Abstract
Intestinal barrier function and microbiota are integrally related and play critical roles in maintenance of host physiology. Sex is a key biologic variable for several disorders. Our aim was to determine sex-based differences in response to perturbation and subsequent recovery of intestinal barrier function and microbiota in healthy humans. Twenty-three volunteers underwent duodenal biopsies, mucosal impedance, and in vivo permeability measurement. Permeability testing was repeated after administration of indomethacin, then 4 to 6 wk after its discontinuation. Duodenal and fecal microbiota composition was determined using 16S rRNA amplicon sequencing. Healthy women had lower intestinal permeability and higher duodenal and fecal microbial diversity than healthy men. Intestinal permeability increases after indomethacin administration in both sexes. However, only women demonstrated decreased fecal microbial diversity, including an increase in Prevotella abundance, after indomethacin administration. Duodenal microbiota composition did not show sex-specific changes. The increase in permeability and microbiota changes normalized after discontinuation of indomethacin. In summary, women have lower intestinal permeability and higher microbial diversity. Intestinal permeability is sensitive to perturbation but recovers to baseline. Gut microbiota in women is sensitive to perturbation but appears to be more stable in men. Sex-based differences in intestinal barrier function and microbiome should be considered in future studies.—Edogawa, S., Peters, S. A., Jenkins, G. D., Gurunathan, S. V., Sundt, W. J., Johnson, S., Lennon, R. J., Dyer, R. B., Camilleri, M., Kashyap, P. C., Farrugia, G., Chen, J., Singh, R. J., Grover, M. Sex differences in NSAID-induced perturbation of human intestinal barrier function and microbiota.
Keywords: lactulose, permeability, indomethacin, gender, microbiome
The intestinal barrier function is a complex physiologic process governed by luminal, mucosal, and submucosal factors. It is a selectively permeable barrier restricting the passage of pathogens, toxins, and intraluminal antigens while allowing passage of nutrients, electrolytes, and water (1). Intestinal microbiota is integral to cross-talk with the intestinal epithelium. Changes in intestinal mucosal barrier function or microbiota can influence each other bidirectionally. Barrier function can be assessed ex vivo by measuring transmucosal resistance (TMR) and flux of macromolecules across tissues in an Ussing chamber (2). For in vivo assessment, urinary excretion of orally ingested substances such as lactulose, mannitol, sucrose, sucralose, rhamnose, polymers of polyethylene glycol, 51Cr-EDTA, and 99mTc-DTPA can be used (3). Lactulose and mannitol are among the most commonly used molecules, in tandem, for assessing intestinal permeability. Lactulose is a disaccharide with a molecular diameter of 9.5 Å, which can only move across larger spaces or defects of tight junctions at the crypt, providing a primary parameter for paracellular permeability (4).
Sex differences in these biologic functions are important, considering the greater predilection for conditions like nonsteroidal antiinflammatory drug (NSAID)-induced upper gastrointestinal bleeding (5) and irritable bowel syndrome in women (6). Men have higher gastroduodenal permeability than women in response to alcohol intake (7). However, it is not known whether there are baseline sex differences in intestinal permeability in healthy humans. Microbiota differences have been reported between healthy women and men (8, 9). In human studies, up to 75% of individuals demonstrate an increase in intestinal permeability after NSAIDs, making it a model for studying dynamics of intestinal barrier function and effect of variables like sex (10–13). Sex was not associated with intestinal inflammation in arthritis patients who received chronic therapy with NSAIDs; however, the effect on intestinal permeability was not studied (14). It is not known whether there are sex-based differences in response to acute perturbation with NSAIDs.
Intestinal microbiota has been proposed to play a role in NSAID-induced intestinal injury (15). Germ-free and antibiotic-treated rats do not develop small intestinal ulcers upon treatment with indomethacin, suggesting that microbiota are essential in mediating NSAID-induced intestinal injury (16, 17). Indomethacin-induced enteropathy in mice has been associated with changes in microbiota composition (18, 19). In a study involving detailed regional profiling of different luminal and mucosal segments of the intestine, indomethacin resulted in microbiota changes across colon and feces but not small bowel (20). Human studies have shown mixed results, including differences in fecal bacterial profiles among chronic NSAID recipients (21), whereas there was no differences after acute administration (22). However, these studies are confounded by primary disease conditions or concomitant use of other medications.
The goal of this study was to characterize sex-based differences at baseline in response to administration of NSAIDs, and to study the subsequent recovery of intestinal barrier function and microbiota in healthy human subjects.
MATERIALS AND METHODS
Subjects
The study population consisted of 23 healthy volunteers (12 women and 11 men). Those with a history of abdominal surgery (except appendectomy or cholecystectomy), inflammatory bowel disease, irritable bowel syndrome, microscopic colitis, or celiac disease were excluded. None of the women was pregnant at the time of the study. Additional exclusion criteria included use of the following drugs, which are known to increase intestinal permeability, before and during the study: tobacco (within 6 mo), oral corticosteroids (within 6 wk), NSAIDs (within 4 wk), and antibiotics (within 1 wk of each permeability test). Any prescription, over-the-counter, or herbal medications that can affect gastrointestinal transit (e.g., osmotic laxatives, antiemetics, prokinetics, anticholinergics, narcotics, peppermint oil, or antidepressants with known effects on intestinal transit) were prohibited 1 wk before study start. Participants were excluded if they had bleeding disorders or were taking medication that could increase risk or bleeding from mucosal biopsy wounds and if they scored higher than 12 for anxiety or depression on the Hospital Anxiety and Depression Scale (HADS). The study was approved by the institutional review board at Mayo Clinic (IRB 15-003603), and all subjects provided written informed consent before participation. The study was listed at U.S. National Library of Medicine (Bethesda, MD, USA; NCT02603822; https://clinicaltrials.gov).
In vivo intestinal permeability measurement
Saccharide excretion assay
Measurement of in vivo intestinal permeability was done using lactulose, 12C mannitol, and 13C mannitol as previously described (23). Briefly, volunteers were instructed to not consume artificial sweeteners (sucralose, aspartame), lactulose, or mannitol for 2 d before testing. Lactulose (1000 mg), 12C mannitol (100 mg), and 13C mannitol (100 mg) were dissolved in 250 ml of water and were orally administered to volunteers. Urine samples were collected before saccharide administration (baseline) and again at 0 to 2 and 2 to 24 h after saccharide administration. HPLC–mass spectrometry was used to measure saccharide concentrations in urine samples. Cumulative concentration of each saccharide was calculated for each time interval. Because 13C makes up 1% of naturally occurring carbon, concentrations of 13C mannitol were corrected for the percentage of 13C in 12C mannitol, as previously described (2). Cumulative lactulose concentrations at 0 to 2 h and 2 to 24 h were used to report proximal small intestinal and distal small intestinal/colonic permeability, respectively (24). All 23 volunteers participated in the saccharide excretion assay at baseline (visit 1), after administration of indomethacin (visit 2), and then 4 to 6 wk later (visit 3). Indomethacin dosing was 75 mg × 2 doses (8 and 0.5 h before permeability testing) for 9 volunteers and 75 mg twice a day for 5 d for 14 volunteers.
Endoscopic duodenal mucosal impedance at baseline
After not eating for 8 h, participants underwent sedated esophagogastroduodenoscopy. Duodenal mucosal impedance was measured as previously described (2). Briefly, a 2-mm diameter catheter was passed through an endoscope and was placed on the duodenal mucosa under direct visualization. Two 360-degree circumferential sensors, placed 2 mm apart on the mucosa, were connected to a voltage transducer that produced a 10 µA current at a frequency of 2 kHz. Measurements were over a time frame of >10 s using a stationary data acquisition system (Insight; Sandhill Scientific, Denver, CO, USA), and data were analyzed with BioView software (Sandhill Scientific). Impedance was measured on all quadrants of the duodenum with a decompressed lumen after all fluid was aspirated. Values were expressed voltage/current (Ω). The 4 readings were averaged for each volunteer.
