Abstract
Over the past 30 years dithiocarbamate ligands have found application in radiopharmaceutical metal-ligand complexes to image a range of disease states. The vast majority of research and applications, and the widest range of complex structures, have involved radionuclides of technetium and rhenium. Considering the extent of coordination chemistry of dithiocarbamate ligands described elsewhere in this issue, the extent of radiopharmaceutical application with metallic radionuclides is surprisingly narrow. Here we summarise the types of radiopharmaceutical complexes studies and the uses, and potential uses, to which they have been put in nuclear medicine.
Keywords: copper, dithiocarbamate, nuclear medicine, radiopharmaceuticals, rhenium, technetium
Introduction
Diagnostic and therapeutic nuclear medicine relies on radionuclides of several different metals from both the main groups and transitions series. Gamma- and positron-emitting radioisotopes are used for imaging molecular processes in the body using gamma camera imaging and positron emission tomography (PET). Beta- and alpha-emitting radioisotopes targeted to tumours are used for radionuclide therapy. Many of the radioisotopes employed are metals and have to be complexed in suitable form for targeted in vivo delivery to specific organs and disease sites. A wide variety of ligands and chelating agents have been used both to link radiometals to biological targeting molecules and to endow them with useful properties by controlling redox potential, lipophilicity etc. This brief review focuses on use of metal-dithiocarbamate complexes as radiopharmaceuticals. Dithiocarbamate (DTC) ligands are ubiquitous in metal coordination chemistry, as two excellent in-depth reviews of both main group [1] and transition metal [2] dithiocarbamate complexes have shown. It is therefore no surprise that use of metal-dithiocarbamate complexes in nuclear medicine has been researched for over 30 years. However, considering the breadth of experience of general metal-dithiocarbamate chemistry in the literature, research on applications in nuclear medicine has been surprisingly limited in scope and depth. The vast majority of work in this field has focussed on technetium and rhenium. In the 1980s and 1990s the in vivo biodistribution and biological targeting properties of simple Tc/Re-dithiocarbamate complexes were evaluated. Over the past decade dithiocarbamate ligands have increasingly been used as co-ligands in mixed ligand ternary complexes used for a variety of purposes such as heart imaging, or for bioconjugation and radiolabelling of biomolecules. Properties of the dithiocarbamate ligands (such as size and lipophilicity) have been varied to optimise the in vivo characteristics of the complexes. Study of dithiocarbamate complexes of other radiometals has been limited to thallium and copper radionuclides. Some speculative applications involving radionuclides of bismuth, cobalt and gold have also been included in this review.
Technetium and Rhenium
Due to its favourable nuclear properties (half life = 6 h, 140 KeV gamma rays) [3] and convenient supply from the 99Mo/99mTc generator, technetium-99m (99mTc) is the most widely used radioisotope in nuclear medicine imaging. 99mTc exists in a range of oxidation states from +1 to +7 and interacts with a wide variety of ligands to form complexes that are used to image many in vivo functions and disease states. Its congener rhenium has, in addition to its two naturally occurring isotopes (185Re and 187Re), two potentially useful β-emitting radioactive isotopes (186Re and 188Re) that can be used for radionuclide therapy applications. 186Re has a half-life of 90.6 h and is produced in nuclear reactors by neutron bombardment of stable 185Re [3]. 188Re has a half-life of 16.9 h and is produced from the decay of 188W, and is thus available from a 188W/188Re generator [4]. Both isotopes produce imageable gamma rays, which are useful for tracking their biodistribution by gamma camera imaging.
In the earliest reports of dithiocarbamate ligands in 99mTc-radiopharmaceuticals, the lipophilic diethyldithiocarbamate (DEDTC) was used (together with formamidine sulfinic acid (FSA) to reduce pertechnetate) to produce a 99mTc complex whose structure was unknown but which was lipophilic enough to cross the blood brain barrier and clear by the hepatobiliary route [5–7]. A similar complex was produced using stannous tartrate as the reducing agent and used to radiolabel mixed leukocytes in vitro [8]. Subsequent studies have placed more emphasis on using structurally well-defined complexes by using well-established Tc and Re cores, such as the pentavalent technetium nitride [TcN]2+ system, as precursors around which the dithiocarbamate ligands are assembled. These core systems are designed to produce complexes with reasonable stability and control of the chemical characteristics. Their applications may be classified into two general categories: those that are targeted by control of the properties of the complex itself, and those where the dithiocarbamate ligand is used as a bifunctional chelator providing a link to a biological targeting molecule. Each class is discussed separately below. An example of the former are the [MN(DTC)2] complexes whose lipophilic properties are controlled via lipophilic side chains of the dithiocarbamate ligand [9–11]. An example of the latter approach is a dithiocarbamate derivative of an antibiotic such as ciprofloxacin [12,13]. Dithiocarbamate ligands are very well suited to both types as they can be easily synthesised by treatment of molecules that have an existing primary or (preferably) secondary amine group with carbon disulfide [2]. Secondary amines are preferred since dithiocarbamates formed from primary amines have a tendency to decompose into an isothiocyanate [2] or a primary amine and carbon disulfide (CS2) under acidic conditions [14].
The Nitrido (M≡N)2+ core
[MN(DTC)2] complexes
The most commonly used core for Tc and Re with dithiocarbamates in the coordination sphere is the Tc(V) nitrido core, [TcN]2+ [15, 16]. Non-radioactive ReN complexes have been known for many decades. [ReN(DEDTC)2] (1) (figure 1) [17] has a square pyramidal structure [18] as does the analogous [TcN(DEDTC)2] (2), the first reported example of a technetium complex with a Tc≡N triple bond [19]. These dithiocarbamate complexes were synthesised at the non-radioactive level in two steps. The rhenium precursor complex [ReNCl2(PPh3)2] [20] was synthesised from [Re2O7] [20] or Na[ReO4] [27] and hydrazine dihydrochloride (as a source of N) and triphenylphosphine (PPh3) in aqueous ethanol or from [ReOCl3(PPh3)2] by treatment with phenylhydrazine dihydrochloride in aqueous ethanol [28]. [ReNCl2(PPh3)2] was then subjected to ligand exchange with the dithiocarbamate ligands to furnish the dithiocarbamate complex [17]. The radiolabelled 99mTc complex was similarly prepared in two steps: a [99mTcN]2+ intermediate was synthesised by reducing 99mTcO4- in saline with stannous chloride in the presence of succinic dihydrazide (as a nitrogen atom donor) and the chelator DPTA (1,2-diaminopropane-N,N,N′,N′-tetraacetic acid) [12]. The intermediate complex was then incubated with the dithiocarbamate ligands to produce the [99mTcN(DTC)2] complexes.
Figure 1. [MN(DTC)2] complexes.
