Abstract
We have previously demonstrated that elevated levels of soluble triggering receptor expressed on myeloid cells–like transcript 1 (sTLT-1) modulate sepsis-induced inflammation and positively correlate with disseminated intravascular coagulation (DIC). Here, we evaluate the clinical implications of plasma sTLT-1 in acute respiratory distress syndrome (ARDS), which is common in sepsis patients. Soluble TLT-1 levels in the plasma of ARDS patients (n = 20) were determined by slot blot analysis and were compared with clinical parameters to identify significant associations. For comparisons to ARDS, we also measured sTLT-1 levels in matched healthy controls (n = 20). Of the 20 plasma samples evaluated from patients with ARDS, 60% were diagnosed with sepsis and 40% were diagnosed with septic shock. The white blood cells (WBCs) of patients with ARDS were found to be significantly elevated over healthy controls with a mean of 13 k/µL over 6.2 k/µL, respectively. The mean plasma levels of sTLT-1 were 148.4 pg/mL ± 16.52 in the patient cohort and 92.45 pg/mL ± 17.12 in the control group (P = .02). No statistically significant correlations were found between plasma levels of sTLT-1 and WBCs, sepsis, septic shock or acute physiologic, and chronic health evaluation II scores. A statistically significant inverse correlation (r2 = .25, P < .05) was found between plasma sTLT-1 and peripheral platelet counts in patients with ARDS. Increased levels of sTLT-1 in ARDS patients suggest that TLT-1 may mediate the pathobiology of ARDS. Moreover, our data are the first to demonstrate a specific platelet marker in the development of ARDS due to sepsis.
Keywords: ARDS, platelets, neutrophils, TLT-1, sTLT-1, α granules, inflammation
Introduction
Inflammation is a natural biological response directed to control potentially harmful insults to the body.1 During the inflammatory response, blood leukocytes infiltrate the tissue to exert their phagocytic role, while blood platelets adhere, activate, and degranulate at the site of inflammation to mediate hemostasis and leukocyte interaction with the endothelium.1,2 Platelets have been shown to accumulate in the lungs of patients with acute respiratory distress syndrome (ARDS). In fact, bronchoalveolar lavage fluid from patients with ARDS exhibit increased concentrations of platelet-specific α-granule products, suggesting an important role for platelets in the development of ARDS.3 Moreover, during the progression of ARDS, platelet–leukocyte aggregates invade the alveoli. If the inflammatory response is not constrained, significant neutrophil-mediated epithelial damage is observed.4–7 This has been evaluated in biopsies of patients suffering from diffuse alveolar damage, the common pathologic finding in the lungs of ARDS patients, in which the patients were found to have increased and uncontrolled neutrophil accumulation in the lungs, together with excessive platelet activation.8
Similarly, increased numbers of activated platelets are found in the organs of patients suffering from sepsis and septic shock.9–11 Accordingly, elevated plasma levels of several platelet degranulation products, such as the adhesion molecule P-selectin, are associated with adverse clinical outcomes.12 The triggering receptor expressed on myeloid cells like transcript-1 (TLT-1) is a platelet receptor that is sequestered inside the platelet α-granule.13 Platelets have a long form of TLT-1, which contains an immunoglobulin-like extracellular domain and a cytoplasmic domain of 126 amino acids. Platelets also contain two variants of TLT-1. One is a splice variant containing an identical extracellular domain of the full-length transcript, but it is only a 16-amino-acid cytoplasmic tail (termed TLT-1sv) and a second variant that is a soluble form containing only the immunoglobulin-like extracellular domain (termed soluble TLT-1 [sTLT-1]).14 Significantly elevated levels of sTLT-1 have been found in the plasma of septic patients compared to healthy individuals.9 The increased plasma concentration of sTLT-1 during sepsis correlated with the presence of disseminated intravascular coagulation (DIC).9 The role of TLT-1 was further supported in animal models, where the presence of TLT-1 was associated with improved survival in sepsis models.9,15 Altogether, these findings support a role for TLT-1 in the body’s systemic response to inflammatory diseases.
