Legionella pneumophila enters cells in a vacuole derived from the plasma membrane, which then sequesters vesicles from the ER in order to support parasite growth and immune evasion. Arasaki et al. now reveal that the Legionella effector DrrA recruits components of the exocyst to promote tethering of host vesicles with the LCV.
Abstract
During the initial stage of infection, Legionella pneumophila secretes effectors that promote the fusion of endoplasmic reticulum (ER)–derived vesicles with the Legionella-containing vacuole (LCV). This fusion leads to a remodeling of the plasma membrane (PM)–derived LCV into a specialized ER-like compartment that supports bacterial replication. Although the effector DrrA has been shown to activate the small GTPase Rab1, it remains unclear how DrrA promotes the tethering of host vesicles with the LCV. Here, we show that Sec5, Sec15, and perhaps Sec6, which are subunits of the exocyst that functions in the tethering of exocytic vesicles with the PM, are required for DrrA-mediated, ER-derived vesicle recruitment to the PM-derived LCV. These exocyst components were found to interact specifically with a complex containing DrrA, and the loss of Sec5 or Sec15 significantly suppressed the recruitment of ER-derived vesicles to the LCV and inhibited intracellular replication of Legionella. Importantly, Sec15 is recruited to the LCV, and Rab1 activation is necessary for this recruitment.
Introduction
Legionella pneumophila is an intracellular pathogen that subverts host membrane transport processes to create a specialized organelle that supports replication (Roy and Tilney, 2002). In nature, these bacteria replicate inside protozoan hosts, but when inhaled by humans, Legionella can replicate inside alveolar macrophages (Horwitz and Silverstein, 1980; Rowbotham, 1980). The cell biology of the vacuole biogenesis is conserved in these evolutionarily distinct hosts. After internalization, the pathogen-occupied vacuole avoids delivery to a late endosome–lysosome compartment (Horwitz, 1983). The plasma membrane (PM)–derived vacuole intimately associates with ER-derived vesicles that ultimately tether and fuse with the Legionella-containing vacuole (LCV; Tilney et al., 2001). As a result of this membrane remodeling, the LCV membrane is converted into an organelle that has similarities to an ER–Golgi intermediate compartment (ERGIC), which can fuse with the ER to create a vacuole that supports bacterial replication (Kagan and Roy, 2002).
To promote infection and replication, Legionella delivers roughly three hundred different bacterial proteins into the host cell cytosol using a type IV secretion system called the Dot/Icm, and these bacterial effectors modulate several host cell processes to promote intracellular replication (Nagai et al., 2002; Luo and Isberg, 2004; de Felipe et al., 2008; Hubber and Roy, 2010; Finsel and Hilbi, 2015). One effector that is involved in the recruitment of ER-derived vesicles to the LCV is DrrA (SidM), which is localized on the PM via its C-terminal phosphatidylinositol 4-phosphate (PI4P)–binding site and is a guanine nucleotide exchange factor (GEF) that activates the host GTPase Rab1 (Machner and Isberg, 2006; Murata et al., 2006; Brombacher et al., 2009). DrrA-mediated activation of Rab1 is sufficient to mediate the recruitment and fusion of host ER-derived vesicles with a PM-derived organelle (Arasaki et al., 2012); however, the tethering mechanism that underlies this association remains unclear. Our previous studies suggested that activated Rab1 on the PM-derived organelle recruits an unidentified host tethering factor that mediates the intimate association of ER-derived vesicles with the PM and promotes fusion by a SNARE-mediated process (Arasaki and Roy, 2010; Arasaki et al., 2012). Given that the exocyst is implicated in tethering vesicles to the PM, we hypothesized that it may be needed by Legionella to remodel the LCV. The exocyst is composed of eight evolutionally conserved subunits consisting of Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84, and this complex functions as a tether that mediates fusion between the exocytic vesicles and the PM (Orlando and Guo, 2009). Although exocyst subunits are fully assembled in yeast (Heider et al., 2016), subcomplexes appear to be present in other organisms including mammals (Moskalenko et al., 2003; Bodemann et al., 2011) and Drosophila melanogaster (Beronja et al., 2005; Mehta et al., 2005). Here, we present evidence that some components of the exocyst are used by Legionella to achieve noncanonical fusion of ER-derived vesicles with the PM-derived vacuole.
Results and discussion
Several exocyst components are important for DrrA-mediated recruitment of ER-derived vesicles to the PM
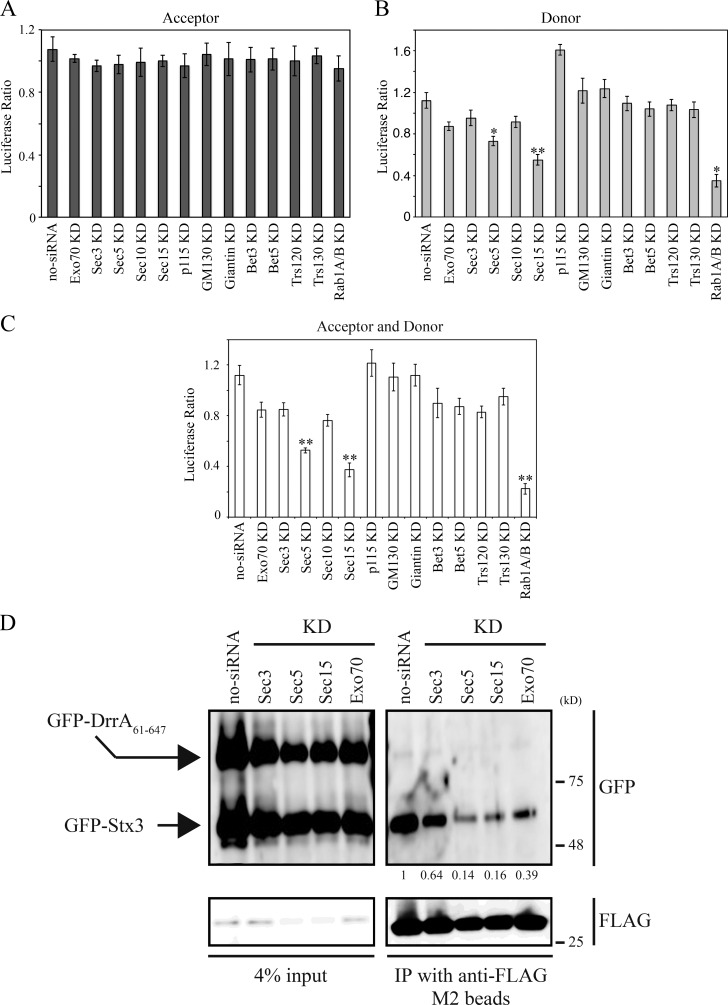
To examine the possibility that a PM tether is responsible for the DrrA–Rab1-mediated association of ER-derived vesicles with the PM, we performed a vesicle recruitment assay that we established in our previous work (Arasaki et al., 2012). In this assay, digitonin-permeabilized cells expressing GFP-DrrA61–647 or untransfected cells incubated with recombinant His6-DrrA were prepared as “acceptor cells.” The acceptor cells were then incubated with GTP and postnuclear supernatant (PNS) fraction from donor cells expressing a luminal ER marker (Luciferase-KDEL) and a v-SNARE (3x-FLAG-Sec22b), the latter of which is localized on the ER-derived vesicles. The efficiency of recruitment of ER-derived vesicles to the PM was determined by measuring luciferase activity or the association of 3x-FLAG-Sec22b with Stx3. A graphic image of this assay is represented in Fig. S1 A. If a tether implicated in this reaction is eliminated, recruitment of vesicles containing Luciferase-KDEL and 3x-FLAG-Sec22b to the PM is significantly blocked (Fig. S1 A, perturbation of step III). To examine whether the function of the exocyst is required for this tethering reaction, we performed siRNA-mediated silencing of several subunits of the exocyst (Fig. S1, B and C). Because Legionella subverts the early secretory pathway, we also analyzed the effect of silencing of tethers that are involved in the early secretory pathway such as p115, GM130, Giantin, Bet3, Bet5, Trs120, and Trs130 (Fig. S1, B and C).