Ex vivo duodenal mucosal barrier function
Sample collection
At baseline esophagogastroduodenoscopy, 12 duodenal mucosal samples were collected via biopsy from the second portion of the duodenum. All endoscopic procedures were done by a single endoscopist (M.G.), and samples were collected using a large-capacity (2.8 mm) biopsy forceps (no pin). Collected samples were placed in either fixatives or in Krebs solution for Ussing chamber studies. All samples were placed on ice and were immediately transported to the laboratory.
Ussing chamber studies
Ussing chamber studies were performed on duodenal mucosa biopsy samples, mounted within 45 min of collection, to measure mucosal barrier function. Samples were mounted in 4 ml Ussing chambers (Physiologic Instruments, San Diego, CA, USA) exposing 0.031 cm2 area with Krebs with 10 mM mannitol and Krebs with 10 mM glucose on the mucosal and submucosal sides, respectively. Samples were allowed to equilibrate for 20 to 30 min, and baseline TMR of each tissue was measured. Average TMR was calculated using the results of 4 to 5 samples per subject. Samples with baseline TMR <10 Ω × cm2 and/or a short circuit current drift of >100 μA cm−2 from baseline were excluded from analysis. At the end of the experiment, samples were treated with forskolin (10 μM) on the mucosal side to check for tissue responsiveness, and samples with no response were excluded from analysis.
Fecal and duodenal microbiota composition
Stool samples were obtained immediately before saccharide administration for permeability testing. Duodenal aspirates (second portion of duodenum) were collected during upper endoscopy in a standardized fashion after urine collection for permeability testing was completed (24 h after single-dose lactulose administration). All samples were stored at −80°C until further use. Stool collection was done at 3 time points (baseline, after indomethacin administration, 4–6 wk later) and duodenal aspirates at 2 time points (baseline and after indomethacin administration). MP Biomedicals FastPrep-24 5G Homogenizer and an M-PVA bead–based automated high-throughput nucleic acid isolation system (Chemagic MSM I; PerkinElmer, Waltham, MA, USA) followed by Mo Bio Laboratories (Carlsbad, CA, USA) PowerSoil DNA Isolation Kit were used. The concentration was measured using Qubit dsDNA HS Kit (PN Q32854; Thermo Fisher Scientific, Waltham, MA, USA). The average yield was 19.0 ng/µl. A 2-step PCR protocol was used to amplify the V3 to V5 region of the 16S rRNA gene and then add Illumina (San Diego, CA, USA) flow cell adaptors containing indices (amplicon size 694 nt). Modified primers were used: V3_341F_Nextera: 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGAGGCAGCAG-3′ and V5_926R_Nextera: 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGCCGTCAATTCMTTTRAGT-3′. The PCR products were diluted, cleaned, quantified, and normalized using a Quant-iT dsDNA HS Assay Kit (Thermo Fisher Scientific). The 16S amplicon pools were quantified using the Kapa SYBR Fast qPCR Kit (Kapa Biosystems, Wilmington, MA, USA) and sequenced using the MiSeq 600 Cycle v.3 Kit (Illumina) and MCS v.2.6.1 (paired reads 2 × 300 bp). DNA extraction from duodenal aspirates was performed using the phenol chloroform method. The V4 region was amplified and barcoded, followed by sequencing with the MiSeq 600 Cycle v.3 Kit (Illumina).
Statistical analysis
Data are presented as means ± sd for continuous variables and as frequencies and percentages for categorical variables. The Mann-Whitney and Wilcoxon signed-rank tests were used to analyze unpaired and paired data, respectively. The Friedman test was used to analyze the repeated-measures data collected during different visits. All analyses, except of microbiome data, were performed by GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA). A value of P < 0.05 was considered statistically significant.
For microbiota composition analysis, after sequencing, adapter-primer sequences were removed from reads as previously described (25). In total, 4,318,493 reads (median 58,429 reads per sample, range 20,492–85,129) passed quality control in study samples. Paired R1 and R2 sequence reads were then processed via the hybrid-denovo bioinformatics pipeline (26), which clustered these good-quality paired-end and single-end reads into operational taxonomic units (OTUs) at 97% similarity level. OTUs were assigned taxonomy using the Ribosomal Database Project (RDP) classifier trained on the GreenGenes 13.5 database (http://greengenes.lbl.gov). Singleton OTU as well as samples with <2000 reads were removed as a quality control step. A total of 1198 OTUs were clustered; these OTUs belonged to 14 phyla, 64 families, and 112 genera.
Alpha and β diversity were analyzed for the OTU data. Alpha diversity reflects species richness and evenness within bacterial populations. Four α-diversity indices (within-sample diversity) were calculated: observed number of OTUs, Chao1 estimator, Shannon, and inverse Simpson index on the rarefied microbiome data (rarefied to 20,000 reads). A linear mixed-effects model was used for testing the covariate effects (i.e., sex, treatment, and sex–treatment interaction) on α diversity, accounting for correlation within subject with different intercepts per subject (random effect for subject). For independent samples, a linear regression model was used. A Wald-type test was used for assessing statistical significance. β diversity reflects the shared diversity between bacterial populations in terms of ecological distance; pairwise distance metrics allow quantification of differences between samples (27). Four β-diversity measures [unweighted, generalized (α = 0.5), weighted UniFrac distances, and Bray-Curtis distance] were calculated using a rarefied OTU table and a phylogenetic tree. To test the association between the covariates and β-diversity measures, permutational multivariate analysis of variance (PERMANOVA) tests were used. Ordination plots were generated using principal coordinate analysis as implemented in R software (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org/) for association of covariates with the β diversities.
Differential abundance analysis was performed at the phylum, class, order, family, and genus levels, and taxa with prevalence <10% or with a maximum proportion <0.2% were excluded from testing to reduce the number of the tests. The count data was normalized into relative abundances by dividing by the geometric mean of pairwise ratio size factor (28). To identify differentially abundant taxa associated with covariates and to account for the nonnormality of the count data, a permutation-based approach was used based on the F statistics of a linear model (square root–transformed taxa relative abundance as the response variable) and within-subject permutation to account for within-subject correlation. Additionally, nonparametric Wilcoxon signed-rank tests between pairs of study visits were conducted. False discovery rate (FDR) control (B-H procedure from “P.adjust” in the R stats package) was used to correct for multiple testing of the permutation based tests, and FDR-adjusted values of P < 0.05 or q < 0.05 were considered significant (pairwise visit tests were not corrected for multiple testing).
RESULTS
Subject demographics
The mean ± sd age of enrolled subjects was 39.8 (10.8) yr; 12 of 23 subjects were women and 11 were men. Two of 23 were receiving therapy for antidepressant, 2 were receiving acetaminophen, 2 were receiving therapy with a proton pump inhibitor, and 2 were receiving allergy medication at the time of the study. The mean anxiety and depression scores on HADS were 2.3 and 0.8, respectively. Mean age, anxiety, and depression scores were similar between women and men.
