Abstract
Macrophage infiltration is common to both emphysema and atherosclerosis, and cigarette smoke down-regulates the macrophage cholesterol efflux transporter ATP binding cassette (ABC)A1. This decreased cholesterol efflux results in lipid-laden macrophages. We hypothesize that cigarette smoke adversely affects cholesterol transport via an ABCA1-dependent mechanism in macrophages, enhancing TLR4/myeloid differentiation primary response gene 88 (Myd88) signaling and resulting in matrix metalloproteinase (MMP) up-regulation and exacerbation of pulmonary inflammation. ABCA1 is significantly down-regulated in the lung upon smoke exposure conditions. Macrophages exposed to cigarette smoke in vivo and in vitro exhibit impaired cholesterol efflux correlating with significantly decreased ABCA1 expression, up-regulation of the TLR4/Myd88 pathway, and downstream MMP-9 and MMP-13 expression. Treatment with liver X receptor (LXR) agonist restores ABCA1 expression after short-term smoke exposure and attenuates the inflammatory response; after long-term smoke exposure, there is also attenuated physiologic and morphologic changes of emphysema. In vitro, treatment with LXR agonist decreases macrophage inflammatory activation in wild-type but not ABCA1 knockout mice, suggesting an ABCA1-dependent mechanism of action. These studies demonstrate an important association between cigarette smoke exposure and cholesterol-mediated pathways in the macrophage inflammatory response. Modulation of these pathways through manipulation of ABCA1 activity effectively blocks cigarette smoke–induced inflammation and provides a potential novel therapeutic approach for the treatment of chronic obstructive pulmonary disease.—Sonett, J., Goldklang, M., Sklepkiewicz, P., Gerber, A., Trischler, J., Zelonina, T., Westerterp, M., Lemaître, V., Okada, V., D’Armiento, J. A critical role for ABC transporters in persistent lung inflammation in the development of emphysema after smoke exposure.
Keywords: COPD, LXR agonist, lipid transporters, cholesterol efflux, macrophage
In emphysema and atherosclerosis, macrophage and lymphocyte infiltrate of the airway and vessel wall, respectively, is a hallmark histopathologic finding (1–3). Smokers display a higher susceptibility and increased severity of cardiovascular disease (4–6), and atherosclerosis is more prominent in smokers with airway obstruction than in smokers with normal lung function (7), potentially linking atherosclerosis and chronic obstructive pulmonary disease (COPD). COPD is the third leading cause of death in the United States and is characterized by inflammation, airflow obstruction, and lung destruction (8). The evolution of lung destruction from emphysema can be explained by a protease/antiprotease imbalance caused by cigarette smoke exposure, which promotes repeated proteolytic injury to the extracellular matrix (9–12), of which the macrophage is a major effector cell. However, the mechanism by which inflammation is maintained within the lungs of patients with emphysema has not been fully elucidated.
Extensive studies have demonstrated that atherosclerotic lesions exhibit increased numbers of lipid-laden macrophages (13), and similar foamy alveolar macrophages accumulate in the lungs of cigarette smoke–exposed mice (14, 15). Primary and second-hand smoke influence plasma lipid concentrations (16–18), significantly decreasing HDL levels and increasing levels of oxidized LDL cholesterol (19), which can damage the vessel endothelium. However, despite the damaging effects of these lipids in the vascular wall, the consequences of systemic lipid changes have not been fully examined within lung tissue. Lowering LDL cholesterol has little or no effect on pulmonary function; on the other hand, HDL suppresses inflammation and could have a protective effect on the lung during smoke exposure (20). HDL binds to bacterial toxins and diminishes inflammation; therefore, in conditions of smoke exposure, decreased HDL could contribute to more inflammation within the lung (21, 22). The major antiatherogenic property of HDL is its ability to stimulate the release of cholesterol from activated cholesterol–filled macrophages (cholesterol efflux), diminishing the inflammatory response (23). Two ATP-binding cassette transporters (ABCs), ABCA1 and ABCG1, control this process of cholesterol efflux, stimulating reverse cholesterol transport (24–27).
Our laboratory observed that apolipoprotein (Apo)E knockout (KO) mice fed a Western-type diet develop severe systemic hypercholesterolemia accompanied by abnormal cholesterol efflux, inducing pulmonary inflammation through a proinflammatory cascade involving TLR4 and matrix metalloproteinases (MMPs), resulting in emphysema formation in these mice (28). ApoE induces cholesterol efflux in macrophages via the ABCA1 and ABCG1 cell surface transporters, initiating the formation of HDL particles; deficiency of ABCA1 and ABCG1 results in a significant decrease in macrophage cholesterol efflux (27). The subsequent accumulation of lipids in macrophages induces an inflammatory response characterized by the secretion of cytokines and proteases (29). To investigate the potential role of cholesterol efflux in emphysema development, the present study was undertaken to examine the direct effect of cigarette smoke on the cholesterol efflux pathway, specifically focusing on the expression of ABCA1 and ABCG1 in the lung after smoke exposure. After identifying an important role for ABCA1 and ABCG1 on macrophage function after smoke exposure, the following experiments revealed a protective role for liver X receptor (LXR) agonists in emphysema with the up-regulation of ABC transporters and attenuation of MMP expression after cigarette smoke exposure. These studies reveal a mechanistic link between cigarette smoke and the development of lung destruction and atherosclerosis.
MATERIALS AND METHODS
Human studies
Our laboratory has stored deidentified human patient lung samples used to study the expression of ABC transporters (ABCA1 and ABCG1). The subjects were classified into 2 categories: 1) normal, obtained from healthy humans, and 2) deidentified COPD, obtained from patients in whom emphysema was clinically confirmed and who had been abstinent from smoking for at least 4 mo. The mRNA was isolated from these samples and used for expression studies.
Cigarette smoke extract preparation
To prepare cigarette smoke extract (CSE), the smoke from one cigarette (Reference Cigarette 3R4F; University of Kentucky, Lexington, KY, USA) (1.1 mg of nicotine, 15 mg of tar) was passed through 25 ml PBS. The pH of the extracted solution was adjusted to 7.4, and the solution was filtered (30). CSE was added to the medium at concentrations up to 5% (v/v).
