Lymph–venous crosstalk supports lymph node functionality at all stages.
Abstract
In this issue, Bovay et al. (https://doi.org/10.1084/jem.20180217) invoke a compelling model of interplay between the venous and lymphatic vasculature in regulating the developmental genesis and early expansion of LNs. This work supports an emerging model that lymph–venous crosstalk supports LN functionality at all stages.
Establishment of LNs and other secondary lymphoid organs occurs during embryogenesis. Our current knowledge indicates that lymphoid tissue development is an interactive sequential system that involves modulation of adhesion molecules, cytokines, and chemokines, which orchestrate the interface of immune “inducer” cells and stromal “organizer” cells (van de Pavert and Mebius, 2010). In this issue of JEM, Bovay et al. advance our understanding of this process in three major ways: (1) by reconciling existing and somewhat opposing literature on the role of lymphatic vessels in LN genesis; (2) by detailing the development of the LN capsule and its investiture with subcapsular macrophages; and (3) by demonstrating a role for interstitial fluid flow in driving the key changes that promote LN maturation.

Insights from Gwendalyn J. Randolph and Rafael S. Czepielewski.
There is a consensus that LNs develop after hematopoietic lymphoid tissue inducer (LTi) cells are recruited to a specific site and are stimulated by nonhematopoietic lymphoid tissue organizer (LTo) cells to promote tissue remodeling, leukocyte recruitment, and the formation of the primordial LN anlagen (van de Pavert and Mebius, 2010). Fetal liver–derived innate lymphoid LTi cells (RORγt+IL7Ra+CD3−CD4+; Spits et al., 2013) follow gradients of LTo-derived CXCL13 through the expression of CXCR5 (Luther et al., 2003). Once in the anlagen, LTi express lymphotoxin-α1β2 (LT α1β2) that interacts with the lymphotoxin-β receptor (LTβR) on LTo, inducing the expression of adhesion molecules (ICAM, VCAM, and MadCAM) and chemokines CCL19/CCL21 and CXCL13 that facilitate the arrival of other leukocytes and the maturation of the LN (van de Pavert and Mebius, 2010).
But what is the role of the lymphatic vasculature that drains into and from every LN? In the first anatomical records of the lymphatic vasculature, Florence R. Sabin stated that LNs arise from primitive lymph sacs (Sabin, 1909). More than a century later, experiments conducted with Prox1-deficient mice (the master transcriptional factor essential for lymphatic cell fate) concluded that lymph sacs or lymphatic vessels were unnecessary for initial LN anlagen formation (Vondenhoff et al., 2009). However, additional investigation regarding the later stages in LN organization in this model could not be verified due to the strain’s embryonic lethality. It is noteworthy that the same authors predicted that lymphatic endothelial cells (LECs) help to position LTi cells favorably to interact with LTo cells. Indeed, last year, Onder et al. (2017) demonstrated that LTβR and RANK signaling in LECs are essential for LN formation. They proposed that LECs play a role in LTi cell retention at developmentally appropriate sites for lymphoid tissue organogenesis and that artificial retention of these cells stimulates ectopic LNs.
One major question is what controls the arrival and anatomical localization of LTi in the fetus. The work of Bovay et al. (2018) is consistent with two papers (van de Pavert et al., 2009; Onder et al., 2017) that had earlier appeared to be in conflict with each other. The authors argue that the apparent lack of LNs in the strains analyzed by Onder et al. (2017) is linked to the fact that lymphatics are essential for LN expansion and maturation, but they also argue that the anlagen can form without lymphatic input. Here, they suggest that it is the blood vasculature that brings in the first LTi cells. Indeed, they provide evidence that LTi cells find “weak spots” in the walls of the venous vasculature, areas with delayed smooth muscle cell coverage, which allows for LTi escape and very early anlagen formation.