To better understand the function of platelets and TLT-1 during inflammatory processes, we measured sTLT-1 levels in the plasma of patients with ARDS. Here, we demonstrate significantly elevated sTLT-1 levels in patients with ARDS compared to controls, and we further compare these results to clinical parameters. Our results suggest a role for TLT-1 in the pathobiology of ARDS.
Methods
Study Population
The University of Utah and Intermountain Medical Center institutional review boards approved this study, and all patients (or a legally authorized representative) provided informed consent. Patients with ARDS who were aged 21 and older were studied within 48 (±12) hours of admission to the intensive care unit (ICU). Acute respiratory distress syndrome was defined according to 8-current consensus guidelines as lung injury of acute onset with bilateral infiltrates chest radiograph, a Pao 2: Fio 2 ≤ 255 mm Hg (corrected the sea-level criterion for Pao 2/Fio 2 ratio of less than 300 mm Hg for the altitude of Salt Lake City (300 × [647 mm Hg Salt Lake City barometric pressure/760 mm Hg sea-level barometric pressure]) and no clinical evidence of left atrial hypertension to account for bilateral pulmonary infiltrates.16–23 Exclusion criteria considered in the ARDS network trials24 were used during the selection process for this study, and it includes the following: neuromuscular diseases that impair the ability to ventilate without assistance, such as C5 or higher spinal cord injury, amyotrophic lateral sclerosis, Guillain-Barré syndrome or myasthenia gravis, pregnancy, severe chronic respiratory disease, burns greater than 40% total body surface area, diffuse alveolar hemorrhage from vasculitis, and severe congestive heart failure, or platelet transfusion. Severe chronic respiratory disease was defined as the following: chronic hypercapnia with Paco 2 > 45 mm Hg, chronic hypoxemia with Pao 2 < 55 mm Hg on Fio 2 = 0.21, hospitalization within the last 6 months for respiratory failure (Paco 2 > 50 mm Hg and/or Pao 2 < 55 mm Hg on 0.21 Fio 2), secondary polycythemia, severe pulmonary hypertension (mean PAP > 40 mm Hg), or chronic ventilator dependency. For comparison, age- and gender-matched, medication-free healthy control subjects (n = 20) were studied.
Following informed consent, whole blood was carefully drawn into 8.6 mL sterile acid-citrate-dextrose (1.4 mL acid-citrate-dextrose/8.6 mL blood) Vacutainer tubes (Becton Dickinson), inverted to ensure adequate mixing, and transported at room temperature to the laboratory within 30 minutes. Platelet-poor plasma was harvested by centrifugation of the whole blood (150 × g for 20 minutes at 20°C) and stored at −80°C until analyzed.
Slot Blot
Levels of sTLT-1 in plasma samples from ARDS patients and healthy control participants were measured by immunoblot technique using a mouse monoclonal antibody against human TLT-1. Plasma obtained from patients with ARDS or healthy control volunteers were subjected to a series of dilutions (1:3), and 100 μL of each dilution was dotted onto a nitrocellulose membrane. The nitrocellulose membrane was allowed to dry for 10 minutes and subjected to 5% milk/Tris-buffered saline with 0.1% Tween-20 (TBST) blocking for 1 hour. The nitrocellulose sheet was then incubated overnight in the presence of antibody (dilution, 1:1000) at 4°C. After thorough washing, the membrane was incubated for another 60 minutes with Horse radish peroxidase (HRP)-conjugated rabbit anti-mouse antibody (dilution, 1:10,000; Jackson Immunoresearch) in 5% milk/TBST blocking solution, washed 3 additional times with TBST, and visualized with substrate (Pierce, Thermo Scientific,Waltham, MA). Each sheet also contained calibration samples of a known concentration of rsTLT-1 (0 to 100 ng/mL). Densitometric determination was achieved by means of the ChemiDoc XRS + and Image Lab Software, version 4.1 (Bio-Rad, Hercules, CA). The level of sTLT-1 in each sample was determined by comparing the optical density of the samples with that of the standard curve. All measurements were performed in duplicate, and the results are expressed as pg/mL plasma.