The [TcN]2+ core has been used to create 99mTc-dithiocarbamate complexes with a range of uses in nuclear medicine. [99mTcN(NOET)2] (3) was the first clinically tested example of a [99mTcN] tracer for imaging the myocardium [9,29]. It was found to be the most effective myocardial imaging agent among a number of lipophilic [99mTcN(DTC)2] complexes including [99mTcN(DEDTC)2] (2) [21]. Although [99mTcN(NOET)2] is uncharged, its myocardial uptake properties in dogs [30] and humans [31,32] are similar to those of the 201Tl+ ion, which behaves as a potassium ion analogue and is taken up by cardiomyocytes via the sodium-potassium ATPase pump. Its subcellular distribution in rat myocardium [33] showed localisation in the hydrophobic parts of the cells. Both the 99mTc complex and its 188Re analogue have been used to radiolabel blood cells [34,35] and a number of lipophilic [99mTcN(DTC)2] complexes including 2 were shown to be taken up in cultured tumour cells in vitro [9]. Lipophilic [99mTcN(DTC)2] complexes containing dithiocarbamate ligands derived from primary amines [10,11,36–45] and a few secondary amines [10,46,47] have been shown to cross the blood brain barrier, suggesting applications in brain perfusion imaging.
More recently the dithiocarbamate ligand has been used as a bifunctional linker to attach technetium to specific targeting molecules. Dithiocarbamate derivatives of a number of antibiotic molecules based on the fluoroquinolene group have been synthesised by addition of CS2 to a native amine group, and their [99mTcN(DTC)2] complexes have been evaluated as infection imaging agents. These include [99mTcN(Ciprofloxacin-DTC)2] (4) [12] and analogues based on Norfloxacin [48], Garenoxacin [49], Trovafloxacin [50], Moxifloxacin [51], Tosufloxacin [52], Sitafloxacin [53] and Gatifloxacin [54]. A [99mTcN(DTC)2] complex based on a benzamide structure (5) was synthesised and evaluated for diagnosis of malignant melanoma [22], however, tumour/organ ratios in mice were lower than for the more established [123I-BZA]. A [99mTcN(DTC)2] complex was synthesised for imaging of sigma receptors, which can be overexpressed in a variety of cancers; but tumour uptake in mice was low [55]. [99mTcN(DTC)2] complexes containing the nitroimidazole functionality (e.g. 6) have also been synthesised for hypoxia imaging but did not show promise [23,24]. [99mTcN(glucosamine-DTC)2] (7) showed tumour uptake in mice [25] and [99mTcN(DTC)2] complexes based on the 2-methoxyphenylpiperazine moiety (8) have been tested for 5-HT1A receptor binding for imaging brain disorders [26].
[TcN(PNP)(DTC)]+ complexes
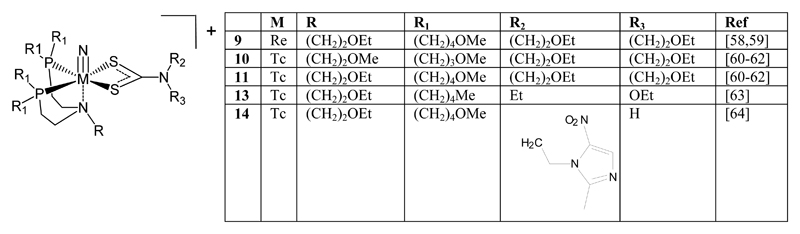
Several mixed ligand cationic complexes with the general structure [MN(PNP)(DTC)]+ (e.g. 9 - 13) (figure 2) have been evaluated (where PNP is a potentially tridentate amino bisphosphine ligand) with dithiocarbamates as co-ligands [56,58,59]. Characterisation of some of the rhenium complexes indicated a square pyramidal geometry with two phosphorus and two sulfur donor atoms as well as the nitride ligand. In addition, a weak interaction between the metal atom and the tertiary nitrogen atom on the backbone of the phosphine ligand [57] is believed to significantly stabilise the complexes; if the diphosphine ligands containing only methylene groups in the chain were used, no pure stable compounds could be isolated [65]. [99mTcN(PNP)(DTC)]+ complexes were stable to transchelation for up to 4 hours in serum and in solutions of excess cysteine or glutathione [61]. They can be the basis of lipophilic cationic tracers for myocardial perfusion imaging and both [99mTcN(PNP3)(DBODC)]+ (10) and [99mTcN(PNP5)(DBODC)]+ (11) show favourable myocardial uptake [60–62], with heart/lung and heart/liver ratios much higher than those of the commercially available agents 99mTc-tetrofosmin and 99mTc-sestamibi [61] and more favourable dosimetry [66]. Rapid myocardial uptake was further improved by incorporating an alicyclic dithiocarbamate ligand (e.g. 12) (figure 3) [67]. The analogous complex [99mTcN(PNP5)(NOET)]+ (13) also shows myocardial uptake [63]. A bifunctional nitroimidazole-dithiocarbamate derivative was used with this ligand system to produce [99mTcN(PNP5)(MNIE-DTC)]+ (14) which has been used to image hypoxia [64].
Figure 2. [MN(PNP)(DTC)]+ complexes.
Figure 3. [MN(PNP)(DTC)]+ and [MNNPh(PNP)(DTC)]+ complexes.
In a related series of complexes the [MN]2+ core was replaced by N-phenylhydrazine to give complexes with the structure [TcNNPh(PNP)(DTC)]+ (15) [68] in which the phenylhydrazine acted as a surrogate for the bifunctional linker HYBA (4-hydrazinobenzoic acid). Combinations of crown ether-derived dithiocarbamates and bisphosphine ligands were evaluated in the metal coordination sphere to optimise the lipophilicity and stabilise the cationic charge [68,69].
TcN(PS)(DTC) complexes
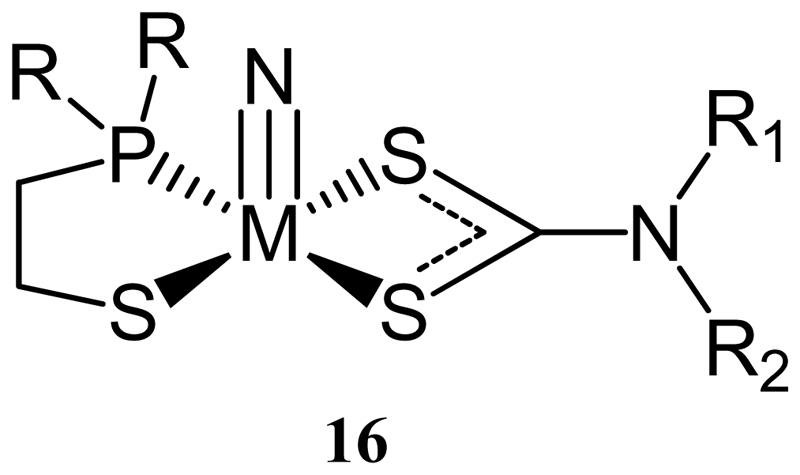
A series of complexes based on the [TcN]2+ core with one bidentate phosphine-thiol (PS) ligand and one dithiocarbamate with the general square pyramidal structure [TcN(PS)(DTC)] (16) (figure 4) has been reported [70–72]. Analogues of these complexes containing lipophilic dithiocarbamates such as DEDTC and dipropyldithiocarbamate (DPDTC) and lipophilic side chains on the PS ligand were able to cross the blood brain barrier [71], but attempts to exploit this by incorporation of dithiocarbamate derivatives of 2-methoxyphenylpiperazine to produce agents for imaging 5HT1A receptors in brain led to reduced blood brain barrier penetration [72].
