Abstract
The muscle membrane, sarcolemma, must be firmly attached to the basal lamina. The failure of proper attachment results in muscle injury, which is the underlying cause of Duchenne muscular dystrophy (DMD), in which mutations in the dystrophin gene disrupts the firm adhesion. In patients with DMD, even moderate contraction causes damage, leading to progressive muscle degeneration. The damaged muscles are repaired through myogenesis. Consequently, myogenesis is highly active in patients with DMD, and the repeated activation of myogenesis leads to the exhaustion of the myogenic stem cells. Therefore, approaches to reducing the risk of the exhaustion are to develop a treatment that strengthens the interaction between the sarcolemma and the basal lamina and increases the efficiency of the myogenesis. Galectin-3 is an oligosaccharide-binding protein and is known to be involved in cell–cell interactions and cell–matrix interactions. Galectin-3 is expressed in myoblasts and skeletal muscle, although its function in muscle remains elusive. In this study, we found evidence that galectin-3 and the monosaccharide N-acetylglucosamine, which increases the synthesis of binding partners (oligosaccharides) of galectin-3, promote myogenesis in vitro. Moreover, in the mdx mouse model of DMD, treatment with N-acetylglucosamine increased muscle-force production. The results suggest that treatment with N-acetylglucosamine might mitigate the burden of DMD.—Rancourt, A., Dufresne, S. S., St-Pierre, G., Lévesque, J.-C., Nakamura, H., Kikuchi, Y., Satoh, M. S., Frenette, J., Sato, S. Galectin-3 and N-acetylglucosamine promote myogenesis and improve skeletal muscle function in the mdx model of Duchenne muscular dystrophy.
Keywords: glycobiology, lectins, monosaccharide
Galectin-3 is a member of the galectin family of soluble, nonglycosylated proteins. Galectins share primary sequence homology in their carbohydrate-recognition domain, which has an affinity for oligosaccharides that contain β-galactoside, in which a galactose (Gal) residue is linked to a saccharide [typically N-acetylglucosamine (GlcNAc)] via a β-linkage (1–3). Galectin-3 contains 2 domains: a C-terminal carbohydrate recognition domain and an N-terminal collagen-like domain consisting of a repeating peptide sequence rich in proline and glycine residues (4). Galectin-3 is expressed in many tissues, including muscle and a subset of proregenerative macrophages (M2), which are required to initiate muscle repair, including adult myogenesis (5–8). Galectin-3 binds to a specific type of oligosaccharide attached to some membrane and extracellular glycoproteins, including cell-adhesion molecules, integrins, some growth factor receptors, such as IGF receptor, and the major component of the basal lamina, laminin (9–12). Upon attachment to the binding partners, galectin-3 molecules oligomerize themselves through the N-terminal, collagen-like domains. Consequently, the self-oligomerized galectin-3 becomes multivalent in oligosaccharide binding and directly cross-links its binding partners on the cell surface or in the cell matrix (2, 9, 13, 14). When galectin-3 cross-links its binding partners to glycoproteins on the surface of a cell, it restricts the movements of those glycoproteins (9). In contrast, when galectin-3 binds to its surface-binding partners on different entities, such as 2 different cells or a cell and cell matrix, that cross-linking results in cell–cell interactions or cell–matrix interactions, respectively (13, 15, 16). Through that cross-linking, galectin-3 exerts multifaceted activities, such as the modulation of signal transduction, membrane receptor dynamics, cell mobility, cell–cell adhesion, and cell–matrix interactions, all of which can be considered necessary steps in myogenesis. However, it remains unknown whether galectin-3, which is expressed in muscle, has any role in myogenesis and in muscle function.
Myogenesis is a part of muscle regeneration processes and is initiated in response to muscle-fiber damage (17, 18). Growth factors are released from injured muscle cells, and leukocytes, such as neutrophils and macrophages, are recruited to the site of injury. Those factors first trigger the differentiation of adult myogenic cells (satellite cells) into myoblasts. The myoblasts elongate, migrate, and make contact with neighboring myoblasts. As the cells adhere to one another, their membranes become intimately aligned, and the myoblasts then fuse together to form multinucleated syncytial myotubes. The mechanism by which mammalian myoblasts undergo fusion remains elusive (19). Nascent myotubes then further fuse with myoblasts to form myofibers. The regenerated myofibers are anchored to laminin in the basal lamina by dystrophin-associated glycoprotein complex (DGC) (20).
Duchenne muscular dystrophy (DMD) is an X-linked, lethal muscular dystrophy (MD) affecting 1 in 5000 males at birth (21). It is the most common childhood form of MD and accounts for 50% of all MD cases (22, 23). Patients usually die in their late 20s. DMD is caused by mutations in the dystrophin gene, which lead to the loss of the cytoskeletal protein dystrophin. Dystrophin connects the cytoskeletal actin filaments to the basal lamina by binding to F-actin through its N terminus and to β-dystroglycan in the DGC through its C terminus (24). DGC binds to laminin in the basal lamina, forming firm attachment of muscle membranes to the basal lamina. That stable anchoring of the muscle fibers to the basal lamina prevents injury associated with normal repeated and unaccustomed eccentric contractions. However, in the muscles of patients with DMD, whose expression of dystrophin is lacking, the myofibers are not properly fixed to the basal lamina and, therefore, detach from the basal lamina during contraction, leading to sarcolemmal instability and damage. Fiber degeneration is then counterbalanced by myogenesis at the expense of adult myogenic cells. The constant degeneration of the muscle fibers in patients with DMD eventually overwhelms the intrinsic capacity for myogenesis. There are very few clinically available therapies for DMD that mitigate disease progression, such as corticosteroids, although some are now in clinical trials, including drugs that can bypass inherited mutations (23, 25). Although gene and cell therapies are on the horizon, it is important to develop new therapeutic approaches for DMD. Two possible approaches for delaying the progression of MD are to strengthen the sarcolemmal attachments to protect them from contractions and to increase the efficiency of myogenesis. In this study, we first investigated whether the efficiency of myogenesis in vitro was increased by galectin-3 or the monosaccharide GlcNAc, which increases the biosynthesis of galectin-3 binding partners. Then, we also studied whether treatment with GlcNAc mitigates DMD in a mouse model of DMD.
MATERIALS AND METHODS
Reagents
Recombinant galectin-3 was purified from an extract of Escherichia coli that overexpressed galectin-3 by affinity chromatography using lactosyl-sepharose (MilliporeSigma, Burlington, MA, USA), as previously described (15, 26, 27). The bound galectin-3 was released with PBS(−) containing 150 mM lactose, and the lactose in the fraction containing galectin-3 was then removed with a HiPrep 26/10 Desalting Column (GE Healthcare Life Sciences, Little Chalfont St. Giles, United Kingdom). The eluate containing the galectin-3 was passed through an ActiClean Etox column (Sterogene Bioseparations, Carlsbad, CA, USA) to ensure that the endotoxin level was <1 pg/µg. The specific (oligosaccharide-binding) activity of galectin-3 was tested before each experiment by using it to induce hemagglutination. Anti-myosin heavy chain (MHC) antibody (MF20), anti–α-tublin antibody (12G10), and anti-myogenin antibody (F5D) were obtained from the Developmental Studies Hybridoma Bank at the University of Iowa (Iowa City, IA, USA). An anti–galectin-3 antibody (M3/38.1.2.8) was purified and biotinylated as previously published (15, 26, 27).
