Abstract
This study illuminates the association between cigarette smoking and adult mortality in the contemporary United States. Recent studies have estimated smoking-attributable mortality using indirect approaches or with sample data that are not nationally representative and that lack key confounders. We use the 1990–2011 National Health Interview Survey Linked Mortality Files to estimate relative risks of all-cause and cause-specific mortality for current and former smokers compared with never smokers. We examine causes of death established as attributable to smoking as well as additional causes that appear to be linked to smoking but have not yet been declared by the U.S. Surgeon General to be caused by smoking. Mortality risk is substantially elevated among smokers for established causes and moderately elevated for additional causes. We also decompose the mortality disadvantage among smokers by cause of death and estimate the number of smoking-attributable deaths for the U.S. adult population ages 35+, net of sociodemographic and behavioral confounders. The elevated risks translate to 481,887 excess deaths per year among current and former smokers compared with never smokers, 14 % to 15 % of which are due to the additional causes. The additional causes of death contribute to the health burden of smoking and should be considered in future studies of smoking-attributable mortality. This study demonstrates that smoking-attributable mortality must remain a top population health priority in the United States and makes several contributions to further illuminate the human costs of this tragedy that has ravaged American society for more than a century.
Keywords: Smoking, Mortality, Cause of death, Smoking-attributable mortality, National Health Interview Survey
Introduction
More than 50 years after the first U.S. Surgeon General report on smoking-attributable mortality (U.S. Department of Health, Education, and Welfare 1964), smoking continues to be the leading behaviorally related cause of death in the United States (GBD 2015 Tobacco Collaborators 2017; Mokdad et al. 2004). A recent surgeon general report (U.S. Department of Health and Human Services (DHHS) 2014) found that smoking causes approximately 437,000 deaths to adults ages 35+ in the United States each year, about one-fifth of all U.S. adult deaths. This estimate is based on 21 causes of death that the surgeon general has formally established to be caused by smoking. Although all studies in the smoking–mortality literature have found that hundreds of thousands of deaths per year are caused by smoking, their findings differ substantially from one another and from the surgeon general report (Gutterman 2015; Rostron and Wilmoth 2011). Clearly, further investigation is warranted on the lethal consequences of smoking to better understand its impact on population health.
Carter and colleagues (2015) examined smoking-attributable mortality from both causes of death with well-established links to smoking and causes that have not yet been declared by the surgeon general to be caused by smoking. They determined that some causes of death that the surgeon general has not yet attributed to cigarette smoking—such as renal failure, hypertensive heart disease, breast cancer, and prostate cancer—exhibit both strong associations with and biologically plausible connections to smoking. Evidence linking these additional causes of death to smoking is suggestive but not yet sufficient for the surgeon general to state definitively that some deaths from these causes are directly attributable to smoking.
The Carter et al. study, while important and influential, was not based on nationally representative data; the five prospective cohort data sets Carter and colleagues analyzed contain a lower percentage of racial/ethnic minority group members and a higher percentage of highly educated individuals than is actually the case in the U.S. population. Both smoking status and mortality risk vary substantially by race/ethnicity and socioeconomic status (Ho and Elo 2013; Lariscy et al. 2015; Rogers et al. 2000). Moreover, Carter et al. were unable to control for some potentially important confounders of the smoking–mortality relationship, including body mass index (BMI) and health insurance coverage. Nationally representative data and statistical controls for confounding characteristics are important for accurately estimating the relationship between smoking and mortality.
The objectives for our study are fourfold. First, we estimate relative risk ratios among current and former smokers compared with never smokers for causes of death established as attributable to smoking as well as the additional causes that Carter and colleagues (2015) compiled. We use the 1990–2011 National Health Interview Survey Linked Mortality Files (NHIS-LMF), a high-quality data set that is nationally representative of the U.S. noninstitutionalized adult population and includes controls for key confounders of the smoking– mortality relationship. Second, we disaggregate current smokers by smoking intensity and former smokers by time since cessation to determine whether the additional causes display similar patterns as established causes according to these two dimensions of smoking behavior. The evidence that these causes are attributable to smoking will be strengthened if mortality risk during follow-up is elevated among heavy compared with light smokers and among those who quit recently compared with long ago. Third, we decompose the mortality disadvantage among smokers by cause of death and express the percentage of the disadvantage that is due to each established and additional cause. Finally, we produce new estimates of the annual number of U.S. adult deaths attributable to smoking in 2010 to determine how many deaths could have been averted in the absence of the substantial smoking burden in the United States.
Background
Smoking-Attributable Causes of Death
The U.S. Surgeon General uses a four-class hierarchy to classify the strength of causal inferences from the existing evidence on smoking and mortality. Causes of death in the first class are considered causally associated with smoking and include cancers of several organs, respiratory diseases, cardiovascular diseases, and diabetes (U.S. DHHS 2014). Lung cancer is the cause of death most prominently associated with smoking and the leading cause of cancer mortality in the United States. However, only about one-quarter of all smoking-attributable deaths are due to lung cancer (Rostron and Wilmoth 2011). Smoking also elevates mortality risk from cancers of the lip and oral cavity, esophagus, stomach, colon and rectum, liver, pancreas, larynx, cervix, urinary bladder, kidney and renal pelvis, and bone marrow (Bonneux 2011; International Agency for Research on Cancer 2004; U.S. DHHS 2014). The remaining established causes are ischemic heart disease; other heart diseases; stroke; atherosclerosis; aortic aneurysm; other arterial diseases; pneumonia, influenza, and tuberculosis; chronic obstructive pulmonary disease (COPD); and diabetes (U.S. DHHS 2014).
For several other causes of death placed into the second class, the surgeon general stated that the “evidence is suggestive but not sufficient to infer a causal relationship” (U.S. DHHS 2014:3), and these causes are not included in the federal government’s official estimates of smoking-attributable mortality.1 Although these causes are not officially recognized by the surgeon general as smoking-attributable, they exhibit strong associations with and biologically plausible connections to cigarette smoking (Carter et al. 2015). These causes include infections, breast cancer, prostate cancer, rare cancers, cancers of unknown site, hypertensive heart disease, essential hypertension and hypertensive renal disease, respiratory diseases (other than pneumonia and influenza), intestinal ischemia, liver cirrhosis, other digestive diseases, and renal failure. The prospect that some breast and prostate cancer deaths may be attributable to smoking is especially important. Although lung cancer is the leading cause of cancer death among U.S. women and men, breast cancer is the second leading cause of cancer death among U.S. women, and prostate cancer is the second leading cause of cancer death among U.S. men (American Cancer Society 2018). Carter and associates (2015) also included deaths from cancers of unknown primary site and deaths from unknown causes because a portion of these deaths may actually be due to smoking-attributable causes but are not identified as such (Anderson 2011).2 For instance, in a prospective study of U.S. veterans, Rogot and Murray (1990) found significantly elevated risk of death among smokers compared with nonsmokers from ill-defined conditions and unknown causes. The additional causes account for a relatively small number of U.S. deaths individually, but when aggregated, they may substantially raise the number of deaths attributable to smoking.
Sociodemographic Confounders of Smoking and Mortality
Smoking varies by sex, age, race/ethnicity, socioeconomic status, and region of residence, and differential smoking prevalence contributes to several of the enduring (and in some cases, growing) sociodemographic disparities in U.S. adult mortality. For example, the U.S. sex gap in life expectancy expanded and then declined throughout the second half of the twentieth century and into the twenty-first century largely because of cohort differences in smoking prevalence between women and men (Preston and Wang 2006; Preston et al. 2014). At present, smoking accounts for about one-fifth of the female advantage in U.S. adult mortality (Rogers et al. 2010). Smoking differences also contribute to racial/ethnic mortality disparities (Blue and Fenelon 2011; Ho and Elo 2013; Lariscy et al. 2015, 2016). For instance, more than 75 % of the Hispanic life expectancy advantage at ages 50+ relative to non-Hispanic whites is due to lower cigarette use among Hispanics (Blue and Fenelon 2011). Perhaps most concerning, smoking has become increasingly concentrated among the lower socioeconomic strata of U.S. society and in specific parts of the country (Link and Phelan 1995; Pampel 2009). As the health consequences of smoking became better understood, highly educated individuals and those in more progressive regions of the country were better equipped to avoid smoking initiation, obtain the support and resources to quit if they started, and live in contexts with stricter anti-smoking laws and policies (de Walque 2010; Fenelon 2013). Thus, using nationally representative data and statistically controlling for these sociodemographic characteristics are essential for accurately estimating mortality attributable to smoking.
Estimating Smoking-Attributable Mortality
Two main approaches are used to estimate smoking-attributable mortality. One approach (which has at least two variants) involves indirect methods (Fenelon and Preston 2012; Peto et al. 1992, 1994; Preston et al. 2010a, b). Indirect approaches rely on the lung cancer death rate of a population as an indicator of the accumulated damage of smoking. Lung cancer mortality is a valid proxy of a population’s smoking burden given that 70 % to 90 % of lung cancer deaths are attributable to smoking in high-income countries (Ezzati and Lopez 2003; Thun et al. 1997), whereas lung cancer mortality is very low among never smokers (Thun et al. 2008). An approach by Peto et al. (1992, 1994), known as the Peto-Lopez method, imports risk ratios from the Cancer Prevention Study II (CPS-II) for causes of death other than lung cancer and arbitrarily reduces the ratios by one-half to account for possible confounding.
Preston, Glei, and Wilmoth (Preston et al. 2010a, b) developed a regression-based approach (hereafter, PGW method) that makes fewer assumptions than the Peto-Lopez method. Specifically, the PGW method estimates smoking-attributable mortality from the association between lung cancer death rates and death rates from all other causes combined, as opposed to importing risk ratios from the CPS-II like the Peto-Lopez method. Preston et al. (2010a) used the PGW indirect approach in a cross-national analysis and estimated that 24 % of U.S. male and female mortality at ages 50+ was due to smoking in 2003. In a follow-up article using the PGW indirect method based on vital statistics data specifically from the United States, Fenelon and Preston (2012) estimated that in 2004, 21 % of U.S. male mortality and 17 % of U.S. female mortality at ages 50–84 was attributable to smoking. Fenelon and Preston (2012) noted that these estimates are modestly lower than those produced by Preston et al. (2010a), most likely because the reliance on U.S. vital statistics data for these U.S.-based estimates more effectively takes the maturity of the U.S. smoking epidemic into account than the Preston et al. (2010a) estimates that are based on data from 20 countries.
Although such findings have tremendous scientific and policy relevance, indirect approaches are limited. Indirect approaches may not accurately model smoking-attributable mortality from causes with different age-related functional forms than lung cancer. Mortality risk for smokers relative to never smokers has increased over time (Mehta and Preston 2012; Thun et al. 2013), but cause-specific incidences and smoker–nonsmoker relative risks change over time in different ways. For example, Thun et al. (2013) found smoker–nonsmoker risk ratios for lung cancer mortality increased from CPS-I (1959–1965) to CPS-II (1982–1988) to a contemporary period (2000–2010): rates increased among smokers and remained stable among nonsmokers. At the same time, death rates for ischemic heart disease decreased among both smokers and nonsmokers, but with a larger proportional decline for nonsmokers so that rate ratios increased. Smoker–nonsmoker rate ratios also increased for COPD mortality. This complex interrelation among death rates for lung cancer and other specific smoking-attributable causes over time in both absolute rates and relative risk ratios by cause of death emphasizes the importance of directly examining smoking-attributable causes individually as opposed to indirectly using all non-lung-cancer smoking deaths combined.
Furthermore, the PGW method performs most successfully at ages 50–84 years but may be less accurate at younger or older ages. The method depends on lung cancer death rates to identify the proportion of deaths attributable to smoking, and lung cancer deaths are relatively rare before age 50 given the lag required for smoking to manifest in physiological damage to the lungs (Gutterman 2015; Rostron and Wilmoth 2011). However, other recent studies have observed smoking-attributable mortality beginning at about age 35 (Rostron 2011; U.S. DHHS 2014). Indirect methods also encounter issues at ages 85+, in part because of comorbidity among older adults (Rosenberg 1999). A number of modifications have been proposed to address these issues—particularly the implausibly high smoking-attributable mortality among older women (Fenelon and Preston 2012; Preston et al. 2010b; Rostron 2010). Indirect approaches are most advantageous when estimating smoking-attributable mortality for populations at advanced stages of the tobacco epidemic and/or for populations without individual-level data on smoking prevalence and cause-specific mortality from a single source.