Macrophage cell culture
Experiments used human monocyte–derived macrophages, murine thioglycolate–elicited peritoneal macrophages, murine alveolar macrophages, and mouse bone marrow–derived macrophages (BMDMs). Human peripheral blood monocytes (THP-1) cells (TIB-202; American Type Culture Collection, Manassas, VA, USA) were differentiated into macrophages by treatment with Phorbol 12-myristate 13-acetate (MilliporeSigma, Burlington, MA, USA) for 72 h. Mouse peritoneal macrophages were isolated from peritoneal cavities of 3% thioglycolate-injected mice 5 d after injection, during which time mice were exposed to room air or smoke (Reference Cigarette 3R4F; University of Kentucky) exposure conditions. Mouse alveolar macrophages were isolated from bronchoalveolar lavage fluid of mice exposed to 5 d of room air or smoke (31). Mouse BMDMs were obtained from LysMCreAbca1flox/flox (ABCA1 KO) and Abca1flox/flox (ABCA1 WT control) mice that were bred in a C57BL/6J background. The obtained cells were then separated by plastic adhesion in Petri dishes containing DMEM and supplemented with 10% fetal bovine serum and 1% antibiotics. Nonadherent progenitor cells were collected after 2 h of incubation in culture medium and seeded at a concentration of 4 × 106 cells/ml in fresh culture medium supplemented with L-cell–conditioned medium containing M-CSF at a concentration of 20%. After ∼7 d, fully differentiated cells were ready for subsequent experiments. Macrophages were stimulated with 5% CSE for 24 h with or without 3 µM of LXR agonist T0901317 (Cayman Chemicals, Ann Arbor, MI, USA), N,N-dimethyl-3β-hydroxycholenamide (DHMCA; Avanti Polar Lipids, Alabaster, AL, USA), and GW3965 (Tocris Bioscience, Minneapolis, MN, USA).
Mouse smoke exposure studies
All animal studies were performed with the approval of the Institutional Animal Care and Use Committee of Columbia University. Mice were chronically exposed to cigarette smoke in a specially designed chamber (Teague Enterprise, Woodland, CA, USA) (32, 33). Eight-week-old mice were exposed to cigarette smoke for 5 h/d, 5 d/wk for 10 d (acute model) and 3 h/d, 5 d/wk for 5 mo (chronic model). The total particulate matter by gravimetric analysis within the smoking chamber was regulated to 100–150 mg/m3. The cigarette used was reference cigarette 3R4F (University of Kentucky). Examined mice included the C57BL/6J and AKR/J and macrophage-specific ABCA1 KO mice (in a C57BL/6J background).
LXR treatment
Eight-week-old AKR/J mice were used in the study and exposed to 3 different conditions: room air (n = 8), cigarette smoke (n = 8), and cigarette smoke with additional treatment with LXR agonist (n = 8). The LXR agonist was delivered in the diet, and the concentration was calculated based on the average mouse weight (30 g) and food consumption (6 g/d). Experimental food was prepared with a 0.015% LXR agonist in food (w/w) concentration corresponding to 30 mg/kg body weight. Both the control group and the custom drug diet were prepared by Research Diets, Inc. (control diet C11000: Purina Rodent Chow 5001; custom diet C13861: Purina Rodent Chow 5001 with 0.015% LXR agonist T0901317; New Brunswick, NJ, USA).
Determination of lung compliance
To determine the pulmonary compliance of the lung, a closed chest model was used (32). Respiratory mechanics were measured using a flexiVent (Scireq, Montreal, QC, Canada) system. Pentobarbital was used for sedation, and mice then underwent neck dissection and tracheostomy. Once on the ventilator, the mice were paralyzed with succinylcholine, and a full assessment of pulmonary mechanics in triplicate was performed using the FlexiVent system. After airway measurements, the animals were euthanized by CO2 inhalation, BAL was performed with sterile PBS, and lung tissue was obtained for later analysis.
Determination of lung inflammation
BAL fluid was collected by perfusing the lungs with 2 ml of PBS. The samples were centrifuged, the BAL supernatant was removed, and the cell pellet was resuspended in 300 μl PBS. Total cell count was obtained with a hemocytometer, and differential cell count was performed on hematoxylin and eosin–stained cytospins. Regular and foamy-like macrophages and lymphocytes were counted under the microscope. A foamy-like phenotype (15) was identified by examining the sequential increase in macrophage size and by quantification with Oil-Red-O staining.
Histology
Histologic evaluation of the inflammation was performed on the left lung, which was pressure perfused to 25 cmH2O and fixed using 10% formalin. Fixed tissue was stained with hematoxylin and eosin, and mean linear intercept was determined as previously described (32).
Real-time PCR
Total RNA was extracted from lung tissue using an RNeasy Mini Kit (Qiagen, Germantown, MD, USA). The RNA was further processed to obtain cDNA, which was used for real-time PCR analysis. TaqMan gene expression assays (Applied Biosystems, Carlsbad, CA, USA) were performed to assess gene transcript levels with the use of an ABI Prism 7900HT Sequence Detection System (Applera, Foster City, CA, USA). The following primers were used to check the respective gene expression: ABCA1 (Hs01059118_m1, Mm00442646_m1), ABCG1 (Hs00245154_m1, Mm00437390_m1), MMP-9 (Mm00442991_m1, Hs00957262_m1), MMP-12 (Mm00500554_m1), MMP-13 (Mm00439491_m1), IL-1β (Mm00434227_g1), TNF-α (Mm00443260_g1), TLR4 (Mm00445273_m1), MYD88 (Mm00440338_m1), and GAPDH (4352932E, 432617E) from TaqMan Gene Expression Assays (Applied Biosystems). β-Actin and GAPDH were used as housekeeping genes, and the results were analyzed using ΔΔCt.
Western blotting
The lungs of mice (∼10 mg) were homogenized in 1 ml of RIPA buffer. Equal protein amounts were loaded into each well. The Western blots were run by standard protocol, and the protein of interest was identified by chemiluminescence. ABCA1 (pAb anti-ABCA1 Antibody-NB400-105) and ABCG1 (pAb anti-ABCG1 Antibody-NB400-132) antibodies were from Novus Biologicals (Littleton, CO, USA). Phos-ERK (T980; Cell Signaling Technology, Danvers, MA, USA), ERK (9102; Cell Signaling Technology), phos-JNK (4668S; Cell Signaling Technology), JNK (sc7345; Santa Cruz Biotechnology, Dallas, TX, USA), IRAK 1 (4504; Cell Signaling Technology), and MyD88 (4283; Cell Signaling Technology) antibodies were used.
Cholesterol efflux assay
Mouse macrophages were placed in a 0.5 ml well of DMEM with fatty acid–free bovine serum albumin (0.2%) and antibiotics (penicillin/streptomycin) and loaded with 3H-cholesterol (1 μCi/ml) together with acetylated LDL (50 μg/ml) for 24 h (27). The macrophages were then washed twice with the medium and treated with the CSE for 2 h. Prior to adding the efflux medium, the cells were washed with PBS. The efflux media [containing serum (2.5%), ApoAI (25–50 μg/ml), and HDL (25 μg/ml)] and the respective smoke components were added. The medium was collected after 6 h of incubation, and the cells were lysed in 0.5 ml of NaOH (1 M). The efflux was calculated as follows: [cpm medium/(cpm medium + cpm lysate)] × 100.