Statistical Analysis
Data analysis was performed with a statistical software package (Graph Pad software-PRISM, 7.01) employing paired, 2-tailed Student t test analysis (2 groups). Mean values and standard error of the means are given. P values < .05 were considered significant. No control for multiple comparisons was performed for exploratory analyses.
Results
Clinical Characteristics
Of the 20 ARDS, all had sepsis; among the group, 60% were diagnosed with severe sepsis and 40% were diagnosed with septic shock (Table 1). Mean age was 49 years (SD: 16.8), and 65% were female. The average ICU length of stay was 11 days (SD: 6.3), and 28-day mortality occurred in 20% (n = 4/20) of the patients. The average acute physiologic and chronic health evaluation (APACHE) II score was 21 (SD: 6.8), and they required ventilation for at least 4 days. As expected, white blood cell counts in patients with ARDS were significantly higher (r2 = 0.001, P = .88) than in healthy participants.
Table 1.
Characteristics of Patient Cohort.a
| Healthy Controls (n = 20) | ARDS Patients (n = 20) | P Value | |
|---|---|---|---|
| Age, years | 51.4 (19.0) | 49.3 (16.8) | .72 |
| Female gender, n (%) | 13 (65%) | 13 (65%) | 1.00 |
| Platelet count, k/µL | 265 (75) | 202 (84) | .02 |
| WBC, k/µL | 6.2 (2.2) | 13.0 (4.2) | <.0001 |
| APACHE II Score | — | 21.2 (6.8) | — |
| Shock,b n (%) | — | 8 (40%) | — |
| 28-day Mortality, n (%) | — | 4 (20%) | — |
| Duration of ventilation, days | — | 4.3 (3.0) | — |
| Length of ICU stay, days | — | 10.7 (6.3) | — |
Abbreviations: ARDS, acute respiratory distress syndrome; APACHE II, acute physiology and chronic health evaluation; ICU, intensive care unit; WBC, white blood cell count.
a Data are Shown as Mean (standard deviation) Unless Otherwise Noted.
b Shock, hypotension requiring vasopressors.
Levels of Plasma sTLT-1 in Patients with ARDS and Healthy Controls
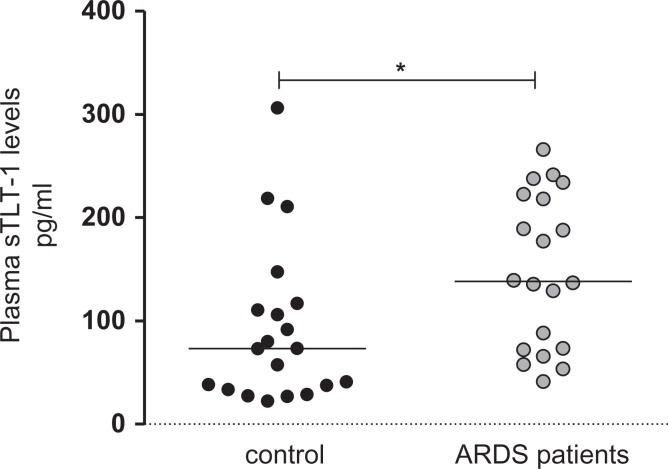
Given that elevated plasma levels of sTLT-1 are implicated in the pathobiology of systemic inflammatory diseases, we measured plasma sTLT-1 in patients diagnosed with ARDS following admission to the ICU. Soluble TLT-1 levels were significantly increased in patients with ARDS compared to matched, healthy control participants (148.4 ± 16.52 vs 92.45 ± 17.12, r2 = 0.1684, P = .01; Figure 1). No significant correlation was observed between patients with ARDS having severe sepsis versus septic shock (P = .44; Figure 2a). To further explore a possible association between sTLT-1 and disease severity, we correlated the plasma levels of sTLT-1 with the APACHE II scoring system and with 28-day mortality. We did not identify any significant correlation between sTLT-1 levels and APACHE II scores and mortality (Figure 2b and c).
Figure 1.