Silencing of host tethers in the acceptor cells did not significantly affect the DrrA–Rab1-mediated vesicle recruitment (Fig. 1 A). In contrast, when host tethers in the donor cells were silenced before the recruitment assay, a strong defect in tethering of ER-derived vesicles with the PM was observed in cells silenced for Sec5 or Sec15, both of which are components of the exocyst (Orlando and Guo, 2009; Fig. 1 B). Moderate suppression of ER-derived vesicle recruitment to the PM was also detected in cells silenced for Exo70, Sec3, or Sec10 (other subunits of the exocyst; Fig. 1 B). Silencing of host tethers in the acceptor cells in addition to the donor cells did not have an additive effect (Fig. 1 C). It should be noted that silencing of components of the TRAPP complex (Bet3, Bet5, Trs120, and Trs130; Sacher et al., 2008), which is the GEF for the host Rab1, or large coiled-coil tethers that participate in tethering between ER-derived vesicles and ERGIC/Golgi with Rab1 (p115, GM130, and Giantin; Goud and Gleeson, 2010) had little, if any, effect on the tethering reaction.
Figure 1.
Loss of Sec5 or Sec15 inhibits the DrrA-mediated recruitment of ER-derived vesicles to the PM. (A–C) Acceptor (A), donor (B), or both acceptor and donor (C) cells were transfected with or without siRNA targeting the indicated proteins. 48 h after transfection, donor cells were transfected with a plasmid encoding Luciferase-KDEL and incubated for 24 h. An ER-derived vesicle recruitment assay using semi-intact cells and recombinant His6-DrrA was conducted as described previously (Arasaki et al., 2012). Values are the mean ± SD (n = 3). *P < 0.05 and **P < 0.01 compared with no-siRNA. (D) HEK293-FcγRII cells (acceptor) and 3x-FLAG-Sec22b–expressing HEK293-FcγRII cells (donor) were transfected with siRNA targeting the indicated proteins. 48 h after transfection, acceptor cells were additionally transfected with plasmids encoding GFP-DrrA61–647 and GFP-Stx3 and incubated for 24 h, and then the cells were permeabilized. The permeabilized cells were incubated with vesicles containing 3x-FLAG-Sec22b prepared from the donor cells, and protein complexes were precipitated using anti-FLAG M2 agarose. The precipitated proteins were analyzed by Western blotting using antibodies against GFP and FLAG. Values below the GFP strip represent the average of the GFP/FLAG intensity ratio (n = 3) normalized to that in no-siRNA. IP, immunoprecipitation; KD, knockdown.
Previous data demonstrated that Legionella promotes formation of a noncanonical SNARE complex consisting of the v-SNARE Sec22b on ER-derived vesicles and t-SNAREs that consist of PM-localized syntaxins (Stx2, 3, or 4) and SNAP23 on the LCV membrane (Arasaki and Roy, 2010). Because in vitro studies have shown that DrrA-mediated activation of Rab1 is sufficient to promote formation of this SNARE complex (Arasaki et al., 2012), we investigated whether Sec5 and Sec15 are required to promote DrrA-mediated SNARE assembly by evaluating functional association of Sec22b with a complex containing Stx3. We first confirmed that the genes encoding components of the exocyst including Sec3, Sec5, Sec15, and Exo70 or other tethering proteins were silenced by individual siRNAs (Fig. S1, B and C), and silencing these genes did not affect protein transport from the ER to Golgi (Fig. S1 D). Next, acceptor cells expressing GFP-Stx3 and GFP-DrrA61–647, the latter of which is a nontoxic DrrA derivative that lacks the N-terminal region of Rab1 AMPylation domain but retains the GEF and PI4P-binding domains (Murata et al., 2006; Müller et al., 2010), were silenced for Sec3, Sec5, Sec15, or Exo70, permeabilized, and incubated with a PNS fraction containing ER-derived vesicles from 3x-FLAG-Sec22b–expressing donor cells in which Sec3, Sec5, Sec15, or Exo70 was silenced. The formation of a functional SNARE complex was determined using immunoprecipitation to evaluate the association of 3x-FLAG-Sec22b on the ER-derived vesicles from the donor cells with GFP-Stx3 in the acceptor cells. The association of GFP-Stx3 with 3x-FLAG-Sec22b was markedly decreased when either Sec5 or Sec15 was silenced (Fig. 1 D).