Healthy women have lower intestinal permeability and greater microbial diversity compared to healthy men
Proximal small intestinal permeability and microbiome
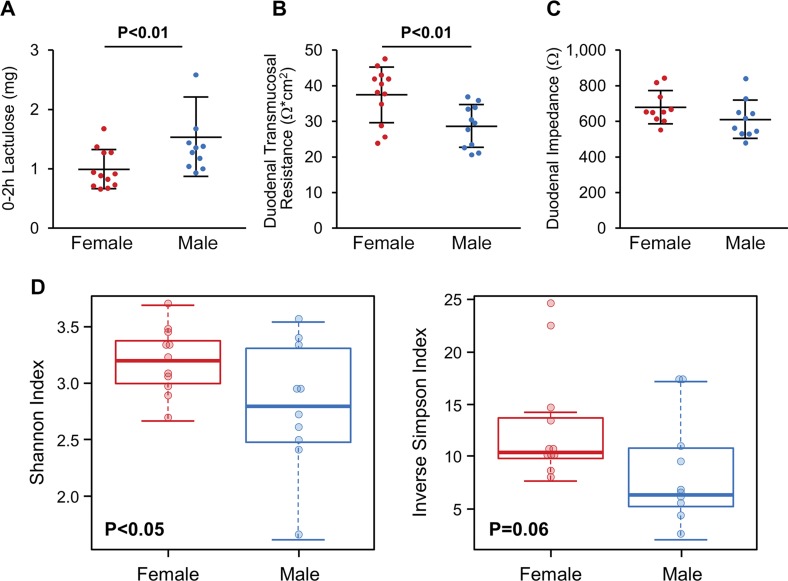
Healthy women have a lower excretion of lactulose (0–2 h) than healthy men [mean ± sd: 1.01 (0.33) vs. 1.54 (0.66) mg, P = 0.009], reflecting lower in vivo proximal small intestinal permeability (Fig. 1A). The average TMR of the duodenal biopsy samples from women was 1.3-fold greater than men [mean ± sd: 37.8 (7.8) vs. 28.9 (6.1) Ω × cm2, P = 0.006] (Fig. 1B). In vivo duodenal mucosal impedance, measured using an endoscopically delivered catheter, also trended to be higher in women than men [mean ± sd: 681 (93) vs. 614 (108) Ω, P = 0.08] (Fig. 1C). Duodenal aspirates showed greater α diversity in women [Shannon index (P = 0.042) and inverse Simpson index (P = 0.063), unpaired Student’s t test] (Fig. 1D). However, no differences were seen for β diversity or taxa abundance in duodenal aspirates from women and men (q < 0.05).
Figure 1.
Sex-based differences in permeability and microbiome of proximal small intestine. A) Healthy women have significantly lower in vivo proximal small intestinal permeability (0–2 h lactulose excretion) than healthy men. B) Healthy women have higher duodenal TMR compared to healthy men. C) Duodenal impedance measured in vivo using endoscopic catheter; results indicated women had tendency to have higher duodenal impedance compared to men. D) Healthy women have greater microbial diversity than healthy men. Data are presented as means ± sd; n = 10–12/group.
Distal small intestinal/colonic permeability and fecal microbiome
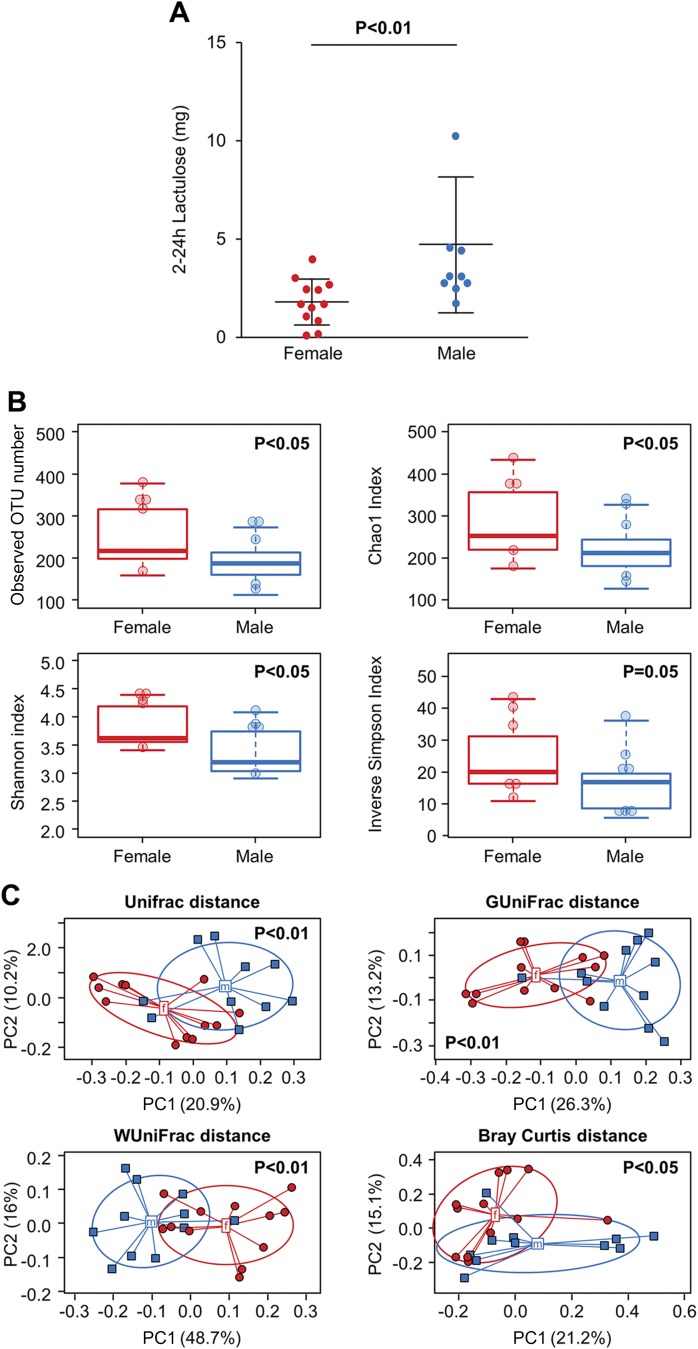
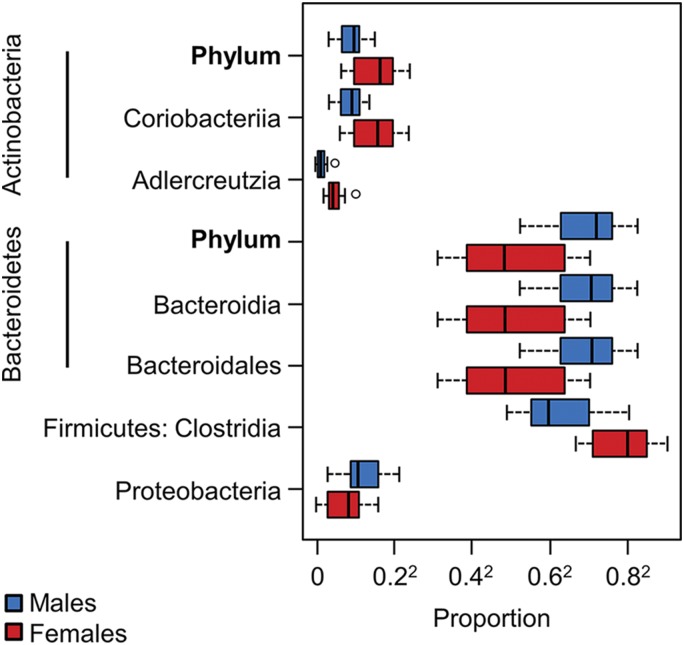
Excretion of lactulose (0–2 h) was also significantly lower in women than men [mean ± sd: 1.84 (1.16) vs. 4.75 (3.44) mg, P = 0.0015] (Fig. 2A). Women had greater α diversity than men [observed OTU number (P = 0.026), Chao1 estimator (P = 0.044), Shannon index (P = 0.011) and inverse Simpson index (P = 0.053), unpaired Student’s t test] (Fig. 2B). Additionally, women and men had different community compositions as determined by β-diversity analysis [UniFrac (P = 0.005), GUniFrac (P = 0.001), WUniFrac (P = 0.001), Bray Curtis (P = 0.030), PERMANOVA] (Fig. 2C). There were several significant differences in taxa abundance between women and men (q < 0.05) (Fig. 3).
Figure 2.
Sex-based differences in permeability of distal small intestine/colon and fecal microbiome. A) Healthy women showed significantly lower in vivo distal small intestinal and colonic permeability (2–24 h lactulose excretion) than healthy men. B) Alpha diversity [observed (P = 0.026), Chao1 estimator (P = 0.044), Shannon index (P = 0.011), Inverse Simpson index (P = 0.053), unpaired t test] was higher in women. C) β diversity [UniFrac (P = 0.005), GUniFrac (P = 0.001), WUniFrac (P = 0.001), Bray Curtis (P = 0.030), PERMANOVA] was higher in women compared to men.
