Transient T-bet expression denotes a unique Tfh cell subset.
Abstract
In this issue, Fang et al. (https://doi.org/10.1084/jem.20180927) report on a subset of T follicular helper (Tfh) cells that transiently expresses T-bet yet continues to produce IFN-γ at late stages of GC reactions following immunization. They find other genes uniquely expressed in this IFN-γ–producing Tfh subset, such as NKG2D, that can be used to better distinguish these functionally distinct Tfh cells.
How T cells develop into the appropriate type of effector T cell during the course of infection has fascinated immunologists for decades. In general, naive T cells are not preconditioned to become a particular type of effector T cell, but as T cells become activated, the signals and cytokines produced by antigen-presenting cells and other local cells provide instructions as to “what type of effector T cell to become.” The T cells then differentiate through a series of coordinated transcriptional and epigenetic changes to open up and “turn on” the appropriate effector loci (e.g., cytokines and chemokines) as needed for that type of effector T cell to optimally respond to the nature of the invading pathogen, be it a virus, a bacterium, a fungus, or a parasite.

Insights from Susan M. Kaech.
Over 30 years ago, the pioneering work by Mosmann and Coffman was the first to clearly demonstrate that there are different types of effector T cells that stably maintain distinct effector functions (Mosmann et al., 1986). By characterizing a panel of CD4+ T cell clonal cell lines, they distinguished the IFN-γ– and IL-2–producing T helper type 1 (Th1) cells from the BSF-1 (or IL-4)–producing T helper type 2 (Th2) cells, which also helped antibody class switching. Since this seminal study, the field has advanced greatly to where we now understand there are not just two, but numerous Th cell subsets that can be distinguished more or less by their dominant effector functions. Of particular importance was the elucidation that there are particular types of Th cells, originally called germinal center (GC) T cells, that helped B cell GC reactions, antibody class switching, and memory B cell development (Zheng et al., 1996). These GC T cells were later found to up-regulate CXCR5 and became known as T follicular helper (Tfh) cells to keep in line with the standard Th nomenclature (Ansel et al., 1999; Breitfeld et al., 2000). Interestingly, while a defining function of Tfh cells is their production of IL-21, they can also produce IL-4 or IFN-γ, Th2 and Th1 signature cytokines, both of which are important for determining to which Ig isotypes the B cell switch (Snapper et al., 1987). How Tfh cells develop these type 1 and type 2 effector profiles has been of great interest. New work by Fang et al. in this issue of JEM shows that by using a “fate-mapping” reporter mouse for the transcription factor T-box expressed in T cells (T-bet, also known as Tbx21), transient expression of T-bet is sufficient to open up the IFN-γ locus to specify IFN-γ production in more mature Tfh cells in the GCs that enhance the development of IgG-2–expressing B cells. Interestingly, sustained expression of T-bet was not needed to maintain IFN-γ–production in the Tfh cells at later stages of GC development.
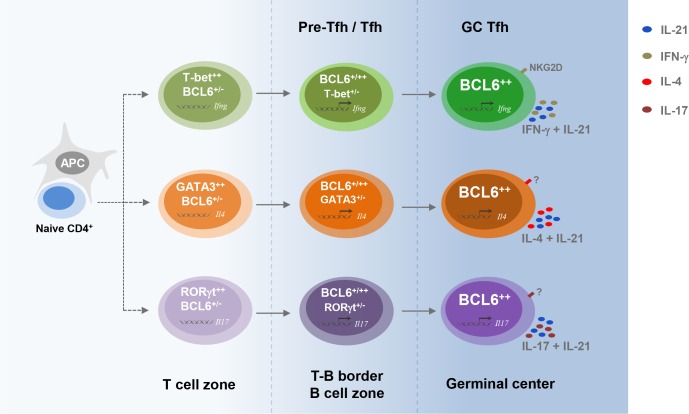
Model of IFN-γ–producing Tfh cell differentiation. In the T cell zone, once naive CD4 T cell is activated, T-bet is transiently expressed by a subset of BCL-6+/– cells. T-bet is then down-regulated by BCL-6 when it migrates into the B cell zone and GC and differentiates into mature Tfh cells. These ex–T-bet Tfh cells have the capability to produce IFN-γ in addition to IL-21 and highly express cell surface protein NKG2D. As a general hypothesis, transient induction of T-bet, GATA3, or RORγt during Tfh cell differentiation may determine the generation of IFN-γ–, IL-4–, or IL-17–producing Tfh cell subsets. However, generalization of this model requires future development of new genetic tools, including mouse strains carrying inducible Cre driven by the Gata3 or Rorc locus.
T-bet has been a widely studied transcription factor since its discovery in 2000 by Dr. Laurie Glimcher’s laboratory (Szabo et al., 2000). Following the seminal discoveries of Mosmann and Coffman, the hunt began for the transcriptional regulators that helped specify Th1 and Th2 cell differentiation. This led to the identification of Stat6, GATA3, and c-maf as critical factors for inducing IL-4 expression and Th2 effector cell development. Then, a few years later, T-bet was discovered to direct the formation of Th1 cells and induce expression of IFN-γ (Szabo et al., 2000). It is now known that, in addition to Th1 cells, T-bet is also expressed in a plethora of other immune cells to help orchestrate type 1 immune responses to viruses and intracellular bacteria, including dendritic cells, CD8+ T cells, natural killer cells, and B cells (Lazarevic et al., 2013).