Elevated levels of soluble triggering receptor expressed on myeloid cells–like transcript 1 (sTLT-1) in patients diagnosed with acute respiratory distress syndrome (ARDS) in a cohort of sepsis. Plasma isolated from patients diagnosed with ARDS or healthy subjects was evaluated for the presence of sTLT-1 by slot blot analysis. Marks represent individual patients. Wilcoxon analysis demonstrate a significant difference between the medians r2 = 0.1684, P = .01. In addition to Wilcoxon statistical evaluation, student t test analysis shows a significant difference between the means (92.45 ± 17.12, N = 20 [control group]; 148.4 ± 16.52, N = 20 [patients with ARDS]; P = .0240), with −55.96 ± 23.79 difference between the means. Horizontal lines represent the median.
Figure 2.
Correlation analysis of plasma soluble triggering receptor expressed on myeloid cells–like transcript 1 (sTLT-1) with disease severity markers. (A) Plasma sTLT-1 levels were correlated with severe sepsis or septic shock incidence among patients with acute respiratory distress syndrome (ARDS), Wilcoxon analysis shows no difference between the means P (ranksum) = .44. (B) Patients with acute respiratory distress syndrome were divided using average acute physiologic and chronic health evaluation II scoring system, and plasma levels of sTLT-1 were correlated between the groups. (C) Statistical analysis demonstrated no significant differences between the means of survivors versus nonsurvivors P (ranksum) = .74. (D) White blood cells peripheral counts (k/µL) were correlated with plasma sTLT-1 levels, r2 = 0.001, P = .88.
Given that one of the hallmarks of ARDS is uncontrolled neutrophil transmigration into the lungs6,25 and as leukocyte (WBC) function is severely disrupted during ARDS,26,27 we evaluated a possible correlation between sTLT-1 and WBC. Our analysis demonstrated no significant correlation between sTLT-1 levels and WBC counts (r2 = 0.001, P = .88) in ARDS (Figure 2d).
As platelets have been identified as key cellular regulators of ARDS,28,29 we correlated platelet count with the levels of sTLT1 in the plasma of patients with ARDS. Our results demonstrated a significant inverse correlation between platelet count and the plasma levels of sTLT-1 (r2 = 0.25, P < .05; Figure 3).
Figure 3.
Plasma levels of soluble triggering receptor expressed on myeloid cells–like transcript 1 (sTLT-1) negatively correlate with platelets counts. The relationship between plasma sTLT-1 and platelet counts was evaluated. The linear regression analysis shows a negative correlation (P < .05) between platelet counts (k/µL) and plasma levels of sTLT-1 (pg/mL).
Discussion
In the present study, plasma levels of sTLT-1 of patients having ARDS were significantly elevated compared to those in the control group. Among control patients, we identified 3 individuals who exhibited high plasma levels of sTLT-1 (>200 pg/mL). Given that high plasma levels of sTLT-1 have been previously associated with sepsis-induced inflammatory processes, we measured the strength of the relationship between plasma sTLT-1 and severe sepsis, septic shock, APACHE II scoring, and mortality. APACHE II scoring system has been widely accepted as a measure of illness severity and found to stratify risk of death in a wide range of disease states and in different clinical settings.30,31 Correlation analysis revealed no significant association between either of the variables considered.
Activation and recruitment of neutrophils are regarded to play a significant role in lung edema and epithelial and endothelial damage in ARDS.6,7,25 Because the neutrophil population was not specifically counted, we evaluated a possible association between plasma sTLT-1 and WBC counts. In contrast to what we expected, correlation analysis revealed no significant association between sTLT-1 and WBC in ARDS. While there were no stratified populations in these studies, we cannot rule out a relationship between sTLT-1 and specific subpopulations of WBCs or severity of the inflammatory response.