Alternatively, direct approaches assess survey respondents’ smoking status and then estimate the mortality burden of smoking through the prospective mortality follow-up of those respondents. Ideally, such survey data cover the full range of adult ages and are generalizable to the U.S. population. Because respondents report their smoking behaviors, the direct approach allows observation of heterogeneity in mortality risk within current and former smoker groups that is due to variation in smoking intensity, duration, and time since cessation. Studies incorporating information on smoking intensity, generally measured as cigarettes smoked per day among current smokers, find that heavy smoking elicits the highest mortality risks (Rogers et al. 2005; Thun et al. 2013). Assessing years since cessation among former smokers is important as well; smoking cessation confers survival advantages, with the mortality risk of former smokers approaching never smokers with greater time since quitting (Doll et al. 2004; Jha et al. 2013; Kenfield et al. 2008). But some former smokers may have quit only after the onset of smoking-attributable morbidity. The ability to observe smoking status, smoking intensity, and time since cessation is key for demonstrating that causes of death are attributable to smoking. We anticipate that if the additional causes compiled by Carter et al. (2015) are indeed attributable to smoking, mortality from these causes will be higher among heavy than light smokers and among recent than long-term quitters, mirroring the pattern for the established smoking-attributable causes.
Prior studies have applied this direct method, but with all causes of death combined, with only a few of the most recognized smoking-attributable causes, or with data containing too few deaths or with cause of death reported too crudely to examine rare causes that are nonetheless attributable to smoking. For example, Rogers et al. (2005) used an earlier release of the NHIS-LMF to directly assess the U.S. all-cause mortality burden of smoking. They found that smoking was responsible for 21 % of adult deaths for men and 13 % for women, translating to 340,000 deaths in the year 2000 that would have been prevented in the absence of smoking. More recently, Rostron (2013) used public-use NHIS-LMF data to estimate that roughly 380,000 U.S. adult deaths per year were caused by smoking—a figure based only on the established smoking-attributable causes of death. Our study is the first to draw on a large, nationally representative data set that directly measures smoking status and key confounders and includes detailed cause-of-death codes; to examine established and additional causes simultaneously; and to employ demographic techniques to assess the longevity consequences of established and additional smoking-attributable causes of death.
Methods
Data
We use a special-request file of the 1990–2011 NHIS-LMF comprising pooled cross-sections of the 1990–2009 NHIS probabilistically linked to death records maintained by the National Death Index through 2011 (National Center for Health Statistics (NCHS) 2015). In years 1990–1995, smoking is measured in supplements to the NHIS core questionnaire that cover such topics as health promotion and disease prevention, cancer control, and the Healthy People 2000 objectives. In years 1997–2009, smoking is measured in the NHIS Sample Adult Files. An important strength of the NHIS-LMF for our study is that it is nationally representative of the U.S. noninstitutionalized population. In contrast, the five samples that Carter and colleagues (2015) analyzed are more highly educated and less racially/ethnically diverse than the U.S. population.
We limit analyses to ages 35+ because these are the ages at which most individuals have established long-term patterns of either smoking or not smoking and, among smokers, are beginning to die from smoking-attributable causes (Rogers and Powell-Griner 1991). Whereas studies using indirect methods restrict ages to 50+ because lung cancer deaths are relatively rare before age 50, several studies have observed smoking-attributable mortality beginning at about age 35 years (Rostron 2011; U.S. DHHS 2014). After excluding respondents with missing values,3 our analytic sample includes 4,191,832 person-years contributed by 456,800 respondents, with 69,142 deaths during the follow-up period. We analyze the restricted-use NHIS-LMF data in Federal Statistical Research Data Centers.
Measures
Cause of Death
Our data set contains restricted-use cause-of-death codes for the 9th and 10th revisions of the International Statistical Classification of Diseases, Injuries, and Causes of Death (ICD-9 and ICD-10; World Health Organization 2010). Prior to 2007, the 113 cause categories in the public-use NHIS-LMF are too inexact for our analysis of the established and additional smoking-attributable causes. Beginning in 2007, the public-use NHIS-LMF reports cause of death in 10 broad categories, which is too imprecise for an analysis of smoking-attributable causes. Additionally, in the public-use mortality data, cause of death is perturbed for some rare causes to protect confidentiality of NHIS respondents (Lochner et al. 2008). Although this perturbation process does not affect results of all-cause or broad cause-specific mortality estimates, this may not be the case for specific smoking-attributable causes of death. The restricted-use mortality file possesses a high level of precision, including cause-of-death codes with a decimal digit to indicate subcategories, which allows us to exactly replicate the Carter et al. (2015) cause-of-death coding scheme. For example, the ICD-10 code C92.0 represents acute myeloid leukemia, a smoking-attributable cause acknowledged by the surgeon general. Researchers using the public-use NHIS-LMF cause-of-death variable would have only a more general leukemia category (ICD-10: C91–C95), even though other forms of leukemia are not linked to smoking (Rostron 2013). Table S1 in the online appendix lists the ICD-9 and ICD-10 codes for each cause of death considered in this study. Deaths occurring in years 1990–1998 are assigned ICD-9 codes, and deaths occurring in years 1999–2011 are assigned ICD-10 codes. The ICD-9 and ICD-10 codes are harmonized based on guidelines recommended by Anderson et al. (2001). Table S2 in the online appendix provides the unweighted number of deaths from each smoking-attributable cause in the 1990–2011 NHIS-LMF.
Smoking Status, Smoking Intensity, and Time Since Quitting
Smoking status is self-reported by respondents and coded as current, former, and never smokers. Current smokers report smoking 100 or more cigarettes in their lifetime and now smoke every day or some days. Former smokers report smoking 100 or more cigarettes in their lifetime but not at all now. Never smokers report smoking fewer than 100 cigarettes in their entire life. Current smokers are further disaggregated by number of cigarettes smoked per day (<10, 10–19, 20–39, and 40+ cigarettes), cut points that correspond to cigarette packages (e.g., less than half a pack of cigarettes, half a pack to less than one whole pack, one pack to less than two packs, and two or more packs, respectively). We disaggregate former smokers by years since they quit smoking (<5, 5–9, 10–19, 20–29, and 30+ years).4 The cross-sectional design of the NHIS allows measurement of smoking status at baseline but does not allow observation of transitions in smoking status between baseline and either death or end of the follow-up period. Some respondents who report smoking in the survey may quit smoking during follow-up, and some former smokers may resume smoking. Such transitions among smoking categories could potentially lead to underestimation of mortality risk ratios for current smokers compared with never smokers and overestimation of mortality risk ratios for former smokers relative to never smokers (Taylor et al. 2002).
Covariates
Models adjust for several sociodemographic and behavioral confounders associated with both smoking status and mortality risk. Covariates include age, race/ethnicity (Hispanic, non-Hispanic black, and non-Hispanic white (reference category)), educational attainment (less than high school, high school graduate (reference category), some college, and college graduate), region of residence (Northeast, Midwest, South, and West (reference category)), marital status (married (reference category), widowed, divorced/separated, and never married), health insurance coverage (insured (reference category) or uninsured), and BMI (underweight (<18.5 kg/m2), healthy weight (18.5–24.9 kg/m2; reference category), overweight (25–29.9 kg/m2), and obese (≥30 kg/m2)).5 We do not adjust for alcohol use in all analyses because it is measured consistently in NHIS Sample Adult File data only beginning in 1997. However, for all causes combined, cirrhosis mortality, and some other causes for which alcohol use is a risk factor, we conduct sensitivity analyses that limit the sample to years 1997 forward and adjust for alcohol use.6
Analytic Approach
In the first analytic step, we use Poisson regression models to estimate relative risk ratios of mortality for current and former smokers relative to never smokers. The outcome variables are all-cause and cause-specific mortality during follow-up. We include 21 causes that are established as attributable to cigarette smoking (according to the surgeon general) and 14 additional causes that Carter et al. (2015) compiled. In these models, we stratify by sex and statistically adjust for confounders. Subsequent models compare mortality risk of current smokers of different smoking intensities with never smokers and mortality risk of former smokers of different durations since cessation with never smokers. Poisson regression models account for whether individuals have survived or died at follow-up, as well as the timing of death. We transform the person-level data into person-year data based on dates of interview and exit through death or end of follow-up. Respondents contribute one-half a person-year for the year of interview and year of death, and one person-year for all intervening years and the final year of follow-up among respondents who survive through December 31, 2011. Poisson regression models are well suited for estimating mortality rates with person-year data. By including a statistical offset of the natural log of exposure time, models estimate the natural log of mortality rates as a linear function of covariates (Powers and Xie 2000). Exponentiation of coefficients produces risk ratios. Generally, discrete- and continuous-time analyses produce practically identical results in mortality research (Allison 2014; Rogers et al. 2000). We apply sample weights adjusted for NDI linkage eligibility so that our results represent the noninstitutionalized U.S. population ages 35+. As recommended by NCHS (2016), we divide supplement and Sample Adult File weights by the number of pooled survey years. We conduct analyses with SAS-callable SUDAAN 11.0.1 software to account for the complex sampling design of the NHIS (Research Triangle Institute 2012).
Next, we estimate smoking-attributable fractions (SAFs) using the formula best suited for risk ratios adjusted for confounders (Williamson 2010):
| (1) |
where pds is the proportion of total deaths occurring among current smokers, RRs is the adjusted all-cause risk ratio of current smokers to never smokers, pdf is the proportion of total deaths occurring among former smokers, and RRf is the adjusted all-cause risk ratio of former smokers to never smokers.
The final portion of the analysis estimates the percentage and number of excess deaths from the established and additional causes attributable to smoking for the U.S. population. These analyses stratify by sex and adjust for all confounders in the relative risk models. We use age distributions from the 2010 U.S. Census as the standard population to produce recent estimates of smoking-attributable mortality (Howden and Meyer 2011). We decompose the mortality disadvantage among smokers by cause of death using the following formula:
| (2) |
where mx,c,s is the death rate from cause c among current smokers in 10-year age group x, mx,c,n is the death rate from cause c among never smokers in age group x, mx,s is the all-cause death rate among smokers in age group x, and mx,n is the all-cause death rate among never smokers in age group x. The resulting percentages (Ps) describe how much of the mortality disadvantage among smokers is due to each established and additional cause of death.
We estimate the annual number of excess deaths attributable to smoking (Ds) with the following formula:
| (3) |
where px,s is the proportion of current smokers in age group x, and Nx is the 10-year age-specific population. This approach is repeated to also estimate the number of excess deaths among former smokers. The excess number of deaths due to smoking is presented for established and additional causes. Ten-year age-specific population counts are drawn from the 2010 U.S. Census (Howden and Meyer 2011). The estimates of excess deaths simulate the annual number of premature deaths that would not have occurred in the absence of smoking (i.e., if current and former smokers experienced the mortality rates of never smokers).
Results
Sample Characteristics
Table 1 presents the distributions of the covariates controlled in regression models, stratified by sex and smoking status. These results reinforce well-known sociodemographic correlates of smoking (Rogers et al. 1995). Among both sexes, we find higher percentages of current smokers who are non-Hispanic black rather than non-Hispanic white, have obtained lower levels of education rather than higher levels, are underweight rather than healthy weight, and are without health insurance rather than insured. Among men, 30.7 % with less than a high school education report never smoking, compared with 57.2 % who graduated college. Cigarette use is much lower among Hispanics relative to non-Hispanic black or white adults for both sexes, but especially so among women. The analyses that follow adjust for these confounders because they are associated with both smoking status and mortality risk. Among current smokers, compared with women, men report higher smoking intensity, with a significantly higher percentage in the groups smoking 20–39 and 40+ cigarettes per day.
Table 1.