Animals
Male mdx dystrophic mice (C57BL/10ScSn-Dmdmdx/J) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and bred at our animal facility. Galectin-3–null mice were obtained from Core F (Mouse Transgenics) of the National Institute of General Medical Sciences (Bethesda, MD, USA)–supported Consortium of Functional Glycomics (Scripps Research Institute, San Diego, CA, USA). Wild-type (C57BL/6) and galectin-3–null mice were bred and maintained at our facility. Based on a previous study by Grigorian et al. (28) on the impact of GlcNAc on T cell–mediated autoimmunity in vivo, 250 mg/kg bodyweight/d was chosen. Mice were randomly assigned to either the control [PBS(−)–treated] or GlcNAc-treated group. GlcNAc (250 mg/kg bodyweight/d) was administered intraperitoneally to the mdx mice for 10 d on d 25–35 after birth. The control mice were injected daily with the same volume of PBS. At the end of the experiment, the mice were euthanized by intraperitoneal injection of 50 mg/kg pentobarbital. All animal breeding and experimental procedures were approved by the Laval University Research Centre Animal Care and Use Committee, and the decisions of which were made in accordance with the Canadian Council on Animal Care guidelines.
Myogenesis
Mouse-derived myoblast cell line, C2C12 (American Type Culture Collection, Manassas, VA, USA) were cultured for propagation in high-glucose DMEM (4.5 g/L of glucose) containing 2 mM glutamine, supplemented with antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin) and 10% heat-inactivated fetal calf serum (GE Healthcare Life Sciences), which is referred to as “growth medium” (GM). For differentiation, C2C12 cells (2.3 × 104 cells in 30 µl of GM) were first plated into a well of µ-Slide VI 0.4 (Ibidi, Planegg, Germany) and incubated at 37°C for 30–40 min to allow the cells to adhere to the well. GM (120 µl) was then added, and the cells were cultured under a constant stream of 95% air and 5% CO2 gas for 18–20 h. To initiate differentiation, the medium was replaced with DMEM containing 1% heat-inactivated horse serum (MilliporeSigma), antibiotics, insulin (10 µg/ml), transferrin (5.5 µg/ml), and sodium selenite (5 ng/ml), which is referred to as “differentiation medium” (DM). The medium was changed every day after the initiation of differentiation. Under these conditions, elongated myotubes typically began to appear after 48 h, and some became thick, mature myotubes after 60 h. The progression of myogenesis was estimated by either counting the number of nuclei or measuring the areas of MHC+ multinuclear myotubes using in-house software. To perform the statistical analyses, the fold increase, compared with the control treatment, was used. The cells were treated with galectin-3 or GlcNAc, and the medium was changed every day after the initiation of differentiation.
Western blot analysis
Cells were exposed to the lysis buffer [20 mM Tris buffer (pH 7.2), 150 mM NaCl, 0.75% Triton X-100, and protease-inhibitor cocktail (MilliporeSigma)] on ice for 30 min. The cell lysates were centrifuged at 14,000 g at 4°C for 15 min. The supernatants (15 µg/well) were subjected to a 10% SDS-PAGE, followed by electrotransfer on a 0.45-µm nitrocellulose filter. Galectin-3, myogenin, and tublin were detected by anti–galectin-3 antibody (anti–Mac-2), anti-myogenin antibody (F5D), and anti–α-tublin antibody (12G10), respectively.
Immunofluorescent staining
The cells were fixed for 15 min in 3.7% paraformaldehyde in PBS (pH 7.4). For MHC staining, the fixed cells were treated with PBS containing 0.25% Triton X-100 for 5 min at room temperature and were then incubated with an anti-MHC antibody (2 µg/ml) for 1 h. After the cells were washed 3 times with PBS, they were incubated with an Alexa Fluor-488– or -546–conjugated anti-mouse IgG antibody (Thermo Fisher Scientific, Waltham, MA, USA) for 1 h. For galectin-3 staining, the cells were incubated with a biotinylated anti–galectin-3 antibody (2.5 µg/ml) for 1 h, and then with streptavidin–Alexa 488 or 546 (Thermo Fisher Scientific). The cell nuclei were counterstained with DAPI (Thermo Fisher Scientific). The images were captured with the WaveFx Spinning Disc Confocal System (Quorum Technologies, Laughton, United Kingdom) with a microscope (Leica Camera, Wetzlar, Germany) controlled by Volocity software (v.4.0; PerkinElmer, Waltham, MA, USA). The Quorum confocal system, with an IX inverted microscope (Olympus, Tokyo, Japan) controlled by MetaMorph software (Molecular Devices, Sunnyvale, CA, USA), was used to capture the images in multiple fields of view (FOVs), and the images were then stitched together with in-house software (29). For immunofluorescence staining of muscle tissue, a frozen 5-µm tissue section was exposed to cold acetone for 10 min, then exposed to 0.3% H2O2–PBS for 5 min, and washed in PBS. After incubation with a blocking buffer containing 0.05% Tween, 0.2% gelatin, 3% bovine serum albumin, 2% horse serum in 100 mM Tris-HCl (pH 7.5), and 150 mM NaCl for 45 min, the sections were exposed to the biotinylated anti–galectin-3 antibody overnight at 4°C. After the sections were washed with PBS, they were incubated with streptavidin–Alexa 488 for 1 h, and the nuclei were stained with DAPI.
Motility analysis with a single-cell tracking system
C2C12 cells (4000 cells/well) were plated onto Lab-Tek 8 well chamber (Thermo Fisher Scientific), and 24 h after plating, the cells were differentiated. Then, the chamber was placed on a microscope stage, and cells were cultured using an environmental chamber at 37°C with 7.5% humidified CO2. The Quorum confocal system, with the Olympus microscope controlled by MetaMorph software, was employed for live cell imaging. Differential interference contrast (DIC) images (2 × 2 FOVs) were taken every 5 min with an Uplsapo Super Apochromat (Olympus) ×10 the dry objective (numerical aperture, 0.4), with a ×1.5 coupler using near-infrared light-emitting diode (pE-100, 740 nm; CoolLED, Andover, United Kingdom). Cell tracking was performed for 300 min after 10 h of differentiation, as previously reported by Sato et al. (29). Briefly, 100 cells were individually tracked, and the moving distances at each time point were calculated by the positions of the cells at each time point with in-house software. The total moving distance of each cell was then calculated.
Isometric contractile properties
Mice were intraperitoneally injected with 0.1 mg/kg bodyweight of buprenorphine to palliate the poor analgesic effect of pentobarbital. After 15 min, the mice were anesthetized with 5 mg/kg body weight of sodium pentobarbital. The soleus and extensor digitorum longus (EDL) muscles were dissected, incubated in buffered physiologic salt solution (Krebs-Ringer), and attached to an electrode and a force sensor (305B-LR; Aurora Scientific, Aurora, ON, Canada) to assess their contractile properties, as previously described by Dufresne et al. (30). The maximum tetanic tension (P0, g) values were obtained using the sensor controlled by dynamic muscle control and data acquisition software (Dynamic Muscle Data Analysis software, v.6.1; Aurora Scientific). The maximum specific tetanic tension (sP0, N/cm2) was calculated by dividing the wet weight by the optimal muscle length, multiplied by the muscle density (1.06 g/cm2), multiplied by the fiber/muscle length ratio. For the eccentric contraction protocol, the muscles were stimulated at 150 Hz for 700 ms. After 500 ms, the muscles were lengthened to 110% L0 at 0.5 L0/s for 200 ms. After the contractile properties were measured, the tendons were removed, and the muscles were weighed.
Photoshop software (Adobe Systems, San Jose, CA, USA) was used to estimate areas of muscle damage and muscle tissues (pixels).
Statistical analysis
Statistical significance was determined with 1-way ANOVA and ANOVA–on ranks (the significances are explained in the figure legends). All statistical analyses were performed with Prism (GraphPad Software, La Jolla, CA, USA), and differences were considered significant at P < 0.05.