Several exocyst components are associated with Stx3 and DrrA
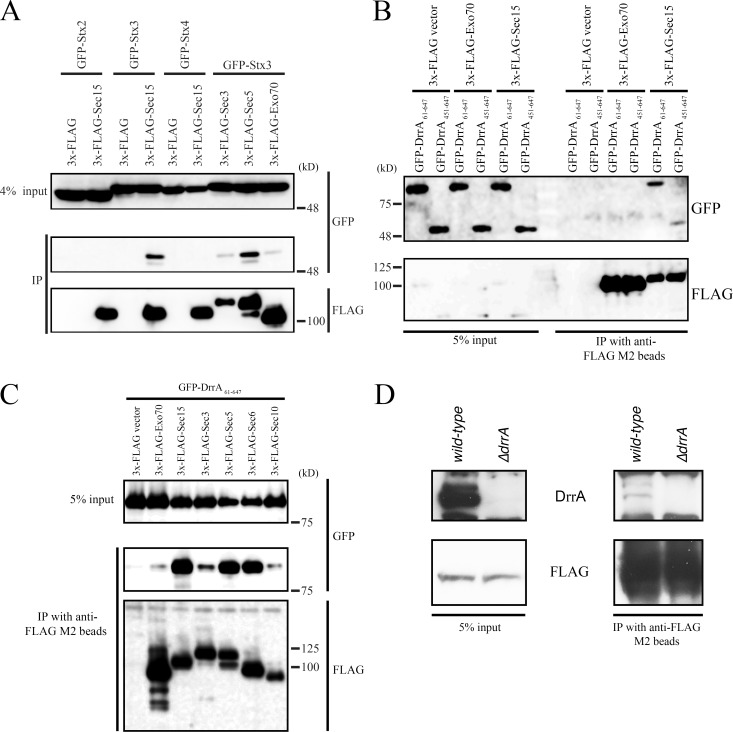
A previous study revealed that Sec6 and Sec8 interact with the neuronal PM-localized t-SNARE, Stx1 (Hsu et al., 1996). Having established that the binding between Stx3 and Sec22b is dependent on Sec5 and Sec15 in the recruitment assay (Fig. 1 D), we investigated whether Stx3 is contained in a complex with these exocyst components. Coexpression experiments revealed that Sec15 as well as Sec5 specifically interact with Stx3 and not with other PM-localized syntaxins, such as Stx2 and Stx 4 (Fig. 2 A). Because DrrA has been shown to form a complex with Stx3 (Arasaki et al., 2012), DrrA might also be a component of a complex containing Sec15 and Stx3. This was tested using a truncated DrrA construct (GFP-DrrA61–647) protein and using the truncated GFP-DrrA451–647 protein that contains the C-terminal PI4P-binding determinant that is sufficient for interaction with Stx3 but is deficient in both AMPylation and Rab1 GEF activity (Arasaki et al., 2012). These GFP-DrrA fusion proteins were produced ectopically in host cells along with 3x-FLAG-Exo70 or Sec15. 3x-FLAG-Sec15 was coprecipitated with the GEF domain–containing GFP-DrrA61–647 protein, but much less with the GEF domain–deficient GFP-DrrA451–647 protein (Fig. 2 B), whereas neither of these truncated DrrA proteins coprecipitated with 3x-FLAG-Exo70. The GFP-DrrA61–647 protein also did not coimmunoprecipitate with the Golgi tethering protein p115 or with an ERGIC/Golgi tether Bet3 (Fig. S2 A), which are both recruited to membranes by active Rab1. Furthermore, other Legionella effector proteins (Lpg2603 and Lpg1101) having a PI4P-binding domain that is closely related to the PI4P-binding domain in DrrA (Hubber et al., 2014) did not coprecipitate with 3x-FLAG-Sec15 (Fig. S2 B).
Figure 2.
Legionella effector DrrA interacts with the exocyst in a component-specific manner. (A) HEK293-FcγRII cells were cotransfected with plasmids encoding 3x-FLAG or 3x-FLAG-exocyst components and GFP-Stx2, 3, or 4. 24 h after transfection, cell lysates were prepared and immunoprecipitated (IP). (B) HEK293-FcγRII cells were cotransfected with plasmids encoding 3x-FLAG, 3x-FLAG-Exo70 or -Sec15, and GFP-DrrA61–647 or -DrrA451–647. 24 h after transfection, cell lysates were prepared and immunoprecipitated. (C) HEK293-FcγRII cells were cotransfected with plasmids encoding GFP-DrrA61–647 and 3x-FLAG constructs. 24 h after transfection, cell lysates were prepared and immunoprecipitated. (D) HEK293-FcγRII cells were transfected with a plasmid encoding 3x-FLAG-Sec15. 24 h after transfection, cells were infected with wild-type Legionella or ΔdrrA mutant strain for 1 h at MOI 50. After infection, cell lysates were prepared and immunoprecipitated.
We next determined the specificity of the interaction of DrrA61–647 with components of the exocyst. GFP-DrrA61–647 also coprecipitated with 3x-FLAG-Sec5 and Sec6 but scarcely with 3x-FLAG-Sec3 or Sec10 (Fig. 2 C). Importantly, a small but significant amount of endogenous DrrA protein translocated into host cells during infection by Legionella was detected in association with 3x-FLAG-Sec15 (Fig. 2 D). These results suggest that some but not all components of the exocyst are members of a complex containing DrrA-Rab1-Stx3 on the LCV.
Sec15 is used for LCV remodeling and Legionella intracellular replication
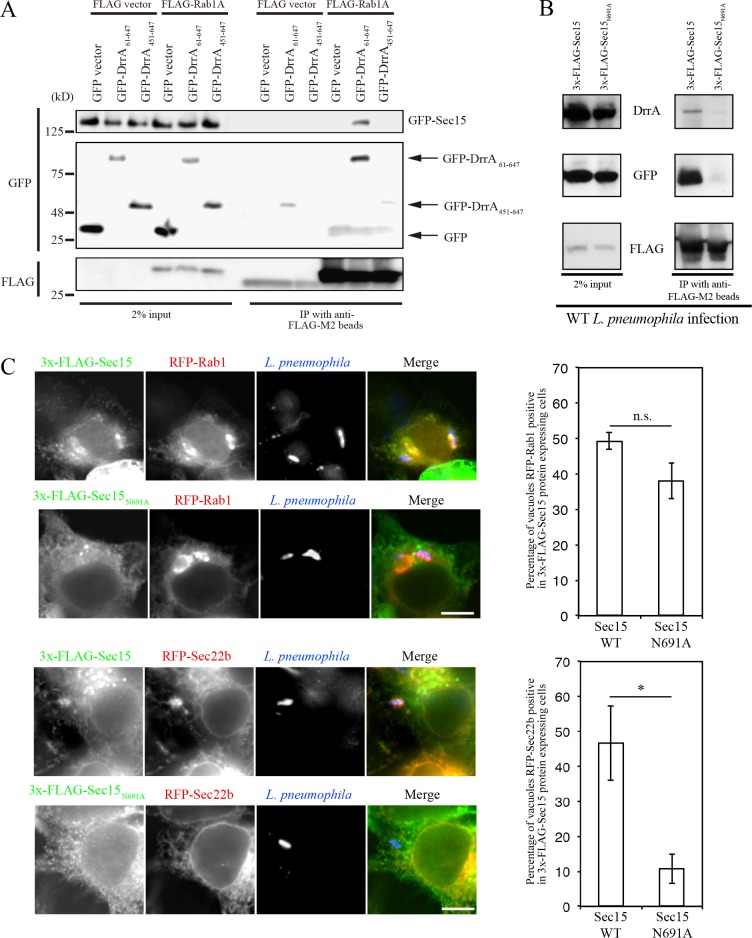
Because Sec15 and Sec5 are required for the DrrA-directed tethering of the ER-derived vesicles to the PM (Fig. 1), DrrA-mediated activation of Rab1 on the LCV might subvert these exocyst components to promote the tethering and fusion of ER-derived vesicles with a PM-derived organelle. Given that Sec15 is an effector of Rab11 that promotes fusion of secretory vesicles with the PM in the exocytic transport pathway (Zhang et al., 2004), it is tempting to speculate that Sec15 is an effector of Rab1, which is activated by DrrA on the PM. This idea was tested by immunoprecipitation showing that Sec15 coprecipitated with Rab1 upon expression of GEF domain–containing DrrA61–647, but not GEF domain–deficient DrrA451–647 (Fig. 3 A). Next, we investigated at which stage Sec15 functions in LCV remodeling. We used a Sec15 variant (Sec15N691A; mammalian N691 residue corresponds to N659 in Drosophila Sec15) that was reported not to bind to Rab11 (Wu et al., 2005). As reported previously, the association of GFP-Rab11 with Sec15N691A was significantly reduced in comparison with wild-type Sec15 (Fig. S2 C). Importantly, this mutation interfered with the association of GFP-Rab1 and the bacteria-secreted DrrA with Sec15 (Fig. 3 B).
