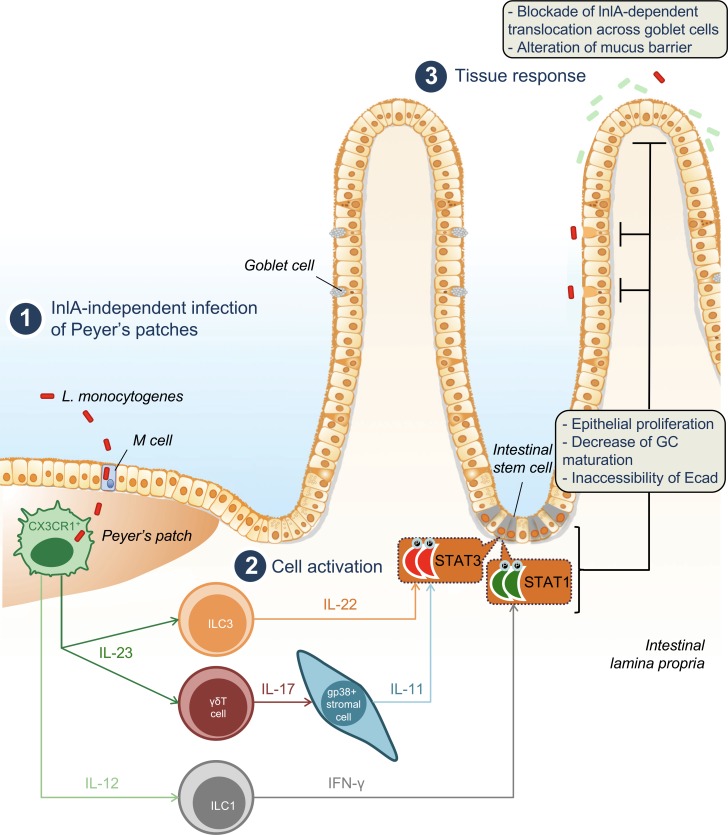
Listeria monocytogenes (Lm) crosses the intestinal villus barrier via goblet cells (GCs). Disson et al. show that Lm infection of Peyer’s patch myeloid cells signals to villus stromal cells, leading to a decrease of GCs expressing luminally accessible E-cadherin, thereby blocking villus infection while favoring colitis.
Abstract
The foodborne pathogen Listeria monocytogenes (Lm) crosses the intestinal villus epithelium via goblet cells (GCs) upon the interaction of Lm surface protein InlA with its receptor E-cadherin. Here, we show that Lm infection accelerates intestinal villus epithelium renewal while decreasing the number of GCs expressing luminally accessible E-cadherin, thereby locking Lm portal of entry. This novel innate immune response to an enteropathogen is triggered by the infection of Peyer’s patch CX3CR1+ cells and the ensuing production of IL-23. It requires STAT3 phosphorylation in epithelial cells in response to IL-22 and IL-11 expressed by lamina propria gp38+ stromal cells. Lm-induced IFN-γ signaling and STAT1 phosphorylation in epithelial cells is also critical for Lm-associated intestinal epithelium response. GC depletion also leads to a decrease in colon mucus barrier thickness, thereby increasing host susceptibility to colitis. This study unveils a novel innate immune response to an enteropathogen, which implicates gp38+ stromal cells and locks intestinal villus invasion, but favors colitis.
Graphical Abstract
Introduction
The intestinal epithelium directly interacts with the external environment and is constantly renewed by stem cells located in intestinal crypts (Barker, 2014). The maintenance of intestinal epithelial homeostasis is tightly regulated by pericryptal stromal cells (Stzepourginski et al., 2017) and the sensing of microbe-associated molecular patterns (MAMPs) from the microbiota (Rakoff-Nahoum et al., 2004; Sommer and Bäckhed, 2013).
Most invasive enteropathogens disrupt gut homeostasis: they can directly modulate epithelial proliferation by inhibiting cell cycle, as does Shigella flexnerii IpaB, which acts on Mad2L2 (Iwai et al., 2007), or promote it, as does the gastric carcinogen Helicobacter pylori (Mimuro et al., 2002). Salmonella enterica, S. flexnerii, and attaching and effacing bacteria such as Citrobacter rodentium, which are all associated with enteritis, lead to epithelial barrier disruption and intestinal inflammation (Sansonetti et al., 1999; Collins et al., 2014). C. rodentium–associated epithelial damage leads to MyD88-dependent epithelial proliferation (Gibson et al., 2008). In a Drosophila melanogaster model of intestinal infection, epithelial proliferation is the consequence of the immune oxidative burst and is JAK/STAT dependent (Buchon et al., 2009). This phenotype is mediated by signaling from the damaged epithelium to stem cells and amplified by visceral muscles (Buchon et al., 2013). In noninfectious models of dextran sodium sulfate (DSS)–induced colitis and methotrexate-induced damage to stem cells, intestinal epithelium wound healing depends on intestinal epithelial cell STAT3 activation by IL-22 (Pickert et al., 2009; Aparicio-Domingo et al., 2015). Upon C. rodentium infection, IL-23 expression by CX3CR1+ cells triggers IL-22 expression by type 3 innate lymphoid cells (ILCs; Longman et al., 2014; Aychek et al., 2015). It has also been shown that IL-22 acts on enterocytes in a STAT3-dependent manner, inducing RegIIIβ and RegIIIγ expression (Zheng et al., 2008; Manta et al., 2013). Epithelial renewal upon infectious- and noninfectious-associated damages may therefore engage the same signaling.
The intestinal phase of listeriosis, a systemic infection caused by the foodborne pathogen Listeria monocytogenes (Lm), is mostly asymptomatic (Charlier et al., 2017), in line with the observation that Lm does not significantly alter the intestinal barrier integrity (Lecuit et al., 2007; Tsai et al., 2013). Lm has the ability to enter epithelial cells through interaction of its surface protein InlA with its receptor E-cadherin (Ecad). As InlA–Ecad interaction is species specific, we generated transgenic (hEcad) and knock-in (KIE16P) humanized Ecad mouse lines to study listeriosis in vivo (Lecuit et al., 2001; Disson et al., 2008). In humanized Ecad mice, Lm is rapidly transcytosed at the small intestinal level in an InlA–Ecad-dependent manner across goblet cells (GCs) expressing luminally accessible Ecad and released into the lamina propria (LP; Fig. S1 A; Lecuit et al., 2001; Nikitas et al., 2011). Lm is also transferred, albeit at a lower efficiency, through M cells in an InlA-independent manner at the Peyer’s patch (PP) level, the only route of infection in nonhumanized mice (Jensen et al., 1998; Chiba et al., 2011; Gessain et al., 2015). We have shown by transcriptomic analysis that the global intestinal host response to Lm is InlA independent and triggered by invasion of PPs (Fig. S1 A; Lecuit et al., 2007). It requires the expression of listeriolysin O (LLO; Lecuit et al., 2007), a major Lm virulence factor involved in Lm escape from its phagocytic vacuole and survival in professional phagocytes (Hamon et al., 2012). We have also shown that Lm induces IL-22 and IFN-γ upon oral infection in humanized Ecad mice (Reynders et al., 2011). Whereas IFN-γ is required to control systemic Lm infection (Harty and Bevan, 1995), IL-22 is not (Graham et al., 2011).
Lm impact on intestinal epithelium homeostasis, although potentially critical for the outcome of the infection, has not been studied. We therefore investigated intestinal epithelium response to orally acquired listeriosis. We show here that Lm induces intestinal epithelial cell proliferation and depletion of GCs expressing accessible Ecad, leading to a complete blockade of Lm intestinal villus invasion. Intestinal epithelium proliferation and GC depletion are independent of Lm intestinal villus invasion, but strictly depend on infection of PP CX3CR1+ cells, which express IL-23 upon infection, leading to STAT3 activation in enterocytes. However, in contrast to host responses to intestinal epithelial damage, Lm-associated IL-23–dependent STAT3 phosphorylation involves not only IL-22 expression, but also induction of IL-11 by LP CD45− gp38+ pericryptal stromal cells. Importantly, we show that IL-23/IL-22/IL-11–dependent intestinal epithelium response to Lm also critically requires IFN-γ–dependent STAT1 phosphorylation. We further demonstrate that this innate immune pathway leads to a decrease of mucus barrier thickness at the colon level, a known promoter of intestinal inflammation (Van der Sluis et al., 2006). Indeed, Lm-associated epithelial response leads to gut sensitization to colitis.
Altogether, these results identify a new innate immune pathway initiated at the PP level against infection, which relies on IL-22– and IL-11–dependent STAT3 activation in epithelial cells and the conjugated action of IFN-γ. This study unveils a new layer of regulation of the intestinal epithelium in response to invasive pathogens and the unexpected role of LP CD45− gp38+ CD34+ stromal cells as innate immune cells responding to pathogen-elicited stimuli.
Results
Lm infection leads to intestinal epithelium proliferation
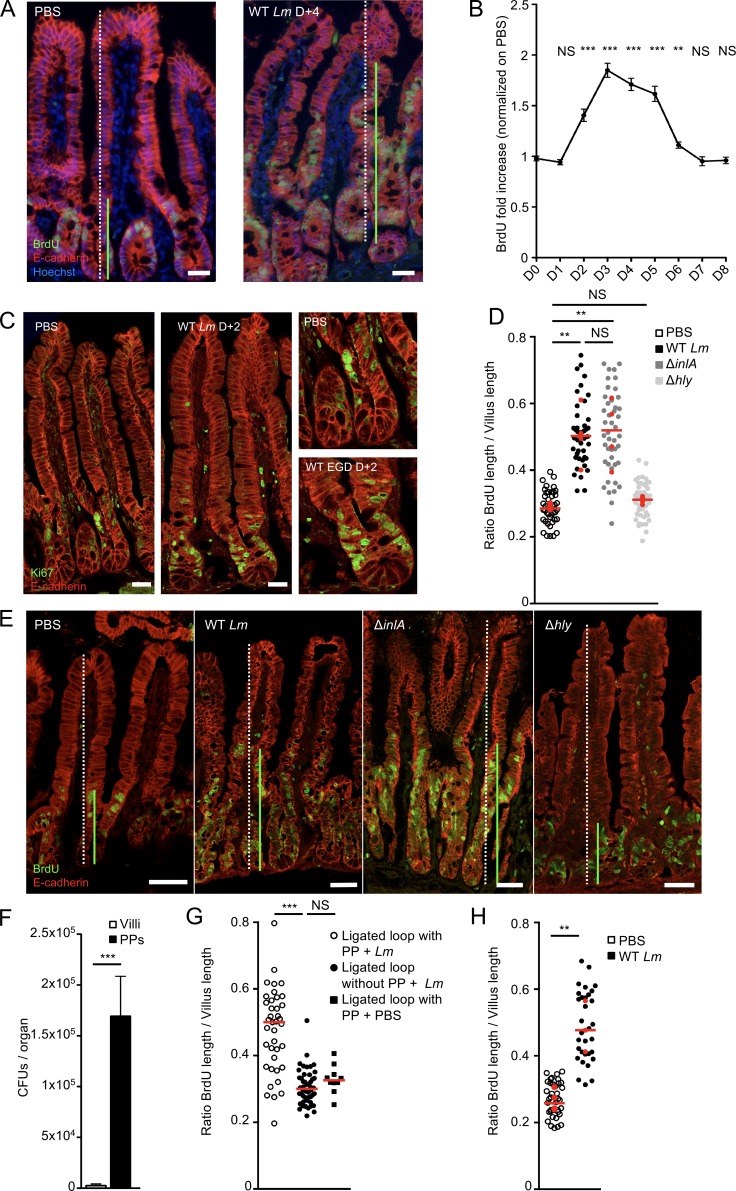
We first investigated intestinal epithelium proliferation upon Lm oral inoculation by quantifying BrdU incorporation in KIE16P humanized mouse intestinal epithelium. Whereas only cells located in intestinal crypts incorporated BrdU at steady-state (Barker et al., 2008), oral infection with two genetically distant WT Lm strains (EGD and EGDe) induced a significant increase in BrdU+ epithelial cells (Fig. 1 A and Fig. S1 B). Increase in enterocyte BrdU incorporation was noticeable as early as day 2 post infection (pi). As BrdU was injected i.p. and incorporated in dividing cells 16 h before tissue sampling, this indicates that proliferation begins in the first day pi. Proliferation peaked between day 3 and 4 pi and returned to basal level at day 6 pi (Fig. 1 B). In line with these results, more Ki67+ cycling cells were counted in crypts and at crypt-villous junctions upon Lm oral infection (Fig. 1 C and Fig. S1 C; Whitfield et al., 2006; Cuylen et al., 2016). No leakage of the epithelial barrier was detected in a biotin barrier assay (Fig. S1 D; Tsai et al., 2013), and no induction of epithelial cell death was observed (Fig. S1 E). We next investigated the dose-dependency of Lm-associated enterocyte proliferation. Epithelial proliferation was induced similarly upon oral inoculation of 5 × 108 and 5 × 109 CFUs, but not 5 × 107 CFUs/animal (Fig. S1 F).
Figure 1.
Lm-induced epithelial cell proliferation in an InlA-independent but PP-dependent manner. (A) Fluorescent imaging of sections of the ileum obtained from KIE16P mice 4 d after oral infection with 5 × 109 Lm. 16 h before euthanasia, mice were injected with BrdU. Sections are stained for proliferating cells (BrdU), epithelial cells (Ecad), and nuclei (Hoechst). Bars, 20 µm. (B) Quantification over time of epithelial proliferation. Fold increase is measured by dividing the length between the first and last BrdU+ epithelial cells by the length of the villi, normalized by the proliferation from the noninfected mice. n = 9–39 villi/point. Data are pooled from three independent experiments. Mann-Whitney test. (C) Confocal imaging of sections of the ileum obtained from KIE16P mice 2 d after oral infection with 5 × 109 Lm. Sections are stained for proliferating cells (Ki67) and epithelial cells (Ecad). Bars, 20 µm. (D) Quantification of epithelial proliferation in KIE16P littermate mice infected with 5 × 109 Lm and isogenic mutants. Each dot corresponds to one quantified villus, except the red dots, which correspond to the mean of each mouse. At least 5 fields/10 villi per mouse. n = 4 mice/bacterial strain. Data are pooled from two independent experiments. A one-way ANOVA test followed by a Sidak’s multiple comparisons was performed on the mean of each mouse. (E) Confocal imaging of frozen sections of the ileum obtained from KIE16P mice treated as in A with 5 × 109 Lm and isogenic mutants. Bars, 20 µm. (F) Lm burden in PPs and villi from mice infected by 5 × 109 CFUs ΔinlA Lm. Eight mice per group. Data are represented as mean ± SEM. Mann-Whitney test. (G) Quantification of epithelial proliferation in ligated loop of KIE16P mice containing one or no PP infected with 1 × 105 ΔinlA Lm 24 h pi. Each dot corresponds to one quantified villus. At least 5 fields/10 villi per mouse. n = 3–4 mice/condition. One for PP PBS control. Data are pooled from two independent experiments. A one-way ANOVA test followed by a Tukey’s multiple comparisons was performed. (H) Quantification of epithelial proliferation in axenic KIE16P littermate mice infected with 5 × 109 Lm. Each dot corresponds to one quantified villus, except the red dots, which correspond to the mean of each mouse. n = 3–4 mice. Data are pooled from two independent experiments. A Student’s t test was performed on the mean of each mouse. **, P < 0.01; ***, P < 0.001.