Oil-red-O assay
BAL from room air control with or without LXR agonist and chronically smoke-exposed AKR/J mice with or without LXR agonist was extracted and plated on Lab-Tek II chamber slides (Thermo Fisher Scientific, Waltham, MA, USA) in DMEM. Cells were incubated for 4 h to allow for adhesion and then washed 3 times with sterile PBS followed by the addition of a 4% paraformaldehyde fixative solution and fixed for 20 min. The cells were then washed with deionized water, and 100% 1,2-propanediol dehydration solution was added to each of the wells for 5 min. This was repeated twice. Oil Red O stain solution (CM-0054; Lifeline Cell Technology, Walkersville, MD, USA) was added to each well and incubated for 30 min at 37°C. After 30 min, 85% 1,2-propanediol stain differential solution (CM-0057; lifeline tech) was added for 1 min. Cells were then washed with water and imaged on a Nikon Eclipse Ti (Nikon, Tokyo, Japan). Results were analyzed by taking a percentage of the stained cells over the total cell number of cells counted.
Statistical analysis
Statistical analysis of the data obtained was performed using the unpaired, 2-tailed Student’s t test when 2 groups were being compared. In the case of a comparison of ≥3 groups, 1-way ANOVA, followed by the Bonferroni post hoc test was performed using GraphPad Prism software (GraphPad Software, La Jolla, CA, USA).
RESULTS
Cigarette smoke blocks ABC transporter expression in macrophages, impairs cholesterol efflux, and correlates with up-regulation of inflammatory markers
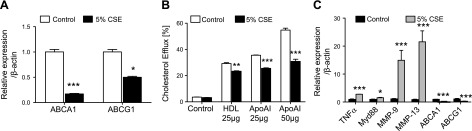
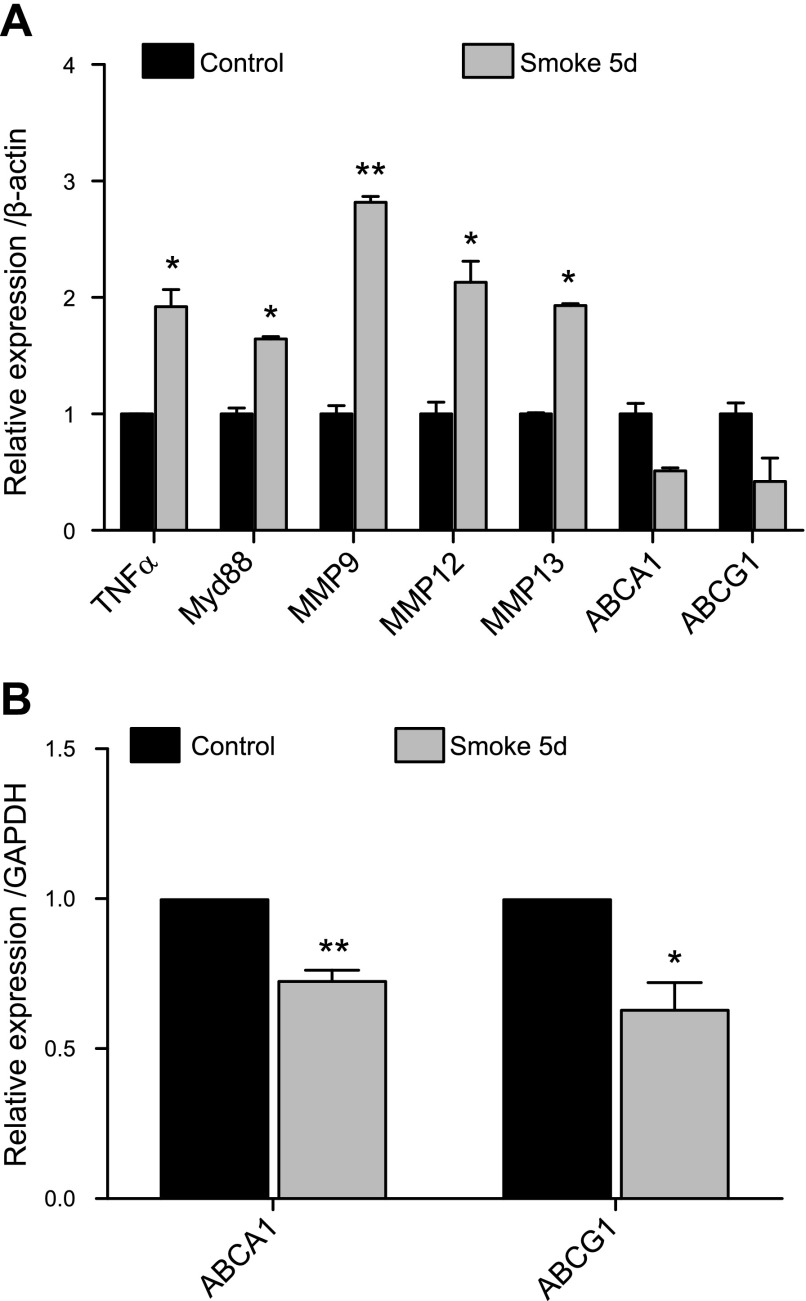
To determine the effect of cigarette smoke exposure on active cholesterol transport in macrophages, thioglycolate-elicited peritoneal macrophages were plated and exposed to CSE. Alternatively, after thioglycolate injection, mice were exposed to cigarette smoke in vivo. A reduction in the expression of the two main active cholesterol transporters (ABCA1 and ABCG1) was observed under smoke exposure conditions in vitro (Fig. 1A). These changes were accompanied by a significant impairment of the cholesterol efflux potential of these macrophages in vitro toward HDL and much more prominently toward ApoAI, which is the main acceptor of cholesterol effluxed by ABCA1 (Fig. 1B). The down-regulation of ABC transporters in macrophages correlated with the increased expression of genes consistently dysregulated under smoke exposure conditions. Genes known to modulate inflammation, such as TNF-α and myeloid differentiation primary response gene 88 (Myd88), were up-regulated, and genes encoding for destructive matrix metalloproteases (MMP-9, MMP-12, and MMP-13), which are critical in emphysema, were increased in macrophages under smoke exposure conditions when ABCA1 and ABCG1 were down-regulated in vitro (Fig. 1C). These findings were repeated with peritoneal macrophages (Fig. 2A) and alveolar macrophages (Fig. 2B) isolated after in vivo smoke exposure, with results demonstrating that cigarette smoke itself without lipid exposure modulates the efflux pathway, correlating with increased inflammation, suggesting that cholesterol transport is altered in cigarette smoke–related lung disease.
Figure 1.