The effects of TLT-1 in immunity and inflammation outside of ARDS have been previously evaluated.9 In humans, increased plasma sTLT-1 is associated with the presence and severity of DIC in septic patients.9 Experimentally, it has been demonstrated a 17 amino acid sequence of the ectodomain of TLT-1 (LR17) modulates the inflammatory reaction of sepsis by dampening leukocyte activation and modulating platelet–neutrophil crosstalk.15 Intriguingly, whereas LR17 treatment was shown to improve survival in mice, treml1 −/− mice were shown to be more susceptible to polymicrobial infection, suggesting a necessity of fine tuning the presence and concentration of sTLT-1 during systemic response to inflammation. Accordingly, ongoing studies in our laboratory demonstrate that TLT-1 plays a role in systemic response to acute lung injury, using a model of lipopolysaccharide (LPS) induced acute lung inflammation. The treml1 −/− mice developed increased inflammatory reaction accompanied by intrapulmonary hemorrhage and uncontrolled neutrophil infiltration to the lungs not observed in their wild-type counterparts. Therefore, it is reasonable to consider that TLT-1 might be implicated in the development of ARDS by modulating platelet-endothelial cell interactions.
Study Limitations and Future Considerations
The study was limited by the number of cases, and therefore, few generalizations with specific disease characteristics were possible from this study group. Indeed, if we used the data obtained in the analysis of our patients with ARDS (148.3 pg/mL ± 17 vs 95.5 ± 17 pg/mL; P = .02) as parameters for a sample size analysis, we conclude that 450 subjects (with a loss factor of 40 subjects) will be needed in order to reach 80% statistical power. To better characterize sTLT-1 involvement in ARDS, plasma levels of sTLT-1 should be evaluated in a larger cohort of patients and should be correlated with cytokines levels, disease severity, and survival rate. This study undoubtedly places TLT-1 as an important factor to be considered to better understand platelet’s function in ARDS.
Footnotes
Authors’ Note: All data are freely available by contacting the corresponding author Washington A. Valance.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by the NIH grants (R01HL090933, HL126547, F31HL136183 and HL112311) from the National Heart Lung and Blood Institute and a NIGMS INBRE grant (P20GM103475). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Rondina MT, Weyrich AS, Zimmerman GA. Platelets as cellular effectors of inflammation in vascular diseases. Circ Res. 2013;112(11):1506–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saboor M, Ayub Q, Ilyas S, Moinuddin Platelet receptors; an instrumental of platelet physiology. Pak J Med Sci. 2013;29(3):891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Idell S, Maunder R, Fein AM, et al. Platelet-specific alpha-granule proteins and thrombospondin in bronchoalveolar lavage in the adult respiratory distress syndrome. Chest. 1989;96(5):1125–1132. [DOI] [PubMed] [Google Scholar]
- 4. Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY, Frenette PS. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med. 2009;15(4):384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robbins RA, Russ WD, Rasmussen JK, Clayton MM. Activation of the complement system in the adult respiratory distress syndrome. Am Rev Respir Dis. 1987;135(3):651–658. [DOI] [PubMed] [Google Scholar]
- 6. Kindt GC, Gadek JE, Weiland JE. Initial recruitment of neutrophils to alveolar structures in acute lung injury. J Appl Physiol (1985). 1991;70(4):1575–1585. [DOI] [PubMed] [Google Scholar]
- 7. Reutershan J, Basit A, Galkina EV, Ley K. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;289(5):L807–L815. [DOI] [PubMed] [Google Scholar]
- 8. Mandal RV, Mark EJ, Kradin RL. Megakaryocytes and platelet homeostasis in diffuse alveolar damage. Exp Mol Pathol. 2007;83(3):327–331. [DOI] [PubMed] [Google Scholar]