Lm-associated intestinal epithelium proliferation is independent of villus epithelial cell invasion, but requires PP infection
We next investigated the bacterial determinants of intestinal epithelial proliferation. Since Lm crosses the villus intestinal epithelial barrier in an InlA-dependent manner (Fig. S1 A), we investigated if cell proliferation was dependent of InlA-mediated villus invasion. Enterocyte proliferation was detected in all intestinal crypts observed, irrespective of local Lm villus invasion, while only 10% of villi are infected (Fig. S1 G). This argues against local proliferation in response to InlA-dependent infection of villi. To directly investigate the role of InlA-dependent villus invasion in Lm-associated intestinal epithelium proliferation, KIE16P humanized mice were infected with an isogenic Lm ΔinlA mutant, which is unable to invade intestinal villi, but crosses via PPs. As expected, intestinal bacterial load was lower upon infection with Lm ΔinlA as compared with WT bacteria (Fig. S1 H). Nevertheless, Lm-associated intestinal epithelium proliferation was comparable in both conditions (Fig. 1, D and E). As previously shown, Lm invades via M cells in an InlA-independent manner the PP (Lecuit et al., 2001; Chiba et al., 2011; Nikitas et al., 2011), where host response to Lm is initiated (Fig. 1 F and Fig. S1 A; Lecuit et al., 2007). To investigate the role of PPs in Lm-associated intestinal epithelium proliferation, we injected Lm ΔinlA in ligated loops containing or not containing a PP. Lm-associated intestinal epithelium proliferation was strictly dependent of the presence of PPs (Fig. 1 G), demonstrating the critical role of PPs in Lm-associated intestinal epithelium proliferation and the absence of contribution of InlA-dependent invasion in this phenomenon. Of note, proliferation was higher in villi near to PPs, suggesting a gradient of induction from PP to LP (Fig. S1 I). We confirmed that proliferation is InlA-independent in nonhumanized C57BL/6J mice, in which Lm entry occurs only through PP M cells (Fig. S1 J).
As intestinal proliferation is regulated by the microbiota (Rakoff-Nahoum et al., 2004), we investigated whether Lm-associated intestinal epithelium proliferation was indirect, as a consequence of an interaction with the microbiota. As in conventional mice, proliferation was induced in germ-free mice mono-associated with Lm, demonstrating the direct impact of Lm on proliferation, rather than via interactions with the microbiota (Fig. 1 H).
Lm-associated intestinal epithelium proliferation depends on epithelial STAT3 activation and leads to a blockade of Lm intestinal villus invasion via GCs
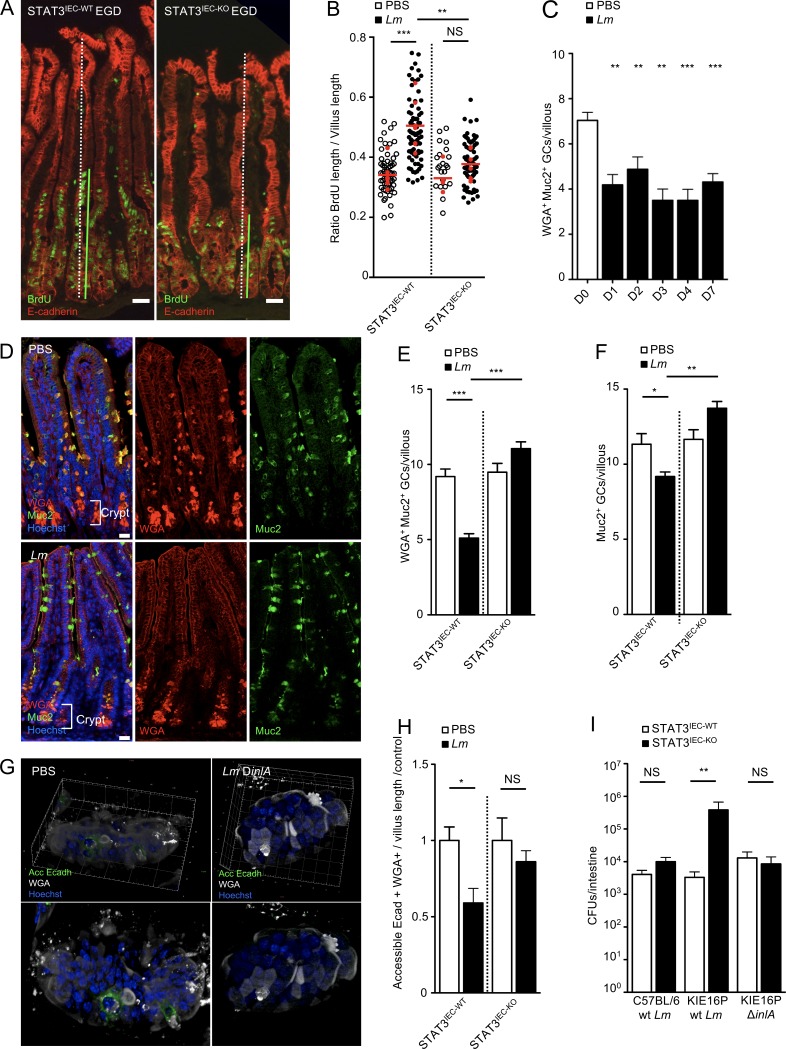
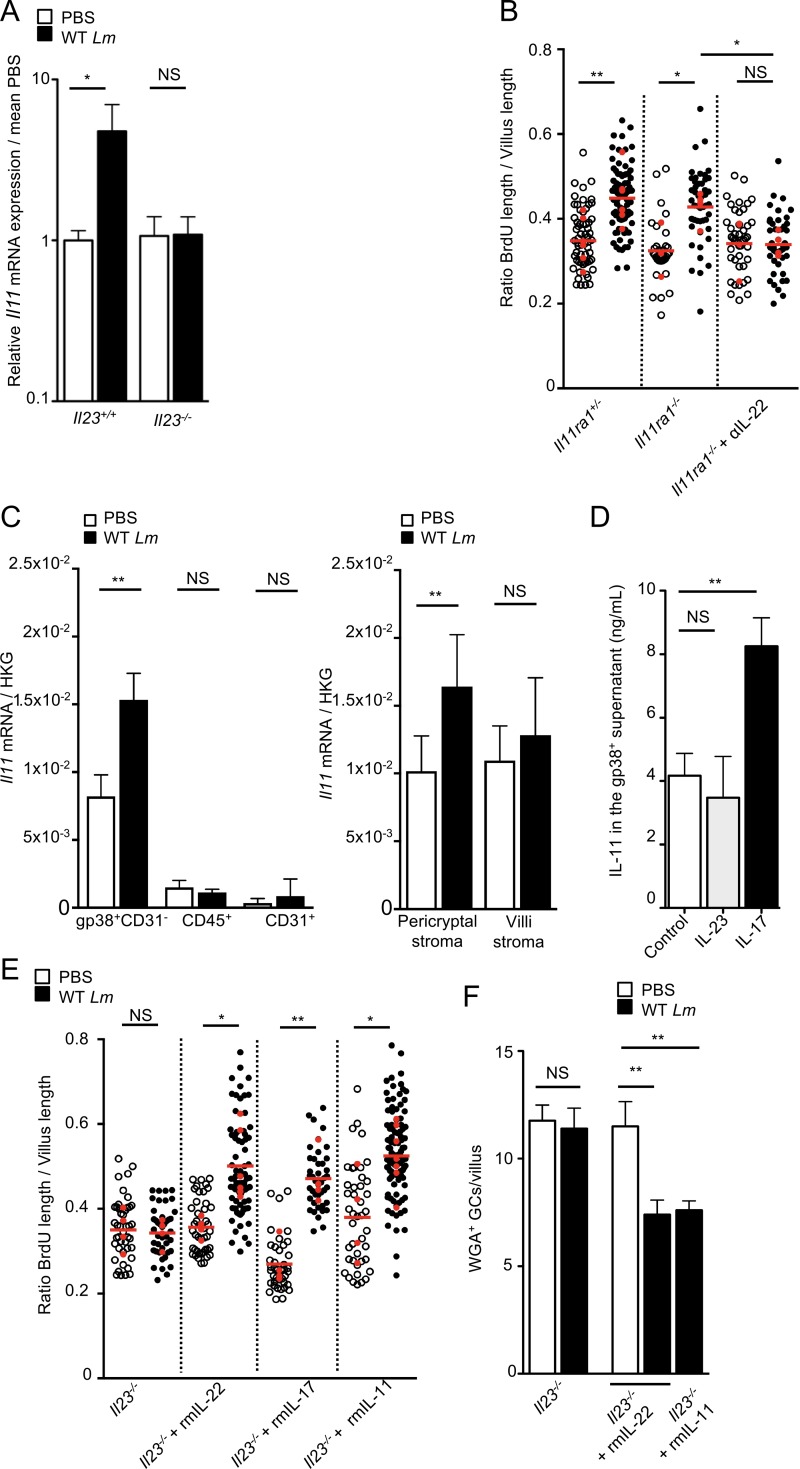
STAT3 activation leads to epithelial cell proliferation upon DSS-induced colitis and methotrexate-induced damage to stem cells (Pickert et al., 2009; Aparicio-Domingo et al., 2015). We generated and infected nonhumanized C57BL/6J Stat3fl/fl vil-Cre-ERT2 mice, in which Stat3 is excised in intestinal epithelial cells (IEC) expressing Villin by hydroxytamoxifen. Proliferation was totally impaired in STAT3IEC-KO mice as compared with STAT3IEC-WT littermates, demonstrating the critical role of epithelial STAT3 in Lm-associated intestinal epithelium proliferation, independently of InlA-dependent entry (Fig. 2, A and B). GCs expressing accessible Ecad are the main sites of InlA-dependent Lm translocation across the intestinal epithelium (Nikitas et al., 2011). Since STAT3-dependent epithelial proliferation may be linked to the turnover and differentiation of GCs (Gonneaud et al., 2016), we investigated the impact of Lm-associated intestinal epithelium proliferation on GCs. We labeled Muc2, the major component of mucin synthesized by GCs, with an anti-MUC2C3 antibody (Johansson et al., 2008). We also stained glycosylated mucins with wheat germ agglutinin (WGA), which specifically binds to sialic acid and N-acetyl-glucosaminyl carbohydrate residues on mature, modified mucins (Fischer et al., 1984). The number of WGA+ Muc2+ GCs was significantly decreased in intestinal villi from infected mice as early as 1 d pi and recovered 14 d pi (Fig. 2, C and D; and Fig. S2 A). The decrease in WGA+ GC number was STAT3-dependent, as excision of STAT3 in Stat3fl/fl vil-Cre-ERT2 mice prevented Lm-induced decrease in GC number (Fig. 2 E and Fig. S2 B). As for proliferation, WGA+ GC number regulation was InlA independent (Fig. S2 C). We next investigated the expression of Muc2 in GCs. Unlike the clear decrease of WGA+ GCs, the number of total Muc2+ GC only slightly decreased in intestine (Fig. 2, D and F). Data from literature indicate that GCs that harbor less glycosylated mucins are not fully mature (Asker et al., 1998; Nowarski et al., 2015). We therefore investigated if the decrease in WGA+ GCs may correlate with other markers of GC differentiation. We have shown previously that WGA+ GCs express luminally accessible Ecad, which is targeted by Lm (Nikitas et al., 2011). We observed a dramatic STAT3-dependent decrease of WGA+ GCs expressing accessible Ecad upon Lm infection (Fig. 2, G and H).
Figure 2.
Lm triggers a STAT3-dependent negative feedback loop blocking its access to intestinal villi via GCs. (A) Confocal imaging of sections of the ileum obtained from STAT3IEC-WT and STAT3IEC-KO mice treated as in Fig. 1 A with 5 × 109 Lm and isogenic mutants. Bars, 20 µm. (B) Quantification of epithelial proliferation in STAT3IEC-WT and STAT3IEC-KO littermate mice 4 d after oral inoculation. Each dot corresponds to one quantified villus, except the red dots, which correspond to the mean of each mouse. n = 3–5 mice (PBS) or 6–7 mice (Lm)/condition. Data are pooled from three independent experiments. A one-way ANOVA test followed by a Sidak’s multiple comparisons was performed on the mean of each mouse. (C) Quantification of WGA+ GCs/villous at different time points after inoculation. n = 8–25 independent villi. Data are pooled from two independent experiments. Data are represented as mean ± SEM. Mann-Whitney test. (D) Confocal imaging of sections of the ileum obtained 4 d pi with 5 × 109 Lm and control. Tissue was fixed in Carnoy. Sections are stained for mucus (WGA), Muc2 mucin (Muc2), and nuclei (Hoechst). WGA+ Muc2− cells located in the crypts are Paneth cells, which are not counted. Bars, 20 µm. (E) Quantification of WGA+ GCs/villous in STAT3IEC-WT and STAT3IEC-KO mice infected by 5 × 109 Lm 4 d pi and noninfected control. n = 53–95 villi. Data are pooled from two independent experiments. Data are represented as mean ± SEM. Mann-Whitney test. (F) Quantification of Muc2+ GCs/villous in STAT3IEC-WT and STAT3IEC-KO mice infected by 5 × 109 Lm 4 d pi and noninfected control. n = 53–95 villi. Data are pooled from two independent experiments. Data are represented as mean ± SEM. Mann-Whitney test. (G) Three-dimensional reconstruction of nonpermeabilized intestinal villi stained with WGA and for accessible Ecad and nuclei. (H) Quantification of WGA+ GCs expressing or not accessible Ecad in STAT3IEC-WT and STAT3IEC-KO mice 4 d after inoculation of 5 × 109 Lm ΔinlA from the upper 100 µm of whole mount staining. n = 17–23 villi. Data are representative of two independent experiments with at least three mice per group. Data are represented as mean ± SEM. Mann-Whitney test. (I) Lm burden in intestine of STAT3IEC-WT and STAT3IEC-KO mice infected by WT Lm or the isogenic mutant ΔinlA. A first 3-d inoculation of 5 × 108 CFUs/animal was followed by a 4-d inoculation of 5 × 109 CFUs/animal. Data are pooled of five independent experiments. 13 mice per group for KIE16P mice infected by WT Lm; 10 mice per group for KIE16P mice infected by Lm ΔinlA and four mice per group for C57BL/6 infected by WT Lm. Wilcoxon test; animals are paired by subgroups for each experiment. *, P < 0.1; **, P < 0.01; ***, P < 0.001.