Descriptive statistics of the analytic sample by sex and smoking status, U.S. adults ages 35+ years
| Female | Male | |||||
|---|---|---|---|---|---|---|
| Variables | Current Smoker | Former Smoker | Never Smoker | Current Smoker | Former Smoker | Never Smoker |
| Age (Years, Mean) | 52.2 (52.1, 52.4) | 59.4 (59.2, 59.6) | 57.0 (56.9, 57.2) | 51.3 (51.2, 51.4) | 60.7 (60.5, 60.8) | 52.0 (51.8, 52.1) |
| Race/Ethnicity | ||||||
| Hispanic | 14.7 (14.0, 15.3) | 13.5 (12.9, 14.0) | 71.9 (71.0, 72.7) | 24.2 (23.5, 24.9) | 23.9 (23.1, 24.7) | 51.9 (51.0, 52.8) |
| Non-Hispanic black | 23.7 (23.0, 24.4) | 14.0 (13.5, 14.6) | 62.2 (61.4, 63.1) | 31.9 (31.0, 32.8) | 21.5 (20.7, 22.2) | 46.6 (45.6, 57.6) |
| Non-Hispanic white | 22.6 (22.3, 22.9) | 23.2 (22.8, 23.5) | 54.2 (53.8, 54.6) | 25.5 (25.2, 25.9) | 33.2 (32.9, 33.6) | 41.2 (40.8, 41.6) |
| Educational Attainment | ||||||
| Less than high school | 26.3 (25.7, 26.8) | 17.7 (17.3, 18.2) | 56.0 (55.3, 56.7) | 35.7 (35.0, 36.4) | 33.6 (32.9, 34.2) | 30.7 (30.1, 31.4) |
| 2002High school diploma | 26.4 (25.9, 26.8) | 20.8 (20.4, 21.2) | 52.8 (52.2, 53.4) | 32.8 (32.3, 33.3) | 31.1 (30.5, 31.6) | 36.1 (35.6, 36.7) |
| Some college | 22.0 (21.6, 22.5) | 23.3 (22.9, 23.8) | 54.6 (54.1, 55.2) | 26.1 (25.5, 26.6) | 31.3 (30.8, 31.9) | 42.6 (42.0, 43.3) |
| College graduate | 11.8 (11.4, 12.2) | 22.6 (22.1, 23.1) | 65.6 (65.1, 66.2) | 12.7 (12.3, 13.1) | 30.1 (29.5, 30.7) | 57.2 (56.6, 57.8) |
| Marital Status | ||||||
| Married | 19.4 (19.1, 19.7) | 22.3 (21.9, 22.6) | 58.3 (57.9, 58.8) | 22.5 (22.2, 22.9) | 34.5 (34.1, 34.9) | 43.0 (42.5, 43.4) |
| Widowed | 15.0 (14.6, 15.5) | 23.7 (23.1, 24.3) | 61.3 (60.6, 62.0) | 19.4 (18.3, 20.5) | 48.7 (47.3, 50.1) | 31.9 (30.6, 33.2) |
| Divorced/separated | 32.8 (32.2, 33.5) | 21.1 (20.6, 21.7) | 46.0 (45.4, 46.7) | 39.7 (38.9, 40.5) | 27.3 (26.6, 28.0) | 33.0 (32.2, 33.8) |
| Never married | 27.6 (26.9, 28.3) | 14.6 (14.0, 15.2) | 57.8 (57.0, 58.5) | 31.5 (30.9, 32.2) | 17.9 (17.4, 18.4) | 50.6 (49.9, 51.3) |
| Region | ||||||
| Northeast | 21.5 (20.9, 22.2) | 24.6 (23.8, 25.3) | 53.9 (53.0, 54.8) | 23.8 (23.1, 24.4) | 33.2 (32.5, 34.0) | 43.0 (42.2, 43.8) |
| Midwest | 23.6 (23.1, 24.2) | 21.0 (20.5, 21.5) | 55.4 (54.7, 56.1) | 27.6 (27.0, 28.3) | 30.9 (30.3, 31.6) | 41.4 (40.9, 42.0) |
| South | 22.7 (22.2, 23.1) | 18.8 (18.3, 19.2) | 58.6 (57.9, 59.2) | 27.8 (27.3, 28.4) | 30.1 (29.6, 30.7) | 42.0 (41.4, 42.7) |
| West | 19.5 (18.9, 20.2) | 22.8 (22.3, 23.4) | 57.6 (56.8, 58.4) | 23.2 (22.4, 24.0) | 31.7 (31.0, 32.4) | 45.2 (44.3, 46.0) |
| Body Mass Index (kg/m2) | ||||||
| Underweight (<18.5) | 35.1 (33.6, 36.6) | 15.8 (14.8, 16.9) | 49.0 (47.5, 50.5) | 48.4 (43.9, 52.8) | 17.1 (13.8, 20.4) | 34.5 (30.4, 38.6) |
| Healthy weight (18.5‒24.9) | 24.1 (23.7, 24.5) | 20.7 (20.3, 21.1) | 55.2 (54.7, 55.7) | 31.7 (31.1, 32.3) | 26.1 (25.6, 26.6) | 42.2 (41.6, 42.8) |
| Overweight (25‒29.9) | 20.1 (19.7, 20.5) | 21.9 (21.5, 22.4) | 58.0 (57.5, 58.5) | 23.7 (23.3, 24.1) | 33.3 (32.8, 33.7) | 43.0 (42.6, 43.5) |
| Obese (30+) | 18.2 (17.8, 18.6) | 22.6 (22.1, 23.1) | 59.2 (58.6, 59.8) | 21.6 (21.1, 22.1) | 35.5 (34.9, 36.1) | 42.9 (42.3, 43.5) |
| Health Insurance Coverage | ||||||
| Uninsured | 35.1 (34.3, 35.9) | 14.4 (13.8, 14.9) | 50.5 (49.6, 51.4) | 44.0 (43.2, 44.8) | 18.0 (17.4, 18.6) | 38.0 (37.2, 38.8) |
| Insured | 20.5 (20.2, 20.8) | 22.1 (21.8, 22.4) | 57.4 (57.0, 57.8) | 23.4 (23.1, 23.7) | 33.2 (32.9, 33.6) | 43.4 (43.0, 43.8) |
| Cigarettes Smoked per Day | ||||||
| Less than 10 cigarettes | 23.3 (22.9, 23.8) | 18.8 (18.3, 19.2) | ||||
| 10‒19 cigarettes | 30.1 (29.5, 30.6) | 22.6 (22.2, 23.1) | ||||
| 20‒39 cigarettes | 41.2 (40.6, 41.8) | 47.3 (46.7, 47.8) | ||||
| 40+ cigarettes | 5.4 (5.1, 5.6) | 11.3 (10.9, 11.7) | ||||
| Years Since Cessationa | ||||||
| Less than 5 years | 23.5 (23.0, 24.0) | 19.7 (19.3, 20.2) | ||||
| 5‒9 years | 16.1 (15.6, 16.5) | 13.7 (13.3, 14.1) | ||||
| 10‒19 years | 28.6 (28.1, 29.2) | 26.9 (26.3, 27.4) | ||||
| 20‒29 years | 18.7 (18.2, 19.2) | 21.6 (21.1, 22.1) | ||||
| 30+ years | 13.1 (12.7, 13.5) | 18.1 (17.7, 18.6) | ||||
| Deaths | 7,781 | 9,595 | 20,473 | 8,759 | 14,269 | 8,265 |
| Person-Years | 531,047 | 497,860 | 1,372,616 | 476,135 | 554,497 | 759,677 |
| Individuals | 54,945 | 49,380 | 152,833 | 52,425 | 56,536 | 90,681 |
Notes: Percentages and means are weighted and total deaths and person-years are unweighted. 95 % confidence intervals are listed in parentheses.
Source: 1990–2011 NHIS-LMF
Survey years 1991 and 1993 do not measure time since smoking cessation among former smokers.
Cause-Specific Relative Risk Ratios
Relative risk ratios show elevated mortality risk among current smokers for all causes combined, causes of death established as smoking-attributable, and the additional causes associated with smoking that Carter and colleagues (2015) compiled (see Table 2). All-cause mortality risk during follow-up is more than twice as high among female and male current smokers compared with never smokers. For former smokers, mortality is 35 % higher among women and 30 % higher among men compared with never smokers. Relative risks for all-cause mortality are similar for both sexes.
Table 2.
Relative risk ratios of all-cause and cause-specific mortality risk among current and former smokers compared with never smokers, U.S. adults ages 35+ years
| Female |
Male |
|||
|---|---|---|---|---|
| Causes of Death | Current Smoker |
Former Smoker |
Current Smoker |
Former Smoker |
| All-Cause Mortality | 2.29*** | 1.35*** | 2.24*** | 1.30*** |
| Diseases Established as Caused by Smoking | ||||
| Lip and oral cavity cancer | 4.34*** | 1.61 | 3.97*** | 1.09 |
| Esophageal cancer | 2.29** | 1.41 | 3.50*** | 1.84*** |
| Stomach cancer | 1.92** | 0.96 | 1.69* | 1.33 |
| Colorectal cancer | 1.52*** | 1.09 | 1.30* | 1.23* |
| Liver cancer | 1.43 | 1.14 | 2.98*** | 1.91*** |
| Pancreatic cancer | 2.04*** | 1.15 | 2.23*** | 1.20 |
| Laryngeal cancer | 26.11*** | 6.45** | 13.23*** | 3.24* |
| Lung cancer | 18.81*** | 6.39*** | 13.79*** | 4.36*** |
| Cervical cancer | 2.28*** | 1.66† | — | — |
| Urinary bladder cancer | 3.97*** | 2.67*** | 6.28*** | 2.93*** |
| Kidney and renal pelvis cancer | 1.77† | 1.82** | 1.91** | 1.30 |
| Acute myeloid leukemia | 2.00* | 1.30 | 1.51 | 1.29 |
| Diabetes | 1.63*** | 1.41*** | 1.50*** | 1.25** |
| Ischemic heart disease | 2.07*** | 1.25*** | 2.17*** | 1.25*** |
| Other heart disease | 1.57*** | 1.12* | 1.57*** | 1.11† |
| Stroke | 1.71*** | 1.06 | 1.56*** | 1.07 |
| Atherosclerosis | 2.39** | 1.28 | 1.95† | 1.12 |
| Aortic aneurysm | 8.05*** | 2.83*** | 8.34*** | 2.59*** |
| Other arterial diseases | 3.52*** | 1.41 | 3.46*** | 1.70* |
| Pneumonia, influenza, and tuberculosis | 2.33*** | 1.33** | 2.02*** | 1.17† |
| Chronic obstructive pulmonary disease | 17.65*** | 7.01*** | 10.58*** | 4.02*** |
| Additional Diseases Associated With Smoking | ||||
| All other infections | 2.18*** | 1.26* | 1.83*** | 1.43*** |
| Breast cancer | 1.21** | 1.16† | — | — |
| Prostate cancer | — | — | 1.33* | 1.13 |
| Rare cancers | 1.57*** | 1.12 | 2.20*** | 1.39** |
| Cancers of unknown site | 2.98*** | 1.47** | 4.15*** | 1.45* |
| Hypertensive heart disease | 1.85*** | 1.16 | 1.78*** | 1.06 |
| Essential hypertension/hypertensive renal disease | 2.13*** | 1.19 | 1.46 | 0.71 |
| All other respiratory diseases | 1.56** | 1.66*** | 1.99*** | 1.36** |
| Intestinal ischemia | 2.86*** | 1.66* | 3.37*** | 1.11 |
| Liver cirrhosis | 2.07*** | 1.21 | 3.64*** | 1.23 |
| All other digestive diseases | 2.16*** | 1.35** | 2.18*** | 1.24† |
| Renal failure | 1.40* | 1.11 | 1.07 | 1.05 |
| Additional rare causes combined | 1.41** | 1.09 | 1.31 | 0.87 |
| Unknown causes | 2.04*** | 0.98 | 1.40* | 1.22 |
Notes: Models adjust for age, race/ethnicity, educational attainment, marital status, region, BMI, and health insurance status. Diseases established as caused by smoking are those recognized by the surgeon general (U.S. DHHS 2014). Additional diseases associated with smoking are those compiled by Carter et al. (2015). Dashes indicate sex-specific causes of death (e.g., cervical and breast cancer among women and prostate cancer among men).
