Deczkowska and Schwartz describe how harnessing the immune system in a well-controlled manner can be used as a universal therapeutic approach to neurodegeneration.
Abstract
Immune cells patrol the brain and can support its function, but can we modulate brain–immune communication to fight neurological diseases? Here, we briefly discuss the mechanisms orchestrating the cross-talk between the brain and the immune system and describe how targeting this interaction in a well-controlled manner could be developed as a universal therapeutic approach to treat neurodegeneration.
The brain–immune axis
For decades, it was accepted that the central nervous system (CNS) is an “immune-privileged site.” In contrast to most of the body’s tissues, the brain and spinal cord were believed to be excluded from immune surveillance, a physiological process whereby immune cells patrol tissues and organs for defense against pathogens and neoplasia. Consequently, immune activities at these sites were completely ignored or considered detrimental, both in the context of the CNS in general and neurological diseases in particular. This view ascribed the inflammation observed in chronic neurodegenerative disease to an autoimmune pathology. As a consequence, attempts were made to treat such conditions with immune-suppressive drugs, all of which failed, leaving researchers baffled (Stower, 2018).
In contrast, emerging studies demonstrate that the CNS requires life-long support from the immune system for its maintenance and repair; specifically, deficiencies in the immune response were shown to aggravate neurological diseases. Here, we briefly discuss the anatomical sites and molecular mechanisms that regulate brain–immune communication, summarize how such communication becomes dysregulated in aging and neurodegenerative disease, and propose approaches to restore it to promote repair.
Neuro–immune checkpoints
The immune response is the body’s defense mechanism, yet robust immune responses may damage the surrounding tissue and could be especially detrimental in the poorly regenerating CNS. With the exception of the microglia, leukocytes are virtually absent from healthy CNS parenchyma. The blood–brain barrier, formed by multiple layers of tightly connected cells, effectively prevents immune cell infiltration into the healthy CNS; “leakiness” in this barrier is associated with pathologies.
Under physiological conditions, a small number of leukocytes continuously patrols the CNS within the cerebrospinal fluid (CSF), and various immune cell types are constitutively present in the blood–CSF barrier and the subarachnoid space of the meninges. The blood–CSF barrier, composed of the choroid plexus (CP) located in the brain’s ventricles, enables controlled trafficking of leukocytes from the blood to the CSF (Kunis et al., 2013). Meningeal spaces encapsulate the brain and the spinal cord and are populated by various immune cell types. Meningeal leukocytes, as well as brain antigens, potentially drain via the dural lymphatics to deep cervical lymph nodes, where they communicate with the peripheral immune system (Louveau et al., 2015). How do neuro–immune communication mechanisms at these sites affect CNS function during aging and age-related neurodegenerative conditions?
Brain–immune communication in aging and neurodegenerative diseases
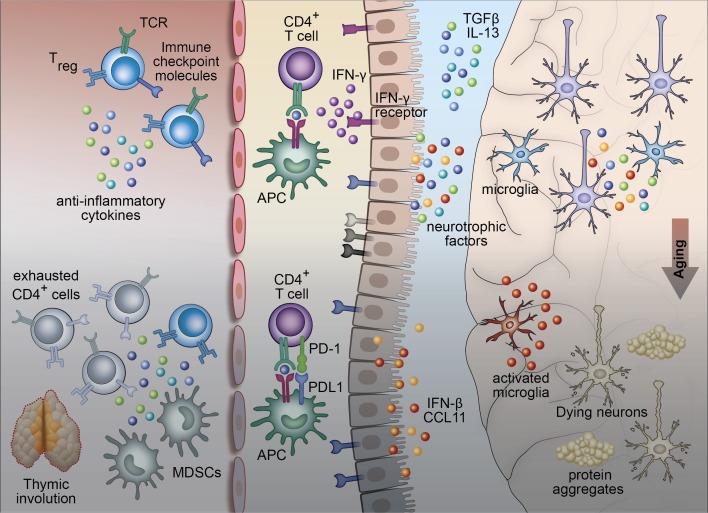
Aging, a primary risk factor of Alzheimer’s disease (AD), can be viewed as a harmful continuous “wear and tear” process in the brain. The mechanisms that promote restoration of homeostasis in a young individual are heavily dependent on the vitality and activity of the immune system (Ziv et al., 2006); if the immune system is compromised, the accumulation of wear and tear continues, and aging-related pathologies become symptomatic (Fig. 1).
Figure 1.