RESULTS
Galectin-3 is expressed in myoblasts and on the sarcolemmal membrane of skeletal muscle
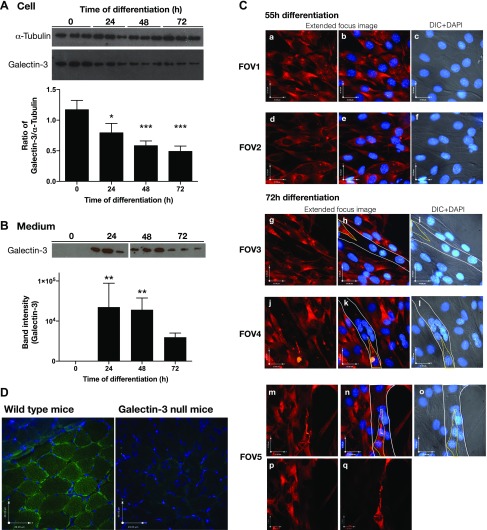
C2C12 cells are murine myoblasts and can be committed to myogenesis by taking them from GM into low-serum DM. We first analyzed the expression of galectin-3 in differentiating murine myoblasts and myotubes. As shown in Fig. 1A, galectin-3 was expressed in both proliferating myoblasts and myotubes, and its expression was significantly reduced 24 h after the initiation of differentiation. The release of galectin-3 to the medium was significantly increased 24 h after the initiation of differentiation and gradually declined thereafter (Fig. 1B). These results suggest that the synthesis and localization of galectin-3 is regulated during myogenesis. Immunostaining of galectin-3 also showed that galectin-3 was highly expressed in both differentiated myoblasts and thin, nascent myotubes (Fig. 1Ca–f; data not shown), whereas mature myotubes did not express high levels of galectin-3 (Fig. 1Cg–q). In skeletal muscles, galectin-3 was mainly found on the sarcolemmal membranes (Fig. 1D).
Figure 1.
Expression of galectin-3 in differentiating myoblasts, myotubes, and muscles. A, B). Differentiation of murine myoblast C2C12 cells was induced by changing the medium from GM to DM. The levels of galectin-3 in the cells (A) and medium (B) were detected with Western blotting using an anti–galectin-3 antibody. Representative images from 3 independent experiments and results of densitometry analyses (means ± sd) are shown. Significance compared to 0 h of differentiation by 1 way-ANOVA is shown. *P < 0.05, **P < 0.01, ***P < 0.001. When ANOVA–on ranks was used, the detected significance was as follows: for cells, 48 h and 72 h; and for medium, 24 h and 48 h. C) Galectin-3 in myoblasts and myotubes after 55 h (FOV 1 and 2) or 72 h (FOV 3, 4, and 5) of differentiation was visualized with immunostaining using an anti–galectin-3 antibody (red). Extended-focus images of the fluorescence (red) and an optical plane image of stained nuclei (DAPI, blue) were merged (a, b, d, e, g, h, j, k, m, n), as were a focal-plane DIC images and DAPI images are merged and shown (c, f, i, l, o). Myotubes are marked with thin, white lines, and myoblasts that attached onto the myotubes are marked with yellow lines in FOVs 3, 4, and 5. A z-optical image close to the bottom of the cover glass (p) and a z-optical image close to the top of a myotube of FOV 5 (images m–o) show a lack of galectin-3 expression in the myotubes and concentrations of galectin-3 on the extended myoblasts attached to the myotubes. Representative images from 2 independent experiments are shown. D) Galectin-3 expression in muscle derived from wild type (C57BL/6) and galectin-3 null mice.
Galectin-3 promotes myoblast differentiation
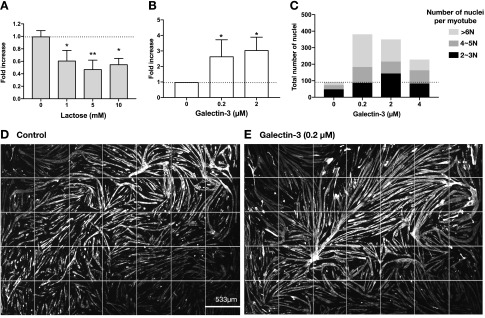
Because the expression and release of galectin-3 are regulated during myogenesis, we next investigated whether galectin-3 increases the efficiency of myogenesis. First, we tested the effect of an antagonist saccharide of galectin-3, lactose, on the myogenesis. The myogenesis was significantly inhibited by the presence of lactose (Fig. 2A). Then, we studied whether exogenously added galectin-3 further increased the formation of myotube. As little as 0.2 µM galectin-3 induced a 2.6-fold increase in myogenesis (Fig. 2B). The myotubes that formed in the presence of galectin-3 contained more nuclei than did those that formed in its absence (Fig. 2C). The stitched images of 7 × 5 FOVs showed that, in the presence of galectin-3, the myotubes were longer (some exceeded 1 mm) than those formed in its absence (Fig. 2D, E).
Figure 2.
Galectin-3 promotes myogenesis. A) Myogenesis was induced in the presence or absence of different concentrations of lactose, an antagonist of galectin-3, for 72 h. Cells were fixed and stained with anti-MHC antibody and DAPI. The total areas of MHC+ multinucleated myotubes were calculated using in-house software, and the fold increase in myogenesis was calculated with cells treated with PBS(−) as 1. Each data point represents the mean of the fold increase obtained by 3 independent experiments with error bars indicating sd. Significance compared with control (0 mM) by 1-way ANOVA is shown. *P < 0.05, **P < 0.01. When ANOVA–on ranks was used, the detected significance was as follows: lactose 5 mM. B, C) Myogenesis was induced in the presence or absence of galectin-3 for 62 h (B) and 72 h (C). The number of nuclei in MHC+ and multinuclear myotubes were counted and the fold increase in myogenesis was calculated taking cells treated with PBS(−) as 1. Each data point represents the mean of the fold increase obtained from 3 independent experiments with error bars indicating sd. Significance compared to control (0 µM) by 1 way -ANOVA is shown. *P < 0.05, **P < 0.01. When ANOVA–on ranks was used, the detected significance was the following: galectin-3, 0.2 µM and 2 µM. C) The number of nuclei in each MHC+ multinucleated myotube found in the stitched FOVs was counted and categorized into 3 groups based on the number of nuclei in each myotube. The representative results obtained from 2 independent experiments are shown. D, E) Stitched images (7 × 5 FOVs) of extended-focus images of myotubes (MHC+ cells are in white) formed in the absence (D) and presence (E) of galectin-3 after differentiation for 72 h are shown together with the size of 1 FOV. Representative images of 3 independent experiments are shown.