Figure 3.
Sex-based differences in fecal microbial composition. Taxa with differences in abundance between healthy women and men (q < 0.05)
Healthy women and men demonstrate increase in proximal small intestinal permeability and decrease in duodenal microbial diversity
Proximal small intestinal permeability
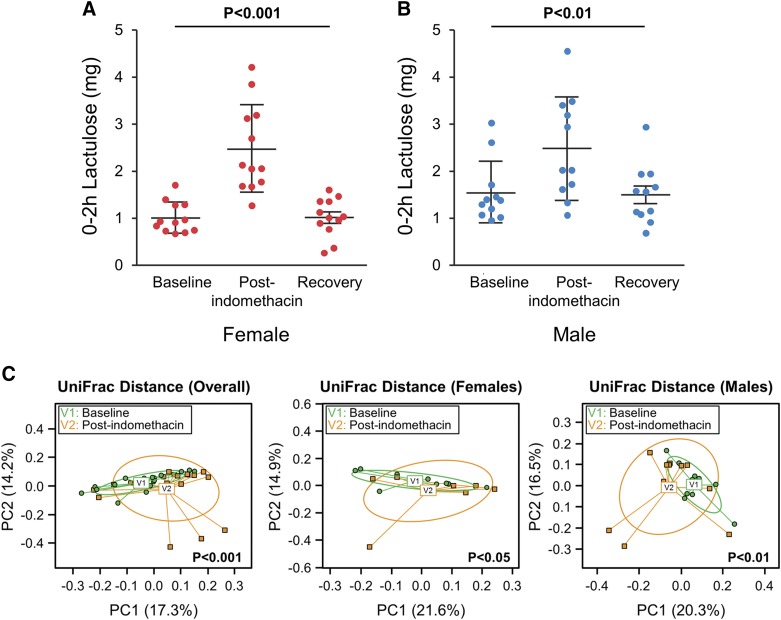
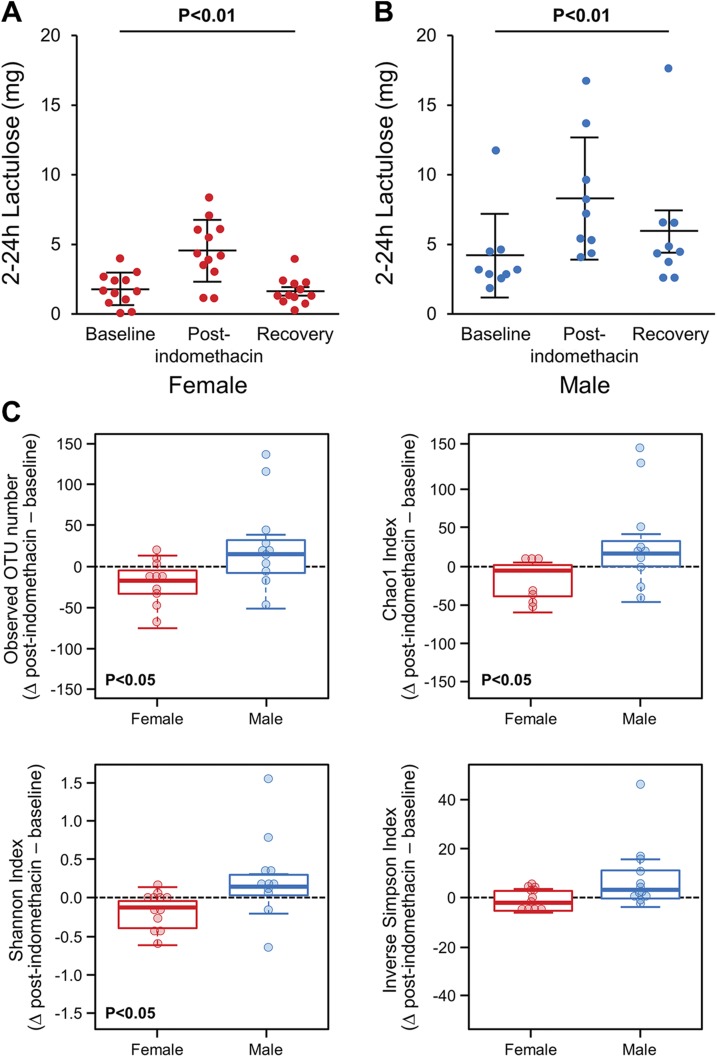
Lactulose excretion (0–2 h) was significantly higher after indomethacin administration compared to baseline and returned to baseline at the third visit 4 to 6 wk later in both women [mean ± sd: 1.01 (0.33), 2.47 (0.93), and 1.01 (0.41) mg, P < 0.0005] (Fig. 4A) and men [mean ± sd: 1.54 (0.66), 2.48 (1.09), and 1.50 (0.62) mg, P < 0.005] (Fig. 4B). Lactulose to 13C mannitol excretion ratio (LMR) also increased after indomethacin administration at 0 to 2 h [mean ± sd: 0.0096 (0.0042), 0.019 (0.0078), and 0.010 (0.0037) mg, P < 0.0001] (Supplemental Fig. 1A). Administration of indomethacin for 1 or 5 d had a similar effect of increased 0 to 2 h lactulose excretion [visit after indomethacin administration, mean ± sd with longer duration 2.47 (1.07) vs. shorter duration: 2.47 (0.90) mg, P = 0.93] (Supplemental Fig. 2A).
Figure 4.
Effect of indomethacin on proximal small intestinal permeability and microbiome. Lactose excretion at 0–2 h was significantly higher after indomethacin administration (visit 2) compared to baseline (visit 1) and returned to baseline at recovery (visit 3). A) Women. B). Men. Data are presented as paired comparisons. Means ± sd; n = 10–11/group. C) β diversity decreased after indomethacin administration. Overall group [UniFrac (P < 0.001), PERMANOVA]; women [UniFrac (P = 0.016)]; men [UniFrac (P = 0.004), PERMANOVA].
Duodenal microbiome
Duodenal aspirates showed no significant difference in α diversity after indomethacin administration in the overall group. However, β-diversity analysis revealed a significant compositional difference [UniFrac (P < 0.001), PERMANOVA]. Subgroup analysis showed that both women and men demonstrated overall compositional shifts after indomethacin administration [UniFrac (P = 0.016 for women, and P = 0.004 for men), PERMANOVA] (Fig. 4C).
Healthy women and men demonstrate increase in distal small intestinal and colonic permeability, but only women showed decreased fecal microbial diversity
Distal small intestinal/colonic permeability
The 2 to 24 h lactulose excretion was significantly higher after indomethacin administration compared to baseline and returned to baseline at the third visit 4 to 6 wk later in both women [mean ± sd: 1.84 (1.17), 4.59 (2.21), and 1.70 (0.98) mg, P < 0.005] (Fig. 5A) and men [mean ± sd: 4.133 (3.01), 8.30 (4.42), and 5.91 (4.64) mg, P < 0.01] (Fig. 5B). LMR also increased after indomethacin administration at 2 to 24 h [mean ±sd: 0.017 (0.01), 0.038 (0.016), and 0.021 (0.012) mg, P < 0.0001] (Supplemental Fig. 1B). One or 5 d indomethacin administration had similar effect of increased 2 to 24 h lactulose excretion [visit after indomethacin administration, mean ± sd with longer duration 6.74 (4.42) vs. shorter duration: 5.43 (2.65) mg, P = 0.70] (Supplemental Fig. 2B).
Figure 5.