Figure 3.
Expression of mutant Sec15 inhibits recruitment of Sec22b to the LCV. (A) HEK293-FcγRII cells were cotransfected with plasmids encoding GFP, GFP-DrrA61–647 or -DrrA451–647, and FLAG or FLAG-Rab1A. 24 h after transfection, cell lysates were prepared and immunoprecipitated (IP). (B) HEK293-FcγRII cells were transfected with plasmids encoding 3x-FLAG-Sec15 (wild-type) or -Sec15N691A and GFP-Rab1. 24 h after transfection, cells were infected with wild-type Legionella for 1 h at MOI 50. After infection, cell lysates were prepared and immunoprecipitated. (C) HEK293-FcγRII cells were cotransfected with 3x-FLAG-Sec15 (wild-type) or -Sec15N691A and RFP-Rab1 or -Sec22b. 24 h after transfection, cells were infected with wild-type Legionella for 1 h at MOI 5, fixed, and stained with antibodies against FLAG and Legionella. Localization of RFP-Rab1 and RFP-Sec22b was assessed for the vacuoles containing wild-type Legionella. Values are the mean ± SD (n = 3, 50 vacuoles in each experiment). n.s., not significant. *P < 0.05 compared with wild-type. Bar, 10 µm.
We next examined whether the Sec15N691A variant interfered with the recruitment of ER-derived vesicles to the LCV during Legionella infection. In cells expressing wild-type Sec15, both Rab1 and Sec22b were localized to the LCV (Fig. 3 C, top and third rows). By contrast, in cells expressing the Sec15N691A variant, there was a defect in Sec22b recruitment to the LCV, although Rab1 recruitment was largely unaffected (Fig. 3 C, second and bottom rows), suggesting that Sec15 function is important for recruitment of ER-derived vesicles, but not Rab1, to the LCV.
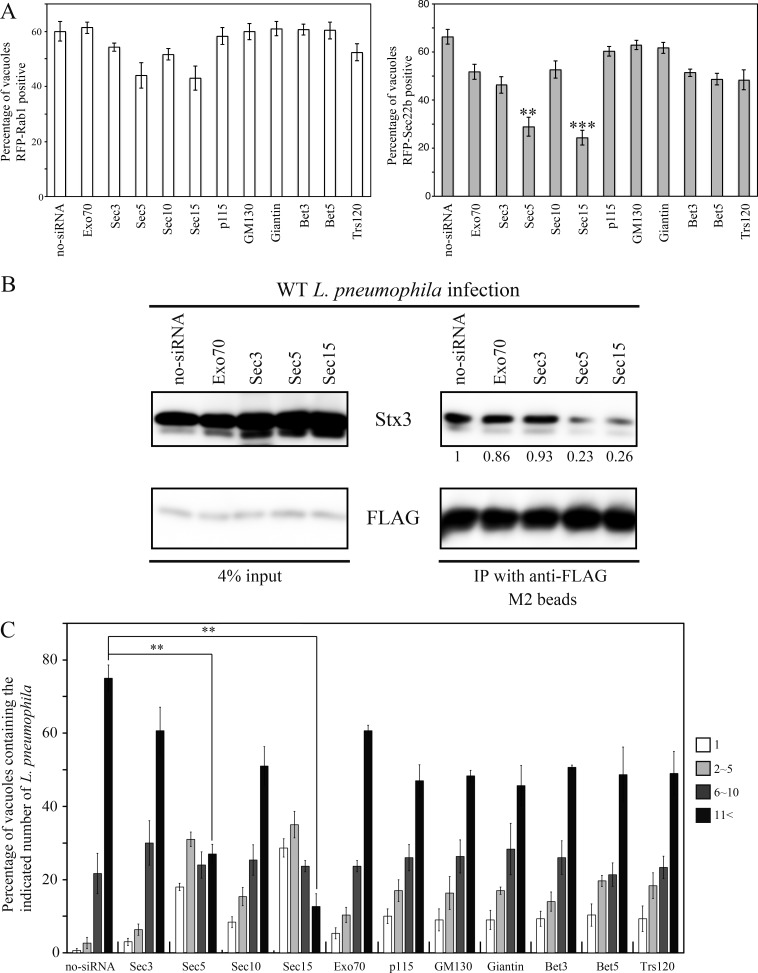
Gene silencing experiments were conducted to further examine the requirement of exocyst components for recruitment of Sec22b to the LCV. First, we confirmed that the silencing of a set of exocyst subunits did not affect the uptake of Legionella (Fig. S1 E). Silencing of Sec5 or Sec15 dramatically reduced the localization of Sec22b to the LCV, with only a minor effect on Rab1 localization (Fig. 4 A). Because recruitment of ER-derived vesicles to the LCV leads to formation of a functional SNARE complex containing Stx3 and Sec22b, we used coimmunoprecipitation to assess whether these exocyst components are needed for SNARE assembly. The levels of Stx3 that coprecipitated with 3x-FLAG-Sec22b were significantly reduced in Legionella-infected cells in which Sec5 or Sec15 had been silenced (Fig. 4 B). By contrast, depletion of Exo70 or Sec3 did not affect the complex formation between Stx3 and Sec22b during infection. Furthermore, the association of the LCV with the ER was suppressed in cells silenced for Sec5 or Sec15 but not Exo70 or Sec3 (Fig. S2 D), suggesting that Sec5 and Sec15 play a key role in remodeling and subsequent ER association of LCV. Additionally, Legionella intracellular replication was inhibited in cells silenced for Sec5 or Sec15 but not Exo70 or Sec3 (Fig. 4 C).
Figure 4.