To better understand how IFN-γ–producing Tfh cells arise during an immune response, Fang et al. (2018) used a dual reporter T-bet fate–mapping mouse strain, T-bet-ZsGreen-T2A-CreERT2-Rosa26-loxP-STOP-loxPtdTomato (ZTCE-tdTomato), in which cells that have expressed T-bet during a period of tamoxifen treatment can be permanently marked by the expression of a fluorescent protein, tdTomato, even after they have turned off T-bet expression, which can be determined by the expression of another fluorescent protein, ZsGreen. In looking at Tfh cells that are produced a couple of weeks following immunization with a peptide emulsified in CFA, the authors noted that many Tfh cells were tdTomato+ ZsGreen− T cells. This indicated that many Tfh cells displayed an “ex–T-bet” cell phenotype, suggesting that at some point in their development, they had expressed T-bet. Upon closer analysis, Fang et al. (2018) found that these Tfh cells with a history of T-bet expression were the only Tfh cells capable of producing IFN-γ. Furthermore, in looking at the regions of open chromatin in the IFN-γ locus in this subset of ex–T-bet Tfh cells, the authors noted that there was unique accessibility at CNS-6 and CNS+29 that was not observed in the other tdTomato– Tfh cells. This indicated that the IFN-γ locus was particularly remodeled in this subset of IFN-γ–producing Tfh cells, and interestingly, CNS-6 and CNS+29 have both been defined as regulatory elements that enhance IFN-γ expression in the absence of T-bet (Schoenborn et al., 2007). Thus, this would fit the transient T-bet expression model in Tfh cells, wherein a brief pulse of T-bet is needed to remodel these regulatory elements, but sustained expression is not necessary to maintain IFN-γ–producing Tfh cells. It should be noted that such transient expression of T-bet in Tfh cells was described earlier during lymphocytic choriomeningitis virus infection (Marshall et al., 2011; Weinstein et al., 2018).
To verify that T-bet was necessary for inducing this IFN-γ–producing subset of Tfh cells, the authors deleted T-bet from the CD4 T cells and found that IFN-γ–Tfh cells were no longer observed after immunization. This result confirmed previous work showing that T-bet was required for IFN-γ–producing Tfh cells during lymphocytic choriomeningitis virus infection (Weinstein et al., 2018). Lastly, the authors show that there are more unique features to the IFN-γ–producing Tfh cells than just IFN-γ. By comparing the gene expression patterns between tdTomato+ (ex–T-bet) and tdTomato– Tfh cells during immunization, Fang et al. (2018) revealed 67 additional genes that are uniquely expressed in this Tfh subset (but are shared with non-Tfh tdTomato+ CD4 T cells). One gene in particular that the authors noted was Klrk1 (also known as NKG2D), which was also more highly expressed on tdTomato+ Tfh cells by flow cytometry. Thus, NKG2D could serve as a good surrogate marker for identifying IFN-γ+ Tfh cells in other types of immunizations and infections.
In summary, this work helps to elucidate how different types of effector functions are established in Tfh cells. While T-bet is required to generate IFN-γ–producing Tfh cells, its expression is only needed transiently to remodel the IFN-γ locus and open regulatory elements that will allow sustained expression of IFN-γ in Tfh cells long-term. It is possible that the transient expression of T-bet is critical to maintaining Tfh cell identity, and perhaps longer periods of expression would lead to the differentiation of Th1 cells that would then lose CXCR5 expression and turn on CXCR3, for example. Thus, as the field moves forward, it will be important to better understand how T-bet’s activity is uniquely controlled across multiple immune cell types to induce both common and cell type–specific genetic circuits that enable the orchestration of a multicellular type 1 immune response.
References
- Ansel K.M., et al. . 1999. J. Exp. Med. 190:1123–1134. 10.1084/jem.190.8.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitfeld D., et al. . 2000. J. Exp. Med. 192:1545–1552. 10.1084/jem.192.11.1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D., et al. J. Exp. Med. 2018 doi: 10.1084/jem.20180927. [DOI] [Google Scholar]
- Lazarevic V., et al. . 2013. Nat. Rev. Immunol. 13:777–789. 10.1038/nri3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall H.D.H., et al. . 2011. Immunity. 35:633–646. 10.1016/j.immuni.2011.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T.R., et al. . 1986. J. Immunol. 136:2348–2357. [PubMed] [Google Scholar]
- Schoenborn J.R., et al. . 2007. Nat. Immunol. 8:732–742. 10.1038/ni1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper C.M., et al. . 1987. Science. 236:944–947. 10.1126/science.3107127 [DOI] [PubMed] [Google Scholar]
- Szabo S.J., et al. . 2000. Cell. 100:655–669. 10.1016/S0092-8674(00)80702-3 [DOI] [PubMed] [Google Scholar]
- Weinstein J.S., et al. . 2018. J. Exp. Med. 215:337–355. 10.1084/jem.20170457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B., et al. . 1996. J. Exp. Med. 184:1083–1091. 10.1084/jem.184.3.1083 [DOI] [PMC free article] [PubMed] [Google Scholar]