We next investigated the impact of decreased accessible Ecad on Lm InlA-dependent villus invasion. We generated humanized Ecad KIE16P STAT3IEC-KO and STAT3IEC-WT mice in which InlA-dependent entry occurs. To this end, we inoculated these mice with WT or Lm ΔinlA at 5 × 108 CFUs to activate STAT3. As expected, proliferation was inhibited in KIE16P STAT3IEC-KO compared with KIE16P STAT3IEC-WT (Fig. S2 D). 3 d later, we reinoculated with the same bacteria at higher dose of 5 × 109 CFUs for 4 d. Strikingly, InlA-dependent Lm entry was fully inhibited in KIE16P STAT3IEC-WT mice, whereas it was not in KIE16P STAT3IEC-KO, showing that Lm infection blocks subsequent InlA-dependent entry in a STAT3-dependent manner (Fig. 2 I, middle). In sharp contrast, no impact was detected on translocation of Lm ΔinlA, suggesting that STAT3 activation inhibits specifically InlA-dependent entry that occurs via GCs (Fig. 2 I, right). As a control, we infected nonhumanized C57BL/6 STAT3IEC-KO and STAT3IEC-WT mice (Fig. 2 I, left) and confirmed that STAT3 has no impact on InlA-independent invasion, whereas proliferation and the number of WGA+ GCs are modulated in these mice.
Together, these results indicate that STAT3 activation, which is induced by Lm in an InlA-independent manner, leads to a complete blockade of InlA-dependent intestinal villus invasion, by depleting specifically GCs expressing luminally accessible Ecad.
Infection of PP CX3CR1 initiates Lm-associated intestinal epithelium response
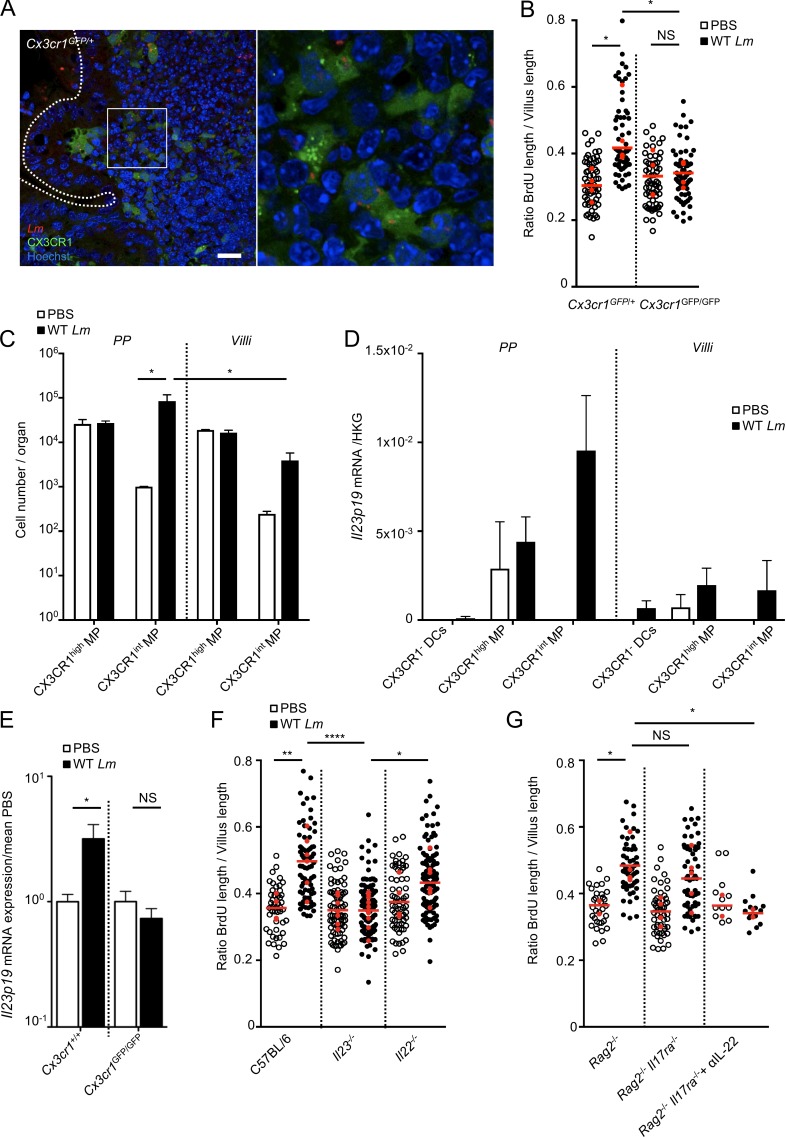
We next investigated how STAT3 is activated in the epithelium upon Lm infection in an InlA-independent but PP-dependent manner. We observed that Lm is rapidly captured mostly by CX3CR1+ cells in PPs upon oral infection with 5 × 109 CFUs, whereas no bacteria was detected in PPs with 5 × 107 CFUs, which fits with the inoculum required for enterocyte proliferation (Fig. 3 A and Fig. S2, E and G). We next investigated the role of PP CX3CR1 in Lm-associated intestinal epithelium response. When compared with Cx3cr1GFP/+ littermates, Lm-associated epithelial cell proliferation in Cx3cr1GFP/GFP mice was totally abolished (Fig. 3 B), even though Lm were present in both CX3CR1GFP/+ and CX3CR1GFP/GFP cells (Fig. 3 A and Fig. S2 F). This indicates that CX3CR1 expression is critically needed for Lm-associated intestinal epithelium proliferation. Proliferation of epithelial cells and WGA+ cell decrease required expression of LLO, which is encoded by hly and is necessary for bacterial escape from phagocytic vacuole (data not shown). LLO is also required for Lm-associated intestinal epithelium proliferation in germ-free mice as well as in C57BL/6 mice, where entry occurs only via PPs (Fig. 1 H and Fig. S1 I). This suggests that Lm access to PP CX3CR1+ cytoplasm is needed for signaling. We next investigated if PP CX3CR1+ cells are involved in signaling upstream of STAT3. IL-23 and its downstream effector IL-22 are expressed in a CX3CR1-dependent manner upon C. rodentium infection (Longman et al., 2014; Aychek et al., 2015), and IL-22 is a potent inducer of epithelial proliferation (Pickert et al., 2009; Lindemans et al., 2015). We investigated if, as do colonic CX3CR1+ cells upon C. rodentium infection, PP CX3CR1+ cells express IL-23 upon Lm infection. At steady-state, most CX3CR1+ cells were CX3CR1high in PPs and villi, as previously reported (Varol et al., 2009; Bonnardel et al., 2015). However, upon 24 h of Lm oral infection, the number of CX3CR1int mononuclear phagocytes (MPs) was significantly increased in PPs compared with noninfected tissue and villi from infected mice (Fig. 3 C). Upon infection, CX3CR1+, and especially PP CX3CR1int MPs, express Il23 (Fig. 3 D and S2 H). We next showed that induction of Il23p19 expression is CX3CR1-dependent in Cx3cr1GFP/GFP mice (Fig. 3 E). These data demonstrate that CX3CR1 expression in PPs is necessary for IL-23 production in response to Lm infection.
Figure 3.
IL-23 produced by CX3CR1 is involved in Lm-associated epithelial proliferation. (A) Confocal imaging of a vibratome section from a PP infected by 5 × 109 Lm 24 h after oral inoculation. Bars, 20 µm. (B) Quantification of epithelial proliferation in Cx3cr1GFP/GFP and Cx3cr1GFP/+ cohoused control mice 4 d after oral inoculation. **, P < 0.01; ***, P < 0.001. Each dot corresponds to one quantified villus, except the red dots, which correspond to the mean of each mouse. n = 4 mice/condition. Data are pooled from three independent experiments. A one-way ANOVA test followed by a Sidak’s multiple comparisons was performed on the mean of each mouse. (C) Cell number per organ counted by flow cytometry. All PP versus entire intestinal tissue. Data are representative of two independent experiments with three mice per group. Data are represented as mean ± SEM. A one-way ANOVA test followed by a Sidak’s multiple comparisons was performed on the mean of each mouse. (D) Il23p19 mRNA quantification by qRT-PCR on sorted PPs and villi CX3CR1− and CX3CR1+ cells 24 h pi. Data are representative of two independent experiments with at least three mice per group. Data are represented as mean ± SEM. (E) Il23p19 mRNA quantification by qRT-PCR from intestinal tissue of Cx3cr1GFP/GFP and Cx3cr1GFP/+ mice 24 h pi. Data are pooled of three independent experiments. Six Cx3cr1+/+ mice; seven Cx3cr1GFP/GFP mice. Data are represented as mean ± SEM. Student’s t test. (F) Quantification of epithelial proliferation in control cohoused C57BL/6 and mutant mice 4 d after oral inoculation. Each dot corresponds to one quantified villus, except the red dots, which correspond to the mean of each mouse. n = 4–9 mice/condition. Data are pooled from four independent experiments. A one-way ANOVA test followed by a Sidak’s multiple comparisons was performed on the mean of each mouse. (G) Quantification of epithelial proliferation in cohoused Rag2−/− and Rag2−/−IL17ra−/− injected or not with a blocking αIL-22 antibody 4 d after oral inoculation. Each dot corresponds to one quantified villus, except the red dots, which correspond to the mean of each mouse. n = 12–57 villi. Data are pooled of two independent experiments. A one-way ANOVA test followed by a Sidak’s multiple comparisons was performed on the mean of each mouse. *, P < 0.05; **, P < 0.01; ****, P < 0.0001.
We next investigated the role of IL-23 on Lm-associated intestinal epithelium proliferation. IL-23 pathway disruption in Il23p19−/− mice fully impaired Lm-associated enterocyte proliferation (Fig. 3 F), indicating that IL-23, while being produced at the PP level, exerts its effect at the villus level. Surprisingly, enterocyte proliferation in response to Lm was still observed in Il22−/− mice (Fig. 3 F). We therefore investigated whether other factors contributing to Lm-associated intestinal epithelium proliferation were involved and induced by IL-23. Lm-associated enterocyte proliferation was not abolished in Rag2−/− mice, devoid of B and T cells, and was partially observed in Rag2−/−Il17ra−/− mice, which lack IL-17–induced signaling (Fig. 3 G). However, Lm-associated enterocyte proliferation was absent in Rag2−/−Il17ra−/− mice when treated with an anti–IL-22 antibody (Fig. 3 G), indicating that both IL-22 and IL-17 are involved in Lm-associated epithelial proliferation.
IL-11 expressed by gp38+ stromal cells is involved in Lm-associated epithelial response
To decipher the complementary roles of IL-22 and IL-17 in Lm-associated epithelial response, we investigated the contribution of other modulators of epithelial proliferation potentially linked to IL-23 and STAT3. IL-11 is involved in gastric and colon tumorigenesis through STAT3 activation (Putoczki et al., 2013) and could therefore be a modulator of enterocyte proliferation. We first showed that Il11 transcription is induced upon Lm infection in an IL-23–dependent manner (Fig. 4 A). We next showed that blocking of IL-22 in Il11ra−/−mice fully inhibited proliferation, while blocking IL-11 or IL-22 alone is not sufficient (Fig. 3 F and Fig. 4 B). This indicates that both IL-11 and IL-22 contribute to IL-23–dependent Lm-associated enterocyte proliferation. We investigated the source of IL-11 during Lm infection. Several cell types in the intestinal LP have been reported to express IL-11, including immune cells, endothelial cells, and mesenchymal stromal cells (Bamba et al., 2003; Andoh et al., 2005; Putoczki and Ernst, 2010). We have previously shown that a majority of mesenchymal stromal cells of the LP, both in the villi and around the crypts, express gp38 (podoplanin; Peduto et al., 2009; Stzepourginski et al., 2015). CD45−CD31−gp38+ stromal cells isolated from the LP of infected mice expressed significantly higher level of Il11 transcripts compared with intestinal immune cells (CD45+) and endothelial cells (CD31+; Fig. 4 C and Fig. S3 A). Among total gp38+ stromal cells, the pericryptal subset coexpressing CD34, which we previously identified as a major component of the intestinal stem cell niche (Stzepourginski et al., 2017), is the major source of IL-11 expression upon Lm infection (Fig. 4 C and Fig. S3 B). In vitro stimulation of gp38+ stromal cells by recombinant IL-23 did not induce expression of IL-11 (Fig. 4 D), consistent with the observation that gp38+ stromal cells do not express IL-23 receptor (Fig. S3 C). These results suggest that another factor bridges IL-23 signaling with IL-11 expression. Since IL-17 plays a role in Lm-associated enterocyte proliferation (Fig. 3 G), we investigated its contribution to gp38+ stromal cells’ IL-11 production. IL-17 was sufficient to increase IL-11 release by gp38+ stromal cells (Fig. 4 D), indicating a role for IL-17 in IL-11 production.
Figure 4.