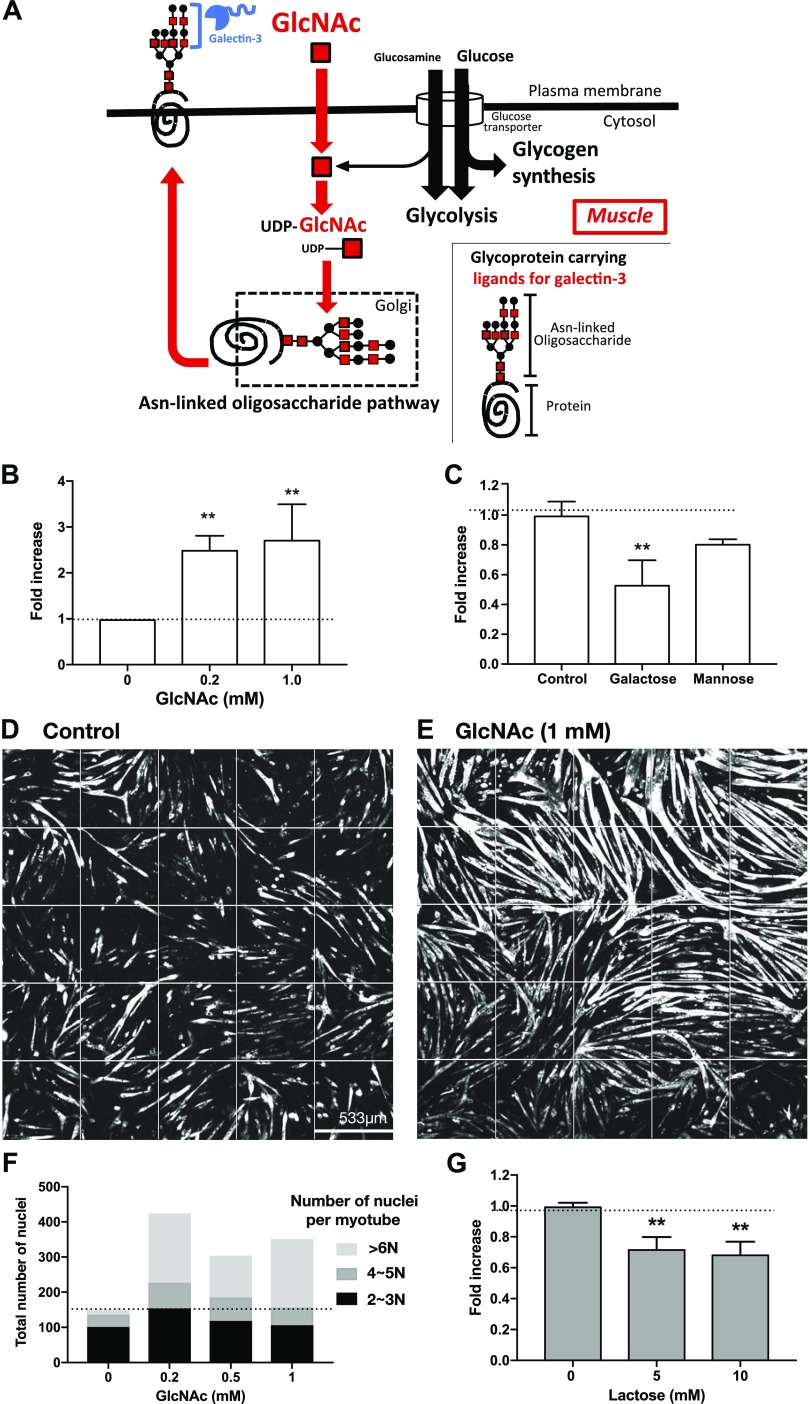
Monosaccharide GlcNAc, which increases the biosynthesis of the glycan ligands of galectin-3, increases myogenesis
Because galectin-3 increased myogenesis, we next tested whether the myogenesis was also enhanced by increasing the expression of galectin-3 binding partners, the ligands. Most galectin-3 ligands are a specific type of asparagine-linked oligosaccharide on membrane glycoproteins (31). Those oligosaccharides carry many N-acetyllactosamine (LacNAc, Galβ1-4GlcNAc) residues on their branches (Fig. 3A), and the affinity and avidity of galectin-3 for those oligosaccharides increases in proportion to the number of LacNAc units (28, 32). The number of LacNAc units strongly depends on the number of GlcNAc branches, especially the β1,6 branches; the enzyme MGAT5 (α1,6-mannosyl glycoprotein 6-βGlcNAc transferase) mediates its synthesis (28, 32). Importantly, MGAT5 catalyzes the rate-limiting step in the synthesis of the oligosaccharide ligands of galectin-3. MGAT5 transfers GlcNAc from uridine diphosphate (UDP)-GlcNAc to the precursor oligosaccharides attached to proteins in the Golgi apparatus, but the reaction is suboptimal because the Michaelis constant (Km) of MGAT5 for UDP-GlcNAc (11 mM) is around 10-fold greater than the intra-Golgi UDP-GlcNAc concentration (32). Therefore, an increase in the UDP-GlcNAc concentration enhances the synthesis of galectin-3 ligands in a linear manner. The intracellular concentration of UDP-GlcNAc can be augmented by increasing the extracellular concentration of GlcNAc (Fig. 3A) (28). Previous studies using immune and cancer cells demonstrated in vitro and in vivo that the treatment of cells with GlcNAc increases the expression of galectin-3 ligands (14). Therefore, we next examined whether increase in the expression of galectin-3 ligands by GlcNAc promotes the myogenesis. As little as 0.2 mM GlcNAc was sufficient to increase myogenesis (Fig. 3B). In contrast, a control saccharide, mannose (10 mM), had no effect on the efficiency of myogenesis, whereas a weak antagonist of galectin-3, Gal, inhibited the myogenesis at 10, but not 5, mM (Fig. 3C and data not shown). Similar to galectin-3, GlcNAc increased both the length and thickness of the myotubes (Fig. 3D, E) and also promoted the formation of myotubes that contained many nuclei (Fig. 3F). An antagonist of galectin-3, lactose, inhibited GlcNAc-induced myogenesis (Fig. 3G).
Figure 3.
GlcNAc promotes myogenesis. A) Regulation of the synthesis of Asn-linked glycans by GlcNAc in muscle cells. Glucosamine is taken up by a glucose transporter. Most glucosamine is used for glycolysis and glycogen synthesis, and only a small percentage is transformed into UDP-GlcNAc. In contrast, extracellular GlcNAc is rapidly endocytosed to synthesize UDP-GlcNAc. UDP-GlcNAc is then incorporated into Asn-linked glycans, which are attached to proteins in the Golgi apparatus. The glycoproteins are then transported to the cell surface. Galectin-3 binds to the glycoproteins. B) Myogenesis was induced for 62 h in the presence or absence of GlcNAc. The number of nuclei in MHC+ multinucleated myotube was counted, and the fold increase in myogenesis was calculated taking cells treated with PBS(−) as 1. Each data point represents the mean of the fold increase obtained in 3 independent experiments, with error bars indicating sd. Significance compared with control (0 mM) by 1-way ANOVA is shown. **P < 0.01. When ANOVA–on ranks was used, the detected significance was as follows: GlcNAc, 0.2 mM and 1 mM, where P < 0.05. C) Myogenesis was induced in the presence or absence of saccharides (Gal and mannose) at a concentration of 10 mM. The total areas of MHC+ multinucleated myotubes were calculated, and the fold increase in the myogenesis was calculated taking cells treated with PBS(−) as 1. Each data point represents the mean of the fold increase obtained in 3 independent experiments with error bars indicating sd. Significance compared with control by 1-way ANOVA is shown. **P < 0.01. When ANOVA–on ranks was used, the detected significance was the following: Gal, where P < 0.05. D, E) Stitched, extended-focus images (5 × 5 FOVs) of myotubes (MHC+ cells are white) formed in the absence (D) or presence (E) of GlcNAc after differentiation for 72 h. Representative images from 3 independent experiments are shown. F) Myogenesis was induced for 72 h, and the number of nuclei in each MHC+ multinucleated myotube found in the stitched FOVs was counted and categorized into 3 groups based on the number of nuclei in each myotube. The representative results obtained from 2 independent experiments are shown. G) Myogenesis was induced in the presence of GlcNAc (1 mM) together with different concentrations of the galectin-3 antagonist, lactose. The total areas of MHC+ multinucleated myotubes were calculated and the fold increase in the myogenesis was calculated taking cells treated with GlcNAc as 1. Each data point represents the mean of the fold increase obtained by 3 independent experiments with error bars indicating sd. Significance compared to control by 1 way -ANOVA is shown. **P < 0.01. When ANOVA–on ranks was used, the detected significance was the following: lactose 10 mM, where P < 0.05.