ABC transporters, cholesterol efflux, and inflammatory markers are regulated by cigarette smoke in macrophages in vitro. A) mRNA expression level analysis by real-time PCR of ABCA1 and G1 24 h after 5% CSE treatment (n = 3). B) Cholesterol efflux toward ApoAI (25–50 μg) and HDL (25 μg) was measured in thioglycolate-elicited macrophages using tritiated cholesterol (n = 3). C) In vitro mRNA expression analysis of TNF-α, Myd88, MMPs, and ABCA1/G1 in macrophages (n = 3). β-Actin was used as a housekeeping gene control for real-time PCR. *P < 0.05, **P < 0.01, ***P < 0.001 when compared with controls (statistically significant).
Figure 2.
Cigarette smoke regulates ABC transporters in conjunction with inflammatory markers and MMPs in macrophages in vivo. A) In vivo mRNA expression analysis of TNF-α, Myd88, MMPs, and ABCA1/G1 from thioglycolate-elicited peritoneal macrophages isolated from mice exposed to smoke for 5 d as compared with mice exposed to room air (n = 3). B) In vivo mRNA expression profile of ABCA1 and ABCG1 from alveolar macrophages from mice exposed to smoke for 5 d as compared with mice exposed to room air (n = 3). β-Actin and GAPDH were used as a housekeeping gene control for real-time PCR. *P < 0.05, **P < 0.01, ***P < 0.001 when compared with controls (statistically significant).
Modulation of ABC transporters by LXR agonist blocks cigarette smoke–induced inflammation in macrophages
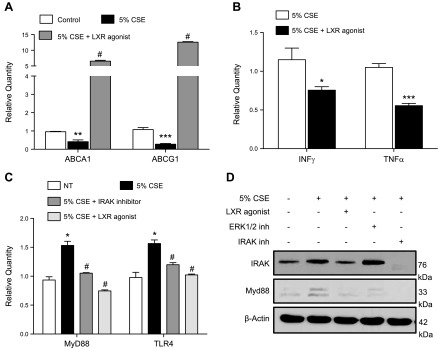
The LXR agonist T0901317 is a potent inducer of ABC transporters (34). We therefore tested the effect of T0901317 on macrophages treated with CSE. T0901317 rescued the expression of both ABC transporters under smoke exposure conditions (Fig. 3A) and reversed the inflammatory up-regulation of IFN-γ and TNF-α due to CSE (Fig. 3B). Activation of TLR4/MyD88, a critical signaling pathway induced by cigarette smoke that leads to increased inflammation and protease production (33, 35, 36), was also inhibited by T0901317 (Fig. 3C, D).
Figure 3.
Reestablishment of ABC transporter expression under CSE conditions by LXR agonist in macrophages. A, B) mRNA expression of macrophage in ABCA1 and ABCG1 (A) and IFN-γ and TNF-α after 24 h with 5% CSE with or without pretreatment with LXR agonist (T0901317) (3 μM) (B). C) mRNA expression of TLR4 and Myd88 in nontreated vs. CSE-treated peritoneal macrophages after pretreatment with LXR agonist (3 μM) and IRAK1/4 inhibitor (5 μM). D) Western blotting of IRAK1 and Myd88 in nontreated vs. CSE-treated peritoneal macrophages after pretreatment with LXR agonist (3 μM) or with ERK1/2 and IRAK1/4 inhibitors (5 μM; n = 3 for each group). Data are presented as fold regulation. *P < 0.05, **P < 0.01, ***P < 0.001 vs. controls, #P < 0.05 vs. 5% CSE-treated macrophages (statistically significant).
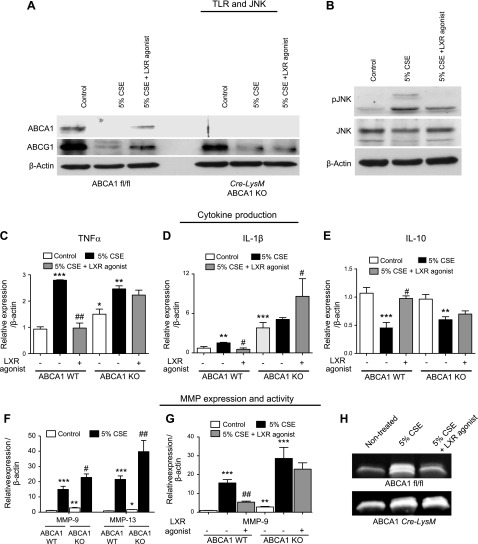
To further explore the relationship between smoke exposure and the regulation of ABC transporters, macrophages were isolated from mice lacking ABCA1 in myeloid cells (Cre-LysM ABCA1 KO). As expected based on previous work (37, 38), ABCA1 was not expressed in ABCA1 KO macrophages (Fig. 4A). Studies were performed to explore the effect of ABCA1 loss on the cigarette smoke–induced inflammatory and protease response in vitro. Initially, mouse bone marrow–derived macrophages were exposed to 5% CSE and treated with the LXR agonist. LXR agonism inhibited cigarette smoke–induced phosphorylation of JNK (Fig. 4B), which correlated with the up-regulation of Myd88 and TLR4 (Fig. 3C, D). These signaling molecules are known to be important in the inflammatory and proteolytic response to cigarette smoke (30, 33). Subsequently, ABCA1 WT and KO mouse macrophages were exposed to 5% CSE with and without LXR agonist treatment. Treatment of WT macrophages with 5% CSE induced the up-regulation of IL-1β and TNF-α and the down-regulation of IL-10; this response was reversed back to baseline with LXR agonist treatment (Fig. 4C–E). Importantly, the LXR agonist did not reverse the induction of cytokines by 5% CSE in the ABCA1 macrophage KO mice (Fig. 4C–E). These findings suggest that the ABCA1 transporter plays a significant anti-inflammatory role in the cigarette smoke–induced activation of macrophages. Most notably, the up-regulation of MMP-9 and MMP-13 by CSE was potentiated in the macrophages lacking ABCA1 (Fig. 4F). In addition, treatment with the LXR agonist reduced mRNA expression and gelatinase activity of MMP-9 only in the presence of the ABCA1 transporter (Fig. 4G, H)
Figure 4.