IL-11 expressed by Gp38+ stromal cells in an IL-23–dependent manner is involved in Lm-associated epithelial proliferation. (A) Il11 mRNA quantification by qRT-PCR from intestinal tissue of Il23+/+ and Il23−/− mice 48 h pi. Data are pooled of two independent experiments. Five Il23+/+ mice; four Il23−/− mice. Data are represented as mean ± SEM. Student’s t test. (B) Quantification of epithelial proliferation in littermate Il11ra1+/− and Il11ra1−/− mice injected or not with a blocking αIL-22 antibody 4 d after oral inoculation. Each dot corresponds to one quantified villus, except the red dots, which correspond to the mean of each mouse. At least 5 fields/10 villi per mouse. n = 3–8 mice/condition. Data are pooled of three independent experiments. A one-way ANOVA test followed by a Sidak’s multiple comparisons was performed on the mean of each mouse. (C) Il11 mRNA quantification by qRT-PCR from sorted LP populations, as indicated, 48 h pi. Data are representative of two independent experiments. Three animals per group. Data are represented as mean ± SEM. Student’s t test. (D) IL-11 protein quantification by an ELISA assay from sorted CD45− gp38+ stromal cells stimulated in vitro with the indicated cytokines. Data are pooled of three independent experiments. Six culture wells per condition. Data are represented as mean ± SEM. Mann-Whitney test. (E) Quantification of epithelial proliferation in Il23−/− littermate mice complemented with rmIL-22, rmIL-17, and rmIL-11 4 d after oral inoculation. Each dot corresponds to one quantified villus except the red dots, which correspond to the mean of each mouse. n = 4–7 mice/condition. Data are pooled of three independent experiments. A one-way ANOVA test followed by a Sidak’s multiple comparisons was performed on the mean of each mouse. (F) Quantification of WGA+ GCs/villous in Il23−/− mice complemented with recombinant IL-11 and IL-22, infected by 5 × 109 Lm 4 d pi and noninfected control. n = 10–30 villi. Data are representative of two independent experiments. Data are represented as mean ± SEM. Mann-Whitney test. *, P < 0.05; **, P < 0.01.
We next investigated in vivo whether IL-11, IL-17, and IL-22 complement IL-23 inhibition to induce Lm-associated enterocyte proliferation. Treatment of Lm-infected Il23p19−/− mice with recombinant IL-11, IL-17, or IL-22 fully restored Lm-associated epithelial proliferation, as well as a decrease in WGA+ GCs (Fig. 4, E and F). Together, these results show that intestinal gp38+ stromal cells are the major producer of IL-11 after Lm infection, which acts in concert with IL-22 downstream of IL-23 and IL-17 to induce Lm-associated intestinal epithelium proliferation. However, injection of recombinant IL-11 or IL-22 does not induce enterocyte proliferation or a decrease in WGA+ GCs in uninfected mice (Fig. 4, E and F), indicating that STAT3 activation is necessary, but not sufficient, to induce epithelial proliferation and requires another pathway which is induced by Lm infection.
IFN-γ production by natural killer (NK) cells is required for Lm-associated epithelial response
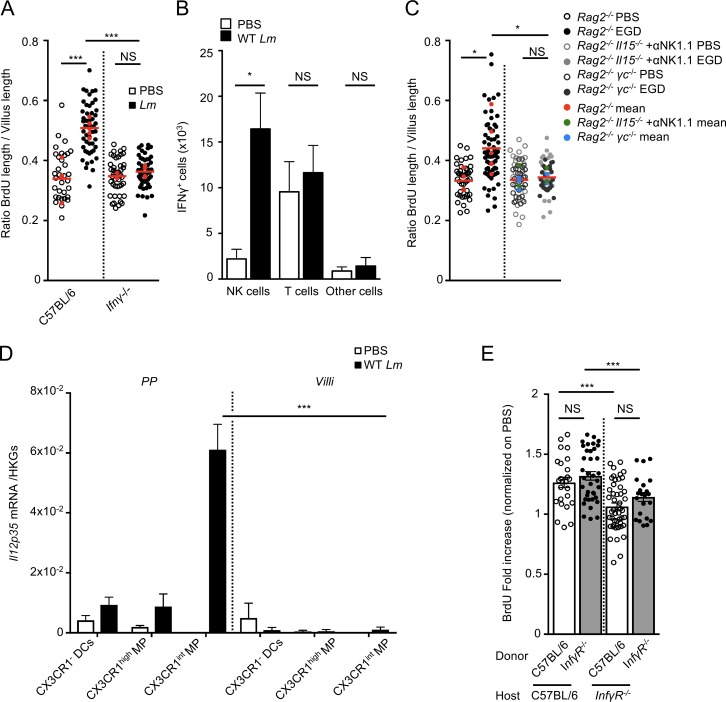
IFN-γ is a major cytokine controlling Lm infection in vivo (Harty and Bevan, 1995). IFN-γ is also involved in tissue homeostasis, in particular in the intestine and liver (Smith et al., 2000; Brooling et al., 2005). We investigated enterocyte proliferation in Ifnγ−/− mice orally inoculated with Lm. As expected, IFN-γ depletion led to an increased Lm systemic burden (Fig. S4 A), yet no enterocyte proliferation was detectable, demonstrating the critical role of IFN-γ in Lm-associated enterocyte proliferation (Fig. 5 A). As previously shown, IFN-γ was mostly produced by NK cells and to a lower degree by T cells (Fig. 5 B and Fig. S4 B; Andersson et al., 1998; Reynders et al., 2011). Lm-associated enterocyte proliferation was still observed in Rag2−/− mice, which lack T and B cells, but was fully abolished in Rag2−/−γC−/− mice, which also lack ILCs and NK cells (Fig. 5 C). To investigate if group 1 ILCs (including ILC1 and NK cells) are the cells involved in Lm-associated enterocyte proliferation, we depleted all ILC1 cells in Rag2−/−Il15−/− mice injected with an antibody against NK1.1, a condition where ILC2s and ILC3s are preserved, but ILC1s are totally absent. Lm-associated epithelial proliferation was completely abrogated, as in Rag2−/−γC−/− mice, indicating the essential role of ILC1s in Lm-associated enterocyte proliferation (Fig. 5 C).
Figure 5.
IFN-γ produced by NK cells is involved in Lm-associated epithelial proliferation. (A) Quantification of epithelial proliferation in cohoused C57BL/6 control and Ifnγ−/− mice 4 d after oral inoculation. Each dot corresponds to one quantified villus, except the red dots, which correspond to the mean of each mouse. At least 5 fields/10 villi per mouse. n = 3–5 mice/condition. Data are pooled of two independent experiments. A one-way ANOVA test followed by a Sidak’s multiple comparisons was performed on the mean of each mouse. (B) Quantification by flow cytometry analysis of IFN-γ+ cells. Data are pooled of three independent experiments. n = 4–5 animals per group. Data are represented as mean ± SEM. Student’s t test. (C) Quantification of epithelial proliferation in cohoused Rag2−/−, Rag2−/− γc−/−, Rag2−/−Il15−/− injected with a blocking αNK1.1 antibody 4 d after oral inoculation. Each dot corresponds to one quantified villus, except the red, green, and blue dots which correspond to the mean of each mouse. n = 3–6 mice per condition. Data are pooled of two independent experiments. A one-way ANOVA test followed by a Sidak’s multiple comparisons was performed on the mean of each mouse. (D) I12p35 mRNA quantification by qRT-PCR on sorted PPs and villi CX3CR1− and CX3CR1+ cells 24 h pi. Data are representative of two independent experiments with at least three mice per group. Data are represented as mean ± SEM. Student’s t test. (E) Quantification of epithelial proliferation in C57BL/6:IfnγR−/− and IfnγR−/−:C57BL/6 chimera mice 4 d after oral inoculation. Data are pooled of two independent experiments with two to four mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We next investigated the signal triggering IFN-γ production by NK cells in response to Lm oral infection. It has been shown that CX3CR1+ cells produce IL-12 during sepsis (Ishida et al., 2008), leading to IFN-γ production by NK cells. Accordingly, CX3CR1+ MPs in PPs, but not in villi, expressed IL-12 in response to Lm infection (Fig. 5 D), and expression of both IFN-γ and IL-12 was CX3CR1-dependent (Fig. S4, C and D). IL-23 is a potent inducer of IFN-γ in Helicobacter hepaticus infection in mice (Buonocore et al., 2010). However, in our model, IFN-γ was induced after Lm infection in both WT and Il23p19−/− mice, indicating that in this context, IFN-γ production is IL-23 independent (Fig. S4 E).
IFN-γ can act both on hematopoietic and nonhematopoietic cells. To decipher if myeloid hematopoietic (radio-sensitive) or nonmyeloid nonhematopoietic (radio-resistant) cells are involved in IFN-γ–dependent Lm-associated enterocyte proliferation, we analyzed bone marrow chimera mice for which donor and recipient were either WT (C57BL/6) or Ifnγr−/− mice. C57BL/6→Ifnγr−/− and Ifnγr−/−→C57BL/6 mice chimeras were generated and infected with Lm. Proliferation was impaired when IFN-γR−/− cells were radio-resistant, but not radio-sensitive, indicating a role of nonhematopoietic cells, likely epithelial cells, in IFN-γ–dependent proliferation (Fig. 5 E).
STAT3 and STAT1 activations lead to intestinal enterocyte proliferation
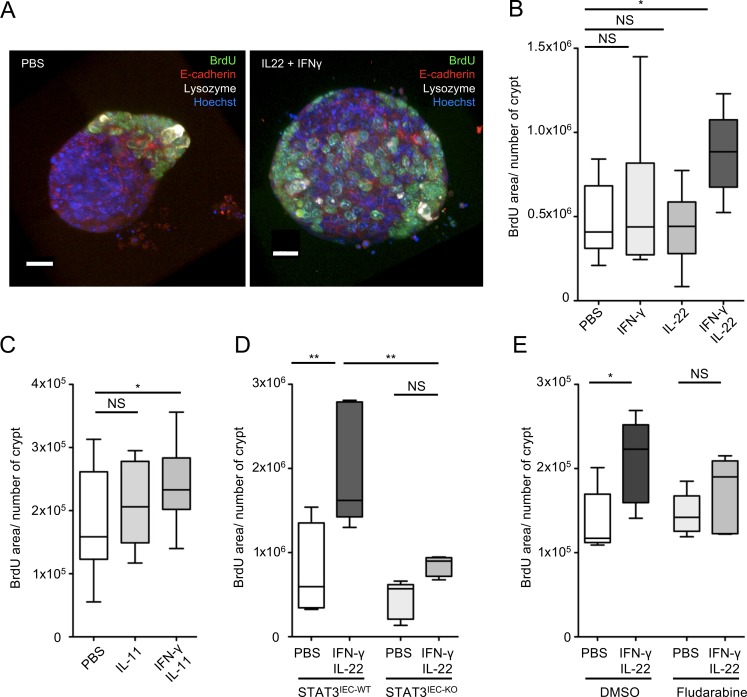
Both IL-22 and IL-11, known to activate STAT3 and IFN-γ, which activates STAT1, are required in vivo for Lm-associated enterocyte proliferation. IL-22/IL-11 and IFN-γ are expressed 24–48 h pi (data not shown; Reynders et al., 2011). To determine whether IL-22, IL-11, and IFN-γ act in an interdependent and direct manner on epithelial cells to induce proliferation, we used intestinal organoids. We first determined by Western blotting that STAT3 is phosphorylated upon IL-22 or IL-11, but not IL-23, treatment of intestinal organoids and that STAT1 is phosphorylated upon IFN-γ treatment, indicating a direct effect of these cytokines on epithelial cells (Fig. S4, F and G). As expected, induction of proliferation in organoids did not occur with IL-23 (data not shown). It also did not occur with IL-22 or IFN-γ alone, indicating that these cytokines are not sufficient to induce proliferation (Fig. 6 A and Fig. S4 I for the method). In contrast, proliferation was observed when both a STAT3 inducer (IL-22 or IL-11) and a STAT1 inducer (IFN-γ) were simultaneously added to intestinal organoids (Fig. 6, A–C). STAT3 excision by the addition of hydroxytamoxifen to Stat3fl/fl Villin-cre intestinal organoids blocked proliferation induced by IL-22 and IFN-γ, indicating the critical requirement for STAT3 in proliferation (Fig. 6 D and Fig. S4 H). STAT1 inhibitor fludarabine (Frank et al., 1999) also led to a decrease in proliferation, indicating that both STAT3 and STAT1 activators are required to induce epithelial cell proliferation (Fig. 6 E).
Figure 6.
IL-23 and IFN-γ pathways complement to induce epithelial proliferation. (A) Maximum intensity projection of Z stacks from organoids incubated with the indicated cytokines. (B) Quantification of BrdU incorporation in organoids incubated with the indicated cytokines and treatment, detailed in the supplemental procedures. n = 8–10 pooled organoids from two independent experiments. A one-way ANOVA test followed by a Sidak’s multiple comparisons was performed. (C) Quantification of BrdU incorporation in organoids incubated with the indicated cytokines and treatment, detailed in the supplemental material. n = 15–17 pooled organoids from three independent experiments. A one-way ANOVA test followed by a Sidak’s multiple comparisons was performed. (D) Quantification of BrdU incorporation in organoids incubated with the indicated cytokines and treatment, detailed in the supplemental material. n = 4 (PBS) or 5 (IFN-γ + IL-22) organoids pooled from two plates, representative of two independent experiments. A one-way ANOVA test followed by a Sidak’s multiple comparisons was performed. (E) Quantification of BrdU incorporation in organoids incubated with the indicated cytokines and treatment, detailed in the supplemental material. n = 5 organoids pooled from two plates for each condition. A one-way ANOVA test followed by a Sidak’s multiple comparisons was performed. *, P < 0.05; **, P < 0.01.