Effect of treatment with either galectin-3 or GlcNAc on the initial stage of myogenesis
We next examined whether the treatment of galectin-3 or GlcNAc had any effect on the commitment into differentiation or stimulation of migration. As shown in Supplemental Fig. 1, the up-regulated expression of myogenin, which is directly involved in the differentiation process and triggers expression of myotube-specific genes, became detectable 48 h after differentiation. Treatment with galectin-3 or GlcNAc did not increase the expression. The cell motility was not enhanced by those treatments, but rather, showed some trends in reduction although it was not statistically significant (Supplemental Fig. 2). Together, those results suggest that galectin-3 and GlcNAc do not have significant effects of the initial stages of myoblast differentiation, suggesting the possibility that galectin-3 facilitates the later stages of myogenesis, which are related to the fusion process.
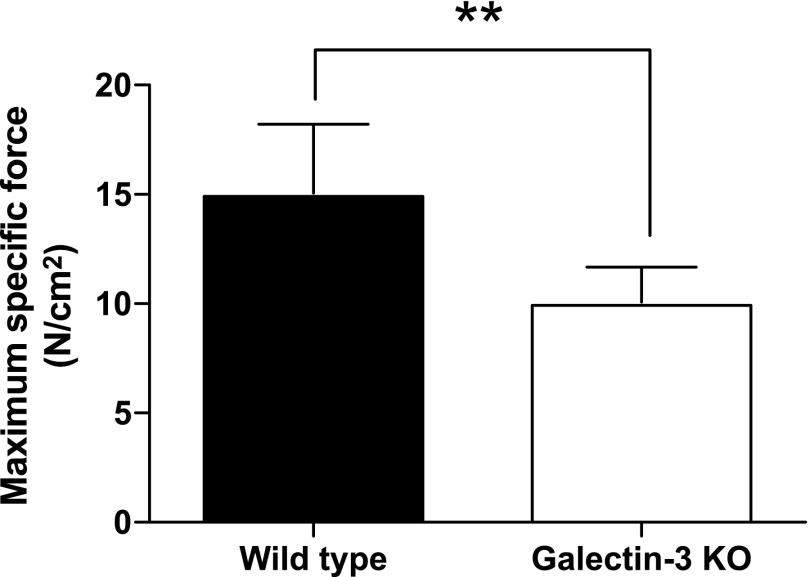
Lack of galectin-3 impairs the force-production capacity of soleus muscles
To examine the role of galectin-3 in muscle physiology, we compared the maximum specific force in both wild-type mice and galectin-3–deficient mice. As shown in Fig. 4, the specific tetanic tension of the soleus muscles in the galectin-3–deficient mice was inferior to that in the wild-type mice, suggesting that the lack of galectin-3 impairs the capacity for muscle-force production.
Figure 4.
Lack of galectin-3 impairs the force-production capacity of soleus muscles. Soleus muscles were isolated from galectin-3 knockout mice, and the maximum specific force was measured. Data represent the mean of n = 6, with error bars representing sd. Significance compared with wild-type by 1-way ANOVA is shown. **P < 0.01.
GlcNAc treatment increases muscle function in mdx mice
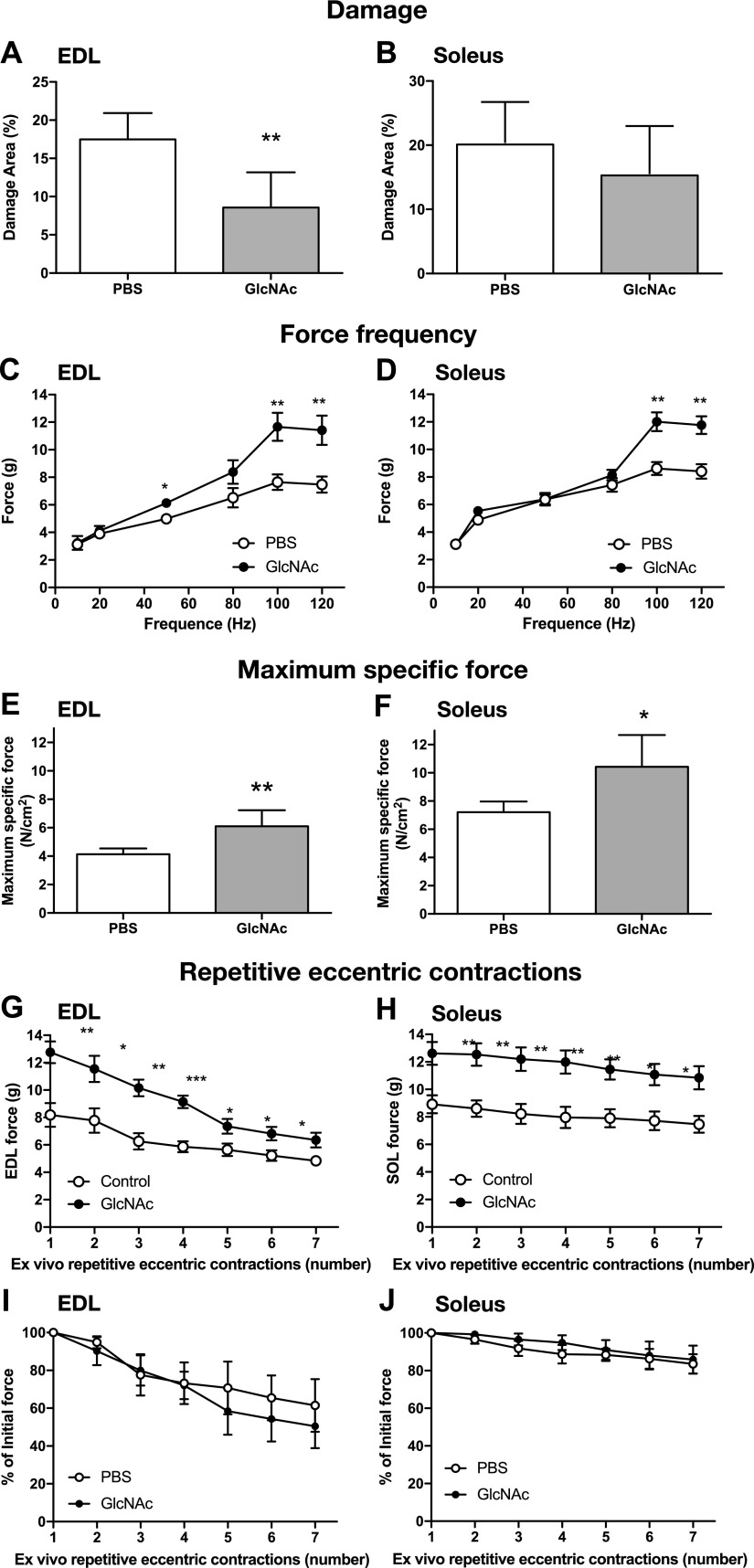
We next assessed the therapeutic potential of GlcNAc in DMD-model mice. In mdx mice, muscle degeneration initiated around the third week, and highly active regeneration occurred between the fourth and fifth weeks (33–38). Beyond 6 wk, the muscle of the mdx mice increased in weight, and muscle strength partially recovered without any treatment (33–37). For example, the difference in force of EDL muscles between control and dystrophic EDL muscles was reduced from 67 to 22.6% between 35 and 56 d of age (30). Thus, we intentionally started a 10-d treatment from 25 d to include the most severe peak of muscle degeneration and regeneration. The mdx mice were intraperitoneally administered either PBS or GlcNAc daily, at a dose of 250 mg/kg bodyweight, for 10 d starting at 25 d after birth, that is, the first and most important peak of muscle degeneration and regeneration in this mouse model. Treatment with GlcNAc significantly reduced the damaged areas in the EDL muscles (Fig. 5A, B). The force-frequency curves and maximum specific force of the fast-twitch fibers of the isolated EDL and the slow-twitch fibers of the isolated soleus muscles were performed after treatment for 10 d (Fig. 5C–F). No significant difference was observed in body weight, muscle weights, peak tension, half-relaxation time, and twitch tension (Supplemental Table S1). At low frequencies, the force-frequency curves were similar between GlcNAc-treated and control mice (Fig. 5C, D). In contrast, at high frequencies, GlcNAc significantly increased force production of both EDL and soleus muscle, relative to PBS-treated controls (Fig. 5C, D). Maximum specific-force generation of both EDL and soleus muscle was also significantly greater in mice treated with GlcNAc compared with that in the PBS-treated mice. We next examined the effect of repetitive eccentric contractions on the muscles. Although force generation by GlcNAc-treated muscles remained significantly greater than control muscles, there was no significant difference between groups when expressed as the percentage of force drop following repetitive eccentric contractions (Fig. 5G–J). These results suggest that GlcNAc treatment improves force production but does not protect muscle against stressful ex vivo repetitive eccentric contractions.