Source: 1990–2011 NHIS-LMF
p < .10
p < .05
p < .01
p < .001
Among established causes, the risk ratios of death due to lung cancer, laryngeal cancer, and COPD for female current smokers exceed 17 compared with never smokers. In addition, the risk ratio of death due to ischemic heart disease for male current smokers is 2.17 compared with never smokers. Although risk ratios are smaller for some heart diseases and cancers other than of the lungs, heart diseases and other cancers are responsible for more total deaths than lung cancer, laryngeal cancer, and COPD (Murphy et al. 2017). We also find elevated mortality risk among smokers from the additional causes, net of confounders. For example, female and male current smokers exhibit rate ratios of death due to hypertensive heart disease that are 85 % and 78 % higher, respectively, than their never-smoker counterparts. Risk ratios for current smokers are also substantial for cancers of unknown site (2.98 among women and 4.15 among men) and intestinal ischemia (2.86 among women and 3.37 among men). Excluding these additional causes from estimates of excess mortality due to smoking may underestimate the smoking burden on mortality.7
We use the numbers of deaths in Table 1 and the adjusted all-cause risk ratios in Table 2 to estimate sex-specific SAFs. We find that 18 % of female deaths and 26 % of male deaths are attributable to cigarette smoking. Our SAF estimates resemble fractions estimated in other recent studies, although they represent a high variant of the range among men. Relative to the 17 % of deaths among women and 21 % of deaths among men that Fenelon and Preston (2012) calculated for U.S. adults ages 50–84 in 2004, our male fraction is 5 percentage points higher. Recall, though, that our estimates are based on ages 35+.
Heterogeneity Among Current and Former Smokers
We next disaggregate current smokers by smoking intensity and former smokers by time since quitting. In this analytic step, we combine women and men and adjust for sex to bolster sample sizes for relatively rare causes of death because rate ratios are similar for women and men for most causes. Table 3 displays rate ratios comparing mortality risk of current smokers of different smoking intensities with never smokers. We find graded risk of mortality for most of the established causes and several (but not all) of the additional causes, with the heaviest smokers exhibiting the highest mortality risk.8 Additional causes that exhibit notable gradients include all other infections, cancers of unknown site, liver cirrhosis, and unknown causes. Death during follow-up is even significantly elevated among the lightest smokers (i.e., those who smoke <10 cigarettes per day) for 24 of the 35 causes, including 8 of the 14 additional causes.
Table 3.
Relative risk ratios of all-cause and cause-specific mortality risk among current smokers (by cigarettes smoked per day) compared with never smokers, U.S. adults ages 35+ years
| Causes of Death | 40+ Cigarettes |
20‒39 Cigarettes |
10‒19 Cigarettes |
<10 Cigarettes |
|---|---|---|---|---|
| All-Cause Mortality | 3.23*** | 2.47*** | 2.04*** | 1.78*** |
| Diseases Established as Caused by Smoking | ||||
| Lip and oral cavity cancer | 6.19*** | 5.50*** | 3.45*** | 2.24* |
| Esophageal cancer | 3.61*** | 3.57*** | 2.13** | 3.46*** |
| Stomach cancer | 3.52*** | 1.63* | 1.76* | 2.21** |
| Colorectal cancer | 1.69** | 1.54*** | 1.49** | 1.24 |
| Liver cancer | 2.21* | 2.41*** | 1.71* | 2.54*** |
| Pancreatic cancer | 2.09** | 2.20*** | 1.98*** | 2.12*** |
| Laryngeal cancer | 36.59*** | 22.28*** | 16.28*** | 7.31*** |
| Lung cancer | 33.65*** | 21.70*** | 15.60*** | 9.04*** |
| Cervical cancer (women only) | 3.10† | 3.57*** | 1.54 | 1.50 |
| Urinary bladder cancer | 8.07*** | 6.36*** | 4.22*** | 4.59*** |
| Kidney and renal pelvis cancer | 1.66 | 1.94** | 1.77* | 1.84* |
| Acute myeloid leukemia | 2.92* | 1.67† | 1.43 | 1.71 |
| Diabetes | 2.53*** | 1.65*** | 1.55*** | 1.15 |
| Ischemic heart disease | 2.83*** | 2.29*** | 1.88*** | 1.72*** |
| Other heart disease | 1.95*** | 1.62*** | 1.43*** | 1.44*** |
| Stroke | 1.60*** | 1.66*** | 1.54*** | 1.63*** |
| Atherosclerosis | 3.59* | 2.67** | 1.40 | 2.02 |
| Aortic aneurysm | 8.69*** | 11.10*** | 7.09*** | 6.35*** |
| Other arterial diseases | 7.37*** | 4.09*** | 2.98*** | 2.85** |
| Pneumonia, influenza, and tuberculosis | 3.22*** | 2.48*** | 1.70*** | 1.94*** |
| Chronic obstructive pulmonary disease | 30.63*** | 19.81*** | 14.27*** | 11.75*** |
| Additional Diseases Associated With Smoking | ||||
| All other infections | 3.42*** | 1.82*** | 1.78*** | 1.59*** |
| Breast cancer (women only) | 0.89 | 1.34** | 1.21 | 1.10 |
| Prostate cancer (men only) | 1.44 | 1.23 | 1.58* | 1.09 |
| Rare cancers | 2.48*** | 2.01*** | 1.52** | 1.58** |
| Cancers of unknown site | 5.64*** | 4.00*** | 3.22*** | 2.16*** |
| Hypertensive heart disease | 2.14** | 1.92*** | 2.02*** | 1.20 |
| Essential hypertension/hypertensive renal disease | 2.83* | 1.98** | 1.00 | 1.73† |
| All other respiratory diseases | 2.24** | 1.77*** | 1.82*** | 1.47* |
| Intestinal ischemia | 2.67* | 3.46*** | 2.66** | 2.65*** |
| Liver cirrhosis | 3.96*** | 2.78*** | 2.75*** | 2.69*** |
| All other digestive diseases | 3.29*** | 2.33*** | 1.77*** | 1.86*** |
| Renal failure | 1.00 | 1.24 | 1.21 | 1.16 |
| Additional rare causes combined | 1.51 | 1.52** | 1.09 | 1.07 |
| Unknown causes | 2.22** | 2.08*** | 1.95*** | 1.10 |
Notes: Models adjust for sex, age, race/ethnicity, educational attainment, marital status, region, BMI, and health insurance status. Diseases established as caused by smoking are those recognized by the surgeon general (U.S. DHHS 2014). Additional diseases associated with smoking are those compiled by Carter et al. (2015).
Source: 1990–2011 NHIS-LMF
p < .10
p < .05
p < .01
p < .001
Table 4 presents risk ratios comparing mortality risk of former smokers of different durations since cessation to never smokers. Risk ratios generally decline as time since quitting increases. Some former smokers who recently quit may have quit only after the onset of smoking-attributable morbidity. Mortality risk among former smokers who quit smoking 30+ years ago does not statistically differ from never smokers for nearly all causes of death. The exceptions are cancers of the liver, lung, and bladder; COPD; and all other respiratory diseases. For these causes, mortality risk is still significantly elevated even among former smokers who quit smoking 30+ years ago.
Table 4.
Relative risk ratios of all-cause and cause-specific mortality risk among former smokers (by years since cessation) compared with never smokers, U.S. adults ages 35+ years
| Causes of Death | <5 Years |
5‒9 Years |
10‒19 Years |
20‒29 Years |
30+ Years |
|---|---|---|---|---|---|
| All-Cause Mortality | 2.01*** | 1.74*** | 1.43*** | 1.16*** | 1.04* |
| Diseases Established as Caused by Smoking | |||||
| Lip and oral cavity cancer | 4.40*** | 2.85* | 1.55 | 0.74 | 0.67 |
| Esophageal cancer | 2.84*** | 1.84† | 1.72* | 1.80* | 0.87 |
| Stomach cancer | 2.01* | 2.55** | 1.91** | 1.03 | 1.14 |
| Colorectal cancer | 1.21 | 1.71*** | 0.94 | 1.11 | 1.02 |
| Liver cancer | 1.39 | 1.33 | 2.06*** | 0.86 | 1.87** |
| Pancreatic cancer | 1.93*** | 1.38 | 1.28 | 1.06 | 1.01 |
| Laryngeal cancer | 12.19*** | 9.59*** | 4.53** | 1.54 | 0.63 |
| Lung cancer | 14.16*** | 10.43*** | 5.99*** | 3.74*** | 1.99*** |
| Cervical cancer (women only) | 2.00 | 1.01 | 1.97 | 1.98 | —a |
| Urinary bladder cancer | 2.58** | 4.89*** | 4.01*** | 2.27*** | 2.41*** |
| Kidney and renal pelvis cancer | 2.27** | 1.66 | 0.95 | 1.48 | 1.26 |
| Acute myeloid leukemia | 1.12 | 2.62** | 1.64 | 1.82† | 1.25 |
| Diabetes | 1.90*** | 1.45** | 1.29** | 1.20† | 1.15 |
| Ischemic heart disease | 1.86*** | 1.53*** | 1.41*** | 1.12** | 0.98 |
| Other heart disease | 1.45*** | 1.36** | 1.19* | 0.91 | 1.08 |
| Stroke | 1.19† | 1.05 | 1.29** | 0.97 | 0.92 |
| Atherosclerosis | 2.36* | 1.10 | 1.54 | 1.31 | 1.17 |
| Aortic aneurysm | 3.74*** | 4.29*** | 2.93*** | 1.88* | 1.03 |
| Other arterial diseases | 1.78 | 2.00† | 2.42** | 1.66 | 0.96 |
| Pneumonia, influenza, and tuberculosis | 1.55** | 1.59** | 1.24† | 1.09 | 0.90 |
| Chronic obstructive pulmonary disease | 15.58*** | 12.10*** | 7.02*** | 4.0*** | 2.23*** |
| Additional Diseases Associated With Smoking | |||||
| All other infections | 1.39* | 1.32 | 1.19 | 1.38* | 1.06 |
| Breast cancer (women only) | 1.08 | 1.82*** | 1.13 | 1.24 | 0.67 |
| Prostate cancer (men only) | 1.20 | 1.21 | 1.31† | 1.09 | 0.89 |
| Rare cancers | 1.84*** | 1.45* | 1.26† | 1.16 | 1.19 |
| Cancers of unknown site | 2.26*** | 2.17** | 1.56* | 0.84 | 1.13 |
| Hypertensive heart disease | 1.27 | 1.20 | 0.85 | 1.09 | 0.75 |
| Essential hypertension/hypertensive renal disease | 1.28 | 1.60 | 1.11 | 0.67 | 1.11 |
| All other respiratory diseases | 1.47† | 1.99** | 1.66*** | 1.55** | 1.48** |
| Intestinal ischemia | 2.67** | 1.68 | 2.16** | 0.84 | 0.84 |
| Liver cirrhosis | 1.42 | 1.10 | 1.41† | 1.46 | 1.19 |
| All other digestive diseases | 1.79** | 1.86*** | 1.12 | 1.19 | 0.90 |
| Renal failure | 1.62** | 1.20 | 1.25† | 0.97 | 0.85 |
| Additional rare causes combined | 1.35 | 1.13 | 0.99 | 0.95 | 0.89 |
| Unknown causes | 2.69*** | 1.27 | 0.97 | 0.89 | 0.97 |
Notes: Survey years 1991 and 1993 do not measure time since smoking cessation among former smokers. Models adjust for sex, age, race/ethnicity, educational attainment, marital status, region, BMI, and health insurance status. Diseases established as caused by smoking are those recognized by the surgeon general (U.S. DHHS 2014). Additional diseases associated with smoking are those compiled by Carter et al. (2015).
Source: 1990–2011 NHIS-LMF
Too few deaths from cervical cancer occurred to estimate a risk ratio among former smokers who quit 30+ years ago.
p < .10
p < .05
p < .01
p < .001
By disaggregating current smokers by smoking intensity and former smokers by time since cessation, we find that the gradients are generally in the expected direction. That is, the gradients are consistent with the premise that the additional causes are attributable to smoking. For most causes, risk ratios are higher among heavy smokers and recent quitters than among light smokers and respondents who quit long ago, respectively.