Figure 4. A [TcN(PS)(DTC)] complex.
The tricarbonyl [M(CO)3]+ core
The [99mTc(CO)3]+ core with a dithiocarbamate ligand has been utilised for radiolabelling biomolecules with 99mTc [73]. The coordination sphere consisted of the three CO ligands plus one monodentate co-ligand such as isonitrile or phosphine (17) (figure 5), and one bidentate dithiocarbamate co-ligand. The link to the biomolecules was through either the monodentate ligand or the dithiocarbamate. Conjugation of the dithiocarbamate ligand to a model amino acid using a dithiocarbamate-carboxylate-NHS ester 18 (figure 6) was only possible if the CS2 functionality was first protected using a non-radioactive [M(CO)3]+ fragment as shown in figure 6 to avoid decomposition under the acidic conditions needed for the conjugation step [73]. Alternatively, a monodentate phosphine ligand could be used as the bifunctional linker (17) [74]. [99mTc(CO)3]+ has also been used to radiolabel a range of dithiocarbamate compounds including brain perfusion agents [75], 5-HT1A receptor imaging agents [26] and dithiocarbamate derivatives of Ciprofloxacin [13], Tosufloxacin [52] and Sitafloxacin [76] for infection imaging. Whilst it is expected that the dithiocarbamate ligand coordinates to the technetium in bidentate mode, the identity of the ligand in the sixth coordination site, and the overall structure of the complex, remains unknown.
Figure 5. A [Tc(CO)3]+ dithiocarbamate complex.
Figure 6. Dithiocarbamate ligand synthesis with [M(CO)3]+ as a protecting group.
Miscellaneous technetium and rhenium complexes
Ternary ligand complexes such as 19 (figure 7) have also been evaluated for the development of new potential rhenium radiopharmaceuticals. The core consisted of a diazenide ligand, triphenylphosphine and some simple dithiocarbamates [77]. The seven-coordinate distorted pentagonal bipyramidal complex [Re(DEDTC)3(CO)] (20) [78] and its technetium analogue (21) [79] have been reported. Further radioactive 99mTc complexes of the general formula [99mTc(DTC)3(CO)] were produced [80]. These highly lipophilic complexes were rapidly cleared by the hepatobiliary system and showed potential as hepatobiliary imaging agents. The same authors have also published the structures of [Tc(DEDTC)2Cl2(NS)] (22) [81] and [Tc(DEDTC)2Br2(NS)] (23) [82].
Figure 7. Miscellaneous technetium and rhenium dithiocarbamate complexes.
Copper
Copper has four radionuclides useful for molecular imaging, 60Cu, 61Cu, 62Cu and 64Cu. 64Cu is the most widely used thanks to its favourable properties for PET imaging and radionuclide therapy (half-life 12.7 h, 18 % β+, 39 % β−, 43 % electron capture). Dithiocarbamate ligands are well known as effective ligands for Cu(II), forming square-planar [Cu(II)(DTC)2] complexes, but their use as chelating groups for copper radionuclides has been somewhat neglected to date [2,83]. The radiolabelled complexes are formed very rapidly in high yield but lack kinetic stability in biological media. [62Cu(DMDTC)2] (24) (figure 8) and [62Cu(DEDTC)2] (25) [84] readily cross the blood brain barrier and have potential utility as brain perfusion imaging agents [85]. Because of their lipophilicity and kinetic lability these complexes are taken up and trapped in cells, and complexes of dimethyldithiocarbamate (DMDTC), DEDTC and DPDTC (26) were used to label a mouse macrophage cell line (J774) with very high efficiency depending on the dithiocarbamate used. [64Cu(DMDTC)2] showed the most rapid and efficient uptake [86]. However, the wash-out rates were too rapid for imaging cell trafficking in vivo and were the same for all the complexes, suggesting rapid intracellular dissociation of all the complexes.
Figure 8. [Cu(DTC)2] complexes.
Bifunctional dithiocarbamate ligands have been developed incorporating a bisphosphonate group for strong binding of copper radionuclides to several inorganic materials of interest to the biomedical imaging and engineering fields [87]. The copper complex [64Cu(DTCBP)2] (27) is obtained instantly at room temperature by simple addition of the radionuclide to the ligand. This is in contrast to most macrocyclic chelators commonly used for 64Cu chemistry, such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) or 4,11-bis(carboxymethyl)-1,4,8,11-tetraazabicyclo[6.6.2]hexadecane (CB-TE2A) in which complex formation is relatively slow and heating (ca. 95 °C) is necessary [83]. When conjugated to surfaces of inorganic materials via its bisphosphonate groups, [64Cu(DTCBP)2] resists transchelation reactions in vivo [87].
Thallium
201Tl-DEDTC was introduced in the 1980s as a lipophilic radiotracer for imaging cerebral blood flow [6,7,88–90]. The structure of the complex was not determined, although thallium is known to form [Tl(I)(DTC)] and [Tl(III)(DTC)3] complexes [1]. The tracer was found to have higher retention in rabbit brain and much lower blood protein binding than the technetium “analogue” 99mTc-DEDTC of unknown structure [7]. The low energy and low abundance of gamma rays from 201Tl along with a long half-life of 3 days make the imaging properties and dosimetry of this radionuclide less ideal for SPECT imaging than the 99mTc complexes such as 99mTc-HMPAO [91] which replaced it.
Bismuth
A selection of bismuth complexes [Bi(DTC)3] have been synthesised and evaluated in vitro and in vivo for their anti-cancer properties [92]. Their in vivo mechanism of action is unknown as there is no known biological target. Nevertheless, if they are able to selectively enter tumour cells then radioactive analogues using the α-emitters 212Bi and 213Bi [93,94] could be potential candidates for radionuclide therapy.
Cobalt
Cobalt-55 is a positron-emitting isotope (t½ = 18.2 h) that has been scarcely explored for nuclear medicine. It has been used to mimic calcium influx in ischaemic tissue and for carbohydrate and platelet labelling [95–97]. Dithiocarbamate ligands are well known to form kinetically inert Co(III) complexes [Co(DTC)3] [2]. Thus, 55Co derivatives could provide stable PET imaging agents with potentially very low transchelation rate in vivo. The extremely long half life of the radioactive daughter isotope (55Fe, t½ = 2.7 years), however, represents a significant obstacle for the successful development of these agents, as it may result in unnecessary long-term radiation doses to subjects and represents an environmental issue.
Gold
Radioisotopes of gold are available and occasionally used in medicine. The 195mHg/195mAu generator was developed to produce 195mAu, a short half-life gamma emitter [98] that can be used as a tracer for imaging blood flow. Beta emitters 198Au and 199Au, produced from either nuclear reactor or cyclotron [99], have potential for radionuclide therapy [100,101]. Chelators for use with gold isotopes are underdeveloped but since gold (III) dithiocarbamate complexes have demonstrated high anti-tumour cytotoxicity with less non-target toxicity than the common anticancer drug cisplatin [102], and have good stability in physiological conditions [103], radioactive gold dithiocarbamate complexes may have potential as radiopharmaceuticals.