Figure 5.
GlcNAc treatment improves the function of dystrophic muscles in mdx mice. DMD mdx mice were treated intraperitoneally with GlcNAc (250 mg/kg bodyweight/d) for 10 d, starting 25 d after birth. Data represents the mean of n = 6, with error bars representing sd. A, B) Soleus and EDL muscles were collected on d 35 and stained with hematoxylin and eosin. The areas of muscle damage (pixels) and muscle tissues (pixels) were estimated with Photoshop software (Adobe Systems, San Jose, CA, USA), and the percentage of damage was calculated. C–H) Soleus and EDL muscles were collected on d 35, and the force-frequency curves (C, D), maximum specific force (sP0) (E, F), and resistance to repetitive eccentric contractions [force (G, H) and percentage of initial force (I, J)] were measured. Significance compared with PBS-treated mice by 1-way ANOVA is shown. *P < 0.05, **P < 0.01.
DISCUSSION
In this study, we have shown that galectin-3 or the monosaccharide GlcNAc, which increases the biosynthesis and expression of the glycan ligands of galectin-3, increases the efficiency of myogenesis. We have also presented evidence in a mouse model of DMD that GlcNAc has therapeutic potential in rescuing the functions of both slow- and fast-twitch dystrophic skeletal muscles during the initial peak of muscle degeneration and regeneration (33–36). It has been reported that mice lacking the key enzyme MGAT5, which is the rate-limiting enzyme in the biosynthesis of galectin-3 ligands, show impairment in muscle repair and growth with aging (39). Our results demonstrate that the maximum specific force of the soleus muscles in galectin-3–deficient mice was inferior to that in wild-type mice, suggesting that the lack of galectin-3 impairs their capacity to generate force. Together, this evidence suggests that galectin-3 has a critical role in muscle biology by interacting with its glycan ligands. These observations raise the possibility that a noninvasive therapy for DMD can be developed using GlcNAc.
The molecular mechanisms by which the interaction between galectin-3 and its glycan ligands promotes myogenesis and mitigates DMD are still unknown. Unlike an interaction between a specific ligand and a receptor, galectin-3–mediated responses are controlled by weak-to-strong binding of galectin-3 to various types of oligosaccharides attached to membrane glycoproteins and by the signals that are emitted from those cell surface proteins. Once galectin-3 molecules attach to their binding partner proteins, they cross-link those proteins. When the cross-links with its binding partners are expressed in 2 different entities, such as 2 cells or a cell and a cell matrix, galectin-3 mediates cell–cell or cell–matrix interactions. When the cross-links with its binding partners is on the cell surface, galectin-3 alters the dynamics of its binding partners or induces signal transduction (1–3). Indeed, in other biologic systems, it has been demonstrated that this oligomerization-driven cross-linking leads to various consequences, including regulation of signal transduction, alteration of membrane dynamics, cell migration, cell–cell adhesion, and cell–matrix interaction. Previous studies in other tissues have suggested that galectin-3 mediates cell–cell interactions, such as the adhesion of neutrophils to endothelial cells and to laminin (13, 15, 40). Therefore, it is possible that galectin-3 facilitates the intimate interactions of differentiating myoblasts that promote fusion in myogenesis. Immediately before the fusion of those adherent myoblasts, phosphatidylserine (PS), a phospholipid that localizes exclusively in the inner cytoplasmic leaflet of membranes, is transiently exposed on the surface of the fusing membranes, and that transient exposure of PS is required for cell fusion (41). Interestingly, galectin-3 reportedly induces the transient exposure of PS on some cells, including lymphocytes and neutrophils, without inducing cell apoptosis (42), implying that galectin-3 induces that PS exposure in myoblasts before the fusion. Galectin-3 is also known to interact with integrins, which are cell-adhesion molecules involved in both cell–matrix interaction and cell migration. Interestingly, α3β1 integrin, which participates in myoblast adhesion, migration, and fusion (43), is reported to interact with galectin-3, promoting lamellipodia formation in epithelial cells (44). Our unpublished results also suggest that galectin-3 interacts with α3β1 integrin expressed in C2C12 cells. Thus, it is possible that galectin-3 promotes migration as well as cell–cell interaction through its interaction with integrins. Therefore, it is likely that galectin-3 is involved in several differentiation steps in myogenesis. Our results show that that galectin-3 and GlcNAc treatment did not increase the expression of myogenin and the motility of differentiating myoblasts, suggesting that galectin-3 is not actively involved in the commitment to the differentiation program, but rather, may have roles in the processes that are linked to cell fusion. In addition to the role that galectin-3 has in myogenesis, it is also possible that galectin-3 is involved in the prevention of muscle degeneration. Dystrophic muscles are subject to repeated injury because the sarcolemmal membrane does not stably adhere to the basal lamina. Galectin-3 is one of the laminin-binding proteins, and that binding is also thought to enhance cell adhesion to the basal lamina. It is possible that the observed reduction in muscle injury in mdx mice treated with GlcNAc was also related to the stabilization of sarcolemmal membrane adhesion to laminin. Our ex vivo muscle contraction profiles show that GlcNAc treatment increased the force production of dystrophic muscles, whereas in a high-stress condition, that treatment failed to protect against repeated, eccentric, contraction-induced muscle dysfunction. Muscle injury induces the recruitment of proinflammatory macrophages. Once recruited, some of those cells switch to the M2 phenotype of regulatory macrophages, which promote myogenesis (8, 45, 46). Interestingly, galectin-3 is involved in the maintenance of the M2 phenotype of macrophages (6). Therefore, it is also possible that both galectin-3 and a treatment that increases the biosynthesis of galectin-3 ligands positively affect the health of dystrophic muscles by several routes. The mechanisms by which GlcNAc mitigates MD warrant further investigation to facilitate the development of a novel therapy for the treatment of DMD.
GlcNAc is related to glucosamine, a compound that is believed to reduce arthritic pain, although its underlying molecular mechanism remains totally unknown. Importantly, the biologic effect of GlcNAc is distinct from that of glucosamine (47). Glucosamine enters cells through glucose transporters, and >95% of it is used for glycolysis, and in the case of muscles, for glycogen synthesis as well (Fig. 3A). In contrast, GlcNAc is taken up by endocytosis, and most of it is then rapidly converted to UDP-GlcNAc, which is incorporated into asparagine-linked glycans on membrane proteins (Fig. 3A). Previous studies using immune and cancer cells have demonstrated in vitro and in vivo that the treatment of cells with high concentrations of GlcNAc (10–20 mM) increases the expression of galectin-3 ligands (9, 15). Interestingly, we found that 0.2 mM GlcNAc was sufficient to promote myogenesis. That result suggests the possibility that the biosynthesis of glycan ligands can be efficiently modified by the administration of GlcNAc to muscle cells, where most glucose and glucosamine is consumed in glycolysis and/or glycogen synthesis, rather than being converted to UDP-GlcNAc for glycan biosynthesis. Because muscle tissue accounts for ∼40–45% of the human body mass, the administration of the monosaccharide GlcNAc as a mitigating therapy for DMD is an interesting therapeutic option. Importantly, GlcNAc does not exert any major adverse effects in humans, even at a dose of 25 mg/kg bodyweight/d for 6 wk (48) or 6 g daily in children (49). The chronic administration of GlcNAc at 2.5 g/kg bodyweight/d to rats for 52 wk induced no apparent adverse effects or histopathologic changes in their tissues (50, 51).