Effect of LXR-dependent ABCA1 re-expression on cigarette smoke–induced proinflammatory and MMP signaling pathways in macrophages. A) Protein analysis of ABCA1 and G1 transporters by Western blot in BMDMs of WT (ABCA1 fl/fl) mice and macrophage-specific ABCA1 KO (ABCA1 Cre-LysM) treated with 5% CSE with or without LXR agonist (T0901317) at 3 μM concentration. B) There is a reduction in smoke-induced JNK phosphorylation with LXR agonist treatment. C–E) mRNA expression analysis of inflammatory cytokines TNF-α (C), IL-1β (D), and IL-10 (E) in BMDMs from WT (ABCA1 fl/fl mice) and macrophage-specific ABCA1 KO treated with 5% CSE with or without LXR agonist (T0901317-Cayman) at 3 μM concentration. F–H) Analysis of MMP activation in BMDMs of WT (ABCA1 fl/fl mice) and macrophage-specific ABCA1 KO treated with 5% CSE with or without LXR agonist (T0901317-Cayman) at 3 μM concentration. F) MMP-9 and MMP-13 mRNA expression. G) MMP-9 mRNA expression with LXR agonist treatment in WT and ABCA1 KO macrophages. H) MMP-9 activity assayed by gelatin zymography (n = 3 for each group). β-Actin was used as a housekeeping gene control for real-time PCR. *P < 0.05, **P < 0.01, ***P < 0.001 when compared with controls, #P < 0.05 compared with 5%CSE (statistically significant).
Cigarette smoke–induced down-regulation of ABC transporters correlates with macrophage activation and an increased inflammatory response in vivo
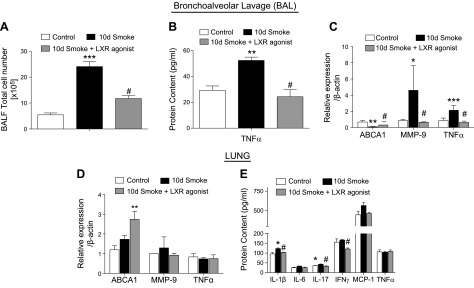
To determine whether the mechanisms identified in vitro contributed to changes in smoke-induced lung inflammation in vivo, cigarette smoke studies were performed in mice. The treatment of mice exposed to cigarette smoke in an acute 10-d model with 25 mg/kg of intraperitoneal injections of an LXR agonist (T0901317) daily during the last 4 d of exposure was examined. LXR agonist T0901317 is detected in the lungs after intraperitoneal administration (Supplemental Fig. S1). Treatment with the LXR agonist blocked the already established inflammation and decreased the total inflammatory cell count (Fig. 5A). Furthermore, cigarette smoke–induced TNF-α protein in the BAL was abrogated in mice treated with the LXR agonist (Fig. 5B). In addition, LXR agonist treatment decreased mRNA levels of TNF-α (Fig. 5C) while inducing ABCA1 expression in alveolar macrophages from BAL (Fig. 5C). Most importantly, cigarette smoke–induced MMP-9 expression was inhibited by treatment with the LXR agonist (Fig. 5C), which correlated with inhibition of inflammation and the inflammatory-driven increase in TNF-α. In whole lung homogenates, LXR agonist treatment of mice increased ABCA1 expression in the setting of acute cigarette smoke exposure (Fig. 5D) but did not alter levels of MMP-9 and TNF-α (Fig. 5E). LXR agonist treatment also inhibited the smoke-induced increase in IL-1β and IL-17 and reduced the overall expression of IFN-γ in lung tissue (Fig. 5E).
Figure 5.
Effect of LXR activation on cigarette smoke–induced acute pulmonary inflammation and MMP activation. Total inflammatory cell counts in BAL fluid (BALF) of mice exposed to cigarette smoke for 10 d with or without LXR agonist treatment vs. room air–exposed control mice (n = 4). A) Protein concentration of TNF-α. B) mRNA analysis of ABCA1, MMP-9, and TNF-α in BAL cells of mice exposed to cigarette smoke for 10 d with or without LXR agonist treatment vs. room air–exposed control mice. C) mRNA analysis of ABCA1, MMP-9, and TNF-α in the lungs of mice exposed to cigarette smoke for 10 d with or without LXR agonist treatment vs. room air–exposed control mice. D) Protein concentrations of IL-1β, IL-6, IL-17, IFN-γ, monocyte chemoattractant protein 1 MCP-1, and TNF-α measured by Luminex cytokines array system in the lungs of mice exposed to cigarette smoke for 10 d with or without LXR agonist treatment vs. room air–exposed control mice. E) β-Actin was used as a housekeeping gene control for real-time PCR. *P < 0.05, **P < 0.01, ***P < 0.001 when compared with controls, #P < 0.05 compared with 10 d of cigarette smoke exposure (significantly significant).
LXR agonism reverses cigarette smoke–induced inflammation and emphysema development in a model of chronic emphysema in mice
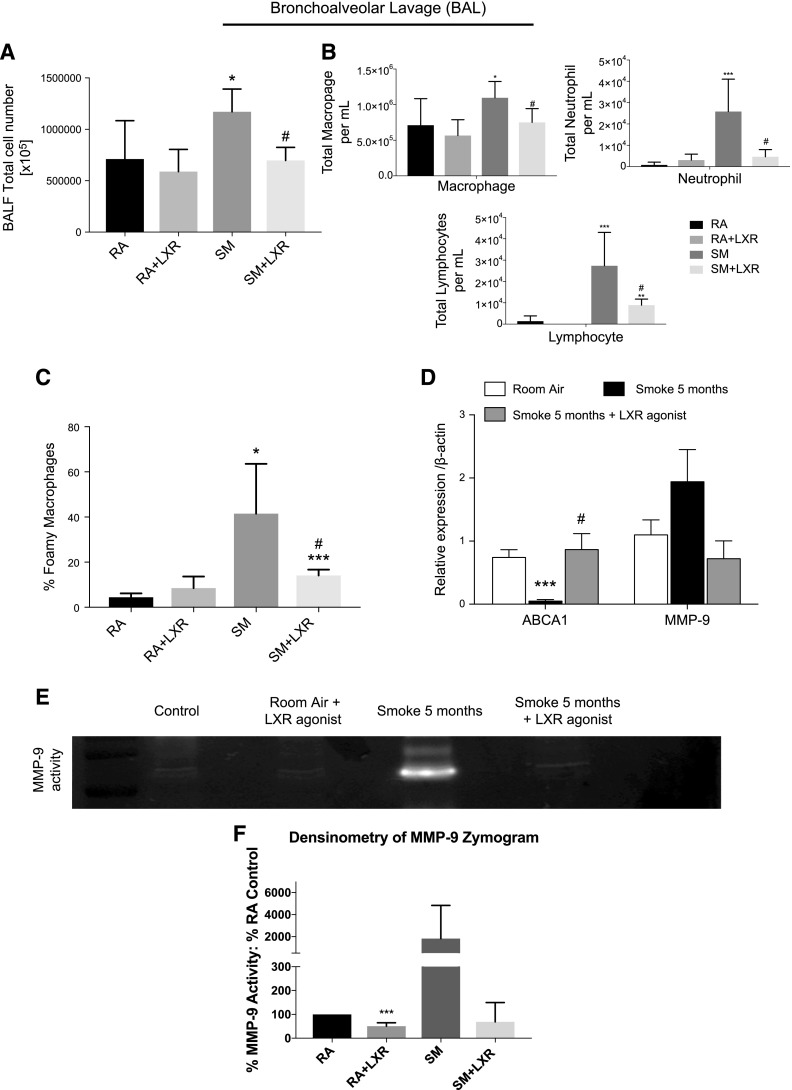
To determine whether the mechanisms identified above contribute to inflammation and tissue destruction observed in a chronic cigarette smoke exposure model, mice were exposed to cigarette smoke for 5 mo. Mice were treated with an LXR agonist in the diet (0.015% w/w; 30 mg/kg daily). LXR agonist was detected in the lungs (Supplemental Fig. 1B). Treatment of mice with the LXR agonist in the chronic 5 mo smoke exposure model resulted in decreased overall inflammatory cell counts (Fig. 6A, B). Furthermore, treatment with the LXR agonist significantly reduced the alveolar macrophage population that exhibited a foam cell–like phenotype detected by Oil-Red-O staining (Fig. 6C). Most importantly, LXR agonist treatment successfully increased the expression of ABCA1 in BAL macrophages (Fig. 6D). Furthermore, whereas mRNA levels of MMP-9 in the BAL cells of the chronic smoke–exposed mice were elevated upon smoke exposure but not statistically significantly lower upon LXR agonist treatment (Fig. 6D), the smoke-induced MMP-9 activity in BAL fluid was attenuated with the LXR agonist treatment (Fig. 6E, F).