Summary
Dithiocarbamate ligands have found application in radiopharmaceuticals containing metallic radionuclides to image a range of disease states. The vast majority of research and applications, and the widest range of complex structures, have involved technetium and rhenium. Considering the extent and variety of coordination chemistry of dithiocarbamate ligands described elsewhere in this issue, the extent of radiopharmaceutical application with metallic radionuclides that has been realised is surprisingly narrow and confined to just a few complex types. The potential for application of these ligands in nuclear medicine remains great, as the field of molecular imaging as whole is evolving and clinical utility and availability of positron emission tomography is growing rapidly. Radionuclide therapy is also a growing field and dithiocarbamate ligands have the potential to contribute, especially with radionuclides of rhenium which offer a particularly wide range of stable, well-defined dithiocarbamate complex types.
Acknowledgements
We thank the Centre of Excellence in Medical Engineering at King’s College London funded by the Wellcome Trust and EPSRC (Grant WT 088641/Z/09/Z) for financial support.
References
- [1].Heard PJ. Main group dithiocarbamate complexes. Prog Inorg Chem. 2005;53:1–69. [Google Scholar]
- [2].Hogarth G. Transition Metal Dithiocarbamates: 1978-2003. Prog Inorg Chem. 2005;53:71–561. [Google Scholar]
- [3].Jurisson S, Berning D, Jia W, Ma D. Coordination compounds in nuclear medicine. Chem Rev. 1993;93:1137–1156. [Google Scholar]
- [4].Boschi A, Bolzati C, Uccelli L, Duatti A. High-yield synthesis of the terminal 188Re≡N multiple bond from generator-produced [188ReO4]- Nucl Med Biol. 2003;30:381–387. doi: 10.1016/s0969-8051(03)00002-7. [DOI] [PubMed] [Google Scholar]
- [5].Pojer PM, Baldas J. Technetium-99m-labelled N,N-diethyldithiocarbamate - A non-polar complex with slow hepatic clearance. Int J Nucl Med Biol. 1981;8:112–114. doi: 10.1016/0047-0740(81)90062-0. [DOI] [PubMed] [Google Scholar]
- [6].de Bruine JF, van Royen EA, Vyth A, de Jong JMBV, van der Schoot JB. Thallium-201 diethyldithiocarbamate: An alternative to iodine-123 N-isopropyl-p-iodoamphetamine. J Nucl Med. 1985;26:925–930. [PubMed] [Google Scholar]
- [7].Ballinger JR, Gerson B, Gulenchyn KY. Technetium-99m diethyldithiocarbamate (DDC): Comparison with thallium-201 DDC as an agent for brain imaging. Int J Radiat Appl Instrum Part A Appl Rad Isot. 1987;38:665–668. doi: 10.1016/0883-2889(87)90136-5. [DOI] [PubMed] [Google Scholar]
- [8].Sampson CB, Solanki C. Techentium-labeled leukocytes using diethyldithiocarbamate - preliminary-report on in vitro studies. Nucl Med Commun. 1988;9:123–127. [PubMed] [Google Scholar]
- [9].Stalteri MA, Parrott SJ, Griffiths VA, Dilworth JR, Mather SJ. Uptake of Tc-99m-nitrido dithiocarbamate complexes by tumour cells. Nucl Med Commun. 1997;18:870–877. doi: 10.1097/00006231-199709000-00012. [DOI] [PubMed] [Google Scholar]
- [10].Mallia MB, Mathur A, Subramanian S, Banerjee S, Kothari K, Koiry SP, Sarma HD, Venkatesh M. Synthesis and evaluation of ether containing 99mTc–nitrido dithiocarbamate complexes as brain perfusion imaging agent. Appl Rad Isot. 2006;64:361–367. doi: 10.1016/j.apradiso.2005.09.003. [DOI] [PubMed] [Google Scholar]
- [11].Zhang J, Yu T, Wang Z, Lin Y, Wen F, Xing M, Wang X. Synthesis and biodistribution of two novel 99mTc nitrido dithiocarbamate complexes containing heterocyclic linkage as potential brain perfusion imaging agents. J Radioanal Nucl Chem. 2007;274:195–197. [Google Scholar]
- [12].Zhang J, Guo H, Zhang S, Lin Y, Wang X. Synthesis and biodistribution of a novel 99mTcN complex of ciprofloxacin dithiocarbamate as a potential agent for infection imaging. Bioorg Med Chem Lett. 2008;18:5168–5170. doi: 10.1016/j.bmcl.2008.08.109. [DOI] [PubMed] [Google Scholar]
- [13].Zhang J, Zhang S, Guo H, Wang X. Synthesis and biological evaluation of a novel 99mTc(CO)3 complex of ciprofloxacin dithiocarbamate as a potential agent to target infection. Bioorg Med Chem Lett. 2010;20:3781–3784. doi: 10.1016/j.bmcl.2010.04.057. [DOI] [PubMed] [Google Scholar]
- [14].Joris SJ, Aspila KI, Chakrabarti Cl. Decomposition of monoalkyl dithiocarbamates. Anal Chem. 1970;42:647–651. [Google Scholar]
- [15].Marchi A, Marchesi E, Marvelli L, Bergamini P, Bertolasi V, Ferretti V. New Water-Soluble Rhenium Complexes with 1,3,5-Triaza-7-phosphaadamantane (PTA) – X-ray Crystal Structures of [ReNCl2(PTA)3], [ReO2Cl(PTA)3], [ReCl3(PTA)2(PPh3)], and [Re2N2Cl3(Et2dtc)(PTA)4] Eur J Inorg Chem. 2008:2670–2679. [Google Scholar]
- [16].Marchi A, Garuti P, Duatti A, Magon L, Rossi R, Ferretti V, Bertolasi V. Synthesis of technetium(V)-nitrido complexes with chelating amines: a novel class of monocationic, octahedral complexes containing the [Tc≡N]2+ core. Crystal structures of [TcN(en)2Cl]+ (en = Ethylenediamine) and [TcN(tad)Cl]+ (tad = 1,5,8,12-Tetraazadodecane) Inorg Chem. 1990;29:2091–2096. [Google Scholar]
- [17].Rowbottom J, Wilkinson G. Dithiocarbamate Complexes of Rhenium(V) and (III) J Chem Soc, Dalton Trans. 1972:826–830. [Google Scholar]
- [18].Fletcher SR, Skapski AC. Crystal and Molecular Structure of Square-Pyramidal Complex Nitridobis(NN-Diethyldithiocarbamato)Rhenium(V) J Chem Soc, Dalton Trans. 1972:1079–1082. [Google Scholar]