Lm infection increases susceptibility to colitis
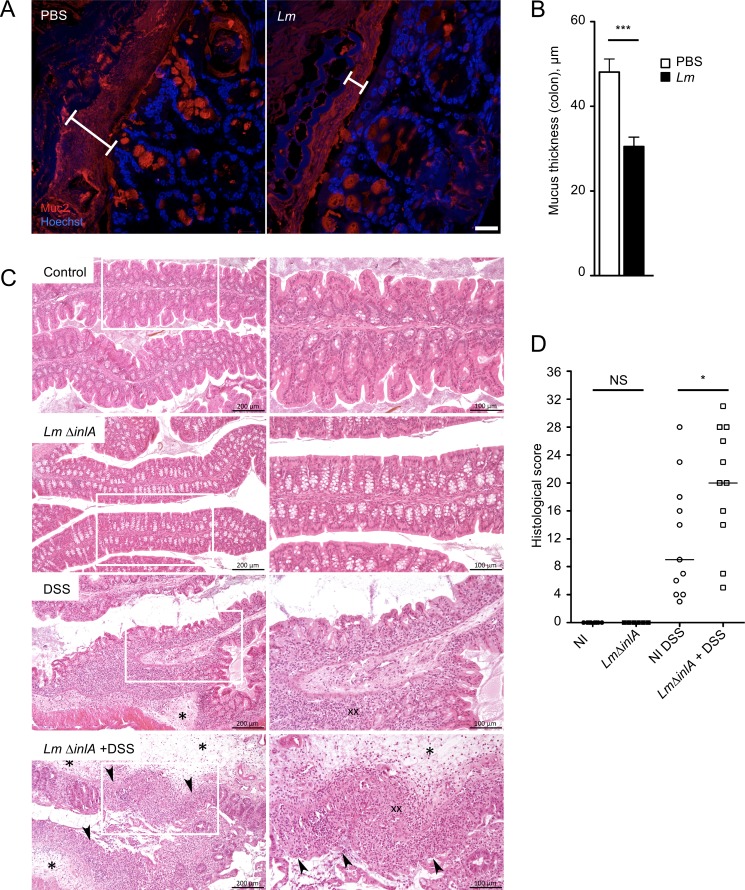
We have previously shown that Lm also targets the cecum and colon (Disson et al., 2008). We investigated if Lm regulates epithelial homeostasis at the cecum and colon level. We observed an increase of proliferation in the colon (Fig. S5 A), as well as a decrease in the number of WGA+ GCs upon Lm infection, while the number of Muc2+ GCs was not modified (Fig. S5, B and C). Depletion of mucus sensitizes mice to colitis (Van der Sluis et al., 2006). We therefore investigated the impact of Lm-associated WGA+ GC depletion on luminal mucus content and its consequences on susceptibility to colitis. The distance between the microflora and the epithelium, which reflects mucus thickness, was strongly decreased in a STAT3-dependent manner in the ileum and cecum (Fig. S5, D–G). At the colon level, the thickness of the highly organized inner mucus layer, measured by a Muc2+ staining, was reduced upon Lm infection (Fig. 7, A and B). Thus, Lm-associated decrease of the differentiation of the GCs inhibits Lm entry, while leading to a significant decrease in the physical separation between the epithelium and the luminal content, a well-established risk factor for colitis. To investigate whether Lm-associated decrease in mucus layer increases susceptibility to colitis, we fed mice with DSS for seven consecutive days, 7 d after Lm ΔinlA infection, when the IL-23 and IFN-γ pathways are no more induced (our observation; Reynders et al., 2011). Mice were sacrificed 14 d pi, and the colitis was evaluated. Infection with Lm ΔinlA alone, which does not invade epithelial cells, did not induce gut lesions 14 d pi, and pathology was similar to noninfected control (Fig. 7 C, top and second from bottom; and Fig. 7 D). As previously described, DSS treatment of noninfected mice induced colitis (Okayasu et al., 1990), as quantified by colonic inflammation and epithelial and mesenchymal lesions (Fig. 7 C, second from top; and Fig. 7 D). Very interestingly, as compared with controls treated by DSS only or with an Lm Δhly strain (Fig. S5 H), with which Lm-associated intestinal response is abolished, animals exposed to Lm ΔinlA before DSS, in which the mucus thickness was reduced, developed significantly more severe colitis (Fig. 7 C, bottom; and Fig. 7 D). Altogether, this indicates that Lm significantly increases susceptibility to colitis.
Figure 7.
Lm-associated sensitization to colitis. (A) Confocal imaging of sections of Carnoy’s fixed colons 4 d pi, 5 × 109 Lm ΔinlA. Sections are stained for Muc2 mucin (Muc2) and nuclei (Hoechst). Bars, 20 µm. (B) Quantification of mucus thickness, in µm. n = 26 to 31 measures. Data are represented as mean ± SEM. Mann-Whitney test. (C) Hematoxylin and eosin staining of Lm ΔinlA-infected and DSS-treated mice. (D) Score of histological lesions induced in DSS-treated mice. One point represents one mouse. NI, not infected; DSS, DSS-treated mice. Data are pooled from two independent experiments. A one-way ANOVA test followed by a Sidak’s multiple comparisons was performed. *, P < 0.05; ***, P < 0.001.
Discussion
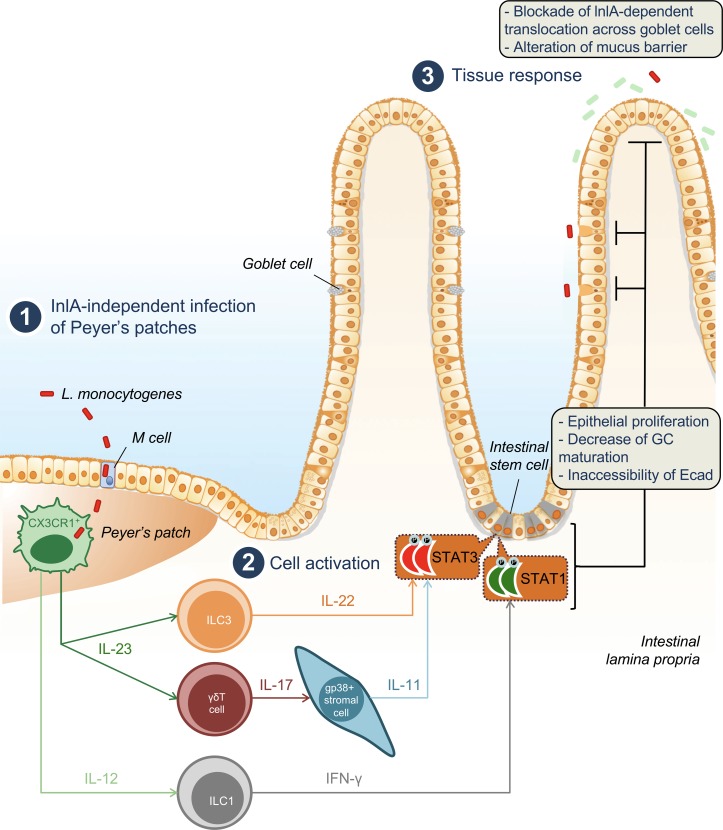
In contrast to other foodborne infections, the intestinal phase of listeriosis is clinically silent in most patients (Charlier et al., 2017). In line with this observation, the crossing of the intestinal barrier by Lm is not associated with epithelial damage, in contrast to other enteropathogens such as S. enterica, S. flexnerii, and C. rodentium, a model for attaching/effacing pathogens in mice inducing epithelial lesions (Collins et al., 2014). Here, we have shown that upon Lm infection, PP CX3CR1+ cells trigger both IL-23/IL-22/IL-11 and IFN-γ pathways, which act in combination to accelerate epithelial cell renewal and decrease the number of WGA+ GCs expressing accessible Ecad. This leads to a complete blockade of intestinal villus invasion by Lm. Unexpectedly, this response, which critically requires IL-23, is partially IL-22–dependent and requires the expression of IL-11 by intestinal gp38+ stromal cells (Fig. 8).
Figure 8.
Model of the mechanisms and downstream effects induced by Lm infection at the villus epithelial level. Lm infects CX3CR1+ cells in PPs (in an InlA-independent manner; 1), triggering both IL-23–IL-22–IL-11 and IL-12–IFN-γ pathways (2). Activation of STAT3 and STAT1 in intestinal epithelial cells leads to an increase of epithelial proliferation and a decrease of goblet cells expressing accessible Ecad. As a consequence, Lm intestinal villus invasion via GCs (which is InlA/Ecad dependent) is blocked, and mucus barrier is altered (3).
The number of GCs and their differentiation are modulated upon infection with other enteropathogens. Indeed, C. rodentium leads to a depletion of GCs and a decrease in the thickness of the mucus layer during the first phase of the infection, which is reversed during the clearance of the infection (Gustafsson et al., 2013). C. rodentium and Salmonella typhimurium modulate GC number in an IFN-γ–dependent manner, and IFN-γ is expressed by CD4 T+ cells in the case of C. rodentium infection (Songhet et al., 2011; Chan et al., 2013; Klose et al., 2013). Lm translocation across intestinal epithelium at the villus level occurs in an InlA-dependent manner via luminally accessible Ecad on GCs (Nikitas et al., 2011). Decrease of WGA+ GC number expressing luminally accessible Ecad in response to Lm therefore selectively blocks InlA-dependent translocation of Lm at the villus level and constitutes a novel and specific innate immune mechanism against an enteropathogen (Fig. S1 A and Fig. 8).
This pathway, which is not initiated upon villus infection, is triggered at the PP level. This is in agreement with previously published results showing that host response to Lm at the intestinal level is fully dependent on Lm PP infection (Lecuit et al., 2007). Our results further illustrate that invasion of villi via GCs in an InlA-dependent manner is silent. This study underlines on one hand the relative unresponsiveness of the villus LP to pro-inflammatory stimuli and on the other hand the critical role of PP in danger detection and propagation of defense signals to the intestinal epithelium.
IL-22 induces epithelial proliferation through STAT3 activation during DSS-induced colitis (Pickert et al., 2009) and is expressed downstream of IL-23 in response to C. rodentium infection in the colon (Aychek et al., 2015). However, although epithelial proliferation is fully IL-23 and STAT3 dependent upon Lm infection, IL-22 only partially relays IL-23 signaling at the intestinal level. We have shown here that IL-17, which is produced by γδ T cells upon Lm infection (Hamada et al., 2008; Sheridan et al., 2013), induces Lm-associated enterocyte proliferation together with IL-22. IL-17 in turn triggers IL-11 expression by LP gp38+CD34+ stromal cells, which activates STAT3 in epithelial cells (Fig. 8). IL-11 is a known activator of STAT3, involved in gastrointestinal tumorigenesis in human and in mouse (Putoczki et al., 2013). IL-11 is also induced upon C. rodentium, by a so-far unknown mechanism and is involved in protection against TLR4-mediated colitis (Gibson et al., 2010). Whereas inhibition of IL-11 does not exacerbate Lm burden in systemic listeriosis (Opal et al., 2000), the role of IL-11 during Lm oral infection had so far not been investigated. Several cell types have been described to express IL-11 in the intestine (Putoczki and Ernst, 2010). We show here that LP gp38+CD34+ stromal cells, a mesenchymal cell subset in close contact with the intestinal crypts, are the major intestinal source of IL-11 upon Lm infection. Several evidences indicate that mesenchymal stromal cells are involved in mucosal immune responses, notably via IL-33 (Owens and Simmons, 2013; Mahapatro et al., 2016). Here, we have uncovered that gp38+CD34+ stromal cells are part of a host response pathway involving CX3CR1-derived IL-23 and γδ T–derived IL-17, which leads to intestinal epithelial cells proliferation and depletion of GCs expressing accessible Ecad in a STAT3-dependent manner in response to an enteric infection. The partially redundant and complementary roles of IL-11 and IL-22 upon Lm infection remain to be characterized in more detail. We have shown that IL-11 effect is compensated by IL-22 in Il11ra−/− mice, but is unmasked when IL-22 is also absent. The induction of STAT3 via the respective receptors of IL-11 and IL-22 may allow the fine tuning in time and space of host tissue responses to infection (Ernst et al., 2014).
IL-22 and IL-11 are not sufficient to induce proliferation and GC depletion in noninfected mice, indicating that a cofactor induced by Lm infection is necessary. IFN-γ is expressed upon Lm infection (Andersson et al., 1998), and both IL-22 and IFN-γ are induced in an InlA-independent manner 24 h pi and peak 48 h pi upon Lm oral infection (Reynders et al., 2011), indicating that they may act together rather than sequentially. We could recapitulate ex vivo stem cells proliferation by simultaneously treating with IL-22 and IFN-γ intestinal organoids. These results indicate that IL-22/IL-11–dependent STAT3 phosphorylation is not sufficient to induce proliferation, which indeed requires STAT1 phosphorylation, in response to IFN-γ receptor engagement. In line with this finding, IL-22–dependent STAT3-induced proliferation is involved in vivo in tissue repair in a graft-versus-host tissue damage model (Lindemans et al., 2015) and DSS-induced colitis (Pickert et al., 2009; Geng et al., 2018). IL-22 and IFN-γ are also induced upon the protozoan Tritrichomonas musculis infection, which increases susceptibility to colorectal cancer induced in Apcmin/+ mice (Chudnovskiy et al., 2016).
WGA+ GC depletion and the decrease of luminally accessible Ecad constitute a specific and very efficient short-term host defense response to Lm infection, by locking its portal of entry (Fig. 8 and Fig. S5 I). But this response is also potentially detrimental for the host by reducing the thickness of the protective mucosal barrier all along the gut and especially in the colon where the mucus layer is highly organized. Decrease of mucus layer thickness has been shown to be associated with the development of colitis (Van der Sluis et al., 2006). In line with these results, we show here that Lm increases host susceptibility to colitis. This provides support to previous report on the association between Lm presence in the gut and the development of inflammatory bowel disease (IBD; Liu et al., 1995; Hugot et al., 2003; Miranda-Bautista et al., 2014). Decrease of mucus layer thickness has also been shown to be associated with colorectal cancer (Velcich et al., 2002) and Crohn’s disease (Niv, 2016). IL-23, IL-22, and IL-11 can promote tumor growth in a mouse model of colorectal tumorigenesis (Grivennikov et al., 2012; Putoczki et al., 2013) and are associated with IBD (Eken et al., 2014). Chronic Lm intestinal carriage is more likely in Western countries where refrigeration favors Lm food contamination and human exposure (Swaminathan and Gerner-Smidt, 2007) and may be associated with gallbladder infection (Begley et al., 2009; Charlier et al., 2014). This study suggests that chronic Lm intestinal carriage may favor both IBD and tumorigenesis, through both decreased mucus layer thickness and production of cytokines such as IL-23 and IL-11, in conjunction with host factor predisposing for these conditions. This hypothesis is in line with the highest incidence of IBD and colon cancer in Western countries (Ng et al., 2013; Kuipers et al., 2015). Assessing the biological and medical relevance of this correlation will require further epidemiological and experimental studies.
Materials and methods
Mice and infection
All the procedures were in agreement with the guidelines of the European Commission for the handling of laboratory animals, directive 86/609/EEC. They were approved by the ethical committees No. 59 under the number 2010-0020 and by the CETEA/CEEA No. 89 under the numbers HA0017, 2015-0014, and 2017-0057. Knock-in E16P mouse line was from our laboratory. Cx3cr1GFP mice (Jung et al., 2000; RRID: MGI:2670353) were provided by the Cryopréservation, Distribution, Typage et Archivage Animal Orléans, Orléans, France. Il23p19−/− mice (Ghilardi et al., 2004) were provided by Genentech through B. Ryffel (CDTA Orléans). Rag2−/−, Rag2−/−γc−/−, Rag2−/−Il17ra−/−, and Rag2−/−Il15−/− were provided by the laboratory of J. Di Santo. Il22−/− mice were provided by R. Flavell (Yale University School of Medicine, New Haven, CT) via C. Leclerc (Institut Pasteur, Paris, France; Zenewicz et al., 2007). Il11ra−/− mice were provided by T. Putoczki (University of Melbourne, Parkville, Australia; Nandurkar et al., 1997). Stat3fl/fl Villin-cre mice are from Stat3fl/fl mice (Takeda et al., 1998) crossed with Villin-Cre (Cre-Ert2) mice (El Marjou et al., 2004). C57BL/6J were provided by Janvier Laboratories. 6–12-wk-old animals were used.