Figure 6.
Effect of LXR activation on cigarette smoke–induced chronic pulmonary inflammation and MMP activation. A) Total inflammatory cell counts in BAL fluid (BALF) of mice exposed to cigarette smoke for 5 mo in AKR/J mice with or without LXR agonist oral treatment in the diet (0.015% w/w, ∼30 mg/kg) vs. room air–exposed control mice without LXR agonist oral treatment in the diet (0.015% w/w, ∼30 mg/kg) (n = 8). B) Cell differentiation counts in BALF of mice exposed to cigarette smoke for 5 mo in AKR/J mice with or without LXR agonist oral treatment in the diet (0.015% w/w, ∼30 mg/kg) vs. room air–exposed control mice without LXR agonist oral treatment in the diet (0.015% w/w, ∼30 mg/kg). C) Differential BAL macrophage cell count describing regular macrophages and foamy-like macrophages performed by Oil-Red-O staining. D) mRNA analysis of ABCA1 and MMP-9 in BAL cells of mice exposed to cigarette smoke for 5 mo in AKR/J mice with or without LXR agonist oral treatment in the diet (0.015% w/w, ∼30 mg/kg) vs. room air–exposed control mice (n = 8). E) MMP-9 activity assayed by gelatin zymography in BALF of mice exposed to cigarette smoke for 5 mo in AKR/J mice with or without LXR agonist oral treatment in the diet (0.015% w/w, ∼30 mg/kg) vs. room air–exposed control mice treated with or without LXR agonist oral treatment in the diet (0.015% w/w, ∼30 mg/kg). This gelatin zymography is representative of all 4 samples per group. F) Densitometry of MMP-9 activity assays (n = 4). GAPDH was used as a housekeeping gene control for real-time PCR. *P < 0.05, **P < 0.01, ***P < 0.001 when compared with controls, #P < 0.05 compared with 5 mo of cigarette smoke exposure (statistically significant).
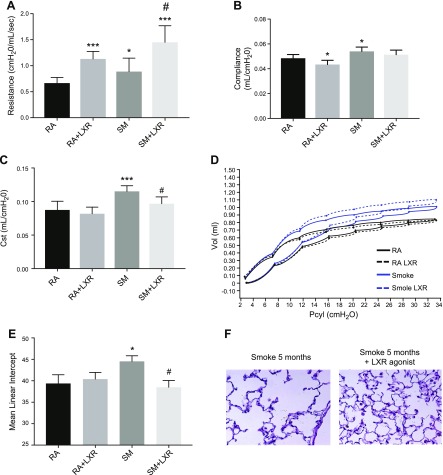
Beyond the inflammatory changes, long-term treatment with LXR agonist in smoke exposure conditions significantly improved lung function as measured by a normalization of lung resistance (Fig. 7A) and compliance (Fig. 7B). Quasi-static compliance, a quantification of pressure-volume loops, demonstrated a normalization of pulmonary elastance in chronic smoke–exposed and LXR agonist–treated mice (Fig. 7C). Representative pressure-volume loops further demonstrate these findings (Fig. 7D). Mean linear intercept quantification revealed that LXR agonist treatment preserved the lung structure and blocked emphysema development in the mice exposed to cigarette smoke for 5 mo (Fig. 7E, F).
Figure 7.
LXR activation significantly alters lung function and structure after chronic cigarette smoke exposure. A–D) Airway resistance (A), pulmonary compliance (B), quasi-static compliance (C), and pressure volume loops (D) were altered in AKR/J mice exposed to cigarette smoke or room air for 5 mo with or without LXR agonist (n = 8) oral treatment in diet. E) Quantitative analysis of lung destruction as represented by mean linear intercept. F) Representative hematoxylin and eosin–stained sections from lungs of mice exposed to cigarette smoke with or without LXR agonist treatment (n = 7–13 per treatment group). *P < 0.05, ***P < 0.001 when compared with room air controls, #P < 0.05 compared with 5 mo of cigarette smoke exposure (statistically significant).
ABC transporter down-regulation in lungs of patients with COPD
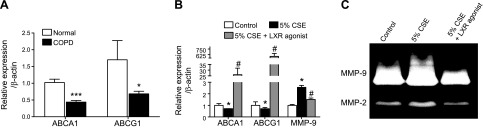
To confirm the relationship of these findings to the human disease, the lung mRNA expression of ABCA1/G1 from patients with or without COPD was evaluated. Down-regulation of ABC transporters, particularly ABCA1, was observed in the lungs of patients diagnosed with COPD (Fig. 8A). In addition, human monocytes that underwent differentiation to macrophages from PBMCs and were exposed to 5% CSE with or without LXR agonist treatment (Fig. 8B, C) were found to down-regulate ABC transporters secondary to cigarette smoke exposure, and the ABC transporter expression was significantly induced by treatment with an LXR agonist (Fig. 8B). Furthermore, exposure to 5% CSE increased MMP-9 expression and activity in these cells, which was subsequently inhibited by treatment with an LXR agonist in vitro (Fig. 8B, C). These studies were repeated with the use of 2 additional LXR agonists, DHMCA and GW3965, which also blocked MMP-9 expression after smoke exposure (Supplemental Fig. S2).
Figure 8.