- [19].Baldas J, Bonnyman J, Pojer PM, Williams GA, Mackay MF. Synthesis and Structure of Bis(Diethyldithiocarbamato)Nitridotechnetium(V) - a Technetium-Nitrogen Triple Bond. J Chem Soc, Dalton Trans. 1981:1798–1801. [Google Scholar]
- [20].Chatt J, Garforth JD, Johnson NP, Rowe GA. 196. Nitrido- and arylimido-complexes of rhenium. J Chem Soc (Resumed) 1964:1012–1020. [Google Scholar]
- [21].Pasqualini R, Duatti A, Bellande E, Comazzi V, Brucato V, Hoffschir D, Fagret D, Comet M. Bis(Dithiocarbamato) Nitrido Technetium-99m Radiopharmaceuticals: A Class of Neutral Myocardial Imaging Agents. J Nucl Med. 1994;35:334–341. [PubMed] [Google Scholar]
- [22].Auzeloux P, Papon J, Masnada T, Borel M, Moreau M-F, Veyre A, Pasqualini R, Madelmont J-C. Synthesis and biodistribution of technetium-99m-labelled N-(diethylaminoethyl)benzamide via a bis(dithiocarbamate) nitridotechnetium(V) complex. J Labelled Compd Radiopharm. 1999;42:325–335. [Google Scholar]
- [23].Giglio J, Fernández S, Rey A, Cerecetto H. Synthesis and biological characterisation of novel dithiocarbamate containing 5-nitroimidazole 99mTc-complexes as potential agents for targeting hypoxia. Bioorg Med Chem Lett. 2011;21:394–397. doi: 10.1016/j.bmcl.2010.10.130. [DOI] [PubMed] [Google Scholar]
- [24].Chu T, Li R, Hu S, Wang Y, Liu X, Wang X. Synthesis and biodistribution of the 99mTc nitrido complex with 2-(4-nitro-1H-imidazolyl)ethyl dithiocarbamate (NIET) J Radioanal Nucl Chem. 2004;261:199–202. [Google Scholar]
- [25].Zhang J, Ren J, Lin X, Wang X. Synthesis and biological evaluation of a novel 99mTc nitrido radiopharmaceutical with deoxyglucose dithiocarbamate, showing tumor uptake. Bioorg Med Chem Lett. 2009;19:2752–2754. doi: 10.1016/j.bmcl.2009.03.131. [DOI] [PubMed] [Google Scholar]
- [26].Yang W, Lin Y, Zhang X, Zhang J, Wang X. Synthesis of several MPP derivatives for 99mTc-labelling and evaluated as potential 5-HT1A receptor imaging agents. Science China Chem. 2011;54:1148–1154. [Google Scholar]
- [27].Chatt J, Rowe GA. 788. Complex compounds of tertiary phosphines and a tertiary arsine with rhenium(V), rhenium(III), and rhenium(II) J Chem Soc (Resumed) 1962:4019–4033. [Google Scholar]
- [28].Sullivan BP, Brewer JC, Gray HB, Linebarrier D, Mayer JM. Inorg Synth. John Wiley & Sons; 2007. pp. 146–150. [Google Scholar]
- [29].Pasqualini R, Duatti A. Synthesis and Characterization of the New Neutral Myocardial Imaging Agent [(99mTcN(noet)2] (noet = N-Ethyl-N-Ethoxydithiocarbamato) J Chem Soc, Chem Commun. 1992:1354–1355. [Google Scholar]
- [30].Ghezzi C, Fagret D, Arvieux CC, Mathieu J-P, Bontron R, Pasqualini R, de Leiris J, Comet M. Myocardial Kinetics of TcN-NOET: A Neutral Lipophilic Complex Tracer of Regional Myocardial Blood Flow. J Nucl Med. 1995;36:1069–1077. [PubMed] [Google Scholar]
- [31].Jeetley P, Sabharwal NK, Soman P, Kinsey C, Raval U, Bhonsle U, Lahiri A. Comparison between Tc-99m N-NOET and Tl-201 in the assessment of patients with known or suspected coronary artery disease. J Nucl Cardiol. 2004;11:664–672. doi: 10.1016/j.nuclcard.2004.05.010. [DOI] [PubMed] [Google Scholar]
- [32].Fagret D, Marie P-Y, Brunotte F, Giganti M, Le Guludec D, Bertrand A, Wolf J-E, Piffanelli A, Chossat F, Bekhechi D, Pasqualini R, et al. Myocardial Perfusion Imaging with Technetium-99m-Tc NOET: Comparison with Thallium-201 and Coronary Angiography. J Nucl Med. 1995;36:936–943. [PubMed] [Google Scholar]
- [33].Uccelli L, Giganti M, Duatti A, Bolzati C, Pasqualini R, Cittanti C, Colamussi P, Piffanelli A. Subcellular Distribution of Technetium-99m-N-NOEt in Rat Myocardium. J Nucl Med. 1995;36:2075–2079. [PubMed] [Google Scholar]
- [34].Mévellec F, Demaimay F, Roucoux A, Moisan A, Noiret N, Patin H. Synthesis, characterization and blood cell labelling evaluation of new 99mTc nitrido radiopharmaceuticals with thioamide [R1C(=S)NHR2] derivatives. J Labelled Compd Radiopharm. 1998;41:863–869. [Google Scholar]
- [35].Demaimay F, Dazord L, Roucoux A, Noiret N, Patin H, Moisan A. Rhenium-188 and Technetium-99m Nitridobis(N-ethoxy-N-ethyldithiocarbamate) Leucocyte Labelling Radiopharmaceuticals: [188ReN(NOET)2] and [99mTcN(NOET)2], NOET = Et(EtO)NCS2: Their In Vitro Localization and Chemical Behaviour. Nucl Med Biol. 1997;24:701–705. doi: 10.1016/s0969-8051(97)00096-6. [DOI] [PubMed] [Google Scholar]
- [36].Zhang J, Wang X, Lu G, Tang Z. Synthesis and biodistribution of a new 99mTc nitrido complex for brain imaging. Appl Rad Isot. 2001;54:745–748. doi: 10.1016/s0969-8043(00)00322-5. [DOI] [PubMed] [Google Scholar]
- [37].Zhang J, Wang X, Li C. Synthesis and biodistribution of a new 99mTc nitrido complex for cerebral imaging. Nucl Med Biol. 2002;29:665–669. doi: 10.1016/s0969-8051(02)00311-6. [DOI] [PubMed] [Google Scholar]
- [38].Wang X. Synthesis of 99mTcN(IPDTC)2 and its biodistribution in mice. J Radioanal Nucl Chem. 2001;249:573–576. [Google Scholar]
- [39].Zhang J, Wang X, Tian C. Synthesis and biodistribution of 99mTcN(PDTC)2 as a potential brain imaging agent. J Radioanal Nucl Chem. 2004;262:505–507. [Google Scholar]
- [40].Zhang J, Wang X, Tian C. Synthesis of a bis-(N-butyl-dithiocarbamato)-nitrido 99mTc complex: A potential new brain imaging agent. J Radioanal Nucl Chem. 2007;273:15–17. [Google Scholar]
- [41].Zhang J, Wang X, Liu J. Synthesis of a bis-(N-sec-butyl-dithiocarbamato)-nitrido-99mTc complex: a potential new radiopharmaceutical for brain perfusion studies. Appl Rad Isot. 2005;62:33–37. doi: 10.1016/j.apradiso.2004.06.001. [DOI] [PubMed] [Google Scholar]