Silencing of Sec5 or Sec15 suppresses intracellular replication of Legionella. (A) HEK293-FcγRII cells were transfected with or without siRNA targeting the indicated proteins. 48 h after transfection, cells were additionally transfected with RFP-Rab1 or RFP-Sec22b for 24 h. After transfection, cells were infected with wild-type Legionella for 1 h at MOI 5, and then localization of RFP-Rab1 and RFP-Sec22b was assessed for vacuoles containing wild-type Legionella. Values are the mean ± SD (n = 3, 100 vacuoles in each experiment). **P < 0.01 and ***P < 0.001 compared with no-siRNA. (B) 3x-FLAG-Sec22b–expressing HEK293-FcγRII cells were transfected with or without siRNA targeting indicated proteins. 72 h after transfection, cells were infected with wild-type Legionella for 1 h at MOI 50. After infection, cell lysates were prepared and immunoprecipitated (IP). Values below the GFP strip represent the average of the Stx3/FLAG intensity ratio (n = 3) normalized to that in no-siRNA. (C) HEK293-FcγRII cells were transfected with or without siRNA targeting the indicated proteins. 72 h after transfection, cells were infected with wild-type Legionella for 10 h at MOI 5. Intracellular replication of Legionella was assessed by counting bacteria residing in a single vacuole in the infected cells. The data are represented as the percentage of vacuoles containing 1 bacterium (white bars), 2–5 bacteria (light gray bars), 6–10 bacteria (dark gray bars), or >11 bacteria (black bars). Values are the mean ± SD (n = 3, 100 vacuoles in each experiment). **P < 0.01 compared with no-siRNA.
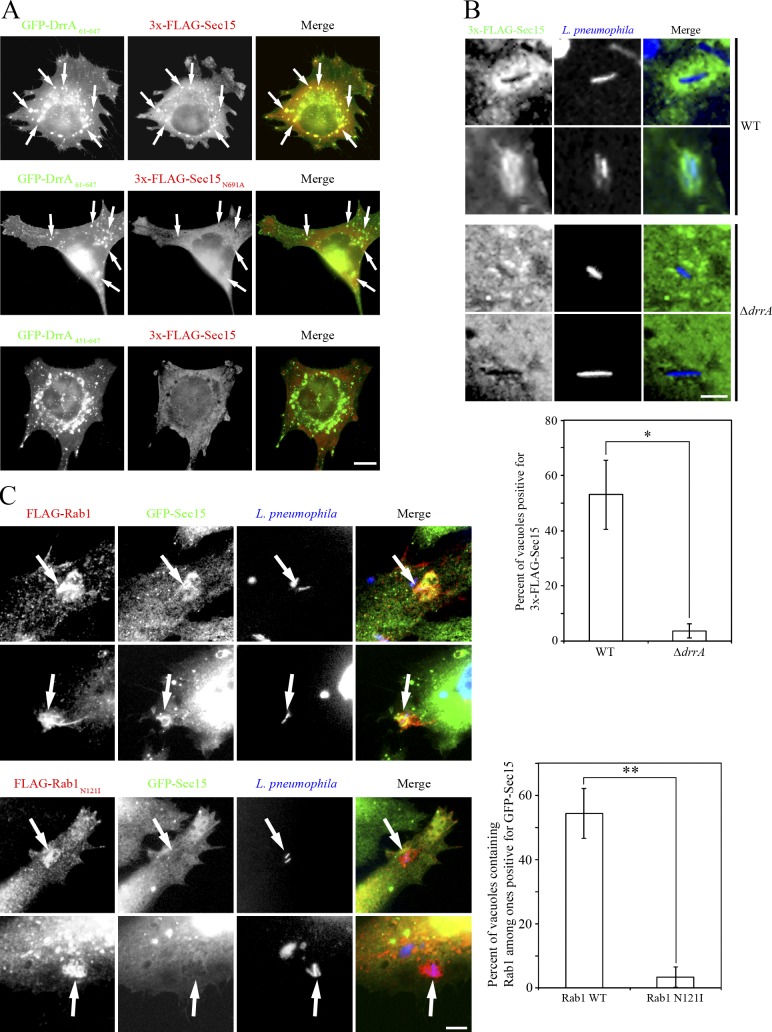
Redistribution of Sec15 to the LCV is dependent on DrrA-mediated Rab1 activation
Finally, because Rab proteins play a critical role in the recruitment of tethering proteins to the target membrane, we asked whether activation of Rab1 by DrrA is required for the recruitment of Sec15 to the LCV. To address this, we examined the colocalization of Sec15 and DrrA61–647. As shown in Fig. 5 A, wild-type Sec15 (top row) but not N691A mutant (middle row) significantly colocalized with the foci of DrrA61–647, whereas noncolocalization of wild-type Sec15 with the foci of DrrA451–647 was observed (bottom row). Furthermore, infection experiments demonstrated that a significant amount of Sec15 was present around the vacuoles containing wild-type (Fig. 5 B, top two rows) but not the isogenic ΔdrrA strain of Legionella (bottom two rows). Consistent with the interaction analysis (Fig. 2 C), Sec5 and Sec6, but not Exo70, Sec3, or Sec10, were recruited to the LCV (Fig. S2 E). Of note, Rab1 activation is required for the LCV distribution of Sec15 because a significant amount of Sec15 was detected on the LCV decorated with wild-type Rab1 (Fig. 5 C, top and second rows), but not dominant negative N121I mutant (Fig. 5 C, third and bottom rows). Rab8A has been implicated in exocyst-mediated tethering on the PM in Dictyostelium discoideum (Essid et al., 2012). Because it is recruited to the LCV in both Dictyostelium amoebae and macrophages (Urwyler et al., 2009; Hoffmann et al., 2014) and is a substrate of Rab1-GAP Legionella effector LepB (Mihai Gazdag et al., 2013), we examined whether Rab8A and also Rab11, a canonical regulator for Sec15, are involved in DrrA-mediated tethering. As shown in Fig. S3 A, Rab1A, but neither Rab8A nor Rab11, immunoprecipitated with GFP-DrrA61–647. Also, neither Rab8A nor Rab11 was colocalized with GFP-DrrA61–647 foci (Fig. S3 B). Moreover, silencing of Rab1A, but not Rab8A or Rab11, suppressed the Legionella-facilitated interaction of Sec22b with Stx3 (Fig. S3, C and D) and the redistribution of Sec15 to the GFP-DrrA61–647 foci (Fig. S3 E), implying that the DrrA–Rab1 complex specifically participates in the interaction of exocyst components with the LCV.
Figure 5.