We present the evidence that galectin-3 and GlcNAc, which increases the level of galectin-3 ligands, have interesting therapeutic potential for mitigating some symptoms associated with DMD. This current study suggests the therapeutic potential of short-term treatment with GlcNAc in the peak of muscle degeneration/regeneration in mdx mice, although it remains to be investigated whether long-term treatment with GlcNAc continuously alleviates the progression of DMD. Although further study is essential to understanding the mechanism by which both galectin-3 and GlcNAc mitigate DMD and promote myogenesis, the present study indicates that using GlcNAc as a supplemental agent may present an interesting class of therapy for DMD, especially because the safety of this inexpensive monosaccharide is relatively established in humans.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors acknowledge the Bioimaging Platform at the Centre de Recherche Centre Hospitalier Universitaire (CHU) de Quebec. The authors thank the Consortium for Functional Glycomics for galectin-3 knockout mice. The mAb directed against MHC was obtained from the Developmental Studies Hybridoma Bank, created by the U.S. National Institutes of Health, Institute of Child Health and Human Development, and maintained at the Department of Biology, University of Iowa (Iowa City, IA, USA). The authors declare no conflicts of interest.
Glossary
- DGC
dystrophin-associated glycoprotein complex
- DM
differentiation medium
- DMD
Duchenne muscular dystrophy
- EDL
extensor digitorum longus
- FOV
field of view
- Gal
galactose
- GlcNAc
N-acetylglucosamine
- GM
growth medium
- LacNAc
N-acetyllactosamine
- MD
muscular dystrophy
- MGAT5
α1,6-mannosyl glycoprotein 6-βGlcNAc transferase
- MHC
myosin heavy chain
- P0
maximum tetanic tension
- PS
phosphatidylserine
- UDP
uridine diphosphate
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
J. Frenette and S. Sato designed the research, analyzed the data, and wrote the paper; A. Rancourt, S. S. Dufresne, G. St-Pierre, H. Nakamura, and S. Sato performed the research and analyzed the data; J.-C. Lévesque and Y. Kikuchi analyzed the data; and M. S. Satoh developed the software necessary to analyze the data and edited the paper.
REFERENCES
- 1.Cummings R. D., Liu F.-T., Vasta G. R. (2017) Galectins. In: Essentials of Glycobiology [Internet], 3rd ed. (Varki, A., Esko, J. D., Stanley, P., Hart, G. W., Aebi, M., Darvill, A.G ., Kinoshita, T., Packer, N. H., Prestegard, J. H., Schnaar, R. L., Seeberger, P. H., Cummings, R. D., Liu, F. T., Vasta, G. R., eds.), Chapter 36, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA
- 2.Sato S., St-Pierre C., Bhaumik P., Nieminen J. (2009) Galectins in innate immunity: dual functions of host soluble β-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs). Immunol. Rev. 230, 172–187 [DOI] [PubMed] [Google Scholar]
- 3.Rabinovich G. A., Toscano M. A. (2009) Turning ‘sweet’ on immunity: galectin–glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 9, 338–352 [DOI] [PubMed] [Google Scholar]
- 4.Hirabayashi J., Kasai K. (1993) The family of metazoan metal-independent β-galactoside-binding lectins: structure, function and molecular evolution. Glycobiology 3, 297–304 [DOI] [PubMed] [Google Scholar]
- 5.Sato S., Hughes R. C. (1994) Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J. Biol. Chem. 269, 4424–4430 [PubMed] [Google Scholar]
- 6.MacKinnon A. C., Farnworth S. L., Hodkinson P. S., Henderson N. C., Atkinson K. M., Leffler H., Nilsson U. J., Haslett C., Forbes S. J., Sethi T. (2008) Regulation of alternative macrophage activation by galectin-3. J. Immunol. 180, 2650–2658 [DOI] [PubMed] [Google Scholar]
- 7.Novak R., Dabelic S., Dumic J. (2012) Galectin-1 and galectin-3 expression profiles in classically and alternatively activated human macrophages. Biochim. Biophys. Acta 1820, 1383–1390 [DOI] [PubMed] [Google Scholar]
- 8.Dumont N., Frenette J. (2010) Macrophages protect against muscle atrophy and promote muscle recovery in vivo and in vitro: a mechanism partly dependent on the insulin-like growth factor-1 signaling molecule. Am. J. Pathol. 176, 2228–2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau K. S., Partridge E. A., Grigorian A., Silvescu C. I., Reinhold V. N., Demetriou M., Dennis J. W. (2007) Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell 129, 123–134 [DOI] [PubMed] [Google Scholar]
- 10.Markowska A. I., Liu F. T., Panjwani N. (2010) Galectin-3 is an important mediator of VEGF- and bFGF-mediated angiogenic response. J. Exp. Med. 207, 1981–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lalancette-Hébert M., Swarup V., Beaulieu J. M., Bohacek I., Abdelhamid E., Weng Y. C., Sato S., Kriz J. (2012) Galectin-3 is required for resident microglia activation and proliferation in response to ischemic injury. J. Neurosci. 32, 10383–10395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato S., Hughes R. C. (1992) Binding specificity of a baby hamster kidney lectin for H type I and II chains, polylactosamine glycans, and appropriately glycosylated forms of laminin and fibronectin. J. Biol. Chem. 267, 6983–6990 [PubMed] [Google Scholar]
- 13.Nieminen J., Kuno A., Hirabayashi J., Sato S. (2007) Visualization of galectin-3 oligomerization on the surface of neutrophils and endothelial cells using fluorescence resonance energy transfer. J. Biol. Chem. 282, 1374–1383 [DOI] [PubMed] [Google Scholar]
- 14.Dennis J. W., Nabi I. R., Demetriou M. (2009) Metabolism, cell surface organization, and disease. Cell 139, 1229–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nieminen J., St-Pierre C., Bhaumik P., Poirier F., Sato S. (2008) Role of galectin-3 in leukocyte recruitment in a murine model of lung infection by Streptococcus pneumoniae. J. Immunol. 180, 2466–2473 [DOI] [PubMed] [Google Scholar]
- 16.Nieminen J., St-Pierre C., Sato S. (2005) Galectin-3 interacts with naive and primed neutrophils, inducing innate immune responses. J. Leukoc. Biol. 78, 1127–1135 [DOI] [PubMed] [Google Scholar]
- 17.Chargé S. B., Rudnicki M. A. (2004) Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 84, 209–238 [DOI] [PubMed] [Google Scholar]
- 18.Bentzinger C. F., Wang Y. X., Rudnicki M. A. (2012) Building muscle: molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 4, a008342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim J. H., Jin P., Duan R., Chen E. H. (2015) Mechanisms of myoblast fusion during muscle development. Curr. Opin. Genet. Dev. 32, 162–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell K. P. (1995) Three muscular dystrophies: loss of cytoskeleton–extracellular matrix linkage. Cell 80, 675–679 [DOI] [PubMed] [Google Scholar]
- 21.Gatheridge M. A., Kwon J. M., Mendell J. M., Scheuerbrandt G., Moat S. J., Eyskens F., Rockman-Greenberg C., Drousiotou A., Griggs R. C. (2016) Identifying non–Duchenne muscular Dystrophy-positive and false negative results in prior Duchenne muscular Dystrophy newborn screening programs: a review. JAMA Neurol. 73, 111–116 [DOI] [PubMed] [Google Scholar]