- [42].Zhang J, Lin X, Ren J, Liu J, Wang X. Synthesis and biodistribution of a novel 99mTc nitrido dithiocarbamate complex containing aromatic group for cerebral imaging. Appl Rad Isot. 2010;68:101–104. doi: 10.1016/j.apradiso.2009.08.019. [DOI] [PubMed] [Google Scholar]
- [43].Zhang J, Wang X, Liu J. Synthesis and biodistribution in mice of a new 99mTc nitrido complex for brain imaging. J Labelled Compd Radiopharm. 2004;47:647–655. [Google Scholar]
- [44].Zhang J, Wang X, Lu G, Tang Z. Synthesis, characterization and biodistribution of a 99mTc nitrido complex as a potential brain perfusion imaging agent. J Labelled Compd Radiopharm. 2000;43:693–700. [Google Scholar]
- [45].Zhang JB, Luo G, Wang XB. Synthesis and biodistribution of a novel Tc-99m nitrido dithiocarbamate complex containing ether group as a potential myocardial and brain imaging agent. J Radioanal Nucl Chem. 2009;279:783–785. [Google Scholar]
- [46].Zhang J, Wang X, Li C. Synthesis and biodistribution of a new 99mTc nitrido complex as a potential myocardial and cerebral imaging agent. Appl Rad Isot. 2002;56:857–861. doi: 10.1016/s0969-8043(01)00273-1. [DOI] [PubMed] [Google Scholar]
- [47].Zhang J, Wang X, Li C. Synthesis and biodistribution of 99mTcN(CHIPDTC)2 as a potential myocardial perfusion imaging agent. J Radioanal Nucl Chem. 2002;254:99–101. [Google Scholar]
- [48].Zhang S, Zhang W, Wang Y, Jin Z, Wang X, Zhang J, Zhang Y. Synthesis and Biodistribution of a Novel 99mTcN Complex of Norfloxacin Dithiocarbamate as a Potential Agent for Bacterial Infection Imaging. Bioconj Chem. 2011;22:369–375. doi: 10.1021/bc100357w. [DOI] [PubMed] [Google Scholar]
- [49].Shah S, Khan A, Khan M. Radiosynthesis and biodistribution of (TcN)-Tc-99m-Garenoxacin dithiocarbamate complex a potential infection imaging agent. J Radioanal Nucl Chem. 2011;288:59–64. [Google Scholar]
- [50].Shah S, Khan M. Radiocomplexation and biological characterization of the 99mTcN-trovafloxacin dithiocarbamate: a novel methicillin-resistant Staphylococcus aureus infection imaging agent. J Radioanal Nucl Chem. 2011;288:215–220. [Google Scholar]
- [51].Shah S, Khan M. Evaluation of 99mTcN–moxifloxacin dithiocarbamate, as a potential radiopharmaceutical for scintigraphic localization of infectious foci. J Radioanal Nucl Chem. 2011;288:357–362. [Google Scholar]
- [52].Shah S, Khan M. 99mTc(CO)3-tosufloxacin dithiocarbamate complexation and radiobiological evaluation in male Wister rat model. J Radioanal Nucl Chem. 2011;288:485–490. [Google Scholar]
- [53].Shah S, Khan A, Khan M. Radiosynthesis and biological evaluation of 99mTcN-sitafloxacin dithiocarbamate as a potential radiotracer for Staphylococcus aureus infection. J Radioanal Nucl Chem. 2011;287:827–832. [Google Scholar]
- [54].Shah S, Khan M. 99mTcN–gatifloxacin dithiocarbamate complex: a novel multi-drug-resistance Streptococcus pneumoniae (MRSP) infection radiotracer. J Radioanal Nucl Chem. 2011;289:903–908. [Google Scholar]
- [55].Lu J, Kong D, Jia H, Deuther-Conrad W, Brust P, Wang X. Preparation and biological evaluation of 99mTcN-4-(cyclohexylpiperazin-1-yl)-dithioformate as a potential sigma receptor imaging agent. J Labelled Compd Radiopharm. 2007;50:1200–1205. [Google Scholar]
- [56].Bolzati C, Boschi A, Uccelli L, Tisato F, Refosco F, Cagnolini A, Duatti A, Prakash S, Bandoli G, Vittadini A. Chemistry of the Strong Electrophilic Metal Fragment [99Tc(N)(PXP)]2+ (PXP = Diphosphine Ligand). A Novel Tool for the Selective Labeling of Small Molecules. J Am Chem Soc. 2002;124:11468–11479. doi: 10.1021/ja0200239. [DOI] [PubMed] [Google Scholar]
- [57].Bolzati C, Refosco F, Cagnolini A, Tisato F, Boschi A, Duatti A, Uccelli L, Dolmella A, Marotta E, Tubaro M. Synthesis, Solution-State and Solid-State Structural Characterization of Monocationic Nitrido Heterocomplexes [M(N)(DTC)(PNP)]+ (M = 99Tc, Re; DTC = Dithiocarbamate; PNP = Heterodiphosphane) Eur J Inorg Chem. 2004:1902–1913. [Google Scholar]
- [58].Marotta E, Tisato F, Refosco F, Bolzati C, Porchia M, Traldi P. Electrospray ionization mass spectrometry in the structural characterization of a mixed nitrido-Tc heterocomplex of interest for myocardial imaging. Rapid Commun Mass Spectrom. 2003;17:1225–1228. doi: 10.1002/rcm.1021. [DOI] [PubMed] [Google Scholar]
- [59].Tubaro M, Marotta E, Traldi P, Bolzati C, Porchia M, Refosco F, Tisato F. ESI/MSn in the structural characterisation of some nitrido-Re heterocomplexes. Int J Mass Spectrom. 2004;232:239–247. [Google Scholar]
- [60].Boschi A, Bolzati C, Uccelli L, Duatti A, Benini E, Refosco F, Tisato F, Piffanelli A. A class of asymmetrical nitrido Tc-99m heterocomplexes as heart imaging agents with improved biological properties. Nucl Med Commun. 2002;23:689–693. doi: 10.1097/00006231-200207000-00014. [DOI] [PubMed] [Google Scholar]
- [61].Boschi A, Uccelli L, Bolzati C, Duatti A, Sabba N, Moretti E, Di Domenico G, Zavattini G, Refosco F, Giganti M. Synthesis and Biologic Evaluation of Monocationic Asymmetric 99mTc-Nitride Heterocomplexes Showing High Heart Uptake and Improved Imaging Properties. J Nucl Med. 2003;44:806–814. [PubMed] [Google Scholar]
- [62].Hatada K, Riou LM, Ruiz M, Yamamichi Y, Duatti A, Lima RL, Goode AR, Watson DD, Beller GA, Glover DK. 99mTc-N-DBODC5, a New Myocardial Perfusion Imaging Agent with Rapid Liver Clearance: Comparison with 99mTc-Sestamibi and 99mTc-Tetrofosmin in Rats. J Nucl Med. 2004;45:2095–2101. [PubMed] [Google Scholar]