- 9. Washington AV, Gibot S, Acevedo I, et al. TREM-like transcript-1 protects against inflammation-associated hemorrhage by facilitating platelet aggregation in mice and humans. J Clin Invest. 2009;119(6):1489–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guclu E, Durmaz Y, Karabay O. Effect of severe sepsis on platelet count and their indices. Afr Health Sci. 2013;13(2):333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Layios N, Delierneux C, Hego A, et al. Sepsis prediction in critically ill patients by platelet activation markers on ICU admission: a prospective pilot study. Intensive Care Med Exp. 2017;5(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu W, Song Y. Biomarkers for patients with trauma associated acute respiratory distress syndrome. Mil Med Res. 2017;4:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Washington AV, Schubert RL, Quigley L, et al. A TREM family member, TLT-1, is found exclusively in the alpha-granules of megakaryocytes and platelets. Blood. 2004;104(4):1042–1047. [DOI] [PubMed] [Google Scholar]
- 14. Gattis JL, Washington AV, Chisholm MM, et al. The structure of the extracellular domain of triggering receptor expressed on myeloid cells like transcript-1 and evidence for a naturally occurring soluble fragment. J Biol Chem. 2006;281(19):13396–13403. [DOI] [PubMed] [Google Scholar]
- 15. Derive M, Bouazza Y, Sennoun N, et al. Soluble TREM-like transcript-1 regulates leukocyte activation and controls microbial sepsis. J Immunol. 2012;188(11):5585–5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cho YJ, Moon JY, Shin ES, et al. Clinical Practice Guideline of Acute Respiratory Distress Syndrome. Tuberc Respir Dis (Seoul). 2016;79(4):214–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cuthbertson BH, Dellinger P, Dyar OJ, et al. UK guidelines for the use of inhaled nitric oxide therapy in adult ICUs. American-European Consensus Conference on ALI/ARDS. Intensive Care Med. 1997;23(12):1212–1218. [DOI] [PubMed] [Google Scholar]
- 18. Fan E, Del Sorbo L, Goligher EC, et al. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(9):1253–1263. [DOI] [PubMed] [Google Scholar]
- 19. ARDS Jsorsf. [Clinical practice guideline for acute lung injury and acute respiratory distress syndrome]. Nihon Kokyuki Gakkai Zasshi. 2010;Suppl:1–101. [PubMed] [Google Scholar]
- 20. Hashimoto S. [Clinical practice in ARDS—the present and the future—]. Masui. 2013;62(5):517–521. [PubMed] [Google Scholar]
- 21. Schönhofer B, Kuhlen R, Neumann P, Westhoff M, Berndt C, Sitter H. [Non-invasive ventilation as treatment for acute respiratory insufficiency. Essentials from the new S3 guidelines]. Anaesthesist. 2008;57(11):1091–1102. [DOI] [PubMed] [Google Scholar]
- 22. Takeuchi M, Tachibana K. Mechanical ventilation for ARDS patients—for a better understanding of the 2012 Surviving Sepsis Campaign Guidelines. Cardiovasc Hematol Disord Drug Targets. 2015;15(1):41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 pt 1):818–824. [DOI] [PubMed] [Google Scholar]
- 24. Thompson BT, Bernard GR. ARDS Network (NHLBI) studies: successes and challenges in ARDS clinical research. Crit Care Clin. 2011;27(3):459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17(3-4):293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gama de Abreu M, Kirschfink M, Quintel M, Albrecht DM. White blood cell counts and plasma C3a have synergistic predictive value in patients at risk for acute respiratory distress syndrome. Crit Care Med. 1998;26(6):1040–1048. [DOI] [PubMed] [Google Scholar]
- 27. Preira P, Forel JM, Robert P, et al. The leukocyte-stiffening property of plasma in early acute respiratory distress syndrome (ARDS) revealed by a microfluidic single-cell study: the role of cytokines and protection with antibodies. Crit Care. 2016;20:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sakamaki F, Ishizaka A, Handa M, et al. Soluble form of P-selectin in plasma is elevated in acute lung injury. Am J Respir Crit Care Med. 1995;151(6):1821–1826. [DOI] [PubMed] [Google Scholar]
- 29. Erlich JM, Talmor DS, Cartin-Ceba R, Gajic O, Kor DJ. Prehospitalization antiplatelet therapy is associated with a reduced incidence of acute lung injury: a population-based cohort study. Chest. 2011;139(2):289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu D, Ai Y, Xu Q. [The clinical features of multiple-trauma patients APACHE II score system]. Hunan Yi Ke Da Xue Xue Bao. 1999;24(3):285–286. [PubMed] [Google Scholar]
- 31. Navarrete-Navarro P, Ruiz-Bailén M, Rivera-Fernández R, et al. Acute respiratory distress syndrome in trauma patients: ICU mortality and prediction factors. Intensive Care Med. 2000;26(11):1624–1629. [DOI] [PubMed] [Google Scholar]