Brain–immune communication points during aging and neurodegenerative disease. In a young individual, the peripheral immune system promotes CNS immune surveillance via the CP. Immune activities are controlled by anti-inflammatory cytokines, T regulatory cell function, and checkpoint receptors and ligands (such as PD-1/PD-L1) expressed on T cells, antigen-presenting cells, and possibly on the CP epithelium itself. With aging, dysregulation of peripheral immunity (thymic involution, increase in the systemic levels of myeloid-derived suppressor cells (MDSCs), and exhausted T cells), and CP-specific mechanisms (IFN-I, decrease in local IFN-γ levels) hamper supportive brain–immune cross-talk and promote accumulation of damage in the brain (neurodegeneration).
The microglia are the primary phagocytic cells within the brain that act by engulfing misfolded proteins, cell debris, aggregated proteins, and toxic lipid products. During aging, microglia gradually lose their phagocytic capacity, resulting in accumulation of waste material and leading to local low-grade chronic inflammation. In parallel, the peripheral immune system shows signs of deterioration, manifested by reduced levels of naive T cells, increased numbers of FoxP3+ T regulatory cells, and an increased proportion of exhausted memory T cells, which express high levels of inhibitory immune checkpoint receptors, such as Programmed Death-1 (PD-1). While these immunosuppressive mechanisms protect against the potential autoimmune reaction of T cells, they may limit immune-mediated repair mechanisms. The age-related changes in the immune system that are relevant to brain maintenance and repair are manifested, among many processes, by the reduction of expression of immune trafficking molecules by the CP. The resultant reduction in immune surveillance, although not a primary cause of brain aging and age-related dementia, might be a factor that determines disease onset or contributes to its escalation. In addition, in both mice and humans, the aged CP expresses IFN-I, shown to negatively affect cognitive ability in mice via its effect on microglia (Baruch et al., 2014; Deczkowska et al., 2017). Additionally, recent studies revealed that the function of dural lymphatics may also be compromised in aged and AD mice (Da Mesquita et al., 2018).
Chronic low-grade inflammation within the brain and the continuous exhaustion of the peripheral immune system that potentially resolves it are reminiscent of the phenomenon of “immune evasion,” a strategy used by pathogens and tumors to evade the host immune response. In cancer treatment, immunotherapy is now routinely used to revive the suppressed T cell response to promote cancer eradication. In the case of aging-related brain pathology, revival of the immune activity could potentially stimulate dormant maintenance mechanisms. What are the mechanisms involved in immune evasion in this context, and can we target them to promote brain rejuvenation?
Targeting neuro–immune checkpoints in neurodegeneration: Challenges and opportunities
Numerous attempts to develop treatments for AD have failed (Stower, 2018), suggesting that there is a major gap in our understanding of the disease mechanism and in the translation of often promising findings from animal models to the human condition.
The animal models used in research of neurodegeneration are mainly genetic, and therefore most closely reflect familial forms of the disease (e.g., 5xFAD, APP/PS1, and others). As a consequence, any therapy based on such models may be limited to the patients suffering from familial forms of the disease bearing similar mutations. By the time the cognitive deficit becomes evident, additional processes go awry in the brain, and therefore, disease modification may require targeting multiple factors. In addition, in such animal models, confounding age-related factors and the effects of lifestyle are not manifested unless purposely included. One such example is the gut microbiome, which profoundly affects both the immune responses and brain function, was shown to affect the brain microglia, and was suggested to modulate development and progression of neurological diseases. Another source of heterogeneity among individuals is the prevalence of latent CNS infections, which was recently linked to AD (Readhead et al., 2018) and appears consistent with aging-related expression of IFN-I at the CP (Baruch et al., 2014; Deczkowska et al., 2017).