- [63].Chu J, Li B, Kong D, Wang X, Zhang J. [99mTcN(PNP5)(NOEt)]+;: A novel potential myocardial perfusion imaging agent. J Radioanal Nucl Chem. 2006;269:175–179. [Google Scholar]
- [64].Kong D, Lu J, Ye S, Wang X. Synthesis and biological evaluation of a novel asymmetrical 99mTc-nitrido complex of metronidazole derivative. J Labelled Compd Radiopharm. 2007;50:1137–1142. [Google Scholar]
- [65].Tisato F, Refosco F, Porchia M, Bolzati C, Bandoli G, Dolmella A, Duatti A, Boschi A, Jung CM, Pietzsch H-J, Kraus W. The Crucial Role of the Diphosphine Heteroatom X in the Stereochemistry and Stabilization of the Substitution-Inert [M(N)(PXP)]2+ Metal Fragments (M = Tc, Re; PXP = Diphosphine Ligand) Inorg Chem. 2004;43:8617–8625. doi: 10.1021/ic049139r. [DOI] [PubMed] [Google Scholar]
- [66].Cittanti C, Uccelli L, Pasquali M, Boschi A, Flammia C, Bagatin E, Casali M, Stabin MG, Feggi L, Giganti M, Duatti A. Whole-Body Biodistribution and Radiation Dosimetry of the New Cardiac Tracer 99mTc-N-DBODC. J Nucl Med. 2008;49:1299–1304. doi: 10.2967/jnumed.108.053132. [DOI] [PubMed] [Google Scholar]
- [67].Bolzati C, Cavazza-Ceccato M, Agostini S, Refosco F, Yamamichi Y, Tokunaga S, Carta D, Salvarese N, Bernardini D, Bandoli G. Biological in Vitro and in Vivo Studies of a Series of New Asymmetrical Cationic [99mTc(N)(DTC-Ln)(PNP)]+ Complex (DTC-Ln = Alicyclic Dithiocarbamate and PNP = Diphosphinoamine) Bioconj Chem. 2010;21:928–939. doi: 10.1021/bc900493e. [DOI] [PubMed] [Google Scholar]
- [68].Kim Y-S, He Z, Hsieh W-Y, Liu S. A Novel Ternary Ligand System Useful for Preparation of Cationic 99mTc-Diazenido Complexes and 99mTc-Labeling of Small Biomolecules. Bioconj Chem. 2006;17:473–484. doi: 10.1021/bc0502715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Liu S. Ether and crown ether-containing cationic 99mTc complexes useful as radiopharmaceuticals for heart imaging. Dalton Trans. 2007:1183–1193. doi: 10.1039/b618406e. [DOI] [PubMed] [Google Scholar]
- [70].Bolzati C, Cavazza-Ceccato M, Agostini S, Tisato F, Bandoli G. Technetium and Rhenium in Five-Coordinate Symmetrical and Dissymmetrical Nitrido Complexes with Alkyl Phosphino-thiol Ligands. Synthesis and Structural Characterization. Inorg Chem. 2008;47:11972–11983. doi: 10.1021/ic801436d. [DOI] [PubMed] [Google Scholar]
- [71].Bolzati C, Benini E, Cavazza-Ceccato M, Cazzola E, Malagò E, Agostini S, Tisato F, Refosco F, Bandoli G. From Symmetrical to Asymmetrical Nitrido Phosphino-thiol Complexes: A New Class of Neutral Mixed-Ligand 99mTc Compounds as Potential Brain Imaging Agents. Bioconj Chem. 2006;17:419–428. doi: 10.1021/bc050358q. [DOI] [PubMed] [Google Scholar]
- [72].Bolzati C, Salvarese N, Carta D, Refosco F, Dolmella A, Pietzsch H, Bergmann R, Bandoli G. Synthesis and biological evaluation of new [Tc(N)(PS)]-based mixed-ligand compounds useful in the design of target-specific radiopharmaceuticals: the 2-methoxyphenylpiperazine dithiocarbamate derivatives as an example. J Biol Inorg Chem. 2011;16:137–155. doi: 10.1007/s00775-010-0712-4. [DOI] [PubMed] [Google Scholar]
- [73].Gorshkov NI, Schibli R, Schubiger AP, Lumpov AA, Miroslavov AE, Suglobov DN. “2+1” Dithiocarbamate–isocyanide chelating systems for linking (M = 99mTc, Re) fragment to biomolecules. J Organomet Chem. 2004;689:4757–4763. [Google Scholar]
- [74].Riondato M, Camporese D, Martín D, Suades J, Alvarez-Larena A, Mazzi U. Synthesis and Characterisation of [Re(CO)3(SS)(P)] Complexes: A [2+1] Concept for 99mTc- and 188Re-Radiopharmaceutical Applications. Eur J Inorg Chem. 2005:4048–4055. [Google Scholar]
- [75].Zhang J, Wang X, Jin C. Synthesis and biodistribution of the 99mTc(CO)3-DEDT complex as a potential new radiopharmaceutical for brain imaging. J Radioanal Nucl Chem. 2007;272:91–94. [Google Scholar]
- [76].Shah S, Khan A, Khan M. Radiosynthesis and biological evolution of 99mTc(CO)3–sitafloxacin dithiocarbamate complex: a promising Staphylococcus aureus infection radiotracer. J Radioanal Nucl Chem. 2011;288:131–136. [Google Scholar]
- [77].Cowley AR, Dilworth JR, Donnelly PS, Ross SJ. Rhenium diazenide ternary complexes with dithiocarbamate ligands: towards new rhenium radiopharmaceuticals. Dalton Trans. 2007:73–82. doi: 10.1039/b611403b. [DOI] [PubMed] [Google Scholar]
- [78].Fletcher SR, Skapski AC. Crystal and molecular structure of carbonyltris(NN-diethyldithiocarbamato)rhenium(III) : a complex of seven-co-ordinate rhenium. J Chem Soc, Dalton Trans. 1974:486–489. [Google Scholar]
- [79].Baldas J, Bonnyman J, Pojer PM, Williams GA, Mackay MF. Preparation and crystal structure of carbonyltris(diethyldithiocarbamato) technetium(III) : an unexpected source of co-ordinated carbon monoxide. J Chem Soc, Dalton Trans. 1982:451–455. [Google Scholar]
- [80].Baldas J, Bonnyman J. Preparation and biological behaviour of some neutral 99mTc-carbonyl dithiocarbamates showing rapid hepatobiliary excretion. Int J Rad Appl Instrum B Nucl Med Biol. 1992;19:741–746. doi: 10.1016/0883-2897(92)90134-k. [DOI] [PubMed] [Google Scholar]
- [81].Baldas J, Bonnyman J, Mackay MF, Williams GA. Structural studies of technetium complexes .5. The preparation and crystal structure of dichlorobis(dirthyldithiocarbamato)-thionitrosyltechnetium(III) Aust J Chem. 1984;37:751–759. [Google Scholar]
- [82].Baldas J, Colmanet SF, Williams GA. Preparation and structure of dibromobis(N,N-diehtyldithiocarbamato)-thionitrosyltechnetium(III) Aust J Chem. 1991;44:1125–1132. [Google Scholar]