DrrA-mediated Rab1 activity is required for the recruitment of Sec15 to the LCV. (A) HeLa-FcγRII cells were cotransfected with GFP-DrrA61–647 or -DrrA451–647 and 3x-FLAG-Sec15 (wild-type) or -Sec15N691A. 24 h after transfection, cells were fixed and stained with an antibody against FLAG. Arrows indicate DrrA-positive structures. Bar, 5 µm. (B) HeLa-FcγRII cells were transfected with 3x-FLAG-Sec15. 24 h after transfection, cells were infected with wild-type Legionella or ΔdrrA mutant strain for 1 h at MOI 10 and fixed, and extracellular Legionella were stained. After staining, cells were permeabilized and stained with an antibody against FLAG. Intracellular Legionella were detected by Hoechst 33342. Bar, 1 µm. Values are the mean ± SD (n = 3, 100 vacuoles in each experiment). *P < 0.05 compared with ΔdrrA. (C) HeLa-FcγRII cells were cotransfected with GFP-Sec15 and FLAG-Rab1 wild-type or -Rab1N121I. 24 h after transfection, cells were infected with wild-type Legionella for 1 h at MOI 10, fixed, and stained with an antibody against FLAG. Legionella were detected by Hoechst 33342. Bar, 2 µm. Values are the mean ± SD (n = 3, 100 vacuoles positive for Rab1 in each experiment). **P < 0.01 compared with Rab1N121I.
In this work, we reveal that the components of the exocyst containing Sec5, Sec6, and Sec15 play a critical role not only in tethering between ER-derived vesicles and LCV but also in the intracellular replication of Legionella. We demonstrate that DrrA-mediated activation of Rab1 promotes the assembly of a complex on the PM-derived LCV that includes components of the exocyst and that these exocyst components then provide an important tethering function that mediates the recruitment of ER-derived vesicles to the LCV.
Intriguingly, Legionella appears not to use the entire exocyst but requires limited components of the exocyst that include Sec5, Sec6, and Sec15 to facilitate the remodeling of LCV. This implies that these components play distinct roles in regulating membrane transport processes. Consistent with this hypothesis, it was shown previously that exocyst components containing Sec5, Sec6, and Sec15 mediate the trafficking of E-cadherin from recycling endosomes to the PM in Drosophila (Langevin et al., 2005). Furthermore, previous work demonstrated that subcomplexes of exocyst including a Sec5-containing subcomplex and an Exo84-containing subcomplex function to suppress and induce autophagy, respectively (Bodemann et al., 2011). Of note is that DrrA binds Sec5, 6, and 15 (Fig. 2 C), all of which are recruited to the LCV (Figs. 5 and S2 E). Therefore, each subunit, not as a subcomplex, may be recruited to the LCV through binding to DrrA.
Previous studies have shown that DrrA-mediated activation of Rab1 is sufficient to promote fusion of ER-derived vesicles with a PM-derived LCV (Arasaki et al., 2012); however, Legionella mutants that lack DrrA are still able to promote remodeling of the LCV by ER-derived vesicles by a process that is dependent on Sec22b (Arasaki et al., 2012). This implies that other effectors encoded by Legionella are capable of “short-circuiting” this pathway by subverting downstream host components important for Sec22b-mediated fusion of ER-derived vesicles with the PM. Our infection studies indicate that Sec5, Sec6, and Sec15 are important for the processes of remodeling of the LCV by Sec22b-positive, ER-derived vesicles, which means that Legionella has likely evolved multiple mechanisms to subvert the tethering functions of the exocyst components to promote the fusion of ER-derived vesicles with the LCV. A likely possibility is that among the hundreds of Legionella effectors of unknown function there is an effector capable of directly recruiting the exocyst components to the LCV to promote vacuole remodeling. Thus, the exocyst represents a host factor that is critical for successful host infection by Legionella, which could be a target for subversion by a Legionella effector of unknown function.
Materials and methods
Cell culture, siRNA, transfection, RT-PCR, and bacterial strain
Maintenance of HEK293-FcγRII and HEK293-FcγRII 3x-FLAG-Sec22b cells was described previously (Arasaki and Roy, 2010). RNA duplexes targeting Exo70, Sec3, Sec5, Sec10, Sec15, p115, GM130, Giantin, Bet3, Bet5, Trs120, and Trs130 were purchased from Dharmacon as siRNAplusSmart Pools. siRNA oligonucleotides against Rab8A (5′-GACAAGUUUCCAAGGAACG-3′) and Rab11 (5′-GAGCUUUUGCAGAAAAGAA-3′ and 5′-CUUGGAUUCCACUAACGUA-3′) were purchased from Japan Bio Services. Transfection of plasmid DNA or siRNA was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s recommendation. For RT-PCR, RNA was prepared using an RNeasy Mini Kit (Qiagen), and cDNA was synthesized with the SuperScript III reverse transcription (Invitrogen) primed by oligo(dT)15. Each cDNA was amplified by PCR with the following primers: β-actin, 5′-GGACTTCGAGCAAGAGATGG-3′ and 5′-AGCACTGTGTTGGCGTACAG-3′; Sec10, 5′-CACCAATCTTTCCAGCAAGC-3′ and 5′-GCAAGTCCTGTTTCCAAAGC-3′; p115, 5′-TGCTTTTGAAAATGCTTTCG-3′ and 5′-ACTACAATTGCCGGTCTTGG-3′; GM130, 5′-CAGCAGAATCAGCAGCTACG-3′ and 5′-CTCTCCAGAAAGCTGGATGC-3′; Giantin, 5′-GTGGAAAGCCAAGTTTCTGC-3′ and 5′-CCATTTCTTGCTCAGCTTCC-3′; Bet3, 5′-CTGAGCTCTTCACCCTGACC-3′ and 5′-ATCCGGATTTCTGTCACACC-3′; Bet5, 5′-TACCTGTTTGACCGGAATGG-3′ and 5′-GGAGAAGAAGGGCAGAGAGC-3′; Trs120, 5′-AATTCAGAAACGGAGCATGG-3′ and 5′-CCTGATCCGACAAGAAGTCC-3′; and Trs130, 5′-CAGCCTCTTAGCCAGTGACC-3′ and 5′-AATCCCAGTCGTGTTCAAGG-3′. Growth of Legionella strains Lp01, the dotA mutant strain CR58, and the ΔdrrA mutant was described previously (Murata et al., 2006).
Antibodies
Rabbit polyclonal antibodies to GFP were purchased from Invitrogen (A-6455). The following antibodies were purchased from Sigma-Aldrich: mouse monoclonal FLAG (F3156), rabbit polyclonal FLAG (F7425), and α-tubulin (T6074). The following antibodies were purchased from Abcam: Sec3 (ab118798), Sec5 (ab64166), Sec15 (ab105075), Exo70 (ab95981), and Stx3 (ab86669). Mouse monoclonal antibodies to Calnexin (610523) and Rab11 (610656) were purchased from BD Bioscience. Mouse monoclonal antibody to Rab8A was purchased from Proteintech (55296-1-AP). Rabbit and mouse polyclonal antibodies to Legionella and rabbit polyclonal antibody to DrrA were prepared in this laboratory (Murata et al., 2006; Arasaki and Roy, 2010).