In contrast to past attempts to treat neurodegenerative diseases, targeting the immune system may overcome the disease heterogeneity among patients and translational obstacles. As described above, based on studies showing that the systemic immune cells support brain plasticity and repair, boosting systemic immunity has been suggested as a way to restore brain–immune communication to modify the course of neurodegenerative diseases (Baruch et al., 2015, 2016). One such promising immunotherapy, used in a variety of cancers, is based on immune checkpoint blockade and is directed at the PD-1 pathway. Anti–PD-1 immunomodulation was recently extended to mouse models of AD, in which it unleashes the peripheral immune response and activates a cascade of events that culminates in mitigation of the brain’s wear and tear. Common immunological factors that contribute to disease escalation and could be modified by the therapy include systemic immunosuppression, loss of IFN-γ signaling at the CP, and altered microglial phenotype (Baruch et al., 2015; Keren-Shaul et al., 2017). Human genome-wide association studies of AD patients versus healthy controls revealed that a large proportion of mutations associated with altered risk of late-onset AD occurs in genes linked to immune signaling (Lambert et al., 2013), further substantiating the idea of targeting the immune system as a comprehensive therapeutic approach. Since immune checkpoint blockade does not directly target the brain pathology, it could be potentially applicable to dementias of multiple etiologies. Notably, intermittent, rather than continuous, exposure to the blocking antibody is required in AD, arguing in favor of a distinct mechanism of action and a better safety profile versus immune checkpoint blockade in cancer (Baruch et al., 2016). Since the treatment evokes a sequential immune-dependent response that together contributes to disease modification, the failure to reproduce the effect of anti–PD-1 on plaque burden can be explained by insufficient peripheral response (Latta-Mahieu et al., 2018).
Follow-up of the growing cohort of patients receiving immunotherapy for cancer should allow identification of biomarkers predicting patients who would respond to such treatment. For example, recent work identified the increased frequency of CD14+CD16−HLA-DRhi blood monocytes as a biomarker accurately predicting outcomes of anti–PD-1 therapy in melanoma patients (Krieg et al., 2018). It would be interesting to test whether this cell subset is also present in the blood of AD or amyotrophic lateral sclerosis patients, in which other changes in the monocyte compartment were previously reported (Thériault et al., 2015).
In conclusion, the development of a therapy that boosts the immune system in a well-controlled way, and thereby restores and/or activates brain–immune communication, is an outcome of a general shift toward the perception of the CNS as a tissue that engages in a constant dialog with peripheral immunity. Such an approach is expected to provide novel treatment modalities in order to harness common immune repair mechanisms to combat AD and perhaps other neurodegenerative diseases.
Acknowledgments
We thank Tal Wiesel for artwork.
A. Deczkowska is supported by Steven and Eden Romick. M. Schwartz is supported by the Advanced European Research Council (ERC-2016-ADG 741744), the Israel Science Foundation Legacy Heritage Biomedical Science Partnership-Research (1354/15), Israel Science Foundation (991/16), the Consolidated Anti-Aging Foundation Chicago (2016–2017), and the Adelis Foundation (2018–2021). M. Schwartz holds the Maurice and Ilse Katz Professorial Chair in Neuroimmunology.
M. Schwartz serves as a consultant to ImmunoBrain Checkpoint, Inc. The authors declare no other competing financial interests.
References
- Baruch K., et al. 2014. Science. 346:89–93. 10.1126/science.1252945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch K., et al. 2015. Nat. Commun. 6:7967 10.1038/ncomms8967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch K., et al. 2016. Nat. Med. 22:135–137. 10.1038/nm.4022 [DOI] [PubMed] [Google Scholar]
- Da Mesquita S., et al. 2018. Nature. 560:185–191. 10.1038/s41586-018-0368-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deczkowska A., et al. 2017. Nat. Commun. 8:717 10.1038/s41467-017-00769-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren-Shaul H., et al. 2017. Cell. 169:1276–1290.e17. 10.1016/j.cell.2017.05.018 [DOI] [PubMed] [Google Scholar]
- Krieg C., et al. 2018. Nat. Med. 24:144–153. 10.1038/nm.4466 [DOI] [PubMed] [Google Scholar]
- Kunis G., et al. 2013. Brain. 136:3427–3440. 10.1093/brain/awt259 [DOI] [PubMed] [Google Scholar]
- Lambert J.C., et al. 2013. Nat. Genet. 45:1452–1458. 10.1038/ng.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latta-Mahieu M., et al. 2018. Glia. 66:492–504. 10.1002/glia.23260 [DOI] [PubMed] [Google Scholar]
- Louveau A., et al. 2015. Trends Immunol. 36:569–577. 10.1016/j.it.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Readhead B., et al. 2018. Neuron. 99:64–82.e7. 10.1016/j.neuron.2018.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stower H. 2018. Nat. Med. 24:894–897. 10.1038/s41591-018-0127-2 [DOI] [PubMed] [Google Scholar]
- Thériault P., et al. 2015. Alzheimers Res. Ther. 7:41 10.1186/s13195-015-0125-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y., et al. 2006. Nat. Neurosci. 9:268–275. 10.1038/nn1629 [DOI] [PubMed] [Google Scholar]