Effect of indomethacin on distal small intestinal/colonic permeability and fecal microbiome. Lactulose excretion (2–24 h) was significantly higher after indomethacin administration (visit 2) compared to baseline (visit 1) and returned to baseline at recovery (visit 3). A) Women. B) Men. Data are presented as paired comparisons. Means ± sd; n = 10–11/group. C). After indomethacin administration, healthy women have evidence of decreased species richness and overall diversity compared to baseline. Observed OTU decreased in women after indomethacin administration (P = 0.012), but not in men. Chao1 estimator decreased in women after indomethacin administration (P = 0.041), but not in men. Shannon index decreased in women after indomethacin administration (P = 0.019), but not in men.
Fecal microbiome
After administration of indomethacin, there were no significant differences in α or β diversity in the overall group. On subgroup analysis, women had evidence of decrease in species richness [observed OTU number (P = 0.012) and Chao1 estimator (P = 0.041), paired Student’s t test] and overall diversity [Shannon index (P = 0.019), paired Student’s t test] compared to the baseline visit (Fig. 5C). Additionally, β-diversity analysis revealed an overall compositional difference in women after indomethacin administration compared to the baseline visit [UniFrac (P = 0.01), PERMANOVA]. However, men did not show these changes.
Healthy women showed increase in fecal Prevotella abundance
Fecal microbiome
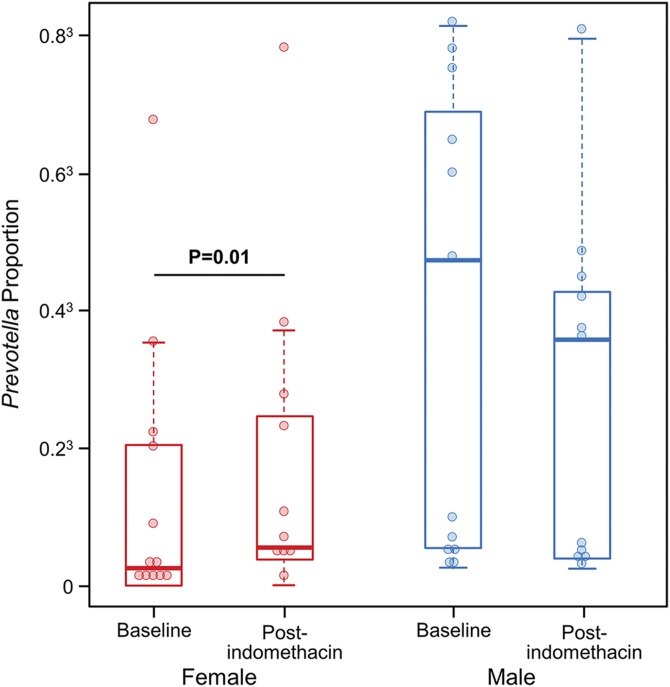
Overall, no taxa were differentially represented after indomethacin administration compared to baseline (q < 0.05). However, the difference in abundance between pre- and postindomethacin administration of Bacteroidetes; Prevotellaceae (q = 3.47E−5), Bacteroidetes; Prevotella (q = 4.79E−5) and Firmicutes; Ruminococcus (q = 4.79E−5) depended on sex. There was an increase in abundance of Bacteroidetes; Prevotellaceae and Bacteroidetes; Prevotella after indomethacin administration in women, while men did not show a significant change; conversely, for Firmicutes; Ruminococcus, an increase was seen in men and a decrease in women. At the species level, the OTU10 of Prevotella, which had 99% sequence homology with Prevotella copri, increased in abundance in women (baseline: 0.027, after indomethacin administration: 0.04, P = 0.01), whereas it remained unchanged in men (baseline: 0.1, after indomethacin administration: 0.07, P = 0.4) (Fig. 6). The α and β microbiota diversity 4 to 6 wk after discontinuation of indomethacin was similar to baseline (data not shown).
Figure 6.
Effect of indomethacin on fecal Prevotella abundance. Prevotella abundance increases after indomethacin administration in women but not men.
Duodenal microbiome
Differential abundance analysis in duodenal aspirates showed decrease in Proteobacteria; Alphaproteobacteria (q = 0.018), Proteobacteria; Rhizobiales (q = 0.024), and Proteobacteria; Pseudomonadaceae (q = 0.047) after indomethacin administration in men. Although changes in abundance of Proteobacteria; Alphaproteobacteria did not reach statistical significance at an FDR of 5% in women, the same trend of decreased abundance was observed (P = 0.016, q = 0.133).
DISCUSSION
This study makes several important observations about intestinal barrier function and microbiota in healthy humans with an emphasis on sex-based differences at baseline and in response to perturbation using NSAIDs. First, in vivo small intestinal and colonic permeability is lower in healthy women than healthy men. Women also had higher in vivo duodenal impedance and ex vivo duodenal TMR. These findings suggest the presence of a stronger intestinal barrier in healthy women. Second, healthy women have greater fecal and duodenal microbial diversity compared to healthy men. Third, the NSAID indomethacin increases both duodenal and colonic permeability, which returns to baseline 4 to 6 wk after discontinuation of indomethacin, thus suggesting resilience in intestinal barrier function. Fourth, indomethacin administration is associated with decrease in microbial diversity in duodenal and fecal contents, especially in women, which recovers 4 to 6 wk later.
A recent study found higher percentage recovery of 0 to 5 h lactulose and mannitol in boys than in girls (3–15 mo old) who are at risk for environmental enteropathy in developing countries (29). Another study based on gas chromatography showed no effect of sex on the measurement of intestinal permeability using lactulose and mannitol excretion (30). A number of animal studies have reflected sex difference in intestinal barrier function. Estrogen receptor knockout mice displayed disruptions in tight junctions and desmosome (31). Macromolecular flux was found to be higher in male Sprague Dawley rat ileum than female (32). Colonic permeability increased in ovariectomized rats, and estradiol decreased colonic permeability by up-regulating occludin and junctional adhesion molecule A in epithelial cells (33). Oral treatment with a soy germ–fermented ingredient rich in phytoestrogen reduced stress-induced intestinal permeability and visceral hypersensitivity in female rats (34). Estrogen has also been found to increase production and viscosity of mucin, which can also reinforce intestinal barrier (35). Our data convincingly suggest lower intestinal permeability or a stronger mucosal barrier in women, which is in concordance with work in animal models. The putative mechanisms for sex-based differences in barrier include a protective role of estrogens in maintenance of barrier function in the female sex, either by direct effects on tight junctions or mucus, or through neuroimmune interactions, which can also affect the intestinal barrier in a sex-dependent manner (36, 37).
We also observed that healthy women have greater microbial diversity than healthy men. Decreased microbial diversity has been described in conditions with increased intestinal permeability, like irritable bowel syndrome and inflammatory bowel disease (38, 39). Hence, it is plausible that a greater microbial diversity is another possible mechanism for a stronger intestinal barrier in women. Our results showed greater abundance of Actinobacteria phylum but lower abundance of Bacteroidetes and Proteobacteria in women compared to men. The class Bacteroidia and order Bacteroidales both had lower abundance in women than men in our study. A study from Europe has shown greater abundance of genus Bacteroides in healthy females compared to healthy males (9). In another study, at the species level, Bacteroides caccae was higher in females, whereas Bacteroides plebeius and Coprococcus catus were higher in males (8). However, our study did not find genus- or species-level differences between female and male subjects. This study also found that females had higher abundance of genus Bilophila, whereas males had higher abundance of Veillonella and Methanobrevibacter genera (8). Duodenal microbiome composition of healthy female and male subjects has not been reported previously. Although we found changes in α diversity, likely as a result of the small sample size, we did not detect differences at FDR <0.05 in taxonomic representation. Future studies in a larger cohort of patients will have to replicate these findings, especially sex-based differences in the duodenal microbiome. The duodenal aspirates were collected 24 h after a single administration of lactulose, and it would be unlikely to observe major shifts in the duodenal microbiome. Moreover, because the procedure was exactly the same at both visits, we expect any variability due to lactulose to be similar at the two time points.