Littermates were used (KIE16P, STAT3IEC, and KIE16P STAT3IEC mice; Il11ra+/−, Il11ra−/−, CX3CR1GFP/+, and CX3CR1GFP/GFP mice; as well as Il23p19−/− mice treated with rmIL-11, rmIL-22, rm-IL-17, and anti–IL-22 and Rag−/− Il15−/− mice treated with anti-NK1.1). When necessary, control C57BL/6J were housed in the same animal facility at least 2 wk before being handling for experiments to avoid a bias coming from microbiota differences and mixed with the other animals in the same cage if possible. In each experiment, control KIE16P or C57BL/6J were used to have an internal control. Germ-free mice were housed in dedicated isolators.
For infection, Lm (EGD, EGDe, and isogenic mutants) fresh colonies were diluted in brain–heart infusion medium to reach mid-log growth phase. For intragastric gavage, 200 µl of bacteria in PBS were mixed with 300 µl of CaCO3 (50 mg/ml–1) before injection in overnight starved animals. For BrdU assays, 150 µl of BrdU-labeling reagent solution (10373283; Life Technologies) were injected i.p. 16 h before sacrifice. To monitor bacterial burden, organs were removed from infected killed animals and homogenized. Before homogenization, intestines were rinsed twice with DMEM and incubated for 2 h at room temperature in DMEM supplemented with gentamicin (100 mg/ml; Sigma-Aldrich). Serial dilutions of cell suspensions in PBS were plated on brain–heart infusion agar plates. After 24 h of incubation at 37°C, CFUs were counted.
For in vivo IL-22 blocking experiments, mice were injected i.p. either with PBS (control) or with 0.3 mg purified anti–IL-22 (AM22.1 provided by J.C. Renauld and L. Dumoutier, Université Catholique de Louvain, Brussels, Belgium) at days −3 and 0 of infection. To deplete NK1.1+ cells, control and Lm-infected Rag2−/−il15−/− mice were injected i.p. with 100 µg of anti-NK1.1 antibody (PK136; Biolegend) in 0.1 ml PBS 1 d before Lm injection.
For in vivo cytokine treatment, mice were injected with 5 ng/g of mouse recombinant IL-22 (R&D; 582-ML-CF) or mouse recombinant IL-11 (R&D; 418-ML-CF) or mouse recombinant IL-17 (R&D; 421-ML-CF) at day −1, 0, 1, 2, and 3 after injection.
Hydroxytamoxifen treatment
Hydroxytamoxifen was dissolved in corn oil at 10 mg/ml. Stat3fl/fl Villin-cre mice were treated for five consecutive days with 1 mg/d, as previously described (Indra et al., 1999), 7 d before infection.
Bone marrow engraftment
Total bone marrow cells were removed from legs (femora and tibiae) of donor WT mice and intravenously injected into 5-wk-old lethally irradiated (1,250 rad) host mice (one donor for four recipient mice) to generate bone marrow chimera. After 10–12 wk of bone marrow reconstitution, chimeras were used to perform experiments.
Biotin penetration experiment
Biotin was used as a molecule to address the integrity of intestinal epithelium as described previously (Tsai et al., 2013). In brief, 2 mg/ml of EZ-link Sulfo-NHS-Biotin (Pierce) in PBS was slowly injected into the lumen of ileum loop via the open end adjacent to cecum immediately after removal of the entire ileum. After 3 min, the loop was opened and followed by PBS wash and 4% paraformaldehyde (PFA) fixation.
Ligated loops
8–12-wk-old mice were fasted for 16 h before surgery. 30 min after injection of the buprenorphine (0.1 mg/kg body weight), deep anesthesia was induced with a mix of ketamine (100 mg/kg body weight; Imalgene 1000; Merial), and xylazine chlorhydrate (10 mg/kg body weight; Rompun; Bayer). Skin was cleaned with Vetadine, a laparotomy was performed, the small intestine was exposed, and ileal loops of 1.5-cm long containing or not containing one PP were prepared. 100 µl of inoculum (105 CFUs) was injected into each loop. Loop was placed back in the peritoneal cavity. PBS was injected subcutaneously to prevent dehydration and BrdU was injected intraperitoneally. Peritoneal membrane and skin were sutured, and animals were returned to their warmed cage. Water was allowed, but not food. 24 h pi, mice were killed. Intestinal loops were harvested, washed in PBS, and fixed in 4% PFA overnight. Tissue staining is described below.
Quantitative real-time PCR (qRT-PCR)
We performed RNA isolation and qRT-PCR as previously described (Dulauroy et al., 2012). In brief, we extracted total RNA from FACS-sorted cells using RNeasy Micro kit (Qiagen) and assessed the quality of total RNA using the 2100 Bioanalyzer system (Agilent Technologies). Transcription was performed using Superscript III reverse transcription (Invitrogen). All procedures were performed according to the manufacturers’ protocols. We performed qRT-PCR using RT2-qPCR primer sets (SABiosciences) and RT2 SYBR-green master mix (SABiosciences) on a PTC-200 thermocycler equipped with a Chromo4 detector (Bio-Rad Laboratories) and analyzed data using Opticon Monitor software (Bio-Rad Laboratories). We normalized Ct values to the mean Ct values obtained for the housekeeping genes Hsp90ab1, Hprt, and Gapdh.
Cell isolation, flow cytometry analysis, and sorting
Isolation of intestinal myeloid and stromal cells were performed as previously described (Satoh-Takayama et al., 2008; Stzepourginski et al., 2015). Cells were blocked and stained with indicated antibodies: CD3 (clone 145-2C11; eBioscience); CD19 (clone 1D3; BD); NKp46 (clone 29A1.4; eBioscience); IFN-γ (clone XMG1.2; BD); NK1.1 (clone PK136; Biolegend); and CD31 (clone 390; eBioscience). Syrian hamster antibody to gp38 was a gift from A. Farr (University of Washington, Seattle, WA).
Cells were analyzed with an LSRFortessa flow cytometer (BD) or sorted with a FACSAria machine (BD). Flow cytometry analysis was done with the FlowJo software (Tree Star). Sorted stromal CD45−gp38+ cells were grown in DMEM containing 10% fetal calf serum at 150,000 cells/well in 24-well plates coated with rat tail collagen (10 µg/cm2). Cells were treated 4 d after sorting with the mouse recombinant IL-17 (R&D; 421-ML) or mouse recombinant IL-23 (R&D; 1887-ML) for 24 h.
Ex vivo organoid culture
Intestinal organoids were obtained as previously described (Sato et al., 2009). Organoids were treated as follows: mouse recombinant IL-11 (R&D; 418-ML) at 50 ng/ml; mouse recombinant IL-22 (R&D; 582-ML) at 50 ng/ml; IFN-γ (eBiosciences 34-8311-85) at 50 ng/ml for 24 h followed by 16 h of BrdU staining (1/100 dilution of the BrdU labeling reagent; 10373283; Life Technologies). For STAT inhibition, fludarabine was added at 50 mM 24 h before cytokine treatment in WT organoid. For STAT3 depletion, Stat3fl/fl Villin-cre organoids were treated with hydroxytamoxifen 24 h before cytokine treatment.
Immunofluorescence staining
Tissues and organoids were labeled as previously described (Disson et al., 2009; Mahe et al., 2013). Intestine or colon was removed from cervical dislocated animals, flushed, and fixed in 4% PFA overnight. Tissues were then washed in PBS and (1) embedded in agarose for 150-µm-thick vibratome section or (2) cryoprotected in sucrose 30%, embedded in optimal cutting temperature compound and nitrogen frozen for 8-µm-thin cryostat section. Organoids were fixed for 30 min in PFA 4% and washed in PBS. For BrdU staining, samples were incubated sequentially in HCl 1N for 10 min at 4°C, HCl 2N for 10 min at room temperature, HCl 2N for 20 min at 37°C, buffered with borate buffer (0.1 M) for 12 min at room temperature, and washed in PBS. Tissues were permeabilized and blocked with 3% BSA in PBS containing 0.4% (thin sections) or 1% (thick sections and organoids) Triton X-100 for at least 1 h. Tissues were then probed with the appropriate primary and secondary antibodies at least 1 h at room temperature or overnight at 4°C. Sections were then mounted in fluorescent mounting medium (Fluoromount G; Beckman Coulter) and dried at 37°C for 30 min. For staining of accessible Ecad, whole mount intestine tissues were labeled with ECCD-2 in PBS BSA 3% as previously described (Nikitas et al., 2011). R12 anti-Lm rabbit polyclonal antibody was provided by P. Cossart (Institut Pasteur, Paris, France). Anti-Ki67 was obtained from Abcam. Anti BrdU clone MoBU-1 and anti-Ecad clone ECCD-2, Alexa Fluor 647 Phalloidin, Alexa Fluor 647, Alexa Fluor 488 WGA, Alexa Fluor 546 and 647 goat anti-rabbit, Alexa Fluor 488 and 647 goat anti-rat, and Hoechst 33342 are from Life Technologies.
For 16S staining, tissues were fixed in Carnoy solution for 24 h and then cut, deparaffinized, and treated as previously described (Canny et al., 2006). For Muc2 staining, tissues were fixed in Carnoy solution for 24 h and then cut and deparaffinized. Sections were boiled in 0.01 M citric acid buffer, pH 6, to retrieve the antigens and were permeabilized, blocked, and stained with the anti-MUC2C3, a gift from G. Hansson, Institute of Biomedicine, Gotheburg, Sweden, at 4°C overnight (Johansson et al., 2008).
TUNEL assay was performed according to manufacturer instructions (click-iT Plus TUNEL Assay; Molecular Probes).
Sections were imaged with an inverted Axiobserver or LSM710 confocal Zeiss microscope (20× or 40× oil objectives). BrdU fold increase was calculated by dividing the distance between basal and upper BrdU+ cells by the length of the villus. When indicated, this ratio was normalized by noninfected animals (PBS) of the experiment to normalize for inter-experiments variations. For each experiment, two to three infected animals and one to two control noninfected animals were tested. Experiments were done in duplicate or triplicate. 5–10 villi were counted for each animal. For organoid, BrdU quantification was obtained by measuring the number of BrdU pixels from three-dimensional acquisition with Icy (de Chaumont et al., 2012), divided by the number of crypts (script in Fig. S4 I). All acquisitions and quantifications were done in a blinded manner.
Immunoblot
Tissue was lysed in lysis buffer containing radioimmunoprecipitation assay 1× (Sigma-Aldrich), anti-phosphatase, and anti-protease (Roche). Protein concentration from total protein extracts was quantified using the BCA Protein Assay kit (Thermo Fisher Scientific). 20 µg of protein for each sample was boiled for 5 min in NuPage buffer (Invitrogen) with reducing agent (Invitrogen) and radioimmunoprecipitation assay 1×. Samples were then subjected to SDS-PAGE and transferred onto polyvinylidene fluoride membranes (Millipore). Membranes were activated by absolute ethanol, followed by electro-transfer and saturation with PBS supplemented with 0.1% Tween 20 (Sigma-Aldrich) and 5% nonfat milk. Membranes were incubated with primary antibodies: rabbit anti–phospho-Stat3 (Tyr705; EP2147Y; ab76315; Abcam), mouse anti–phospho-Stat1 (Tyr701; M135; ab29045; Abcam), and mouse anti–β-Actin (clone AC-15; A1978; Sigma-Aldrich). Membranes were washed three times in PBS supplemented with 0.1% Tween 20 (Sigma-Aldrich), incubated with mouse (NA931; GE Healthcare) or rabbit (NA934; GE Healthcare) secondary antibody coupled to peroxidase, washed three times in PBS supplemented with 0.1% Tween 20 (Sigma-Aldrich), and revealed by chemiluminescence with ECL Western blotting detection system (GE Healthcare). Images were acquired and quantified with the PXi4 GeneSys software version 1.3.9.0.
ELISA
IFN-γ, IL-12, and IL-11 were measured with ELISA kits from Abcam from supernatant of intestinal tissue or in vitro cells in culture. Assays were performed according to the manufacturers’ instructions.
Histological analysis of DSS-treated mice
DSS was added to drinking water at a concentration of 2.5% 36–50 kD DSS (MP Biomedicals) for seven consecutive days (Chassaing et al., 2014) after Lm infection. Histopathological examination of colon was done after fixation of the tissues in PFA 4% and embedding in paraffin. Tissue sections were cut and stained with hematoxylin and eosin. All slides were coded and evaluated in a blinded manner as previously described (Chassaing et al., 2014). In brief, each section was assigned four scores based on the degree of epithelial damage and inflammatory infiltration into the mucosa, submucosa, and muscularis/serosa (0–3). Each of the four scores was multiplied by 1 if the change was focal, 2 if it was patchy, and 3 if it was diffuse. The four individual scores per colon were added, resulting in a total scoring range of 0–36 per mouse.
Statistical analysis
Student’s t tests, nonparametric Mann-Whitney, or one-way ANOVA tests were routinely used for statistical analysis. Figure legends show the minimum number of mice used in the experiment and the statistical test used. Statistical significance is expressed as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Online supplemental material
Fig. S1 shows that Lm-induced epithelial cell proliferation is Lm strain independent, is not associated to barrier damage, is dose-dependent, and is initiated at the PP level, but not at the villus level. Fig. S2 illustrates STAT3’s role on WGA+ GC number decrease and shows that the phenotype is reversible and LLO dependent, but InlA independent. It shows that infected cells are PP CX3CR1+ myeloid cells, which express IL-23 upon infection. Fig. S3 defines the studied gp38+ CD45− CD31− stromal cells population. Fig. S4 shows that IFN-γ is expressed by sNK cells in a CX3CR1-dependent, but IL-23–independent, manner. It also shows that STAT-3 phosphorylation in ex vivo organoid is IL-11 or IL-22 dependent, while STAT1 phosphorylation is IFN-γ dependent. It illustrates the script used to quantify BrdU incorporation in organoids. Fig. S5 shows that Lm-induced epithelial cell proliferation is observed in colon, where the mucus layer is smaller. It also illustrates the model we propose.