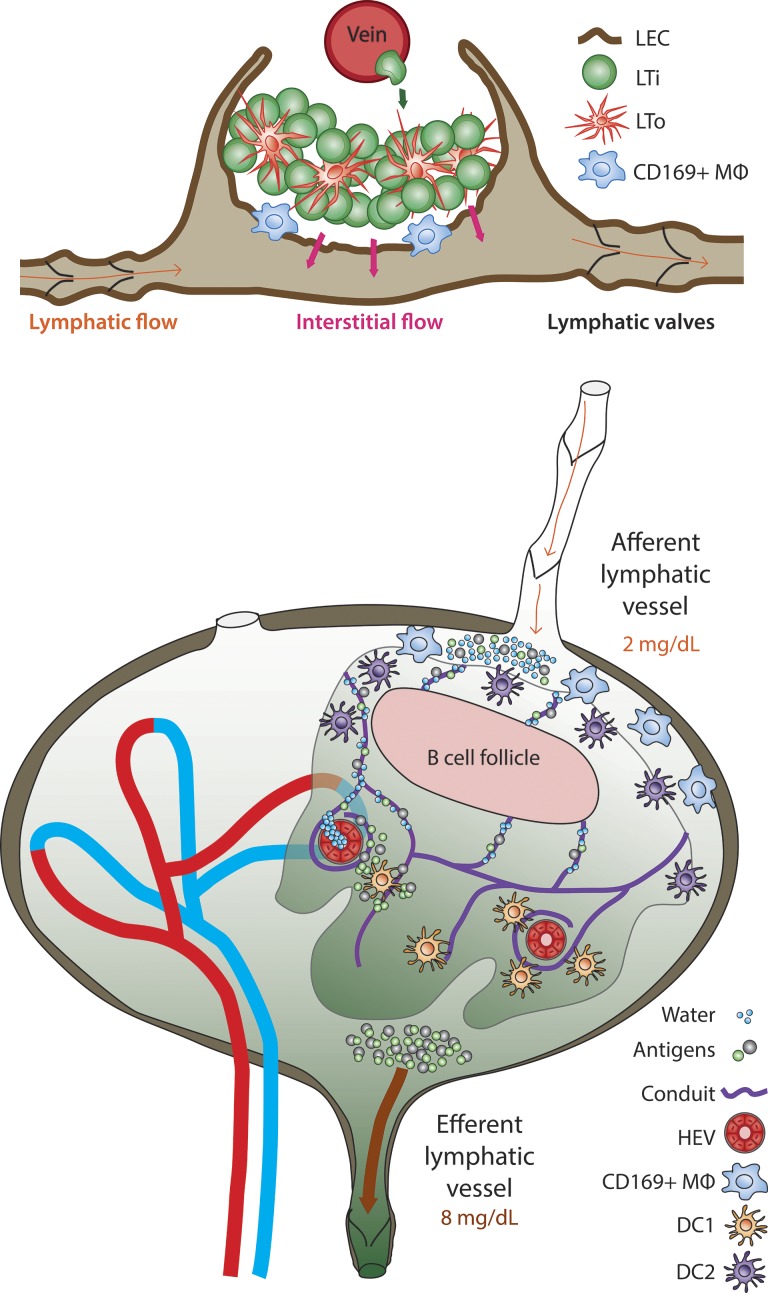
Lymphatic vessel and venous vessel cooperation in immunity includes LTi trafficking from a vein to establish the primordial LN (top), which then expands and matures in response to lymphatic collecting vessel maturation and flow, as per the work and renderings of Bovay et al. (2018) in this issue. These findings add on to a second lymphatic vessel and vein cooperation that promotes optimal immunity in fully mature LNs. Here, afferent collecting lymphatic vessels feed antigens into the adult LN at low concentrations (bottom). Transfer of small molecules and water via the conduits to high endothelial venules results in substantial concentration of these antigens, which in turn fuels improved antigen presentation by the DC1 type of DCs deep in LNs.
But if the venous vasculature drives the formation of LN anlagen, what is the role of the lymphatic vasculature? The role of the lymphatic vasculature supports not so much initiation but expansion and maturation of the developing node. Bovay et al. (2018) observed that LTi cells, presumably just having exited the venous vasculature, became harbored in a sheet of lymphatic endothelium expressing low levels of NRP2, a sheet that would become the LN subcapsular sinus. Interestingly, the sheet consisted of a double layer of lymphatic endothelium that proliferated in a specialized manner to eventually engulf the LTi cluster within its borders, forming a cup-like structure. These changes coincided with the formation of lymphatic valves and the development of vascular smooth muscle cells covering the lymphatic vessels, all hallmarks of a lymphatic collecting vessel. Consequently, lymphatic collecting vessel formation appeared highly connected with LN expansion and maturation. In compelling experiments in which lymphatic collecting vessel maturation was prevented due to genetic loss of the transcription factor FOXC2, the maturation and growth of the anlagen were stunted, with evidence of scattered LTi cells that never made it to the growing lymphoid collection.
Because unidirectional lymph flow cannot be sustained in the absence of FOXC2, these data prompted the authors to consider whether fluid flow through the maturing lymphatic vessels was, in turn, an important event in LN maturation. Noting that the critical chemokine CXCL13 was poorly expressed by fibroblastic stromal cells within the anlagen in the absence of FOXC2 in lymphatics, the authors wondered if the lymph flow created by functional FOXC2+ lymphatic vessels might itself drive the LN expansion and maturation process, including the expression of CXCL13. The authors configured a specialized chamber to generate an in vitro flow system that modeled that of the early lymphatic vessel–LN connection. Indeed, such flow was able to stimulate CXCL13 expression from stromal cells embedded in a three-dimensional matrix. The authors went on to make a compelling case that interstitial fluid flow allowed for LN growth due to stimulating expression of critical chemokines, while also driving the expansion and completion of the subcapsular sinus, including recruitment and positioning of the first specialized subcapsular macrophages. Thus, the work of Bovay et al. (2018) points to another role that interstitial flow plays in driving the establishment of immune organs, starting with the seminal work of Boardman and Swartz (2003) on this topic in establishing vascular and immune cell transit through tissues.
