knapp WH, Potter E. Establishment of radioactive astatine and iodine uptake in cancer cell lines expressing the human sodium/iodide symporter. Eur J Nucl Med Mol Imaging. 2002;29:842–854. doi: 10.1007/s00259-002-0784-7. [DOI] [PubMed] [Google Scholar]
- 21.Petrich T, Quintanilla-Martinez L, Korkmaz Z, Samson E, Helmeke HJ, Meyer GJ, et al. Effective cancer therapy with the alpha-particle emitter [At-211]astatine in a mouse model of genetically modified sodium/iodide symporter-expressing tumors. Clin Cancer Res. 2006;12:1342–1348. doi: 10.1158/1078-0432.CCR-05-1576. [DOI] [PubMed] [Google Scholar]
- 22.Willhauck MJ, Sharif Samani B-R, Gildehaus FJ, Wolf I, Senekowitsch-Schmidtke R, Stark H-J, et al. Application of 188Rhenium as an alternative radionuclide for treatment of prostate cancer after tumor-specific sodium iodide symporter gene expression. J Clin Endocrinol Metab. 2007;92:4451–4458. doi: 10.1210/jc.2007-0402. [DOI] [PubMed] [Google Scholar]
- 23.Groot-Wassink T, Aboagye EO, Wang YH, Lemoine NR, Reader AJ, Vassaux G. Quantitative imaging of Na/I symporter transgene expression using positron emission tomography in the living animal. Mol Ther. 2004;9:436–442. doi: 10.1016/j.ymthe.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Dingli D, Kemp BJ, O'Connor MK, Morris JC, Russell SJ, Lowe VJ. Combined I-124 positron emission tomography/computed tomography Imaging of NIS gene expression in animal models of stably transfected and intravenously transfected tumor. Mol Imaging Biol. 2006;8:16–23. doi: 10.1007/s11307-005-0025-0. [DOI] [PubMed] [Google Scholar]
- 25.Anbar M, Guttmann S. The accumulation of fluoroborate ions in thyroid glands of rats. Endocrinol. 1960;66:888–890. doi: 10.1210/endo-66-6-888. [DOI] [PubMed] [Google Scholar]
- 26.Eskandari S, Loo DDF, Dai G, Levy O, Wright EM, Carrasco N. Thyroid Na+/I- symporter: mechanism, stoichiometry and specificity. J Biol Chem. 1997;272:27230–27238. doi: 10.1074/jbc.272.43.27230. [DOI] [PubMed] [Google Scholar]
- 27.Lewitus Z, Guttmann S, Anbar M. Effect of thyroid-stimulating hormone (TSH) on accumulation of perchlorate and fluoroborate ions in thyroid glands of rats. Endocrinol. 1962;70:295–297. doi: 10.1210/endo-70-3-295. [DOI] [PubMed] [Google Scholar]
- 28.Jauregui-Osoro M, Sunassee K, Weeks AJ, Berry DJ, Paul RL, Cleij M, et al. Synthesis and biological evaluation of [(18)F]tetrafluoroborate: a PET imaging agent for thyroid disease and reporter gene imaging of the sodium/iodide symporter. Eur J Nucl Med Mol Imaging. doi: 10.1007/s00259-010-1523-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bjorke H, Andersson K. Automated, high-resolution cellular retention and uptake studies in vitro. Appl Radiat Isot. 2006;64:901–905. doi: 10.1016/j.apradiso.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Smanik PA, Liu Q, Furminger TL, Ryu K, Xing S, Mazzaferri EL, et al. Cloning of the Human Sodium Iodide Symporter. Biochem Biophys Res Commun. 1996;226:339–345. doi: 10.1006/bbrc.1996.1358. [DOI] [PubMed] [Google Scholar]
- 31.Brattain MG, Fine WD, Khaled FM, Thompson J, Brattain DE. Heterogeneity of Malignant Cells from a Human Colonic Carcinoma. Cancer Res. 1981;41:1751–1756. [PubMed] [Google Scholar]
- 32.Kang JH, Chung JK, Lee YL, Shin JH, Jeong JM, Lee DS, et al. Establishment of a human hepatocellular carcinoma cell line highly expressing sodium iodide symporter for radionuclide gene therapy. J Nucl Med. 2004;45:1571–1576. [PubMed] [Google Scholar]
- 33.Scholz IV, Cengic N, Baker CH, Harrington KJ, Maletz K, Bergert ER, et al. Radioiodine therapy of colon cancer following tissue-specific sodium iodide symporter gene transfer. Gene Ther. 2005;12:272–280. doi: 10.1038/sj.gt.3302410. [DOI] [PubMed] [Google Scholar]