- [83].Wadas TJ, Wong EH, Weisman GR, Anderson CJ. Coordinating Radiometals of Copper, Gallium, Indium, Yttrium, and Zirconium for PET and SPECT Imaging of Disease. Chem Rev. 2010;110:2858–2902. doi: 10.1021/cr900325h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Matsumoto K, Fujibayashi Y, Konishi J, Yokoyama A. Radiolabeling and biodistribution of 62Cu-dithiocarbamate-an application for the new 62Zn/62Cu generator. Radioisotopes. 1990;39:482–486. doi: 10.3769/radioisotopes.39.11_482. [DOI] [PubMed] [Google Scholar]
- [85].Dearling JLJ, Mullen GED, Lewis JS, Welch MJ, Blower PJ. Dithiocarbamate copper complexes as blood flow tracers. J Labelled Compd Radiopharm. 1999;42:835. [Google Scholar]
- [86].Charoenphun PR, Paul R, Weeks A, Berry D, Shaw K, Mullen G, Ballinger J, Blower P. PET tracers for cell labelling with the complexes of copper 64 with lipophilic ligands. Eur J Nucl Med Mol Imaging. 2011;38:S294–S294. [Google Scholar]
- [87].Torres Martin de Rosales R, Tavaré R, Paul RL, Jauregui-Osoro M, Protti A, Glaria A, Varma G, Szanda I, Blower PJ. Synthesis of 64CuII–Bis(dithiocarbamatebisphosphonate) and Its Conjugation with Superparamagnetic Iron Oxide Nanoparticles: In Vivo Evaluation as Dual-Modality PET–MRI Agent. Angew Chem Int Ed. 2011;50:5509–5513. doi: 10.1002/anie.201007894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lear JL, Navarro D. Autoradiographic Comparison of Thallium-201 Diethyldithiocarbamate, Isopropyliodoamphetamine and Iodoantipyrine as Cerebral Blood Flow Tracers. J Nucl Med. 1987;28:481–486. [PubMed] [Google Scholar]
- [89].Limburg M, van Royen EA, Hijdra A, Verbeeten B. rCBF-SPECT in Brain Infarction: When Does It Predict Outcome? J Nucl Med. 1991;32:382–387. [PubMed] [Google Scholar]
- [90].van Royen EA, de Bruïne JF, Hill TC, Vyth A, Limburg M, Byse BL, O'Leary DH, de Jong JMBV, Hijdra A, van der Schoot JB. Cerebral Blood Flow Imaging with Thallium-201 Diethyldithiocarbamate SPECT. J Nucl Med. 1987;28:178–183. [PubMed] [Google Scholar]
- [91].Sharp PF, Smith FW, Gemmell HG, Lyall D, Evans NTS, Gvozdanovic D, Davidson J, Tyrell DA, Pickett RD, Neirinckx RD. Technetium-99m HM-PAO Stereoisomers as Potential Agents for Imaging Regional Cerebral Blood Flow: Human Volunteer Studies. J Nucl Med. 1986;27:171–177. [PubMed] [Google Scholar]
- [92].Li H, Lai CS, Wu J, Ho PC, de Vos D, Tiekink ERT. Cytotoxicity, qualitative structure-activity relationship (QSAR), and anti-tumor activity of bismuth dithiocarbamate complexes. J Inorg Biochem. 2007;101:809–816. doi: 10.1016/j.jinorgbio.2007.01.010. [DOI] [PubMed] [Google Scholar]
- [93].Yang N, Sun H. Biocoordination chemistry of bismuth: Recent advances. Coord Chem Rev. 2007;251:2354–2366. [Google Scholar]
- [94].Hassfjell S, Brechbiel MW. The Development of the α-Particle Emitting Radionuclides 212Bi and 213Bi, and Their Decay Chain Related Radionuclides, for Therapeutic Applications. Chem Rev. 2001;101:2019–2036. doi: 10.1021/cr000118y. [DOI] [PubMed] [Google Scholar]
- [95].Schmaljohann J, Karanikas G, Sinzinger H. Synthesis of cobalt-55/57-complexes for radiolabelling of platelets as a potential PET imaging agent. J Labelled Compd Radiopharm. 2001;44:395–403. [Google Scholar]
- [96].De Reuck J, Santens P, Strijckmans K, Lemahieu I. Cobalt-55 positron emission tomography in vascular dementia: significance of white matter changes. J Neurol Sci. 2001;193:1–6. doi: 10.1016/s0022-510x(01)00606-2. [DOI] [PubMed] [Google Scholar]
- [97].Ferreira CL, Lapi S, Steele J, Green DE, Ruth TJ, Adam MJ, Orvig C. 55Cobalt complexes with pendant carbohydrates as potential PET imaging agents. Appl Rad Isot. 2007;65:1303–1308. doi: 10.1016/j.apradiso.2007.06.003. [DOI] [PubMed] [Google Scholar]
- [98].Brihaye C, Guillaume M, Lavi N, Cogneau M. Development of a Reliable Hg-195m → Au-195m Generator for the Production of Au-195m, a Short-lived Nuclide for Vascular Imaging. J Nucl Med. 1982;23:1114–1120. [PubMed] [Google Scholar]
- [99].Tárkányi F, Hermanne A, Takács S, Shubin YN, Dityuk AI. Cross sections for production of the therapeutic radioisotopes 198Au and 199Au in proton and deuteron induced reactions on 198Pt. Radiochim Acta. 2004;92:223–228. [Google Scholar]
- [100].Kannan R, Zambre A, Chanda N, Kulkarni R, Shukla R, Katti K, Upendran A, Cutler C, Boote E, Katti KV. Functionalized radioactive gold nanoparticles in tumor therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011 doi: 10.1002/wnan.161. [DOI] [PubMed] [Google Scholar]
- [101].Molho P, Verrier P, Stieltjes N, Schacher JM, Ounnoughène N, Vassilieff D, Menkes CJ, Sultan Y. A retrospective study on chemical and radioactive synovectomy in severe haemophilia patients with recurrent haemarthrosis. Haemophilia. 1999;5:115–123. doi: 10.1046/j.1365-2516.1999.00287.x. [DOI] [PubMed] [Google Scholar]
- [102].Ronconi L, Giovagnini L, Marzano C, Bettìo F, Graziani R, Pilloni G, Fregona D. Gold Dithiocarbamate Derivatives as Potential Antineoplastic Agents: Design, Spectroscopic Properties, and in Vitro Antitumor Activity. Inorg Chem. 2005;44:1867–1881. doi: 10.1021/ic048260v. [DOI] [PubMed] [Google Scholar]
- [103].Ronconi L, Marzano C, Zanello P, Corsini M, Miolo G, Maccà C, Trevisan A, Fregona D. Gold(III) Dithiocarbamate Derivatives for the Treatment of Cancer: Solution Chemistry, DNA Binding, and Hemolytic Properties. J Med Chem. 2006;49:1648–1657. doi: 10.1021/jm0509288. [DOI] [PubMed] [Google Scholar]