Down-regulation of ABC transporters in COPD as a potential therapeutic target with LXR agonist. A) mRNA expression analysis of ABCA1/G1 in lungs of patients with moderate and severe COPD (n = 7). B) mRNA analysis of ABCA1, ABCG1, and MMP-9. C) MMP-2 and MMP-9 activity assayed by gelatin zymography in human peripheral blood monocytes differentiated to macrophages in vitro. Macrophages were treated with 5% CSE with or without LXR agonist treatment (3 μM concentration). β-Actin was used as a housekeeping gene control for real-time PCR. *P < 0.05, **P < 0.01, ***P < 0.001 when compared with controls, #P < 0.05 compared with LXR-treated human macrophages (statistically significant).
DISCUSSION
The previously described studies demonstrate that cigarette smoke can down-regulate ABCA1- and ABCG1-dependent cholesterol transport molecules in macrophages and links this change in expression with cigarette smoke–induced pulmonary inflammation and the induction of detrimental MMPs in the lung. The down-regulation of ABC transporter expression in macrophages after cigarette smoke exposure in vivo and in vitro accompanied by the increase in inflammation and MMP-9 activity suggests that cigarette smoke regulation of ABC transporters is a key mechanism regulating emphysema progression. Both the in vivo studies combined with the cell culture experiments demonstrate an important association between cigarette smoke exposure and cholesterol-mediated pathways in macrophages that is regulated by ABC transporters. Importantly, modulation of these pathways through manipulation of ABCA1 with the LXR agonist T0901317 effectively blocked smoke-induced inflammation; other LXR agonists also rescue ABC transporter expression in vitro. The in vitro and in vivo studies clearly demonstrate that LXR agonists, which maintain ABC transporter expression under smoke exposure conditions, can shield the lung from the destructive effects of cigarette smoke. Both short-term and long-term smoke exposure studies document the blunted inflammatory and proteolytic response in animals exposed to smoke and treated with an LXR agonist. Most importantly, the attenuation of inflammation by the LXR agonist was found to protect the animals from the destructive functional and structural changes seen in the lung secondary to chronic smoke exposure. Overall these studies suggest that LXR agonists have the potential to be used as therapeutic agents in COPD.
Over the past several years, the lipid transfer proteins ABCA1 and ABCG1 were demonstrated to be critical molecules in the pathogenesis of atherosclerosis (26, 39). Studies in atherosclerosis demonstrate that ABCA1 activity can suppress macrophage inflammatory cytokine secretion (27, 40, 41). Recent work indicates that the loss of ABCA1 function in macrophages leads to foam cell formation and inflammatory cell recruitment (37). ABCG1 and ABCA1 significantly affect macrophage reverse cholesterol transport but also play a primary role in the maintenance of lipid homeostasis in the lung (42, 43). Although the primary role for ABCA1 is in macrophages, ABCA1 was recently found to be up-regulated in bronchial epithelial cells in response to ozone exposure, but the role for ABCA1 in epithelial cells in the lung under injury conditions is otherwise not described (44). The results of the present study suggest that loss of these transport proteins secondary to cigarette smoke exposure can alter lipid homeostasis in the lung, leading to a heightened inflammatory state. Deficiency in both ABCA1 and ABCG1 in macrophages reduces the protective role of HDL and leads to increased secretion of inflammatory cytokines (27). Consistent with the findings in our study, ABCA1 KO (45) and ABCG1 KO (42) mice manifest abnormal cholesterol efflux, pulmonary inflammation, and other lung structural abnormalities. Macrophages from these mice exhibit an increase in the expression of inflammatory and oxidative stress genes via TLR signaling, suggesting that TLR4 signaling is responsible for both alterations in cholesterol efflux and lung inflammation (23, 46). Induction of inflammatory signaling pathways, mobilization of macrophages to the lung, and tissue-destructive MMP expression in macrophages are hallmarks of cigarette smoke–related pulmonary diseases, such as emphysema. Furthermore, prior studies from our laboratory demonstrate that hypercholesterolemia leads to emphysema development through TLR4 signaling in ApoE KO mice, with mice demonstrating increased inflammatory cells in the lung (28). Here we demonstrate that cigarette smoke can lead to such an inflammatory state simply by disrupting lipid homeostasis through down-regulation of the ABCA1 transport protein.
The use of LXR agonists in the present study demonstrates that these compounds are potentially beneficial for protection against the inflammation and destruction seen in emphysema. Although key LXR target genes include ABC transporters, including ABCA1/G1, LXRs have been described as negative regulators of inflammatory genes and can counteract the LPS-induced expression of MMP-9 (47). In this study, we also demonstrate that several LXR agonists protect from cigarette smoke–induced MMP-9 expression and show that this protection does not occur in the absence of ABCA1, suggesting that the direct effect of LXR agonists on ABCA1 gene expression results in decreased protease expression. Although LXR agonists have been proposed as potential therapeutic agents in atherosclerosis, they have not been tested in clinical trials due to their adverse effects on the liver, leading to hepatic lipogenesis, hypertriglyceridemia, and hepatosteatosis (48). Newer synthetic LXR agonists with limited liver effects are available (49–51) and can be tested in cigarette smoke models in addition to developing inhaled compounds that would likely not target the liver.
Mutations in ABCA1 are linked to low serum HDL levels in Tangiers disease and alter coronary artery disease risk (52, 53). Genome-wide studies in the general population have consistently identified polymorphisms in the ABCA1 loci as a determinant of HDL cholesterol (54, 55); however, whether these are associated with pulmonary disease is not as clear. ApoA1, a major component of HDL, is decreased in the lungs of patients with COPD; overexpression of ApoA1 in the epithelial cell attenuates smoke-induced emphysema development (56), and loss of ApoA1 increases airway inflammation in a smoke exposure model (56), further supporting the immunomodulatory role of ApoA1 and HDL in lung disease. Future studies examining large COPD cohorts, such as COPD gene and Spiromics focusing on HDL and ABC transport genes, could potentially reveal an association with disease susceptibility.