Supplementary Material
Acknowledgments
We thank the members of the Biology of Infection Unit for their support. We thank Hélène Bierne for her implication in the initial phase of the project, Elena Tomasello and Eric Vivier for helpful discussions, Stéphane Dallongeville for image analysis, Sascha Cording, Morgane Lavina for technical help, and Hana Kammoun for critical reading. We thank Edith Gouin and Pascale Cossart for anti-Lm antibodies, Tracy Putoczki for Il11ra1−/− mice, Richard Flavell for Il22−/− mice, Bernhard Ryffel for IL23p19−/− mice, Laure Dumoutier, Jean-Christophe Renauld for anti–IL-22 antibody, and Gunnar C. Hansson for anti-MUC2C3 serum.
This work was financed by Institut Pasteur, Institut National de la Santé et de la Recherche Médicale, Institut Carnot Pasteur Microbes et Santé, LabEx IBEID, the European Research Council grant Invadis, and the Agence Nationale de Recherche Organolist.
The authors declare no competing financial interests.
Author contributions: conceptualization: O. Disson and M. Lecuit; methodology: O. Disson, G. Eberl, J.P. Di Santo, L. Peduto, and M. Lecuit; investigation: O. Disson, C. Blériot, J.-M. Jacob, N. Serafini, G. Gessain, C. Fevre, P. Thouvenot, G. Jouvion, and S. Dulauroy; writing (original draft): O. Disson and M. Lecuit; writing (review and editing): C. Blériot, N. Serafini, G. Eberl, J.P. Di Santo, and L. Peduto; funding acquisition: M. Lecuit; supervision: M. Lecuit; additional mentorship and feedback: G. Eberl, J.P. Di Santo, and L. Peduto.
References
- Andersson A., Dai W.J., Di Santo J.P., and Brombacher F.. 1998. Early IFN-gamma production and innate immunity during Listeria monocytogenes infection in the absence of NK cells. J. Immunol. 161:5600–5606. [PubMed] [Google Scholar]
- Andoh A., Zhang Z., Inatomi O., Fujino S., Deguchi Y., Araki Y., Tsujikawa T., Kitoh K., Kim-Mitsuyama S., Takayanagi A., et al. . 2005. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 129:969–984. 10.1053/j.gastro.2005.06.071 [DOI] [PubMed] [Google Scholar]
- Aparicio-Domingo P., Romera-Hernandez M., Karrich J.J., Cornelissen F., Papazian N., Lindenbergh-Kortleve D.J., Butler J.A., Boon L., Coles M.C., Samsom J.N., and Cupedo T.. 2015. Type 3 innate lymphoid cells maintain intestinal epithelial stem cells after tissue damage. J. Exp. Med. 212:1783–1791. 10.1084/jem.20150318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asker N., Axelsson M.A., Olofsson S.O., and Hansson G.C.. 1998. Dimerization of the human MUC2 mucin in the endoplasmic reticulum is followed by a N-glycosylation-dependent transfer of the mono- and dimers to the Golgi apparatus. J. Biol. Chem. 273:18857–18863. 10.1074/jbc.273.30.18857 [DOI] [PubMed] [Google Scholar]
- Aychek T., Mildner A., Yona S., Kim K.W., Lampl N., Reich-Zeliger S., Boon L., Yogev N., Waisman A., Cua D.J., and Jung S.. 2015. IL-23-mediated mononuclear phagocyte crosstalk protects mice from Citrobacter rodentium-induced colon immunopathology. Nat. Commun. 6:6525 10.1038/ncomms7525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamba S., Andoh A., Yasui H., Makino J., Kim S., and Fujiyama Y.. 2003. Regulation of IL-11 expression in intestinal myofibroblasts: role of c-Jun AP-1- and MAPK-dependent pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 285:G529–G538. 10.1152/ajpgi.00050.2003 [DOI] [PubMed] [Google Scholar]
- Barker N. 2014. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 15:19–33. 10.1038/nrm3721 [DOI] [PubMed] [Google Scholar]
- Barker N., van de Wetering M., and Clevers H.. 2008. The intestinal stem cell. Genes Dev. 22:1856–1864. 10.1101/gad.1674008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley M., Kerr C., and Hill C.. 2009. Exposure to bile influences biofilm formation by Listeria monocytogenes. Gut Pathog. 1:11 10.1186/1757-4749-1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnardel J., Da Silva C., Henri S., Tamoutounour S., Chasson L., Montañana-Sanchis F., Gorvel J.P., and Lelouard H.. 2015. Innate and adaptive immune functions of peyer’s patch monocyte-derived cells. Cell Reports. 11:770–784. 10.1016/j.celrep.2015.03.067 [DOI] [PubMed] [Google Scholar]
- Brooling J.T., Campbell J.S., Mitchell C., Yeoh G.C., and Fausto N.. 2005. Differential regulation of rodent hepatocyte and oval cell proliferation by interferon gamma. Hepatology. 41:906–915. 10.1002/hep.20645 [DOI] [PubMed] [Google Scholar]
- Buchon N., Broderick N.A., Poidevin M., Pradervand S., and Lemaitre B.. 2009. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 5:200–211. 10.1016/j.chom.2009.01.003 [DOI] [PubMed] [Google Scholar]
- Buchon N., Broderick N.A., and Lemaitre B.. 2013. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nat. Rev. Microbiol. 11:615–626. 10.1038/nrmicro3074 [DOI] [PubMed] [Google Scholar]
- Buonocore S., Ahern P.P., Uhlig H.H., Ivanov I.I., Littman D.R., Maloy K.J., and Powrie F.. 2010. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 464:1371–1375. 10.1038/nature08949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canny G., Swidsinski A., and McCormick B.A.. 2006. Interactions of intestinal epithelial cells with bacteria and immune cells: methods to characterize microflora and functional consequences. Methods Mol. Biol. 341:17–35. [DOI] [PubMed] [Google Scholar]
- Chan J.M., Bhinder G., Sham H.P., Ryz N., Huang T., Bergstrom K.S., and Vallance B.A.. 2013. CD4+ T cells drive goblet cell depletion during Citrobacter rodentium infection. Infect. Immun. 81:4649–4658. 10.1128/IAI.00655-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier C., Fevre C., Travier L., Cazenave B., Bracq-Dieye H., Podevin J., Assomany D., Guilbert L., Bossard C., Carpentier F., et al. . 2014. Listeria monocytogenes-associated biliary tract infections: a study of 12 consecutive cases and review. Medicine (Baltimore). 93:e105 10.1097/MD.0000000000000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier C., Perrodeau É., Leclercq A., Cazenave B., Pilmis B., Henry B., Lopes A., Maury M.M., Moura A., Goffinet F., et al. MONALISA study group . 2017. Clinical features and prognostic factors of listeriosis: the MONALISA national prospective cohort study. Lancet Infect. Dis. 17:510–519. 10.1016/S1473-3099(16)30521-7 [DOI] [PubMed] [Google Scholar]
- Chassaing B., Aitken J.D., Malleshappa M., and Vijay-Kumar M.. 2014. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 104:Unit 15.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Nagai T., Hayashi T., Baba Y., Nagai S., and Koyasu S.. 2011. Listerial invasion protein internalin B promotes entry into ileal Peyer’s patches in vivo. Microbiol. Immunol. 55:123–129. 10.1111/j.1348-0421.2010.00292.x [DOI] [PubMed] [Google Scholar]
- Chudnovskiy A., Mortha A., Kana V., Kennard A., Ramirez J.D., Rahman A., Remark R., Mogno I., Ng R., Gnjatic S., et al. . 2016. Host-Protozoan Interactions Protect from Mucosal Infections through Activation of the Inflammasome. Cell. 167:444–456.e14. 10.1016/j.cell.2016.08.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J.W., Keeney K.M., Crepin V.F., Rathinam V.A., Fitzgerald K.A., Finlay B.B., and Frankel G.. 2014. Citrobacter rodentium: infection, inflammation and the microbiota. Nat. Rev. Microbiol. 12:612–623. 10.1038/nrmicro3315 [DOI] [PubMed] [Google Scholar]
- Cuylen S., Blaukopf C., Politi A.Z., Müller-Reichert T., Neumann B., Poser I., Ellenberg J., Hyman A.A., and Gerlich D.W.. 2016. Ki-67 acts as a biological surfactant to disperse mitotic chromosomes. Nature. 535:308–312. 10.1038/nature18610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chaumont F., Dallongeville S., Chenouard N., Hervé N., Pop S., Provoost T., Meas-Yedid V., Pankajakshan P., Lecomte T., Le Montagner Y., et al. . 2012. Icy: an open bioimage informatics platform for extended reproducible research. Nat. Methods. 9:690–696. 10.1038/nmeth.2075 [DOI] [PubMed] [Google Scholar]
- Disson O., Grayo S., Huillet E., Nikitas G., Langa-Vives F., Dussurget O., Ragon M., Le Monnier A., Babinet C., Cossart P., and Lecuit M.. 2008. Conjugated action of two species-specific invasion proteins for fetoplacental listeriosis. Nature. 455:1114–1118. 10.1038/nature07303 [DOI] [PubMed] [Google Scholar]
- Disson O., Nikitas G., Grayo S., Dussurget O., Cossart P., and Lecuit M.. 2009. Modeling human listeriosis in natural and genetically engineered animals. Nat. Protoc. 4:799–810. 10.1038/nprot.2009.66 [DOI] [PubMed] [Google Scholar]
- Dulauroy S., Di Carlo S.E., Langa F., Eberl G., and Peduto L.. 2012. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat. Med. 18:1262–1270. 10.1038/nm.2848 [DOI] [PubMed] [Google Scholar]
- Eken A., Singh A.K., and Oukka M.. 2014. Interleukin 23 in Crohn’s disease. Inflamm. Bowel Dis. 20:587–595. 10.1097/01.MIB.0000442014.52661.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Marjou F., Janssen K.P., Chang B.H., Li M., Hindie V., Chan L., Louvard D., Chambon P., Metzger D., and Robine S.. 2004. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 39:186–193. 10.1002/gene.20042 [DOI] [PubMed] [Google Scholar]
- Ernst M., Thiem S., Nguyen P.M., Eissmann M., and Putoczki T.L.. 2014. Epithelial gp130/Stat3 functions: an intestinal signaling node in health and disease. Semin. Immunol. 26:29–37. 10.1016/j.smim.2013.12.006 [DOI] [PubMed] [Google Scholar]
- Fischer J., Klein P.J., Vierbuchen M., Skutta B., Uhlenbruck G., and Fischer R.. 1984. Characterization of glycoconjugates of human gastrointestinal mucosa by lectins. I. Histochemical distribution of lectin binding sites in normal alimentary tract as well as in benign and malignant gastric neoplasms. J. Histochem. Cytochem. 32:681–689. 10.1177/32.7.6330198 [DOI] [PubMed] [Google Scholar]
- Frank D.A., Mahajan S., and Ritz J.. 1999. Fludarabine-induced immunosuppression is associated with inhibition of STAT1 signaling. Nat. Med. 5:444–447. 10.1038/7445 [DOI] [PubMed] [Google Scholar]
- Geng H., Bu H.F., Liu F., Wu L., Pfeifer K., Chou P.M., Wang X., Sun J., Lu L., Pandey A., et al. . 2018. In Inflamed Intestinal Tissues and Epithelial Cells, Interleukin 22 Signaling Increases Expression of H19 Long Noncoding RNA, Which Promotes Mucosal Regeneration. Gastroenterology. 155:144–155. 10.1053/j.gastro.2018.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessain G., Tsai Y.H., Travier L., Bonazzi M., Grayo S., Cossart P., Charlier C., Disson O., and Lecuit M.. 2015. PI3-kinase activation is critical for host barrier permissiveness to Listeria monocytogenes. J. Exp. Med. 212:165–183. 10.1084/jem.20141406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghilardi N., Kljavin N., Chen Q., Lucas S., Gurney A.L., and De Sauvage F.J.. 2004. Compromised humoral and delayed-type hypersensitivity responses in IL-23-deficient mice. J. Immunol. 172:2827–2833. 10.4049/jimmunol.172.5.2827 [DOI] [PubMed] [Google Scholar]
- Gibson D.L., Ma C., Bergstrom K.S., Huang J.T., Man C., and Vallance B.A.. 2008. MyD88 signalling plays a critical role in host defence by controlling pathogen burden and promoting epithelial cell homeostasis during Citrobacter rodentium-induced colitis. Cell. Microbiol. 10:618–631. 10.1111/j.1462-5822.2007.01071.x [DOI] [PubMed] [Google Scholar]
- Gibson D.L., Montero M., Ropeleski M.J., Bergstrom K.S., Ma C., Ghosh S., Merkens H., Huang J., Månsson L.E., Sham H.P., et al. . 2010. Interleukin-11 reduces TLR4-induced colitis in TLR2-deficient mice and restores intestinal STAT3 signaling. Gastroenterology. 139:1277–1288. 10.1053/j.gastro.2010.06.057 [DOI] [PubMed] [Google Scholar]
- Gonneaud A., Turgeon N., Boudreau F., Perreault N., Rivard N., and Asselin C.. 2016. Distinct Roles for Intestinal Epithelial Cell-Specific Hdac1 and Hdac2 in the Regulation of Murine Intestinal Homeostasis. J. Cell. Physiol. 231:436–448. 10.1002/jcp.25090 [DOI] [PubMed] [Google Scholar]
- Graham A.C., Carr K.D., Sieve A.N., Indramohan M., Break T.J., and Berg R.E.. 2011. IL-22 production is regulated by IL-23 during Listeria monocytogenes infection but is not required for bacterial clearance or tissue protection. PLoS One. 6:e17171 10.1371/journal.pone.0017171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S.I., Wang K., Mucida D., Stewart C.A., Schnabl B., Jauch D., Taniguchi K., Yu G.Y., Osterreicher C.H., Hung K.E., et al. . 2012. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 491:254–258. 10.1038/nature11465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson J.K., Navabi N., Rodriguez-Piñeiro A.M., Alomran A.H., Premaratne P., Fernandez H.R., Banerjee D., Sjövall H., Hansson G.C., and Lindén S.K.. 2013. Dynamic changes in mucus thickness and ion secretion during Citrobacter rodentium infection and clearance. PLoS One. 8:e84430 10.1371/journal.pone.0084430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Umemura M., Shiono T., Tanaka K., Yahagi A., Begum M.D., Oshiro K., Okamoto Y., Watanabe H., Kawakami K., et al. . 2008. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J. Immunol. 181:3456–3463. 10.4049/jimmunol.181.5.3456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon M.A., Ribet D., Stavru F., and Cossart P.. 2012. Listeriolysin O: the Swiss army knife of Listeria. Trends Microbiol. 20:360–368. 10.1016/j.tim.2012.04.006 [DOI] [PubMed] [Google Scholar]