Cause-Specific Excess Mortality Attributable to Smoking
Table 5 presents percentages of cause-specific excess mortality attributable to smoking.9 These percentages describe how much of the mortality disadvantage among smokers is due to each established and additional cause of death. Percentages of smoking-attributable mortality among women are 80.8 % for established causes and 14.8 % for additional causes; these figures for men are 80.3 % and 14.4 %, respectively. The most sizable percentages are observed for the established causes: lung cancer and COPD, which each account for a higher percentage of excess deaths than all additional causes combined. Although none of the additional causes have particularly large percentages individually, together they account for 14 % to 15 % of the excess deaths among smokers. These percentages further demonstrate that studies that do not include the additional causes of death miss a nonnegligible amount of the mortality burden due to smoking. Interestingly, the established and additional causes do not account for 100 % of the smoker mortality disadvantage; 4.4 % and 5.3 % of the smoker disadvantage persists for women and men, respectively. These remaining percentages suggest that either (1) other causes that are yet to be determined or not included (e.g., residential house fires) are attributable to smoking, or (2) risk factors associated with smoking but not adjusted for in this study account for this pattern.
Table 5.
Percentage of excess mortality attributable to smoking, U.S. adults ages 35+ years
| Female |
Male |
|||||
|---|---|---|---|---|---|---|
| Causes of Death | Death Rates Among Current Smokers |
Death Rates Among Never Smokers |
% of Excess Death due to Each Cause |
Death Rates Among Current Smokers |
Death Rates Among Never Smokers |
% of Excess Death due to Each Cause |
| Diseases Established as Caused by Smoking | 80.8 | 80.3 | ||||
| Lip and oral cavity cancer | 5.9 | 1.7 | 0.5 | 24.2 | 1.1 | 1.7 |
| Esophageal cancer | 4.5 | 1.5 | 0.4 | 29.7 | 8.7 | 1.5 |
| Stomach cancer | 5.1 | 2.5 | 0.3 | 8.1 | 7.2 | 0.1 |
| Colorectal cancer | 33.6 | 23.9 | 1.2 | 42.8 | 30.8 | 0.9 |
| Liver cancer | 7.5 | 2.4 | 0.6 | 19.1 | 6.8 | 0.9 |
| Pancreatic cancer | 26.6 | 16.4 | 1.2 | 58.2 | 24.3 | 2.4 |
| Laryngeal cancer | 3.9 | 0.2 | 0.4 | 7.1 | 2.2 | 0.4 |
| Lung cancer | 255.0 | 15.6 | 28.8 | 377.3 | 43.1 | 24.0 |
| Cervical cancer | 3.4 | 0.1 | 0.4 | — | — | — |
| Urinary bladder cancer | 8.8 | 2.0 | 0.8 | 49.7 | 8.0 | 3.0 |
| Kidney and renal pelvis cancer | 3.6 | 1.2 | 0.3 | 11.3 | 5.2 | 0.4 |
| Acute myeloid leukemia | 5.9 | 2.3 | 0.4 | 16.1 | 4.5 | 0.8 |
| Diabetes | 17.9 | 15.7 | 0.3 | 35.0 | 15.8 | 1.4 |
| Ischemic heart disease | 190.1 | 101.1 | 10.7 | 424.4 | 249.9 | 12.5 |
| Other heart disease | 75.7 | 58.7 | 2.0 | 99.6 | 67.1 | 2.3 |
| Stroke | 97.5 | 81.8 | 1.9 | 159.4 | 74.9 | 6.1 |
| Atherosclerosis | 5.3 | 2.3 | 0.4 | 7.1 | 5.0 | 0.2 |
| Aortic aneurysm | 10.2 | 3.3 | 0.8 | 31.8 | 5.0 | 1.9 |
| Other arterial diseases | 8.2 | 1.4 | 0.8 | 10.1 | 3.5 | 0.5 |
| Pneumonia, influenza, and tuberculosis | 43.2 | 21.2 | 2.6 | 91.3 | 34.9 | 4.0 |
| Chronic obstructive pulmonary disease | 227.2 | 12.3 | 25.8 | 246.4 | 30.5 | 15.5 |
| Additional Diseases Associated With Smoking | 14.8 | 14.4 | ||||
| All other infections | 20.8 | 12.3 | 1.0 | 35.7 | 15.8 | 1.4 |
| Breast cancer | 44.4 | 39.5 | 0.6 | — | — | — |
| Prostate cancer | — | — | — | 54.0 | 44.2 | 0.7 |
| Rare cancers | 36.8 | 23.1 | 1.6 | 40.9 | 17.8 | 1.7 |
| Cancers of unknown site | 21.2 | 6.2 | 1.8 | 40.2 | 9.4 | 2.2 |
| Hypertensive heart disease | 13.7 | 12.7 | 0.1 | 23.6 | 6.6 | 1.2 |
| Essential hypertension/hypertensive renal disease | 15.5 | 5.4 | 1.2 | 6.1 | 2.3 | 0.3 |
| All other respiratory diseases | 08.0 | 6.6 | 0.2 | 24.9 | 23.3 | 0.1 |
| Intestinal ischemia | 18.9 | 3.7 | 1.8 | 19.8 | 3.4 | 1.2 |
| Liver cirrhosis | 12.6 | 4.9 | 0.9 | 27.4 | 7.6 | 1.4 |
| All other digestive diseases | 31.3 | 16.2 | 1.8 | 57.3 | 15.1 | 3.0 |
| Renal failure | 5.2 | 4.9 | 0.0 | 20.3 | 20.4 | 0.0 |
| Additional rare causes combined | 33.1 | 15.3 | 2.1 | 18.5 | 11.8 | 0.5 |
| Unknown causes | 15.1 | 2.6 | 1.5 | 15.0 | 5.5 | 0.7 |
| Causes not Linked to Smoking | 256.7 | 219.9 | 4.4 | 417.6 | 344.0 | 5.3 |
| Total Deaths | 1,572.2 | 740.5 | — | 2,550.0 | 1,155.7 | — |
Notes: Rates and percentages are age-adjusted using the 2010 U.S. population (Howden and Meyer 2011). Death rates are expressed per 100,000. Models adjust for age, race/ethnicity, educational attainment, marital status, region, BMI, and health insurance status. Diseases established as caused by smoking are those recognized by the surgeon general (U.S. DHHS 2014). Additional diseases associated with smoking are those compiled by Carter et al. (2015). Dashes indicate sex-specific causes of death (e.g., cervical and breast cancer among women and prostate cancer among men).
Source: 1990–2011 NHIS-LMF
Table 6 and Fig. 1 present estimates for the annual number of excess deaths attributable to smoking, net of sociodemographic and behavioral confounders. We identify 417,524 excess deaths at ages 35+ from established causes, based on the U.S. population in 2010. This estimate is lower than the Centers for Disease Control and Prevention (CDC) unadjusted estimate of 437,400 annual adult deaths due to smoking during 2005–2009. We estimate 64,363 deaths from the additional causes, bringing the total annual number of smoking-attributable deaths among U.S. adults to 481,887.10 When we examine the excess number of deaths by age group, we find that other studies that exclude respondents younger than 50 years omit 23,036 smoking-attributable deaths: 8,328 deaths among women and 14,708 deaths among men.11 Former smokers contribute more excess deaths than current smokers at ages 85+ among women and 75+ among men, whereas current smokers contribute more excess deaths than former smokers at younger ages. Although current smokers exhibit higher risk ratios than former smokers for most causes (Table 2), former smokers still contribute substantial numbers of excess deaths, especially at older ages (Table 6).
Table 6.
Estimated number of excess deaths from smoking that would have been averted in 2010, net of confounders, by sex, U.S. adults ages 35+ years
| Female | Male | Total | |
|---|---|---|---|
| If Current Smoker Rates Were Replaced by Never Smoker Rates | |||
| Established causes | |||
| 35−44 | 1,902 | 3,896 | 5,798 |
| 45−54 | 9,495 | 19,450 | 28,945 |
| 55−64 | 22,738 | 44,609 | 67,347 |
| 65−74 | 27,905 | 49,489 | 77,394 |
| 75−84 | 22,979 | 24,713 | 47,692 |
| 85+ | 4,796 | 4,169 | 8,965 |
| Total | 89,815 | 146,326 | 236,141 |
| Additional causes | |||
| 35−44 | 1,206 | 1,538 | 2,744 |
| 45−54 | 2,580 | 5,664 | 8,244 |
| 55−64 | 4,808 | 11,140 | 15,948 |
| 65−74 | 4,720 | 7,509 | 12,229 |
| 75−84 | 3,328 | 3,764 | 7,092 |
| 85+ | 893 | 497 | 1,390 |
| Total | 17,535 | 30,112 | 47,647 |
| If Former Smoker Rates Were Replaced by Never Smoker Rates | |||
| Established causes | |||
| 35−44 | 333 | 289 | 622 |
| 45−54 | 1,473 | 3,385 | 4,858 |
| 55−64 | 7,255 | 13,015 | 20,270 |
| 65−74 | 18,080 | 37,390 | 55,470 |
| 75−84 | 20,497 | 47,616 | 68,113 |
| 85+ | 10,069 | 21,981 | 32,050 |
| Total | 57,707 | 123,676 | 181,383 |
| Additional causes | |||
| 35−44 | 289 | 268 | 557 |
| 45−54 | 279 | 718 | 997 |
| 55−64 | 987 | 1,574 | 2,561 |
| 65−74 | 1,568 | 1,224 | 2,792 |
| 75−84 | 2,899 | 5,301 | 8,200 |
| 85+ | 1,779 | –170 | 1,609 |
| Total | 7,801 | 8,915 | 16,716 |
| Total | 172,858 | 309,029 | 481,887 |
Note: Estimated number of excess deaths are based on 2010 U.S. population size and age composition, drawn from 2010 U.S. Census Summary File 1 (Howden and Meyer 2011). Models adjust for age, race/ethnicity, educational attainment, marital status, region, BMI, and health insurance status. Diseases established as caused by smoking are those recognized by the surgeon general (U.S. DHHS 2014). Additional diseases associated with smoking are those compiled by Carter et al. (2015).
Source: 1990–2011 NHIS-LMF
Fig. 1.
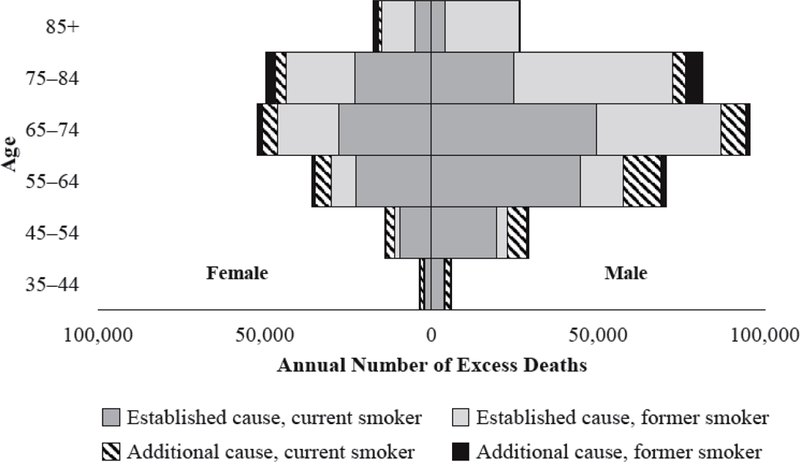
Estimated number of excess deaths attributable to smoking by age group in 2010, net of confounders, U.S. adults ages 35+ years
Comparability of NHIS-LMF and Carter and Colleagues Estimates
We compare the race/ethnicity and educational attainment profiles in the NHIS-LMF and the five cohort studies Carter et al. (2015) used to demonstrate that the nationally representative NHIS-LMF includes greater proportions of racial/ethnic minority and lower-educated respondents relative to the nonrepresentative samples of the five cohort studies (Table 7). Although our overall study examines U.S. adults ages 35+, we limit the sample to ages 55+ here to match the age range of the five cohort studies Carter and colleagues analyzed. A direct comparison by race/ethnicity is not possible because Carter and colleagues do not consider Hispanic ethnicity; their racial categories include “White,” “Black,” and “Other or missing data.” We find very large differences in the educational attainment of respondents between the NHIS-LMF and the five cohort studies. For example, only 11.8 % of female smokers are college graduates in the NHIS-LMF, whereas 37.7 % of female smokers are college graduates in the five cohort studies.
Table 7.