Preparation of permeabilized cells and PNS fractions
HEK293-FcγRII cells were grown on poly-l-lysine (MW 75,000–150,000; Sigma-Aldrich)–coated tissue culture plates. Cells were washed with permeabilization buffer (125 mM KOAc, 2.5 mM Mg(OAc)2, 25 mM Hepes-KOH, pH 7.2, 1 mg/ml glucose, and 1 mM DTT) and treated with 30 µg/ml digitonin in permeabilization buffer for 5 min at room temperature. After treatment, permeabilized cells were washed with permeabilization buffer. To prepare PNS fractions, cells were resuspended in homogenization buffer (125 mM KOAc, 2.5 mM Mg(OAc)2, 25 mM Hepes-KOH, pH 7.2, 250 mM sucrose, 1 mM DTT, and protease inhibitors) and disrupted using a ball bearing homogenizer. Homogenized cells were centrifuged at 1,000 g for 5 min to remove unbroken cells and cell debris, and the resulting supernatant was used as the PNS fraction.
Luciferase-KDEL recruitment assay
HEK293-FcγRII cells grown on poly-l-lysine–coated 24-well plates (105 cells/well) were permeabilized as described previously (Arasaki et al., 2012). Permeabilized cells were incubated with or without 3 µg purified His-DrrA for 1 h at room temperature. Cells were washed, and 100 µl of a PNS fraction from HEK293-FcγRII cells was added with GTP at 0.5 mM final concentration. Cells were incubated for 1 h at room temperature and then washed extensively. To assay tethering, 100 µl lysis buffer (100 mM NaCl, 1 mM MgCl2, 20 mM Hepes-KOH, pH 7.2, 1% Triton X-100, and protease inhibitors) was added to the cells to liberate Luciferase-KDEL from vesicles, and activity was measured using a Luciferase assay kit (New England Biolabs).
Infection, immunoprecipitation, and immunofluorescence analysis
Infection of HEK293-FcγRII cells with Legionella and generation of cell lysates for immunoprecipitation studies were conducted as described previously (Arasaki and Roy, 2010). For immunoprecipitation studies, lysates were incubated with anti-FLAG M2 agarose (Sigma-Aldrich) for 1 h at 4°C. After incubation, beads were washed extensively, and 3x-FLAG fusion proteins were eluted using 3x-FLAG peptide (Sigma-Aldrich; final concentration of 100 µg/ml). For immunofluorescence microscopy, cells were fixed with 4% PFA for 20 min at room temperature and permeabilized with 0.2% Triton X-100 for 15 min at room temperature. In the Legionella infection experiments, extracellular bacteria were detected using rabbit or mouse anti-Legionella antibodies and stained. After staining, cells were permeabilized and further stained for host proteins and intracellular bacteria.
Quantification and statistics
The results from each experiment were averaged and expressed as the mean with SD and analyzed by a paired Student’s t test. In the Luciferase-KDEL recruitment assay, 105 cells were analyzed. The recruitment of Rab1 or Sec22b to the LCV in wild-type or mutant Sec15–expressing cells was measured three times. The recruitment of Rab1 or Sec22b to the LCV in cells with silenced tethering factors and the number of Legionella in replicative vacuoles were measured three times. The recruitment of 3x-FLAG-Sec15 to the vacuole containing wild-type or ΔdrrA mutant Legionella was measured three times. The recruitment of GFP-Sec15 to the LCV decorated by FLAG-Rab1 wild-type or FLAG-Rab1N121I was measured three times.
Online supplemental material
Fig. S1 shows a scheme of the semi-intact assay and siRNA efficiency. Fig. S2 shows the specificity of the Sec15–DrrA interaction and of the exocyst components in LCV recruitment as well as LCV–ER association. Fig. S3 shows that the DrrA–Rab1 complex specifically facilitates exocyst-dependent tethering.
Supplementary Material
Acknowledgments
We thank Dr. Shaeri Mukherjee, Dr. Stanimier Ivanov, and Dr. Andree Hubber for helpful discussions and comments in manuscript preparation.
This work was supported by the National Institutes of Health grant R01AI041699 (to C.R. Roy) and in part by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research 24790425 and 26713016 (to K. Arasaki) and 25291029 (to M. Tagaya); the MEXT-Supported Program for the Strategic Research Foundation at Private Universities (to K. Arasaki and M. Tagaya) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and the Uehara Memorial Foundation.
The authors declare no competing financial interests.
Author contributions: This study was directed by K. Arasaki and C.R. Roy and was conceived and designed by K. Arasaki, H. Kimura, M. Tagaya, and C.R. Roy. K. Arasaki and H. Kimura performed experiments.
References
- Arasaki K., and Roy C.R.. 2010. Legionella pneumophila promotes functional interactions between plasma membrane syntaxins and Sec22b. Traffic. 11:587–600. 10.1111/j.1600-0854.2010.01050.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasaki K., Toomre D.K., and Roy C.R.. 2012. The Legionella pneumophila effector DrrA is sufficient to stimulate SNARE-dependent membrane fusion. Cell Host Microbe. 11:46–57. 10.1016/j.chom.2011.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beronja S., Laprise P., Papoulas O., Pellikka M., Sisson J., and Tepass U.. 2005. Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells. J. Cell Biol. 169:635–646. 10.1083/jcb.200410081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodemann B.O., Orvedahl A., Cheng T., Ram R.R., Ou Y.H., Formstecher E., Maiti M., Hazelett C.C., Wauson E.M., Balakireva M., et al. 2011. RalB and the exocyst mediate the cellular starvation response by direct activation of autophagosome assembly. Cell. 144:253–267. 10.1016/j.cell.2010.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brombacher E., Urwyler S., Ragaz C., Weber S.S., Kami K., Overduin M., and Hilbi H.. 2009. Rab1 guanine nucleotide exchange factor SidM is a major phosphatidylinositol 4-phosphate-binding effector protein of Legionella pneumophila. J. Biol. Chem. 284:4846–4856. 10.1074/jbc.M807505200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Felipe K.S., Glover R.T., Charpentier X., Anderson O.R., Reyes M., Pericone C.D., and Shuman H.A.. 2008. Legionella eukaryotic-like type IV substrates interfere with organelle trafficking. PLoS Pathog. 4:e1000117 10.1371/journal.ppat.1000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essid M., Gopaldass N., Yoshida K., Merrifield C., and Soldati T.. 2012. Rab8a regulates the exocyst-mediated kiss-and-run discharge of the Dictyostelium contractile vacuole. Mol. Biol. Cell. 23:1267–1282. 10.1091/mbc.e11-06-0576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsel I., and Hilbi H.. 2015. Formation of a pathogen vacuole according to Legionella pneumophila: how to kill one bird with many stones. Cell. Microbiol. 17:935–950. 10.1111/cmi.12450 [DOI] [PubMed] [Google Scholar]