A number of uncertainties remain that will be important to seek answers to in the future. From the beautiful images in the work of Bovay et al. (2018), asymmetric distribution of subcapsular lymphatics and CD169+ macrophages seems evident. The face of the LN without these characteristics, we assume, will evolve to become the LN hilum, the medulla, and the location where efferent lymphatic trunks emerge robustly. Yet, the authors did not pursue how the specialization of the medulla versus the cortex arises, though it seems quite possible that lymphatic flow guides these important steps as well.
Finally, and with the concept of fluid flow in mind, we want to return to the model proposed by the authors that the venous and lymphatic vascular beds crucially interact to promote LN morphogenesis. This concept adds to the emergence of evidence that lymphatic vessel and venous crosstalk shapes multiple phases of LN biology, from development to function. Recently, Gerner et al. (2017) upended the notion that LN conduits supply antigen to dendritic cells (DCs; Sixt et al., 2005) by showing that antigen access to DCs was greater if the antigen possessed properties that prevented it from entering the conduit system. Indeed, others have recognized that the conduit network in LNs leads to rapid delivery of small molecules like chemokines from the lymph to the venous blood at the high endothelial venule (Palframan et al., 2001). Too often forgotten is the fact that water is included in the list of small molecules, despite the documented removal of water by what must be the blood vasculature when lymph passes through LNs and emerges with more than a threefold elevation in protein content (Adair et al., 1982). For years, we puzzled over why the LN would evolve to promote lymphatic–venous crosstalk that leads to the concentration of lymph within the LN itself. Gerner et al. (2017) provide an answer in the demonstration that cross-presenting DCs need a higher concentration of antigen to be triggered. Thus, their findings suggest that the mature LN, allowing for antigen to be concentrated right around the very location where DC1 reside near the high endothelial venules, relies on the specialized cooperation between lymphatic and blood vasculature in order to optimize immunity. Now, with Bovay et al. (2018) providing a model as to how both blood and lymphatic vessels may work together to promote LN formation and growth, it becomes possible to see the beauty in how an important developmental process lays out communication between the two vascular beds that remain at the very heart of the LN’s secret to promoting immunity.
Acknowledgments
The authors are supported by National Institutes of Health grants R37AI049653 and DP1DK109668 and a Synergy Award from the Kenneth Rainin Foundation.
References
- Adair T.H., et al. 1982. Am. J. Physiol. 243:H351–H359. 10.1152/ajpheart.1982.243.3.H351 [DOI] [PubMed] [Google Scholar]
- Boardman K.C., and Swartz M.A.. 2003. Circ. Res. 92:801–808. 10.1161/01.RES.0000065621.69843.49 [DOI] [PubMed] [Google Scholar]
- Bovay E., et al. J. Exp. Med. 2018 doi: 10.1084/jem.20180217. [DOI] [Google Scholar]
- Gerner M.Y., et al. 2017. J. Exp. Med. 214:3105–3122. 10.1084/jem.20170335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther S.A., et al. 2003. J. Exp. Med. 197:1191–1198. 10.1084/jem.20021294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder L., et al. 2017. Immunity. 47:80–92.e4. 10.1016/j.immuni.2017.05.008 [DOI] [PubMed] [Google Scholar]
- Palframan R.T., et al. 2001. J. Exp. Med. 194:1361–1373. 10.1084/jem.194.9.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin F.R. 1909. Am. J. Anat. 9:43–91. 10.1002/aja.1000090104 [DOI] [Google Scholar]
- Sixt M., et al. 2005. Immunity. 22:19–29. 10.1016/j.immuni.2004.11.013 [DOI] [PubMed] [Google Scholar]
- Spits H., et al. 2013. Nat. Rev. Immunol. 13:145–149. 10.1038/nri3365 [DOI] [PubMed] [Google Scholar]
- van de Pavert S.A., et al. 2009. Nat. Immunol. 10:1193–1199. 10.1038/ni.1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Pavert S.A., and Mebius R.E.. 2010. Nat. Rev. Immunol. 10:664–674. 10.1038/nri2832 [DOI] [PubMed] [Google Scholar]
- Vondenhoff M.F., et al. 2009. Development. 136:29–34. 10.1242/dev.028456 [DOI] [PMC free article] [PubMed] [Google Scholar]