Comparison of covariate distributions in NHIS-LMF and the five cohort studies analyzed by Carter et al. (2015), U.S. adults ages 55+ years
| Female |
Male |
|||||
|---|---|---|---|---|---|---|
| Variables | Current Smoker | Former Smoker | Never Smoker | Current Smoker | Former Smoker | Never Smoker |
| NHIS-LMF | ||||||
| Age (mean, SD) | 65.6(7.4) | 68.5(8.3) | 71.1(9.2) | 64.7(7.0) | 68.3(8.4) | 68.5(9.0) |
| Race/ethnicity | ||||||
| Hispanic | 4.3 (3.9, 4.7) | 4.1 (3.8, 4.4) | 7.8 (7.3, 8.3) | 6.1 (5.7, 6.5) | 5.0 (4.7, 5.4) | 7.8 (7.4, 8.3) |
| Non-Hispanic black | 10.8(10.2, 11.4) | 7.6 (7.2, 8.1) | 10.4(9.9, 11.0) | 12.5(11.7, 13.2) | 6.4 (6.1, 6.8) | 9.1 (8.5, 9.6) |
| Non-Hispanic white | 84.9(84.2, 85.5) | 88.3(87.8, 88.8) | 81.8(81.1, 82.5) | 81.4(80.6, 82.2) | 88.5(88.0, 89.0) | 83.1(82.4, 83.8) |
| Educational | ||||||
| attainment | ||||||
| Less than high | ||||||
| school | 25.5(24.8, 26.3) | 19.6(19.0, 20.3) | 25.1(24.6, 25.7) | 26.9(26.1, 27.8) | 21.4(20.8, 22.0) | 17.5(17.0, 18.1) |
| High school diploma | 41.3(40.5, 42.1) | 36.5(35.8, 37.2) | 37.4(36.9, 38.0) | 35.8(35.0, 36.6) | 30.8(30.2, 31.5) | 27.0(26.3, 27.6) |
| Some college | 21.3(20.6, 22.0) | 23.8(23.2, 24.4) | 19.6(19.2, 20.0) | 21.5(20.8, 22.2) | 21.5(21.0, 21.9) | 19.5(18.9, 20.0) |
| College graduate | 11.8(11.3, 12.4) | 20.1(19.5, 20.7) | 17.9(17.4, 18.3) | 15.7(15.0, 16.5) | 26.3(25.6, 27.0) | 36.1(35.3, 36.8) |
| Total | 17.1(16.8, 17.4) | 25.8(25.4, 26.1) | 57.1(56.7, 57.6) | 20.7(20.3, 21.0) | 45.5(45.0, 45.9) | 33.8(33.4, 34.3) |
| Carter et al. (2015) | ||||||
| Age (mean, SD) | 64.3(6.0) | 66.1(6.4) | 66.4(6.6) | 64.3(5.7) | 66.8(6.1) | 66.0(6.5) |
| Racea | ||||||
| Black | 6.2 | 4.9 | 5.2 | 3.6 | 2.1 | 2.1 |
| White | 90.3 | 91.7 | 89.1 | 92.6 | 94.2 | 93.4 |
| Other or missing | 3.5 | 3.5 | 5.7 | 3.8 | 3.7 | 4.5 |
| Educational | ||||||
| attainment | ||||||
| High school or less | 26.7 | 18.1 | 21.8 | 29.3 | 22.6 | 15.7 |
| Some college | 33.4 | 28.9 | 27.4 | 35.1 | 30.1 | 21.4 |
| College graduate | 37.7 | 51.6 | 49.4 | 32.9 | 45.3 | 61.5 |
| Total | 9.5 | 41.8 | 48.7 | 9.0 | 58.5 | 32.4 |
Notes: Percentages and means are weighted. 95 % confidence intervals are listed in parentheses. Although our overall study examines U.S. adults ages 35+ years, we limit the sample to ages 55+ here to directly compare our NHIS-LMF covariate distributions to the distributions in the five cohort studies presented by Carter et al. (2015).
Hispanic ethnicity is not reported in Carter and colleagues’ (2015) results.
Table 8 compares our risk ratios to those Carter and colleagues estimated. This comparison generally shows agreement in magnitude and significance levels despite the differences in study design, period, and generalizability, providing further evidence that the list of 21 causes of death the surgeon general recognizes as smoking-attributable should be amended to incorporate the additional causes. Our risk ratios tend to be more modest than those of Carter and associates. However, confidence intervals for the risk ratios from the NHIS-LMF and five cohort studies overlap for most causes, with a few exceptions. Estimates are significantly lower for the NHIS-LMF for all causes combined, lung cancer, ischemic heart disease, and COPD among women and men. We find significantly elevated risk among female smokers from the additional cause category “rare cancers,” whereas Carter et al. did not. Furthermore, Carter and colleagues did not estimate risk ratios for the established cause cervical cancer because their data contained too few deaths. We find significantly higher mortality risk among female current smokers relative to female never smokers from cervical cancer at ages 35+ (Tables 2 and 3) but not at ages 55+ (Table 8). Thus, the nonrepresentative survey design of the CPS-II cohort and other prospective data sets Carter et al. used appears to exaggerate smoker–nonsmoker relative risks compared with the nationally representative NHIS-LMF, perhaps because of the very low mortality among the disproportionately white and college-educated never smokers in the five cohort studies.
Table 8.
Comparison of relative risk ratios of all-cause and cause-specific mortality risk among current smokers compared with never smokers in NHIS-LMF and the five cohort studies analyzed by Carter et al. (2015), U.S. adults ages 55+ years
| Female |
Male |
|||
|---|---|---|---|---|
| Causes of Death | NHIS-LMF | Carter et al. | NHIS-LMF | Carter et al. |
| All-Cause Mortality | 2.3(2.2, 2.4) | 2.8 (2.7, 2.9) | 2.3(2.2, 2.4) | 2.8 (2.8, 2.9) |
| Diseases Established as Caused by Smoking | ||||
| Lip and oral cavity cancer | 4.3(2.4, 7.8) | 5.6 (3.7, 8.6) | 3.7(2.1, 6.6) | 5.7 (4.1, 8.1) |
| Esophageal cancer | 2.2(1.1, 4.1) | 5.1 (3.5, 7.4) | 3.0(2.0, 4.5) | 3.9 (3.0, 5.0) |
| Stomach cancer | 2.3(1.4, 3.7) | 1.7 (1.2, 2.5) | 1.6(1.0, 2.4) | 1.9 (1.4, 2.7) |
| Colorectal cancer | 1.6(1.3, 1.9) | 1.6 (1.4, 1.9) | 1.4(1.0, 1.7) | 1.4 (1.2, 1.7) |
| Liver cancer | 1.5(1.0, 2.4) | 1.8 (1.3, 2.5) | 2.6(1.7, 4.0) | 2.3 (1.8, 3.0) |
| Pancreatic cancer | 2.0(1.5, 2.5) | 1.9 (1.6, 2.2) | 2.2(1.6, 2.9) | 1.6 (1.4, 1.9) |
| Laryngeal cancer | 22.0 (7.3, 66.5) | 103.8 (24.2, 445.5) | 12.2 (4.7, 31.6) | 13.9 (8.3, 23.3) |
| Lung cancer | 17.2 (14.9, 19.8) | 22.9 (21.0, 25.0) | 13.3 (10.9, 16.1) | 25.3 (22.8, 28.1) |
| Cervical cancer | 1.6(0.8, 3.2) | —a | — | — |
| Urinary bladder cancer | 3.6(2.1, 6.1) | 3.9 (2.8, 5.5) | 6.3(4.0, 10.0) | 3.9 (3.0, 5.1) |
| Kidney and renal pelvis cancer | 2.2(1.2, 4.0) | 1.2 (0.9, 1.8) | 2.1(1.3, 3.5) | 1.8 (1.4, 2.4) |
| Acute myeloid leukemia | 1.4(0.7, 3.0) | 1.1 (0.7, 1.7) | 1.3(0.7, 2.5) | 1.9 (1.4, 2.7) |
| Diabetes | 1.6(1.3, 2.0) | 1.5 (1.3, 1.9) | 1.4(1.1, 1.8) | 1.6 (1.3, 1.9) |
| Ischemic heart disease | 2.0(1.9, 2.2) | 3.0 (2.8, 3.2) | 2.0(1.9, 2.2) | 2.6 (2.4, 2.7) |
| Other heart disease | 1.6(1.4, 1.8) | 1.9 (1.7, 2.1) | 1.7(1.4, 2.0) | 2.0 (1.8, 2.2) |
| Stroke | 1.71.5, 1.9) | 2.1 (1.8, 2.3) | 1.5(1.3, 1.8) | 1.9 (1.7, 2.2) |
| Atherosclerosis | 2.4(1.3, 4.2) | 2.1 (1.1, 4.0) | 2.1(1.1, 4.1) | 5.0 (3.2, 7.9) |
| Aortic aneurysm | 7.6(5.0, 11.6) | 10.1 (7.4, 13.6) | 8.4(5.2, 13.5) | 7.5 (5.8, 9.7) |
| Other arterial diseases | 3.6(2.3, 5.8) | 5.6 (3.9, 8.2) | 4.2(2.3, 7.8) | 5.3 (3.4, 8.2) |
| Pneumonia, influenza, and tuberculosis | 2.4(1.9, 2.9) | 1.9 (1.6, 2.4) | 2.0(1.5, 2.6) | 2.0 (1.6, 2.6) |
| Chronic obstructive pulmonary disease | 16.8 (14.6, 19.3) | 25.0 (21.2, 28.1) | 10.0 (8.1, 12.4) | 27.8 (24.1, 32.0) |
| Additional Diseases Associated With Smoking |
||||
| All other infections | 2.1(1.6, 2.6) | 2.5 (2.1, 3.0) | 1.9(1.4, 2.4) | 2.2 (1.8, 2.7) |
| Breast cancer | 1.3(1.1, 1.5) | 1.3 (1.2, 1.5) | — | — |
| Prostate cancer | — | — | 1.3(1.0, 1.6) | 1.4 (1.2, 1.7) |
| Rare cancers | 1.6(1.3, 2.0) | 1.1 (0.9, 1.3) | 2.3(1.7, 3.0) | 1.6 (1.2, 2.0) |
| Cancers of unknown site | 3.2(2.4, 4.2) | 2.7 (2.3, 3.2) | 4.0(2.8, 5.7) | 3.2 (2.8, 3.7) |
| Hypertensive heart disease | 1.7(1.2, 2.3) | 1.9 (1.4, 2.7) | 1.7(1.2, 2.3) | 2.9 (2.2, 3.9) |
| Essential hypertension/hypertensive renal disease |
2.0(1.3, 3.1) | 2.4 (1.7, 3.4) | 1.7(1.0, 3.0) | 2.6 (1.9, 3.6) |
| All other respiratory diseases | 1.6(1.2, 2.1) | 1.9 (1.5, 2.5) | 2.0(1.5, 2.8) | 2.0 (1.5, 2.6) |
| Intestinal ischemia | 2.8(1.8, 4.5) | 6.1 (4.2, 8.7) | 3.8(1.9, 7.5) | 5.6 (3.5, 9.0) |
| Liver cirrhosis | 1.7(1.1, 2.5) | 2.6 (2.0, 3.5) | 2.9(2.1, 4.0) | 3.6 (2.8, 4.6) |
| All other digestive diseases | 2.2(1.7, 2.8) | 2.1 (1.7, 2.5) | 2.3(1.7, 3.1) | 2.6 (2.0, 3.2) |
| Renal failure | 1.4(1.1, 1.9) | 1.9 (1.5, 2.5) | 1.3(0.9, 1.8) | 2.1 (1.6, 2.6) |
| Additional rare causes combined | 1.5(1.2, 1.9) | 2.0 (1.8, 2.3) | 1.4(0.9, 2.1) | 1.9 (1.5, 2.2) |
| Unknown causes | 2.4(1.7, 3.5) | 2.2 (1.9, 2.5) | 1.7(1.1, 2.8) | 1.9 (1.6, 2.2) |
Notes: Our NHIS-LMF data contain sufficient numbers of death to include deaths from cervical cancer. Although our overall study examines U.S. adults ages 35+, we limit the sample to ages 55+ here to directly compare our NHIS-LMF rate ratios to those of the five cohort studies presented by Carter and colleagues. Diseases established as caused by smoking are those recognized by the surgeon general (U.S. DHHS 2014). Additional diseases associated with smoking are those compiled by Carter et al. (2015). Dashes indicate sex-specific causes of death (e.g., cervical and breast cancer among women and prostate cancer among men).