- Harty J.T., and Bevan M.J.. 1995. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 3:109–117. 10.1016/1074-7613(95)90163-9 [DOI] [PubMed] [Google Scholar]
- Hugot J.P., Alberti C., Berrebi D., Bingen E., and Cézard J.P.. 2003. Crohn’s disease: the cold chain hypothesis. Lancet. 362:2012–2015. 10.1016/S0140-6736(03)15024-6 [DOI] [PubMed] [Google Scholar]
- Indra A.K., Warot X., Brocard J., Bornert J.M., Xiao J.H., Chambon P., and Metzger D.. 1999. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 27:4324–4327. 10.1093/nar/27.22.4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y., Hayashi T., Goto T., Kimura A., Akimoto S., Mukaida N., and Kondo T.. 2008. Essential involvement of CX3CR1-mediated signals in the bactericidal host defense during septic peritonitis. J. Immunol. 181:4208–4218. 10.4049/jimmunol.181.6.4208 [DOI] [PubMed] [Google Scholar]
- Iwai H., Kim M., Yoshikawa Y., Ashida H., Ogawa M., Fujita Y., Muller D., Kirikae T., Jackson P.K., Kotani S., and Sasakawa C.. 2007. A bacterial effector targets Mad2L2, an APC inhibitor, to modulate host cell cycling. Cell. 130:611–623. 10.1016/j.cell.2007.06.043 [DOI] [PubMed] [Google Scholar]
- Jensen V.B., Harty J.T., and Jones B.D.. 1998. Interactions of the invasive pathogens Salmonella typhimurium, Listeria monocytogenes, and Shigella flexneri with M cells and murine Peyer’s patches. Infect. Immun. 66:3758–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M.E., Phillipson M., Petersson J., Velcich A., Holm L., and Hansson G.C.. 2008. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA. 105:15064–15069. 10.1073/pnas.0803124105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S., Aliberti J., Graemmel P., Sunshine M.J., Kreutzberg G.W., Sher A., and Littman D.R.. 2000. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20:4106–4114. 10.1128/MCB.20.11.4106-4114.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose C.S., Kiss E.A., Schwierzeck V., Ebert K., Hoyler T., d’Hargues Y., Göppert N., Croxford A.L., Waisman A., Tanriver Y., and Diefenbach A.. 2013. A T-bet gradient controls the fate and function of CCR6-RORγt+ innate lymphoid cells. Nature. 494:261–265. 10.1038/nature11813 [DOI] [PubMed] [Google Scholar]
- Kuipers E.J., Grady W.M., Lieberman D., Seufferlein T., Sung J.J., Boelens P.G., van de Velde C.J., and Watanabe T.. 2015. Colorectal cancer. Nat. Rev. Dis. Primers. 1:15065 10.1038/nrdp.2015.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit M., Vandormael-Pournin S., Lefort J., Huerre M., Gounon P., Dupuy C., Babinet C., and Cossart P.. 2001. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science. 292:1722–1725. 10.1126/science.1059852 [DOI] [PubMed] [Google Scholar]
- Lecuit M., Sonnenburg J.L., Cossart P., and Gordon J.I.. 2007. Functional genomic studies of the intestinal response to a foodborne enteropathogen in a humanized gnotobiotic mouse model. J. Biol. Chem. 282:15065–15072. 10.1074/jbc.M610926200 [DOI] [PubMed] [Google Scholar]
- Lindemans C.A., Calafiore M., Mertelsmann A.M., O’Connor M.H., Dudakov J.A., Jenq R.R., Velardi E., Young L.F., Smith O.M., Lawrence G., et al. . 2015. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 528:560–564. 10.1038/nature16460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., van Kruiningen H.J., West A.B., Cartun R.W., Cortot A., and Colombel J.F.. 1995. Immunocytochemical evidence of Listeria, Escherichia coli, and Streptococcus antigens in Crohn’s disease. Gastroenterology. 108:1396–1404. 10.1016/0016-5085(95)90687-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longman R.S., Diehl G.E., Victorio D.A., Huh J.R., Galan C., Miraldi E.R., Swaminath A., Bonneau R., Scherl E.J., and Littman D.R.. 2014. CX3CR1+ mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J. Exp. Med. 211:1571–1583. 10.1084/jem.20140678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahapatro M., Foersch S., Hefele M., He G.W., Giner-Ventura E., Mchedlidze T., Kindermann M., Vetrano S., Danese S., Günther C., et al. . 2016. Programming of Intestinal Epithelial Differentiation by IL-33 Derived from Pericryptal Fibroblasts in Response to Systemic Infection. Cell Reports. 15:1743–1756. 10.1016/j.celrep.2016.04.049 [DOI] [PubMed] [Google Scholar]
- Mahe M.M., Aihara E., Schumacher M.A., Zavros Y., Montrose M.H., Helmrath M.A., Sato T., and Shroyer N.F.. 2013. Establishment of Gastrointestinal Epithelial Organoids. Curr. Protoc. Mouse Biol. 3:217–240. 10.1002/9780470942390.mo130179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manta C., Heupel E., Radulovic K., Rossini V., Garbi N., Riedel C.U., and Niess J.H.. 2013. CX(3)CR1(+) macrophages support IL-22 production by innate lymphoid cells during infection with Citrobacter rodentium. Mucosal Immunol. 6:177–188. 10.1038/mi.2012.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimuro H., Suzuki T., Tanaka J., Asahi M., Haas R., and Sasakawa C.. 2002. Grb2 is a key mediator of helicobacter pylori CagA protein activities. Mol. Cell. 10:745–755. 10.1016/S1097-2765(02)00681-0 [DOI] [PubMed] [Google Scholar]
- Miranda-Bautista J., Padilla-Suárez C., Bouza E., Muñoz P., Menchén L., and Marín-Jiménez I.. 2014. Listeria monocytogenes infection in inflammatory bowel disease patients: case series and review of the literature. Eur. J. Gastroenterol. Hepatol. 26:1247–1252. 10.1097/MEG.0000000000000188 [DOI] [PubMed] [Google Scholar]
- Nandurkar H.H., Robb L., Tarlinton D., Barnett L., Köntgen F., and Begley C.G.. 1997. Adult mice with targeted mutation of the interleukin-11 receptor (IL11Ra) display normal hematopoiesis. Blood. 90:2148–2159. [PubMed] [Google Scholar]
- Ng S.C., Bernstein C.N., Vatn M.H., Lakatos P.L., Loftus E.V. Jr., Tysk C., O’Morain C., Moum B., and Colombel J.F.. Epidemiology and Natural History Task Force of the International Organization of Inflammatory Bowel Disease (IOIBD) . 2013. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 62:630–649. 10.1136/gutjnl-2012-303661 [DOI] [PubMed] [Google Scholar]
- Nikitas G., Deschamps C., Disson O., Niault T., Cossart P., and Lecuit M.. 2011. Transcytosis of Listeria monocytogenes across the intestinal barrier upon specific targeting of goblet cell accessible E-cadherin. J. Exp. Med. 208:2263–2277. 10.1084/jem.20110560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv Y. 2016. Mucin Genes Expression in the Intestine of Crohn’s Disease Patients: a Systematic Review and Meta-analysis. J. Gastrointestin. Liver Dis. 25:351–357. [DOI] [PubMed] [Google Scholar]
- Nowarski R., Jackson R., Gagliani N., de Zoete M.R., Palm N.W., Bailis W., Low J.S., Harman C.C., Graham M., Elinav E., and Flavell R.A.. 2015. Epithelial IL-18 Equilibrium Controls Barrier Function in Colitis. Cell. 163:1444–1456. 10.1016/j.cell.2015.10.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayasu I., Hatakeyama S., Yamada M., Ohkusa T., Inagaki Y., and Nakaya R.. 1990. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 98:694–702. 10.1016/0016-5085(90)90290-H [DOI] [PubMed] [Google Scholar]
- Opal S.M., Keith J.C., Palardy J.E., and Parejo N.. 2000. Recombinant human interleukin-11 has anti-inflammatory actions yet does not exacerbate systemic Listeria infection. J. Infect. Dis. 181:754–756. 10.1086/315247 [DOI] [PubMed] [Google Scholar]
- Owens B.M., and Simmons A.. 2013. Intestinal stromal cells in mucosal immunity and homeostasis. Mucosal Immunol. 6:224–234. 10.1038/mi.2012.125 [DOI] [PubMed] [Google Scholar]
- Peduto L., Dulauroy S., Lochner M., Späth G.F., Morales M.A., Cumano A., and Eberl G.. 2009. Inflammation recapitulates the ontogeny of lymphoid stromal cells. J. Immunol. 182:5789–5799. 10.4049/jimmunol.0803974 [DOI] [PubMed] [Google Scholar]
- Pickert G., Neufert C., Leppkes M., Zheng Y., Wittkopf N., Warntjen M., Lehr H.A., Hirth S., Weigmann B., Wirtz S., et al. . 2009. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 206:1465–1472. 10.1084/jem.20082683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putoczki T., and Ernst M.. 2010. More than a sidekick: the IL-6 family cytokine IL-11 links inflammation to cancer. J. Leukoc. Biol. 88:1109–1117. 10.1189/jlb.0410226 [DOI] [PubMed] [Google Scholar]
- Putoczki T.L., Thiem S., Loving A., Busuttil R.A., Wilson N.J., Ziegler P.K., Nguyen P.M., Preaudet A., Farid R., Edwards K.M., et al. . 2013. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell. 24:257–271. 10.1016/j.ccr.2013.06.017 [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S., Paglino J., Eslami-Varzaneh F., Edberg S., and Medzhitov R.. 2004. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 118:229–241. 10.1016/j.cell.2004.07.002 [DOI] [PubMed] [Google Scholar]
- Reynders A., Yessaad N., Vu Manh T.P., Dalod M., Fenis A., Aubry C., Nikitas G., Escalière B., Renauld J.C., Dussurget O., et al. . 2011. Identity, regulation and in vivo function of gut NKp46+RORγt+ and NKp46+RORγt- lymphoid cells. EMBO J. 30:2934–2947. 10.1038/emboj.2011.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P.J., Tran Van Nhieu G., and Egile C.. 1999. Rupture of the intestinal epithelial barrier and mucosal invasion by Shigella flexneri. Clin. Infect. Dis. 28:466–475. 10.1086/515150 [DOI] [PubMed] [Google Scholar]
- Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., and Clevers H.. 2009. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 459:262–265. 10.1038/nature07935 [DOI] [PubMed] [Google Scholar]
- Satoh-Takayama N., Vosshenrich C.A., Lesjean-Pottier S., Sawa S., Lochner M., Rattis F., Mention J.J., Thiam K., Cerf-Bensussan N., Mandelboim O., et al. . 2008. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 29:958–970. 10.1016/j.immuni.2008.11.001 [DOI] [PubMed] [Google Scholar]
- Sheridan B.S., Romagnoli P.A., Pham Q.M., Fu H.H., Alonzo F. III, Schubert W.D., Freitag N.E., and Lefrançois L.. 2013. γδ T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity. 39:184–195. 10.1016/j.immuni.2013.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.G., Mitchell S.C., Nash T., and Rhind S.. 2000. Gamma interferon influences intestinal epithelial hyperplasia caused by Lawsonia intracellularis infection in mice. Infect. Immun. 68:6737–6743. 10.1128/IAI.68.12.6737-6743.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer F., and Bäckhed F.. 2013. The gut microbiota--masters of host development and physiology. Nat. Rev. Microbiol. 11:227–238. 10.1038/nrmicro2974 [DOI] [PubMed] [Google Scholar]
- Songhet P., Barthel M., Stecher B., Müller A.J., Kremer M., Hansson G.C., and Hardt W.D.. 2011. Stromal IFN-γR-signaling modulates goblet cell function during Salmonella Typhimurium infection. PLoS One. 6:e22459 10.1371/journal.pone.0022459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stzepourginski I., Eberl G., and Peduto L.. 2015. An optimized protocol for isolating lymphoid stromal cells from the intestinal lamina propria. J. Immunol. Methods. 421:14–19. 10.1016/j.jim.2014.11.013 [DOI] [PubMed] [Google Scholar]
- Stzepourginski I., Nigro G., Jacob J.M., Dulauroy S., Sansonetti P.J., Eberl G., and Peduto L.. 2017. CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc. Natl. Acad. Sci. USA. 114:E506–E513. 10.1073/pnas.1620059114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan B., and Gerner-Smidt P.. 2007. The epidemiology of human listeriosis. Microbes Infect. 9:1236–1243. 10.1016/j.micinf.2007.05.011 [DOI] [PubMed] [Google Scholar]
- Takeda K., Kaisho T., Yoshida N., Takeda J., Kishimoto T., and Akira S.. 1998. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J. Immunol. 161:4652–4660. [PubMed] [Google Scholar]
- Tsai Y.H., Disson O., Bierne H., and Lecuit M.. 2013. Murinization of internalin extends its receptor repertoire, altering Listeria monocytogenes cell tropism and host responses. PLoS Pathog. 9:e1003381 10.1371/journal.ppat.1003381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Sluis M., De Koning B.A., De Bruijn A.C., Velcich A., Meijerink J.P., Van Goudoever J.B., Büller H.A., Dekker J., Van Seuningen I., Renes I.B., and Einerhand A.W.. 2006. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 131:117–129. 10.1053/j.gastro.2006.04.020 [DOI] [PubMed] [Google Scholar]
- Varol C., Vallon-Eberhard A., Elinav E., Aychek T., Shapira Y., Luche H., Fehling H.J., Hardt W.D., Shakhar G., and Jung S.. 2009. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 31:502–512. 10.1016/j.immuni.2009.06.025 [DOI] [PubMed] [Google Scholar]
- Velcich A., Yang W., Heyer J., Fragale A., Nicholas C., Viani S., Kucherlapati R., Lipkin M., Yang K., and Augenlicht L.. 2002. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 295:1726–1729. 10.1126/science.1069094 [DOI] [PubMed] [Google Scholar]
- Whitfield M.L., George L.K., Grant G.D., and Perou C.M.. 2006. Common markers of proliferation. Nat. Rev. Cancer. 6:99–106. 10.1038/nrc1802 [DOI] [PubMed] [Google Scholar]
- Zenewicz L.A., Yancopoulos G.D., Valenzuela D.M., Murphy A.J., Karow M., and Flavell R.A.. 2007. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 27:647–659. 10.1016/j.immuni.2007.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Valdez P.A., Danilenko D.M., Hu Y., Sa S.M., Gong Q., Abbas A.R., Modrusan Z., Ghilardi N., de Sauvage F.J., and Ouyang W.. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14:282–289. 10.1038/nm1720 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.