- 22.Mercuri E., Muntoni F. (2013) Muscular dystrophies. Lancet 381, 845–860 [DOI] [PubMed] [Google Scholar]
- 23.Fairclough R. J., Wood M. J., Davies K. E. (2013) Therapy for Duchenne muscular dystrophy: renewed optimism from genetic approaches. Nat. Rev. Genet. 14, 373–378 [DOI] [PubMed] [Google Scholar]
- 24.Rahimov F., Kunkel L. M. (2013) The cell biology of disease: cellular and molecular mechanisms underlying muscular dystrophy. J. Cell Biol. 201, 499–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung D. G., Wagner K. R. (2013) Therapeutic advances in muscular dystrophy. Ann. Neurol. 74, 404–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelletier I., Sato S. (2002) Specific recognition and cleavage of galectin-3 by Leishmania major through species-specific polygalactose epitope. J. Biol. Chem. 277, 17663–17670 [DOI] [PubMed] [Google Scholar]
- 27.St-Pierre C., Ouellet M., Tremblay M. J., Sato S. (2010) Galectin-1 and HIV-1 infection. Methods Enzymol. 480, 267–294 [DOI] [PubMed] [Google Scholar]
- 28.Grigorian A., Lee S. U., Tian W., Chen I. J., Gao G., Mendelsohn R., Dennis J. W., Demetriou M. (2007) Control of T cell-mediated autoimmunity by metabolite flux to N-glycan biosynthesis. J. Biol. Chem. 282, 20027–20035 [DOI] [PubMed] [Google Scholar]
- 29.Sato S., Rancourt A., Sato Y., Satoh M. S. (2016) Single-cell lineage tracking analysis reveals that an established cell line comprises putative cancer stem cells and their heterogeneous progeny. Sci. Rep. 6, 23328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dufresne S. S., Dumont N. A., Bouchard P., Lavergne É., Penninger J. M., Frenette J. (2015) Osteoprotegerin protects against muscular dystrophy. Am. J. Pathol. 185, 920–926 [DOI] [PubMed] [Google Scholar]
- 31.Patnaik S. K., Potvin B., Carlsson S., Sturm D., Leffler H., Stanley P. (2006) Complex N-glycans are the major ligands for galectin-1, -3, and -8 on Chinese hamster ovary cells. Glycobiology 16, 305–317 [DOI] [PubMed] [Google Scholar]
- 32.Sasai K., Ikeda Y., Fujii T., Tsuda T., Taniguchi N. (2002) UDP-GlcNAc concentration is an important factor in the biosynthesis of β1,6-branched oligosaccharides: regulation based on the kinetic properties of N-acetylglucosaminyltransferase V. Glycobiology 12, 119–127 [DOI] [PubMed] [Google Scholar]
- 33.Duddy W., Duguez S., Johnston H., Cohen T. V., Phadke A., Gordish-Dressman H., Nagaraju K., Gnocchi V., Low S., Partridge T. (2015) Muscular dystrophy in the mdx mouse is a severe myopathy compounded by hypotrophy, hypertrophy and hyperplasia. Skelet. Muscle 5, 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGreevy J. W., Hakim C. H., McIntosh M. A., Duan D. (2015) Animal models of DDuchenne muscular dystrophy: from basic mechanisms to gene therapy. Dis. Model. Mech. 8, 195–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carnwath J. W., Shotton D. M. (1987) Muscular dystrophy in the mdx mouse: histopathology of the soleus and extensor digitorum longus muscles. J. Neurol. Sci. 80, 39–54 [DOI] [PubMed] [Google Scholar]
- 36.Grady R. M., Teng H., Nichol M. C., Cunningham J. C., Wilkinson R. S., Sanes J. R. (1997) Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell 90, 729–738 [DOI] [PubMed] [Google Scholar]
- 37.Deconinck A. E., Rafael J. A., Skinner J. A., Brown S. C., Potter A. C., Metzinger L., Watt D. J., Dickson J. G., Tinsley J. M., Davies K. E. (1997) Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell 90, 717–727 [DOI] [PubMed] [Google Scholar]
- 38.Manning J., O’Malley D. (2015) What has the mdx mouse model of Duchenne muscular dystrophy contributed to our understanding of this disease? J. Muscle Res. Cell Motil. 36, 155–167 [DOI] [PubMed] [Google Scholar]
- 39.Cheung P., Pawling J., Partridge E. A., Sukhu B., Grynpas M., Dennis J. W. (2007) Metabolic homeostasis and tissue renewal are dependent on β1,6GlcNAc-branched N-glycans. Glycobiology 17, 828–837 [DOI] [PubMed] [Google Scholar]
- 40.Kuwabara I., Liu F. T. (1996) Galectin-3 promotes adhesion of human neutrophils to laminin. J. Immunol. 156, 3939–3944 [PubMed] [Google Scholar]
- 41.Van den Eijnde S. M., van den Hoff M. J., Reutelingsperger C. P., van Heerde W. L., Henfling M. E., Vermeij-Keers C., Schutte B., Borgers M., Ramaekers F. C. (2001) Transient expression of phosphatidylserine at cell–cell contact areas is required for myotube formation. J. Cell Sci. 114, 3631–3642 [DOI] [PubMed] [Google Scholar]
- 42.Stowell S. R., Qian Y., Karmakar S., Koyama N. S., Dias-Baruffi M., Leffler H., McEver R. P., Cummings R. D. (2008) Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J. Immunol. 180, 3091–3102 [DOI] [PubMed] [Google Scholar]
- 43.Brzóska E., Bello V., Darribère T., Moraczewski J. (2006) Integrin α3 subunit participates in myoblast adhesion and fusion in vitro. Differentiation 74, 105–118 [DOI] [PubMed] [Google Scholar]
- 44.Saravanan C., Liu F. T., Gipson I. K., Panjwani N. (2009) Galectin-3 promotes lamellipodia formation in epithelial cells by interacting with complex N-glycans on α3β1 integrin. J. Cell Sci. 122, 3684–3693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arnold L., Henry A., Poron F., Baba-Amer Y., van Rooijen N., Plonquet A., Gherardi R. K., Chazaud B. (2007) Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saclier M., Yacoub-Youssef H., Mackey A. L., Arnold L., Ardjoune H., Magnan M., Sailhan F., Chelly J., Pavlath G. K., Mounier R., Kjaer M., Chazaud B. (2013) Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells 31, 384–396 [DOI] [PubMed] [Google Scholar]
- 47.Wellen K. E., Lu C., Mancuso A., Lemons J. M., Ryczko M., Dennis J. W., Rabinowitz J. D., Coller H. A., Thompson C. B. (2010) The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev. 24, 2784–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simon R. R., Marks V., Leeds A. R., Anderson J. W. (2011) A comprehensive review of oral glucosamine use and effects on glucose metabolism in normal and diabetic individuals. Diabetes Metab. Res. Rev. 27, 14–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salvatore S., Heuschkel R., Tomlin S., Davies S. E., Edwards S., Walker-Smith J. A., French I., Murch S. H. (2000) A pilot study of N-acetyl glucosamine, a nutritional substrate for glycosaminoglycan synthesis, in paediatric chronic inflammatory bowel disease. Aliment. Pharmacol. Ther. 14, 1567–1579 [DOI] [PubMed] [Google Scholar]
- 50.Takahashi M., Inoue K., Yoshida M., Morikawa T., Shibutani M., Nishikawa A. (2009) Lack of chronic toxicity or carcinogenicity of dietary N-acetylglucosamine in F344 rats. Food Chem. Toxicol. 47, 462–471 [DOI] [PubMed] [Google Scholar]
- 51.Lee K. Y., Shibutani M., Takagi H., Arimura T., Takigami S., Uneyama C., Kato N., Hirose M. (2004) Subchronic toxicity study of dietary N-acetylglucosamine in F344 rats. Food Chem. Toxicol. 42, 687–695 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.