Source: 1990–2011 NHIS-LMF
Carter et al. (2015) excluded cervical cancer deaths since the five cohort studies they analyze do not include enough deaths from this established cause.
Discussion
This study improves understanding of the linkage between cigarette smoking and all-cause and cause-specific adult mortality in the United States. We find compelling evidence for several additional causes of death that are not currently recognized by the U.S. Surgeon General as attributable to smoking. By estimating relative risk ratios with a large, nationally representative data set and adjusting for sociodemographic and behavioral confounders, our study provides further support that current and former smokers experience greater risk of death from these additional causes at follow-up than never smokers.
The key contributions of this study are fourfold. First, we find substantially higher mortality among smokers than never smokers from causes established as attributable to smoking and modestly higher mortality among smokers from additional causes that emerging evidence suggests are due to smoking. Although the diseases established as caused by smoking generally have higher relative risk ratios, the additional diseases associated with smoking are still important, contribute to additional risk of death, are relatively common, and should be considered in future studies that examine smoking and mortality. Thus, we replicate Carter et al.’s (2015) findings but with nationally representative data adjusting for a wider array of sociodemographic and behavioral factors that confound the smoking-mortality association.
Second, we find generally graded patterns of mortality risk by smoking intensity among current smokers and by time since cessation among former smokers. Mortality risk is higher among heavy than light smokers and higher among recent quitters than those who quit longer ago. However, compared with never smokers, even the lightest smokers (those who smoke less than half a package of cigarettes per day) exhibit elevated mortality risk from 24 of the 35 causes of death. Our results also show that smoking cessation confers substantial survival advantages. These gradients by smoking intensity and time since cessation observed for the additional causes of death resemble the graded patterns for the causes established as linked to smoking, providing further evidence that the additional causes are attributable to smoking.
Third, we decompose the mortality disadvantage among smokers by cause of death and find that the additional causes account for 14.8 % and 14.4 % of smoking-attributable deaths among U.S. women and men, respectively. These relatively rare causes account for small percentages of U.S. deaths individually but a considerable percentage of smoking-attributable deaths when aggregated. Although lung cancer mortality is often used as a proxy for a population’s smoking burden, it accounts for only about one-quarter of the smoker mortality disadvantage (28.8 % among women and 24.0 % among men).
Fourth, we provide new, current estimates of the excess number of adult deaths due to smoking in the United States in 2010. Our estimate of 481,887 annual deaths for adults 35+, adjusted for sociodemographic and behavioral confounders, exceeds the CDC’s estimate as well as an earlier estimate with NHIS-LMF data (Rogers et al. 2005), likely because we account for causes of death established as attributable to smoking as well as the additional causes Carter and colleagues compiled. Our estimate of the number of premature deaths attributable to smoking reinforces that smoking is the leading cause of preventable mortality in the United States.
Comparability With Prior Research
Our results using the direct approach generally agree with and complement studies using indirect approaches. Our SAFs (18 % of female deaths and 26 % of male deaths) are similar to those using the PGW indirect method, although our estimate for men is somewhat higher. Ho and Elo (2013) also found that SAFs among men from the direct method tend to be slightly higher than when using the indirect method. Our number of excess deaths from established causes is modestly lower than the CDC’s recent estimate. However, when we incorporate the 64,363 deaths from additional causes, our total number of smoking-attributable deaths among U.S. adults aged 35+ is 481,887. This estimate is less than the 514,369 deaths that Preston et al. (2010a: online supplement) identified among U.S. adults ages 50+ in 2003 as well as the 520,000 deaths Rostron (2011) reported based on an older public-use version of the NHIS-LMF data and all-cause hazard ratios. Our estimate may be lower because we adjust for sociodemographic and behavioral confounders. In supplemental models that adjust for age but no other confounders, we estimate 518,804 smoking-attributable deaths.
At the same time, our study contributes new findings beyond the existing literature. Most importantly, we provide compelling evidence that the additional causes of death are associated with smoking in nationally representative data. We find significantly elevated relative risk ratios for all of the additional causes even after adjusting for sociodemographic and behavioral confounders. Although other studies have found that adjustment for confounders only modestly attenuates risk ratios (Malarcher et al. 2000; Rogers et al. 2005), adjustment rules out the possibility that other risk factors underlie the smoking-mortality association. Prior medical research has demonstrated plausible physiological pathways between smoking and these additional causes of death resulting from the carcinogenic and other toxic substances in cigarette smoke (U.S. DHHS 2004). We also find evidence suggesting that these additional causes are attributable to smoking when we disaggregate smokers into four current smoker groups and five former smoker groups—a level of detail absent from most prior studies. The generally graded patterns by intensity and time since cessation mirror the pattern for established causes.
Our results merit further research on the additional causes of death to further solidify the causal link between smoking and mortality from these causes. Other researchers must consider the full array of smoking-attributable causes in future studies of the contribution of smoking to patterns and disparities of adult mortality. If studies continue to demonstrate a significantly higher risk of death from these additional causes among smokers relative to never smokers and biological mechanisms are plausible, then the surgeon general should upgrade the evidence linking these causes of death to smoking from “suggestive” to “sufficient,” and these causes should be factored into future estimates of the number of premature deaths due to smoking (U.S. DHHS 2014).
Limitations
Three limitations may affect our results. First, the restricted-use cause-of-death variables used in this study include underlying cause of death without incorporating information on contributing causes of death. Underlying cause refers to the medical condition that initiates the sequence of morbid events that ultimately lead to death (Anderson 2011). If a smoking-attributable cause of death is identified as a contributing but not underlying cause on the death certificate, deaths from that cause are not considered as attributable to smoking. This may particularly be an issue among older decedents with multiple chronic conditions. An analysis that considers total mentions of smoking-attributable causes would potentially expand the number and percentage of deaths that result from smoking (Nam et al. 1994). Second, we estimate mortality attributable to cigarette smoking but not to other forms of tobacco use because consumption of these other tobacco products is intermittently assessed in the NHIS. Although cigarette smoking is the most common form of tobacco use in the United States, young adults also use other products, such as little cigars, electronic cigarettes, hookah, and smokeless tobacco (Kasza et al. 2017; Lariscy et al. 2013)—all health risk behaviors that may play substantial roles in future morbidity and mortality among young adult cohorts. Finally, although our multivariate analyses adjust for several key sociodemographic and behavioral confounders, we do not adjust for alcohol use throughout because it is consistently measured in the NHIS only beginning in 1997. Without controls for alcohol use, we potentially overestimate relative risk of mortality among current smokers relative to never smokers if smoking is correlated with long-term heavy drinking.
Conclusion
Estimating smoking-attributable mortality remains a challenge. Neither “tobacco” nor “smoking” is an official ICD code as a cause of death, and social survey measurement of smoking patterns does not accurately assess full smoking history: smoking initiation, development of nicotine addiction, changes in smoking status, changes in smoking intensity, successful or failed cessation attempts, secondhand smoke exposure, onset of smoking-attributable morbidity, and final cessation or death from smoking-attributable or other causes of death. Continued refinement and evaluation of both direct and indirect methods used to study smoking and mortality are necessary to advance science in this critical area of study. Studies of smoking and mortality using direct methods like those used here would benefit from further advancements in measuring complete smoking histories of individuals and in longitudinal measurement of smoking behavior to improve the accuracy of estimates of smoking-attributable mortality in the United States.
Smoking is a national tragedy that continues to kill nearly half a million American adults prematurely every year, as well as millions more internationally. We urge continued research on this topic and continued aggressive policymaking aimed at reducing and eventually eliminating cigarette and other tobacco use. This study demonstrates that smoking-attributable mortality must remain a top population health priority in the United States and makes several contributions to further illuminate the human costs of this tragedy that has ravaged American society for more than a century.
Supplementary Material
Acknowledgments
An earlier draft of this article was presented at the 2016 annual meeting of the Population Association of America, Washington, DC. We are grateful to the Carolina Population Center, University of North Carolina at Chapel Hill and its Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) center grant (P2C HD050924), University of Colorado Population Center, University of Colorado–Boulder and its NICHD center grant (P2C HD066613), and Duke Population Research Institute, Duke University and its National Institute on Aging (NIA) training grant (T32 AG000139) for general support. We thank Charles Nam, Arun Hendi, Negasi Beyene, three anonymous Demography reviewers, and seminar participants at Florida State University, Syracuse University, and the University of Missouri for helpful comments. We thank NCHS for providing a special-request version of the NHIS-LMF data. The research in this article was conducted in the Missouri Federal Statistical Research Data Center (RDC) in Columbia, Missouri, and Triangle RDC in Durham, North Carolina, while the first author was a Special Sworn Status researcher of the U.S. Census Bureau at the Center for Economic Studies. The content of this article is the sole responsibility of the authors and does not necessarily represent the official views of the U.S. Census Bureau, NCHS, NIA, NICHD, or the National Institutes of Health. This article was screened to ensure that no confidential data are revealed.
Footnotes
Causes of death are placed into class 3 if “evidence is inadequate to infer the presence or absence of a causal relationship” and class 4 if “evidence is suggestive of no causal relationship (U.S. DHHS 2014:3). We do not examine class 3 and 4 causes because they are not associated with smoking.
Naghavi et al. (2010) presented a strategy to redistribute “garbage codes” into meaningful cause-of-death categories. Nonetheless, we include ill-defined conditions and unknown causes in our analyses in order to replicate the causes of death that Carter and colleagues (2015) identified.
We exclude from analysis those respondents missing on smoking status (1.1 %); missing on race/ethnicity or identifying with a group other than Hispanic, non-Hispanic black, or non-Hispanic white (3.8 %); or missing on other confounders (<1.2 %).
We examine time since cessation among former smokers based on reports at baseline.
BMI and health insurance should be viewed as proximate factors that lie along the causal chain from smoking to mortality. Smoking tends to suppress appetite, and smokers may be less likely to have health insurance because of higher premiums (Berman et al. 2014; Krueger et al. 2004). Similarly, smoking initiation may precede completion of education (Maralani 2014). However, because many of the causes of death we examine have complex etiologies, controlling for proximate factors is necessary to isolate the effects of smoking on cause-specific mortality. Sensitivity analyses show that results are robust to excluding BMI and health insurance from the models.
Sensitivity analyses include dummy variables for heavy, former, and never drinkers (reference = light/moderate drinkers). Among women, respondents who drink one or fewer drinks per day are coded as light/moderate drinkers, and respondents who drink more than one drink per day are coded as heavy drinkers. Among men, respondents who drink two or fewer drinks per day are coded as light/moderate drinkers, and respondents who drink more than two drinks per day are coded as heavy drinkers.
In supplemental models, all-cause risk ratios are robust to adjustment for alcohol use (measured only in years 1997 forward); ratios are 2.32 and 2.21 among female and male current smokers, respectively, and 1.41 and 1.32 among female and male former smokers, respectively (p < .001). Net of alcohol use, cirrhosis mortality risk ratios among women are attenuated to nonsignificance: 1.47 (p = .12) among current smokers and 1.03 (p = .90) among former smokers. Corresponding cirrhosis mortality risk ratios among male current smokers and male former smokers are 3.29 (p < .001) and 1.83 (p < .01), respectively.
Importantly, we observe a graded relationship between smoking intensity and all-cause mortality and most causes of death. Further, for most causes, each category of smoking intensity exhibits significantly higher mortality than never smokers (the referent). Nonetheless, a clear gradient between smoking intensity and mortality risk is not present for every cause of death partly because of small numbers of deaths from some rare causes of death after the data are restricted to current smokers and stratified by smoking intensity.
The sex-, age-, and cause-specific mortality rates used to calculate the percentage and number of excess deaths attributable to smoking are available in Tables S3–S8 of the online appendix.
Calculations that adjust for age but no other confounders increase the number of excess deaths due to smoking by approximately 37,000 deaths to a total of 518,804 excess deaths (319,443 among men and 199,361 among women).