Acute administration of NSAIDs can increase intestinal permeability. Observations that germ-free mice are protected against NSAID-induced intestinal injury have demonstrated an important role of intestinal microbiota in this process (16, 17). Acute administration of indomethacin can induce intestinal injury and changes in small bowel and colonic microbiota (18, 40). These effects on the microbiota can also be seen upon chronic administration (20). Outside of direct and local effects on the epithelium, the microbiota can influence NSAID-induced intestinal injury through changes in bile acid milieu (41), drug metabolism, and enterohepatic circulation (42, 43). In general, NSAID enteropathy is characterized by increased abundance of gram-negative bacteria (40, 44–46), plausibly inducing injury through bacterial LPS in a Toll-like receptor 4-dependent manner (47). Antibiotics against gram-negative flora have been shown to be effective against prevention of NSAID enteropathy both in animal models (16, 40, 48, 49) and in humans (50–52). Conversely, vancomycin, an agent that targets gram-positive bacteria, was associated with relative increase in abundance of gram-negative bacteria and worsening of NSAID-induced intestinal injury (19). Additionally, Bifidobacterium (44, 45)- and Lactobacillus (53–55)-based probiotics have been found to be protective against NSAID enteropathy.
Our study showed that women demonstrated a decrease in microbiota diversity and an associated increase in fecal Bacteroidetes; Prevotellaceae, a gram-negative family, and decrease in Firmicutes; Ruminococcus, a gram-positive genus, after indomethacin administration. At the species level, OTU10, which shares a 99% sequence homology with Prevotella copri, was significantly increased after indomethacin administration in women but remained unchanged in men. Women had lower abundance of Prevotella than men before indomethacin administration (baseline visit). This plausibly suggests that permeability increase after NSAIDs is microbiome dependent in women but not in men. Additionally, changes in the duodenal microbiota did not reveal any sex-specific differences, and in fact showed a decrease in Proteobacteria, a gram-negative phylum, providing observations contrary to findings from animal studies. The relative contribution of duodenal and colonic microbiota in the pathophysiology of NSAID enteropathy needs to be further clarified. Interestingly, although the use of proton pump inhibitors has helped reduce upper gastrointestinal complications from NSAID use, the incidence of lower gastrointestinal bleeding increased, suggesting a compounding role of dysbiosis induced by proton pump inhibitors in NSAID enteropathy (56). A limitation of the study is that microbiota changes are associations with NSAID administration rather than clarifying the contribution of small bowel and colon microbiota on NSAID enteropathy, which can be hard to study in humans. Additionally, the sample size is relatively small. Regardless, our findings provide insight into shifts in microbiota composition after acute administration of NSAIDs in an otherwise healthy population, as well as insight into the association of microbiota changes with changes in intestinal permeability.
The measurement of intestinal permeability has been performed using various saccharides and methods for quantitative detection in the urine. A significant variation in permeability measurement was observed over repeated measurements in a group of patients with celiac disease treated with placebo (57). This raised the question of temporal stability and resilience of intestinal permeability measurement that had not been examined previously. A longitudinal cohort study of African adults showed significant longitudinal and seasonal variability in villous height, xylose excretion, and LMR; however, a significant proportion of these were HIV positive and had undiagnosed intestinal infections (58). To study this, we used a rigorous protocol of measuring in vivo intestinal barrier function longitudinally and assessing response to perturbation using NSAID challenge and subsequent recovery. This demonstrated that the measurements made by saccharide excretion are temporally stable in healthy individuals, considering their complete recovery to baseline values 4 to 6 wk after acute perturbation. Changes in permeability over the course of stable or fluctuating disease activity in nonhealth states (celiac disease, inflammatory bowel disease, irritable bowel syndrome) remain to be further studied.
CONCLUSIONS
There is a significant difference in small intestinal and colonic permeability and mucosal barrier function between women and men. The intestinal barrier function is sensitive to perturbation with indomethacin but recovers after discontinuation of indomethacin. Finally, gut microbiota composition and diversity among women and men is also different at baseline and is more affected by NSAIDs in women. Future studies will need to confirm these findings in a larger cohort and examine whether specific microbial signatures can predict vulnerability to NSAID-induced intestinal injury. However, sex-based differences in baseline intestinal permeability and microbiome should be considered in future studies.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank L. Anderson (Mayo Clinic) for administrative assistance. This project was funded by grants from the Mayo Clinic Department of Laboratory Medicine and Pathology, Mayo Clinic Division of Gastroenterology and Hepatology, and by the U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant K23 DK103911 (to M.G.). J.C. is supported by Mayo Clinic Center for Individualized Medicine. M.G. has served on the advisory board or received research support from Takeda, DongA, Ironwood, and Napo. The remaining authors declare no conflicts of interest.
Glossary
- FDR
false discovery rate
- HADS
Hospital Anxiety and Depression Scale
- LMR
lactulose/mannitol excretion ratio
- NSAID
nonsteroidal antiinflammatory drug
- OTU
operational taxonomic unit
- PERMANOVA
permutational multivariate analysis of variance
- TMR
transmucosal resistance
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. Edogawa, S. A. Peters, W. J. Sundt, R. J. Singh, and M. Grover implemented the study concept and design; S. Edogawa, S. A. Peters, G. D. Jenkins, S. V. Gurunathan, S. Johnson, R. B. Dyer, J. Chen, and M. Grover acquired the data; S. Edogawa, S. A. Peters, G. D. Jenkins, S. Johnson, R. B. Dyer, M. Camilleri, P. C. Kashyap, G. Farrugia, J. Chen, R. J. Singh, and M. Grover analyzed the data; S. Edogawa, S. A. Peters, G. D. Jenkins, S. Johnson, R. B. Dyer, M. Camilleri, P. C. Kashyap, G. Farrugia, J. Chen, R. J. Singh, and M. Grover interpreted the data; S. Edogawa, S. A. Peters, G. D. Jenkins, R. J. Lennon, J. Chen, and M. Grover performed statistical analysis; S. Edogawa, S. A. Peters, S. V. Gurunathan, and M. Grover drafted the report; G. D. Jenkins, W. J. Sundt, S. Johnson, R. J. Lennon, R. B. Dyer, M. Camilleri, P. C. Kashyap, G. Farrugia, J. Chen, R. J. Sing, and M. Grover critically revised the manuscript; and M. Grover supervised the study.