- Goud B., and Gleeson P.A.. 2010. TGN golgins, Rabs and cytoskeleton: regulating the Golgi trafficking highways. Trends Cell Biol. 20:329–336. 10.1016/j.tcb.2010.02.006 [DOI] [PubMed] [Google Scholar]
- Heider M.R., Gu M., Duffy C.M., Mirza A.M., Marcotte L.L., Walls A.C., Farrall N., Hakhverdyan Z., Field M.C., Rout M.P., et al. 2016. Subunit connectivity, assembly determinants and architecture of the yeast exocyst complex. Nat. Struct. Mol. Biol. 23:59–66. 10.1038/nsmb.3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C., Finsel I., Otto A., Pfaffinger G., Rothmeier E., Hecker M., Becher D., and Hilbi H.. 2014. Functional analysis of novel Rab GTPases identified in the proteome of purified Legionella-containing vacuoles from macrophages. Cell. Microbiol. 16:1034–1052. [DOI] [PubMed] [Google Scholar]
- Horwitz M.A. 1983. The Legionnaires’ disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J. Exp. Med. 158:2108–2126. 10.1084/jem.158.6.2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M.A., and Silverstein S.C.. 1980. Legionnaires’ disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J. Clin. Invest. 66:441–450. 10.1172/JCI109874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S.C., Ting A.E., Hazuka C.D., Davanger S., Kenny J.W., Kee Y., and Scheller R.H.. 1996. The mammalian brain rsec6/8 complex. Neuron. 17:1209–1219. 10.1016/S0896-6273(00)80251-2 [DOI] [PubMed] [Google Scholar]
- Hubber A., and Roy C.R.. 2010. Modulation of host cell function by Legionella pneumophila type IV effectors. Annu. Rev. Cell Dev. Biol. 26:261–283. 10.1146/annurev-cellbio-100109-104034 [DOI] [PubMed] [Google Scholar]
- Hubber A., Arasaki K., Nakatsu F., Hardiman C., Lambright D., De Camilli P., Nagai H., and Roy C.R.. 2014. The machinery at endoplasmic reticulum-plasma membrane contact sites contributes to spatial regulation of multiple Legionella effector proteins. PLoS Pathog. 10:e1004222 10.1371/journal.ppat.1004222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J.C., and Roy C.R.. 2002. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat. Cell Biol. 4:945–954. 10.1038/ncb883 [DOI] [PubMed] [Google Scholar]
- Langevin J., Morgan M.J., Sibarita J.B., Aresta S., Murthy M., Schwarz T., Camonis J., and Bellaïche Y.. 2005. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev. Cell. 9:365–376. 10.1016/j.devcel.2005.07.013 [DOI] [PubMed] [Google Scholar]
- Luo Z.Q., and Isberg R.R.. 2004. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc. Natl. Acad. Sci. USA. 101:841–846. 10.1073/pnas.0304916101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machner M.P., and Isberg R.R.. 2006. Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev. Cell. 11:47–56. 10.1016/j.devcel.2006.05.013 [DOI] [PubMed] [Google Scholar]
- Mehta S.Q., Hiesinger P.R., Beronja S., Zhai R.G., Schulze K.L., Verstreken P., Cao Y., Zhou Y., Tepass U., Crair M.C., and Bellen H.J.. 2005. Mutations in Drosophila sec15 reveal a function in neuronal targeting for a subset of exocyst components. Neuron. 46:219–232. 10.1016/j.neuron.2005.02.029 [DOI] [PubMed] [Google Scholar]
- Mihai Gazdag E., Streller A., Haneburger I., Hilbi H., Vetter I.R., Goody R.S., and Itzen A.. 2013. Mechanism of Rab1b deactivation by the Legionella pneumophila GAP LepB. EMBO Rep. 14:199–205. 10.1038/embor.2012.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalenko S., Tong C., Rosse C., Mirey G., Formstecher E., Daviet L., Camonis J., and White M.A.. 2003. Ral GTPases regulate exocyst assembly through dual subunit interactions. J. Biol. Chem. 278:51743–51748. 10.1074/jbc.M308702200 [DOI] [PubMed] [Google Scholar]
- Müller M.P., Peters H., Blümer J., Blankenfeldt W., Goody R.S., and Itzen A.. 2010. The Legionella effector protein DrrA AMPylates the membrane traffic regulator Rab1b. Science. 329:946–949. 10.1126/science.1192276 [DOI] [PubMed] [Google Scholar]
- Murata T., Delprato A., Ingmundson A., Toomre D.K., Lambright D.G., and Roy C.R.. 2006. The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat. Cell Biol. 8:971–977. 10.1038/ncb1463 [DOI] [PubMed] [Google Scholar]
- Nagai H., Kagan J.C., Zhu X., Kahn R.A., and Roy C.R.. 2002. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science. 295:679–682. 10.1126/science.1067025 [DOI] [PubMed] [Google Scholar]
- Orlando K., and Guo W.. 2009. Membrane organization and dynamics in cell polarity. Cold Spring Harb. Perspect. Biol. 1:a001321 10.1101/cshperspect.a001321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham T.J. 1980. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 33:1179–1183. 10.1136/jcp.33.12.1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy C.R., and Tilney L.G.. 2002. The road less traveled: transport of Legionella to the endoplasmic reticulum. J. Cell Biol. 158:415–419. 10.1083/jcb.200205011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher M., Kim Y.G., Lavie A., Oh B.H., and Segev N.. 2008. The TRAPP complex: insights into its architecture and function. Traffic. 9:2032–2042. 10.1111/j.1600-0854.2008.00833.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L.G., Harb O.S., Connelly P.S., Robinson C.G., and Roy C.R.. 2001. How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane. J. Cell Sci. 114:4637–4650. [DOI] [PubMed] [Google Scholar]
- Urwyler S., Nyfeler Y., Ragaz C., Lee H., Mueller L.N., Aebersold R., and Hilbi H.. 2009. Proteome analysis of Legionella vacuoles purified by magnetic immunoseparation reveals secretory and endosomal GTPases. Traffic. 10:76–87. 10.1111/j.1600-0854.2008.00851.x [DOI] [PubMed] [Google Scholar]
- Wu S., Mehta S.Q., Pichaud F., Bellen H.J., and Quiocho F.A.. 2005. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat. Struct. Mol. Biol. 12:879–885. 10.1038/nsmb987 [DOI] [PubMed] [Google Scholar]
- Zhang X.M., Ellis S., Sriratana A., Mitchell C.A., and Rowe T.. 2004. Sec15 is an effector for the Rab11 GTPase in mammalian cells. J. Biol. Chem. 279:43027–43034. 10.1074/jbc.M402264200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.