References
- Allison PD (2014). Event history and survival analysis: Regression for longitudinal event data (2nd ed.) Beverly Hills, CA: Sage Publications. [Google Scholar]
- American Cancer Society. (2018). Cancer facts and figures 2018 Atlanta, GA: American Cancer Society; Retrieved from https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf [Google Scholar]
- Anderson RN (2011). Coding and classifying causes of death: Trends and international differences. In Rogers RG & Crimmins EM (Eds.), International handbook of adult mortality (pp. 467–489). Dordrecht, The Netherlands: Springer. [Google Scholar]
- Anderson RN, Miniño AM, Hoyert DL, & Rosenberg HM (2001). Comparability of cause of death between ICD-9 and ICD-10: Preliminary estimates. National Vital Statistics Reports, 49(2), 1–32. [PubMed] [Google Scholar]
- Berman M, Crane R, Seiber E, & Munur M (2014). Estimating the cost of a smoking employee. Tobacco Control, 23, 428–433. [DOI] [PubMed] [Google Scholar]
- Blue L, & Fenelon A (2011). Explaining low mortality among U.S. immigrants relative to native-born Americans: The role of smoking. International Journal of Epidemiology, 40, 786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneux L (2011). Mortality affected by health care and public health policy interventions. In Rogers RG & Crimmins EM (Eds.), International handbook of adult mortality (pp. 583–607). Dordrecht, the Netherlands: Springer. [Google Scholar]
- Carter BD, Abnet CC, Feskanich D, Freedman ND, Hartge P, Lewis CE, … Jacobs EJ (2015). Smoking and mortality—Beyond established causes. New England Journal of Medicine, 372, 631–640. [DOI] [PubMed] [Google Scholar]
- de Walque D (2010). Education, information, and smoking decisions: Evidence from smoking histories in the United States, 1940–2000. Journal of Human Resources, 45, 682–717. [Google Scholar]
- Doll R, Peto R, Boreham J, & Sutherland I (2004). Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ, 328, 1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati M, & Lopez AD (2003). Estimates of global mortality attributable to smoking in 2000. Lancet, 362, 847–852. [DOI] [PubMed] [Google Scholar]
- Fenelon A (2013). Geographic divergence in mortality in the United States. Population and Development Review, 39, 611–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenelon A, & Preston SH (2012). Estimating smoking-attributable mortality in the United States. Demography, 49, 797–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2015 Tobacco Collaborators. (2017). Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: A systematic analysis from the Global Burden of Disease Study 2015. Lancet, 389, 1885–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutterman S (2015). Mortality of smoking by gender. North American Actuarial Journal, 19, 200–223. [Google Scholar]
- Ho JY, & Elo IT (2013). The contribution of smoking to black-white differences in U.S. mortality. Demography, 50, 545–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden LM, & Meyer JA (2011). Age and sex composition: 2010 (2010 Census Briefs No. C2010BR-03) Washington, DC: U.S. Census Bureau; Retrieved from http://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf [Google Scholar]
- International Agency for Research on Cancer. (2004). Tobacco smoke and involuntary smoking (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Vol. 83). Lyon, France: International Agency for Research on Cancer; Retrieved from http://monographs.iarc.fr/ENG/Monographs/vol83/mono83.pdf [PMC free article] [PubMed] [Google Scholar]
- Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun MJ, Anderson RN, … Peto R (2013). 21st-century hazards of smoking and benefits of cessation in the United States. New England Journal of Medicine, 368, 341–350. [DOI] [PubMed] [Google Scholar]
- Kasza KA, Ambrose BK, Conway KP, Borek N, Taylor K, Goniewicz ML, … Hyland AJ (2017). Tobacco-product use by adults and youths in the United States in 2013 and 2014. New England Journal of Medicine, 376, 342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenfield SA, Stampfer MJ, Rosner BA, & Colditz GA (2008). Smoking and smoking cessation in relation to mortality in women. JAMA, 299, 2037–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger PM, Rogers RG, Hummer RA, & Boardman JD (2004). Body mass, smoking, and overall and cause-specific mortality among older U.S. adults. Research on Aging, 26, 82–107. [Google Scholar]
- Lariscy JT, Hummer RA, & Hayward MD (2015). Hispanic older adult mortality in the United States: New estimates and an assessment of factors shaping the Hispanic paradox. Demography, 52, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariscy JT, Hummer RA, Rath JM, Villanti AC, Hayward MD, & Vallone DM (2013). Race/ethnicity, nativity, and tobacco use among U.S. young adults: Results from a nationally representative survey. Nicotine & Tobacco Research, 15, 1417–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariscy JT, Nau C, Firebaugh G, & Hummer RA (2016). Hispanic-white differences in lifespan variability in the United States. Demography, 53, 215–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link BG, & Phelan JC (1995). Social conditions as fundamental causes of disease. Journal of Health and Social Behavior, 35(Extra Issue), 80–94. [PubMed] [Google Scholar]
- Lochner K, Hummer RA, Bartee S, Wheatcroft G, & Cox C (2008). The public-use National Health Interview Survey linked mortality files: Methods of reidentification risk avoidance and comparative analysis. American Journal of Epidemiology, 168, 336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malarcher AM, Schulman J, Epstein LA, Thun MJ, Mowery P, Pierce B, … Giovino GA (2000). Methodological issues in estimating smoking-attributable mortality in the United States. American Journal of Epidemiology, 152, 573–584. [DOI] [PubMed] [Google Scholar]
- Maralani V (2014). Understanding the links between education and smoking. Social Science Research, 48, 20–34. [DOI] [PubMed] [Google Scholar]
- Mehta NK, & Preston SH (2012). Continued increases in the relative risk of death from smoking. American Journal of Public Health, 102, 2181–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, & Gerberding JL (2004). Actual causes of death in the United States, 2000. JAMA, 291, 1238–1245. [DOI] [PubMed] [Google Scholar]
- Murphy SL, Xu J, Kochanek KD, Curtin SC, & Arias E (2017). Deaths: Final data for 2015. National Vital Statistics Reports, 66(6), 1–75. [PubMed] [Google Scholar]
- Naghavi M, Makela S, Foreman K, O’Brien J, Pourmalek F, & Lozano R (2010). Algorithms for enhancing public health utility of national causes-of-death data. Population Health Metrics, 8(9), 1–14. 10.1186/1478-7954-8-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam CB, Hummer RA, & Rogers RG (1994). Underlying and multiple causes of death related to smoking. Population Research and Policy Review, 13, 305–325. [Google Scholar]
- National Center for Health Statistics (NCHS). (2015). National health interview survey [electronic resource]. Hyattsville, MD: National Center for Health Statistics. Retrieved from https://www.cdc.gov/nchs/nhis/index.htm [Google Scholar]
- National Center for Health Statistics (NCHS). (2016). Variance estimation guidance, NHIS 2006–2015 Hyattsville, MD: National Center for Health Statistics; Retrieved from https://www.cdc.gov/nchs/data/nhis/2006var.pdf [Google Scholar]
- Pampel FC (2009). The persistence of educational disparities in smoking. Social Problems, 56, 526–542. [Google Scholar]
- Peto R, Lopez AD, Boreham J, Thun MJ, & Heath C (1992). Mortality from tobacco in developed countries: Indirect estimation from national vital statistics. Lancet, 339, 1268– 1278. [DOI] [PubMed] [Google Scholar]
- Peto R, Lopez AD, Boreham J, Thun MJ, & Heath C (1994). Mortality from smoking in developed countries, 1950–2000 Oxford, UK: Oxford University Press. [Google Scholar]
- Powers DA, & Xie Y (2000). Statistical methods for categorical data analysis San Diego, CA: Academic Press. [Google Scholar]
- Preston SH, Glei DA, & Wilmoth JR (2010a). A new method for estimating smoking-attributable mortality in high-income countries. International Journal of Epidemiology, 39, 430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SH, Glei DA, & Wilmoth JR (2010b). Contribution of smoking to international differences in life expectancy. In Crimmins EM, Preston SH, & Cohen B (Eds.), International differences in mortality at older ages: Dimensions and sources (pp. 105– 131). Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Preston SH, Stokes A, Mehta NK, & Cao B (2014). Projecting the effect of changes in smoking and obesity on future life expectancy in the United States. Demography, 51, 27– 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SH, & Wang H (2006). Sex mortality differences in the United States: The role of cohort smoking patterns. Demography, 43, 631–646. [DOI] [PubMed] [Google Scholar]
- Research Triangle Institute. (2012). SUDAAN language manual, volumes 1 and 2, release 11.0 Research Triangle Park, NC: Research Triangle Institute. [Google Scholar]
- Rogers RG, Everett BG, Saint Onge JM, & Krueger PM (2010). Social, behavioral, and biological factors, and sex differences in mortality. Demography, 47, 555–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RG, Hummer RA, Krueger PM, & Pampel FC (2005). Mortality attributable to cigarette smoking in the United States. Population and Development Review, 31, 259– 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RG, Hummer RA, & Nam CB (2000). Living and dying in the USA: Behavioral, health, and social differentials of adult mortality San Diego, CA: Academic Press. [Google Scholar]
- Rogers RG, Nam CB, & Hummer RA (1995). Demographic and socioeconomic links to cigarette smoking. Social Biology, 42(1–2), 1–21. [DOI] [PubMed] [Google Scholar]
- Rogers RG, & Powell-Griner E (1991). Life expectancies of cigarette smokers and nonsmokers in the United States. Social Science & Medicine, 32, 1151–1159. [DOI] [PubMed] [Google Scholar]
- Rogot E, & Murray JL (1990). Smoking and causes of death among U.S. veterans: 16 years of observation. Public Health Reports, 95, 213–222. [PMC free article] [PubMed] [Google Scholar]
- Rosenberg HM (1999). Cause of death as a contemporary problem. Journal of the History of Medicine and Allied Sciences, 54, 133–153. [DOI] [PubMed] [Google Scholar]
- Rostron B (2010). A modified new method for estimating smoking-attributable mortality in high-income countries. Demographic Research, 23(article 14), 399–420. 10.4054/DemRes.2010.23.14 [DOI] [Google Scholar]
- Rostron B (2011). Smoking-attributable mortality in the United States. Epidemiology, 22, 350– 355. [DOI] [PubMed] [Google Scholar]
- Rostron B (2013). Smoking-attributable mortality by cause in the United States: Revising the CDC’s data and estimates. Nicotine & Tobacco Research, 15, 238–246. [DOI] [PubMed] [Google Scholar]
- Rostron BL, & Wilmoth JR (2011). Estimating the effect of smoking on slowdowns in mortality declines in developed countries. Demography, 48, 461–479. [DOI] [PubMed] [Google Scholar]
- Taylor DH, Hasselblad V, Henley J, Thun MJ, & Sloan FA (2002). Benefits of smoking cessation for longevity. American Journal of Public Health, 92, 990–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice RL, Lopez AD, … Gapstur SM (2013). 50-year trends in smoking-related mortality in the United States. New England Journal of Medicine, 368, 351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thun MJ, Day-Lally C, Myers DG, Calle EE, Flanders WD, Zhu B-P, … Heath CW (1959 through 1965) and II (1982 through 1988). In Burns D, Garkinkel L, & Samet JD (Eds.), Changes in cigarette-related disease risks and their implications for prevention and control, smoking and tobacco control (Tobacco Control Monograph, Series No. 8). Bethesda, MD: Cancer Control and Population Sciences, National Cancer Institute, U.S. National Institutes of Health. [Google Scholar]
- Thun MJ, Hannan LM, Adams-Campbell LL, Boffetta P, Buring JE, Feskanich D, … Samet JM (2008). Lung cancer occurrence in never-smokers: An analysis of 13 cohorts and 22 cancer registry studies. PLoS Medicine, 5(9), e185 10.1371/journal.pmed.0050185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health, Education, and Welfare. (1964). Smoking and health: Report of the Advisory Committee to the surgeon general of the Public Health Service (Public Health Service Publication No. 1103). Washington, DC: U.S. Government Printing Office. [Google Scholar]
- U.S. Department of Health and Human Services (DHHS). (2004). The health consequences of smoking: A report of the surgeon general Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. [Google Scholar]
- U.S. Department of Health and Human Services (DHHS). (2014). The health consequences of smoking—50 years of progress: A report of the surgeon general Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. [Google Scholar]
- Williamson DF (2010). The population attributable fraction and confounding: Buyer beware. International Journal of Clinical Practice, 64, 1019–1023. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO). (2010). ICD-10: International statistical classification of diseases and related health problems (10th revision). Geneva, Switzerland: WHO; Retrieved from http://www.who.int/classifications/apps/icd/icd10online [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