REFERENCES
- 1.France M. M., Turner J. R. (2017) The mucosal barrier at a glance. J. Cell Sci. 130, 307–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters S. A., Edogawa S., Sundt W. J., Dyer R. B., Dalenberg D. A., Mazzone A., Singh R. J., Moses N., Smyrk T. C., Weber C., Linden D. R., MacNaughton W. K., Turner J. R., Camilleri M., Katzka D. A., Farrugia G., Grover M. (2017) Constipation-predominant irritable bowel syndrome females have normal colonic barrier and secretory function. Am. J. Gastroenterol. 112, 913–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camilleri M., Madsen K., Spiller R., Greenwood-Van Meerveld B., Verne G. N. (2012) Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 24, 503–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjarnason I., MacPherson A., Hollander D. (1995) Intestinal permeability: an overview. Gastroenterology 108, 1566–1581 [DOI] [PubMed] [Google Scholar]
- 5.Hernández-Díaz S., Rodríguez L. A. (2000) Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation: an overview of epidemiologic studies published in the 1990s. Arch. Intern. Med. 160, 2093–2099 [DOI] [PubMed] [Google Scholar]
- 6.Cremonini F., Talley N. J. (2005) Irritable bowel syndrome: epidemiology, natural history, health care seeking and emerging risk factors. Gastroenterol. Clin. North Am. 34, 189–204 [DOI] [PubMed] [Google Scholar]
- 7.Farhadi A., Keshavarzian A., Kwasny M. J., Shaikh M., Fogg L., Lau C., Fields J. Z., Forsyth C. B. (2010) Effects of aspirin on gastroduodenal permeability in alcoholics and controls. Alcohol 44, 447–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haro C., Rangel-Zúñiga O. A., Alcalá-Díaz J. F., Gómez-Delgado F., Pérez-Martínez P., Delgado-Lista J., Quintana-Navarro G. M., Landa B. B., Navas-Cortés J. A., Tena-Sempere M., Clemente J. C., López-Miranda J., Pérez-Jiménez F., Camargo A. (2016) Intestinal microbiota is influenced by gender and body mass index. PLoS One 11, e0154090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mueller S., Saunier K., Hanisch C., Norin E., Alm L., Midtvedt T., Cresci A., Silvi S., Orpianesi C., Verdenelli M. C., Clavel T., Koebnick C., Zunft H. J., Doré J., Blaut M. (2006) Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl. Environ. Microbiol. 72, 1027–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein J. L., Eisen G. M., Lewis B., Gralnek I. M., Zlotnick S., Fort J. G.; Investigators (2005) Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin. Gastroenterol. Hepatol. 3, 133–141 [DOI] [PubMed] [Google Scholar]
- 11.Maiden L., Thjodleifsson B., Theodors A., Gonzalez J., Bjarnason I. (2005) A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology 128, 1172–1178 [DOI] [PubMed] [Google Scholar]
- 12.Sugimori S., Watanabe T., Tabuchi M., Kameda N., Machida H., Okazaki H., Tanigawa T., Yamagami H., Shiba M., Watanabe K., Tominaga K., Fujiwara Y., Oshitani N., Koike T., Higuchi K., Arakawa T. (2008) Evaluation of small bowel injury in patients with rheumatoid arthritis by capsule endoscopy: effects of anti–rheumatoid arthritis drugs. Digestion 78, 208–213 [DOI] [PubMed] [Google Scholar]
- 13.Edogawa S., Takeuchi T., Kojima Y., Ota K., Harada S., Kuramoto T., Narabayashi K., Inoue T., Higuchi K. (2015) Current topics of strategy of NSAID-induced small intestinal lesions. Digestion 92, 99–107 [DOI] [PubMed] [Google Scholar]
- 14.Sigthorsson G., Tibble J., Hayllar J., Menzies I., Macpherson A., Moots R., Scott D., Gumpel M. J., Bjarnason I. (1998) Intestinal permeability and inflammation in patients on NSAIDs. Gut 43, 506–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjarnason I., Scarpignato C., Holmgren E., Olszewski M., Rainsford K. D., Lanas A. (2018) Mechanisms of damage to the gastrointestinal tract from nonsteroidal anti-inflammatory drugs. Gastroenterology 154, 500–514 [DOI] [PubMed] [Google Scholar]
- 16.Koga H., Aoyagi K., Matsumoto T., Iida M., Fujishima M. (1999) Experimental enteropathy in athymic and euthymic rats: synergistic role of lipopolysaccharide and indomethacin. Am. J. Physiol. 276, G576–G582 [DOI] [PubMed] [Google Scholar]
- 17.Robert A., Asano T. (1977) Resistance of germfree rats to indomethacin-induced intestinal lesions. Prostaglandins 14, 333–341 [DOI] [PubMed] [Google Scholar]
- 18.Basivireddy J., Jacob M., Ramamoorthy P., Balasubramanian K. A. (2005) Alterations in the intestinal glycocalyx and bacterial flora in response to oral indomethacin. Int. J. Biochem. Cell Biol. 37, 2321–2332 [DOI] [PubMed] [Google Scholar]
- 19.Syer S. D., Blackler R. W., Martin R., de Palma G., Rossi L., Verdu E., Bercik P., Surette M. G., Aucouturier A., Langella P., Wallace J. L. (2015) NSAID enteropathy and bacteria: a complicated relationship. J. Gastroenterol. 50, 387–393 [DOI] [PubMed] [Google Scholar]
- 20.Liang X., Bittinger K., Li X., Abernethy D. R., Bushman F. D., FitzGerald G. A. (2015) Bidirectional interactions between indomethacin and the murine intestinal microbiota. eLife 4, e08973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers M. A. M., Aronoff D. M. (2016) The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin. Microbiol. Infect. 22, 178.e1–178.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bokulich N. A., Battaglia T., Aleman J. O., Walker J. M., Blaser M. J., Holt P. R. (2016) Celecoxib does not alter intestinal microbiome in a longitudinal diet-controlled study. Clin. Microbiol. Infect. 22, 464–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grover M., Camilleri M., Hines J., Burton D., Ryks M., Wadhwa A., Sundt W., Dyer R., Singh R. J. (2016) (13)C mannitol as a novel biomarker for measurement of intestinal permeability. Neurogastroenterol. Motil. 28, 1114–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao A. S., Camilleri M., Eckert D. J., Busciglio I., Burton D. D., Ryks M., Wong B. S., Lamsam J., Singh R., Zinsmeister A. R. (2011) Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome–diarrhea and controls. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G919–G928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baldwin E. A., Walther-Antonio M., MacLean A. M., Gohl D. M., Beckman K. B., Chen J., White B., Creedon D. J., Chia N. (2015) Persistent microbial dysbiosis in preterm premature rupture of membranes from onset until delivery. PeerJ 3, e1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeraldo P., Kalari K., Chen X., Bhavsar J., Mangalam A., White B., Nelson H., Kocher J. P., Chia N. (2014) IM-TORNADO: a tool for comparison of 16S reads from paired-end libraries. PLoS One 9, e114804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J., Bittinger K., Charlson E. S., Hoffmann C., Lewis J., Wu G. D., Collman R. G., Bushman F. D., Li H. (2012) Associating microbiome composition with environmental covariates using generalized UniFrac distances. Bioinformatics 28, 2106–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J., Chen L. (In press) GMPR: a novel normalization method for microbiome sequencing data. bioRxiv [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kosek M. N., Lee G. O., Guerrant R. L., Haque R., Kang G., Ahmed T., Bessong P., Ali A., Mduma E., Peñataro Yori P., Faubion W. A., Lima A. A. M., Paredes Olortegui M., Mason C., Babji S., Singh R., Qureshi S., Kosek P. S., Samie A., Pascal J., Shrestha S., McCormick B. J. J., Seidman J. C., Lang D. R., Zaidi A., Caulfield L. E., Gottlieb M.; MAL-ED Network (2017) Age and sex normalization of intestinal permeability measures for the improved assessment of enteropathy in infancy and early childhood: results from the MAL-ED study. J. Pediatr. Gastroenterol. Nutr. 65, 31–39 [DOI] [PubMed] [Google Scholar]
- 30.Shaikh M., Rajan K., Forsyth C. B., Voigt R. M., Keshavarzian A. (2015) Simultaneous gas-chromatographic urinary measurement of sugar probes to assess intestinal permeability: use of time course analysis to optimize its use to assess regional gut permeability. Clin. Chim. Acta 442, 24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wada-Hiraike O., Imamov O., Hiraike H., Hultenby K., Schwend T., Omoto Y., Warner M., Gustafsson J. A. (2006) Role of estrogen receptor beta in colonic epithelium. Proc. Natl. Acad. Sci. USA 103, 2959–2964Erratum in: Proc. Natl. Acad. Sci. USA (2006) 103, 8299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Homma H., Hoy E., Xu D. Z., Lu Q., Feinman R., Deitch E. A. (2005) The female intestine is more resistant than the male intestine to gut injury and inflammation when subjected to conditions associated with shock states. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G466–G472 [DOI] [PubMed] [Google Scholar]
- 33.Braniste V., Leveque M., Buisson-Brenac C., Bueno L., Fioramonti J., Houdeau E. (2009) Oestradiol decreases colonic permeability through oestrogen receptor beta–mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J. Physiol. 587, 3317–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moussa L., Bézirard V., Salvador-Cartier C., Bacquié V., Houdeau E., Théodorou V. (2013) A new soy germ fermented ingredient displays estrogenic and protease inhibitor activities able to prevent irritable bowel syndrome–like symptoms in stressed female rats. Clin. Nutr. 32, 51–58 [DOI] [PubMed] [Google Scholar]
- 35.Diebel M. E., Diebel L. N., Manke C. W., Liberati D. M. (2015) Estrogen modulates intestinal mucus physiochemical properties and protects against oxidant injury. J. Trauma Acute Care Surg. 78, 94–99 [DOI] [PubMed] [Google Scholar]
- 36.Klein S. L., Flanagan K. L. (2016) Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638 [DOI] [PubMed] [Google Scholar]
- 37.Pigrau M., Rodiño-Janeiro B. K., Casado-Bedmar M., Lobo B., Vicario M., Santos J., Alonso-Cotoner C. (2016) The joint power of sex and stress to modulate brain–gut–microbiota axis and intestinal barrier homeostasis: implications for irritable bowel syndrome. Neurogastroenterol. Motil. 28, 463–486 [DOI] [PubMed] [Google Scholar]
- 38.Simrén M., Barbara G., Flint H. J., Spiegel B. M., Spiller R. C., Vanner S., Verdu E. F., Whorwell P. J., Zoetendal E. G.; Rome Foundation Committee (2013) Intestinal microbiota in functional bowel disorders: a Rome Foundation report. Gut 62, 159–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ni J., Wu G. D., Albenberg L., Tomov V. T. (2017) Gut microbiota and IBD: causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 14, 573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kent T. H., Cardelli R. M., Stamler F. W. (1969) Small intestinal ulcers and intestinal flora in rats given indomethacin. Am. J. Pathol. 54, 237–249 [PMC free article] [PubMed] [Google Scholar]
- 41.Blackler R. W., Motta J. P., Manko A., Workentine M., Bercik P., Surette M. G., Wallace J. L. (2015) Hydrogen sulphide protects against NSAID-enteropathy through modulation of bile and the microbiota. Br. J. Pharmacol. 172, 992–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reuter B. K., Davies N. M., Wallace J. L. (1997) Nonsteroidal anti-inflammatory drug enteropathy in rats: role of permeability, bacteria, and enterohepatic circulation. Gastroenterology 112, 109–117 [DOI] [PubMed] [Google Scholar]
- 43.LoGuidice A., Wallace B. D., Bendel L., Redinbo M. R., Boelsterli U. A. (2012) Pharmacologic targeting of bacterial β-glucuronidase alleviates nonsteroidal anti-inflammatory drug–induced enteropathy in mice. J. Pharmacol. Exp. Ther. 341, 447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallace J. L., Syer S., Denou E., de Palma G., Vong L., McKnight W., Jury J., Bolla M., Bercik P., Collins S. M., Verdu E., Ongini E. (2011) Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology 141, 1314–1322, 1322.e1–1322.e5 [DOI] [PubMed] [Google Scholar]
- 45.Kinouchi T., Kataoka K., Bing S. R., Nakayama H., Uejima M., Shimono K., Kuwahara T., Akimoto S., Hiraoka I., Ohnishi Y. (1998) Culture supernatants of Lactobacillus acidophilus and Bifidobacterium adolescentis repress ileal ulcer formation in rats treated with a nonsteroidal antiinflammatory drug by suppressing unbalanced growth of aerobic bacteria and lipid peroxidation. Microbiol. Immunol. 42, 347–355 [DOI] [PubMed] [Google Scholar]
- 46.Hagiwara M., Kataoka K., Arimochi H., Kuwahara T., Ohnishi Y. (2004) Role of unbalanced growth of gram-negative bacteria in ileal ulcer formation in rats treated with a nonsteroidal anti-inflammatory drug. J. Med. Invest. 51, 43–51 [DOI] [PubMed] [Google Scholar]
- 47.Watanabe T., Higuchi K., Kobata A., Nishio H., Tanigawa T., Shiba M., Tominaga K., Fujiwara Y., Oshitani N., Asahara T., Nomoto K., Takeuchi K., Arakawa T. (2008) Non-steroidal anti-inflammatory drug–induced small intestinal damage is Toll-like receptor 4 dependent. Gut 57, 181–187 [DOI] [PubMed] [Google Scholar]
- 48.Leite A. Z., Sipahi A. M., Damião A. O., Coelho A. M., Garcez A. T., Machado M. C., Buchpiguel C. A., Lopasso F. P., Lordello M. L., Agostinho C. L., Laudanna A. A. (2001) Protective effect of metronidazole on uncoupling mitochondrial oxidative phosphorylation induced by NSAID: a new mechanism. Gut 48, 163–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uejima M., Kinouchi T., Kataoka K., Hiraoka I., Ohnishi Y. (1996) Role of intestinal bacteria in ileal ulcer formation in rats treated with a nonsteroidal antiinflammatory drug. Microbiol. Immunol. 40, 553–560 [DOI] [PubMed] [Google Scholar]
- 50.Scarpignato C., Dolak W., Lanas A., Matzneller P., Renzulli C., Grimaldi M., Zeitlinger M., Bjarnason I. (2017) Rifaximin reduces the number and severity of intestinal lesions associated with use of nonsteroidal anti-inflammatory drugs in humans. Gastroenterology 152, 980–982.e3 [DOI] [PubMed] [Google Scholar]
- 51.Bjarnason I., Hayllar J., Smethurst P., Price A., Gumpel M. J. (1992) Metronidazole reduces intestinal inflammation and blood loss in non-steroidal anti-inflammatory drug induced enteropathy. Gut 33, 1204–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lanas A., Scarpignato C. (2006) Microbial flora in NSAID-induced intestinal damage: a role for antibiotics? Digestion 73(Suppl 1), 136–150 [DOI] [PubMed] [Google Scholar]
- 53.Watanabe T., Nishio H., Tanigawa T., Yamagami H., Okazaki H., Watanabe K., Tominaga K., Fujiwara Y., Oshitani N., Asahara T., Nomoto K., Higuchi K., Takeuchi K., Arakawa T. (2009) Probiotic Lactobacillus casei strain Shirota prevents indomethacin-induced small intestinal injury: involvement of lactic acid. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G506–G513 [DOI] [PubMed] [Google Scholar]
- 54.Endo H., Higurashi T., Hosono K., Sakai E., Sekino Y., Iida H., Sakamoto Y., Koide T., Takahashi H., Yoneda M., Tokoro C., Inamori M., Abe Y., Nakajima A. (2011) Efficacy of Lactobacillus casei treatment on small bowel injury in chronic low-dose aspirin users: a pilot randomized controlled study. J. Gastroenterol. 46, 894–905 [DOI] [PubMed] [Google Scholar]
- 55.Montalto M., Gallo A., Curigliano V., D’Onofrio F., Santoro L., Covino M., Dalvai S., Gasbarrini A., Gasbarrini G. (2010) Clinical trial: the effects of a probiotic mixture on non-steroidal anti-inflammatory drug enteropathy—a randomized, double-blind, cross-over, placebo-controlled study. Aliment. Pharmacol. Ther. 32, 209–214 [DOI] [PubMed] [Google Scholar]
- 56.Lué A., Lanas A. (2016) Protons pump inhibitor treatment and lower gastrointestinal bleeding: balancing risks and benefits. World J. Gastroenterol. 22, 10477–10481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leffler D. A., Kelly C. P., Abdallah H. Z., Colatrella A. M., Harris L. A., Leon F., Arterburn L. A., Paterson B. M., Lan Z. H., Murray J. A. (2012) A randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge. Am. J. Gastroenterol. 107, 1554–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelly P., Menzies I., Crane R., Zulu I., Nickols C., Feakins R., Mwansa J., Mudenda V., Katubulushi M., Greenwald S., Farthing M. (2004) Responses of small intestinal architecture and function over time to environmental factors in a tropical population. Am. J. Trop. Med. Hyg. 70, 412–419 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.