Smoke regulation of cholesterol transport, inflammation, and lung tissue destruction by MMPs appear to be mechanistically linked to changes in transporter expression, playing a role in the increased risk of emphysema development in smokers and potentially presenting a therapeutic target. This study describes a common overlapping mechanism in the development of atherosclerosis and emphysema. Correlative and epidemiologic studies suggest a link between cardiovascular disease and the destruction of the lung from cigarette smoke exposure; however, a unifying pathogenic link has not been described. The effect of cigarette smoke on expression of the major ABC transporters within the lung suggest that the disruption of lipid homeostasis could be an initial event leading to chronic inflammation after smoke exposure. The increased production of proteases by macrophages with lower levels of ABC transporter activity additionally supports the critical role of lipid homeostasis in smoke-induced lung disease. Most significantly, the present study identifies a novel therapeutic approach, already developed in the field of atherosclerosis, that has the potential to protect from the development of emphysema after smoke exposure.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Alan Tall (Columbia University) for the donation of LysmCreAbca1flox/flox mice, and Dr. Emilio Arteaga-Solis (Columbia University) for assistance with the airway mechanics assessment. This work was supported by the U.S. National Institutes of Health (NIH) National Heart, Lung, and Blood Institute Grants R01 HL086936 (to J.D.), K08 HL126071 (to M.G.), T32 HL007343 (Principal Investigator: Dr. Ira Goldberg, supported M.G.), NIH National Institute of General Medical Sciences Grant T32 GM008464 (Principal Investigator: Dr. Charles Emala, supported A.G.), and the Stony Wold-Herbert Fund (to A.G.). M.W. was supported by the Netherlands Organization of Scientific Research VIDI grant 917.15.350 and a Rosalind Franklin Fellowship from the University of Groningen (Groningen, The Netherlands). J.S., M.G., and P.S. contributed equally to this work.
Glossary
- ABC
ATP binding cassette
- Apo
apolipoprotein
- BMDM
bone marrow–derived macrophage
- COPD
chronic obstructive pulmonary disease
- CSE
cigarette smoke extract
- KO
knockout
- LXR
liver X receptor
- MMP
matrix metalloproteinase
- Myd88
myeloid differentiation primary response gene 88
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
J. D’Armiento conceived the study; J. Sonett, M. Goldklang, P. Sklepkiewicz, and J. D’Armiento designed the research experiments; J. Sonett, M. Goldklang, P. Sklepkiewicz, Y. Okada, and J. D’Armiento, analyzed data; J. Sonett, M. Goldklang, P. Sklepkiewicz, A. Gerber, J. Trischler, T. Zelonina, M. Westerterp, and V. Lemaître performed the experiments; J. Sonett, M. Goldklang, P. Sklepkiewicz, and J. D’Armiento wrote the manuscript; and Y. Okada reviewed the histology slides.
REFERENCES
- 1.Finkelstein R., Fraser R. S., Ghezzo H., Cosio M. G. (1995) Alveolar inflammation and its relation to emphysema in smokers. Am. J. Respir. Crit. Care Med. 152, 1666–1672 [DOI] [PubMed] [Google Scholar]
- 2.Finkelstein R., Ma H. D., Ghezzo H., Whittaker K., Fraser R. S., Cosio M. G. (1995) Morphometry of small airways in smokers and its relationship to emphysema type and hyperresponsiveness. Am. J. Respir. Crit. Care Med. 152, 267–276 [DOI] [PubMed] [Google Scholar]
- 3.Hansson G. K. (2005) Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352, 1685–1695 [DOI] [PubMed] [Google Scholar]
- 4.Barnoya J., Glantz S. A. (2005) Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation 111, 2684–2698 [DOI] [PubMed] [Google Scholar]
- 5.Freund K. M., Belanger A. J., D’Agostino R. B., Kannel W. B. (1993) The health risks of smoking. The Framingham Study: 34 years of follow-up. Ann. Epidemiol. 3, 417–424 [DOI] [PubMed] [Google Scholar]
- 6.Howard G., Wagenknecht L. E., Burke G. L., Diez-Roux A., Evans G. W., McGovern P., Nieto F. J., Tell G. S. (1998) Cigarette smoking and progression of atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) study. JAMA 279, 119–124 [DOI] [PubMed] [Google Scholar]
- 7.Iwamoto H., Yokoyama A., Kitahara Y., Ishikawa N., Haruta Y., Yamane K., Hattori N., Hara H., Kohno N. (2009) Airflow limitation in smokers is associated with subclinical atherosclerosis. Am. J. Respir. Crit. Care Med. 179, 35–40 [DOI] [PubMed] [Google Scholar]
- 8.Mannino D. M., Buist A. S. (2007) Global burden of COPD: risk factors, prevalence, and future trends. Lancet 370, 765–773 [DOI] [PubMed] [Google Scholar]
- 9.Gadek J. E., Fells G. A., Crystal R. G. (1979) Cigarette smoking induces functional antiprotease deficiency in the lower respiratory tract of humans. Science 206, 1315–1316 [DOI] [PubMed] [Google Scholar]
- 10.Fujita J., Nelson N. L., Daughton D. M., Dobry C. A., Spurzem J. R., Irino S., Rennard S. I. (1990) Evaluation of elastase and antielastase balance in patients with chronic bronchitis and pulmonary emphysema. Am. Rev. Respir. Dis. 142, 57–62 [DOI] [PubMed] [Google Scholar]
- 11.Imai K., Dalal S. S., Chen E. S., Downey R., Schulman L. L., Ginsburg M., D’Armiento J. (2001) Human collagenase (matrix metalloproteinase-1) expression in the lungs of patients with emphysema. Am. J. Respir. Crit. Care Med. 163, 786–791 [DOI] [PubMed] [Google Scholar]
- 12.Hautamaki R. D., Kobayashi D. K., Senior R. M., Shapiro S. D. (1997) Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 277, 2002–2004 [DOI] [PubMed] [Google Scholar]
- 13.Aqel N. M., Ball R. Y., Waldmann H., Mitchinson M. J. (1985) Identification of macrophages and smooth muscle cells in human atherosclerosis using monoclonal antibodies. J. Pathol. 146, 197–204 [DOI] [PubMed] [Google Scholar]
- 14.Kratzer A., Salys J., Nold-Petry C., Cool C., Zamora M., Bowler R., Koczulla A. R., Janciauskiene S., Edwards M. G., Dinarello C. A., Taraseviciene-Stewart L. (2013) Role of IL-18 in second-hand smoke-induced emphysema. Am. J. Respir. Cell Mol. Biol. 48, 725–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirama N., Shibata Y., Otake K., Machiya J., Wada T., Inoue S., Abe S., Takabatake N., Sata M., Kubota I. (2007) Increased surfactant protein-D and foamy macrophages in smoking-induced mouse emphysema. Respirology 12, 191–201 [DOI] [PubMed] [Google Scholar]
- 16.De Padua Mansur A., Caramelli B., Vianna C. B., Chamone D., Ramires J. A. (1997) Smoking and lipoprotein abnormalities on platelet aggregation in coronary heart disease. Int. J. Cardiol. 62, 151–154 [DOI] [PubMed] [Google Scholar]
- 17.Moskowitz W. B., Mosteller M., Schieken R. M., Bossano R., Hewitt J. K., Bodurtha J. N., Segrest J. P. (1990) Lipoprotein and oxygen transport alterations in passive smoking preadolescent children. The MCV Twin Study. Circulation 81, 586–592 [DOI] [PubMed] [Google Scholar]
- 18.Feldman J., Shenker I. R., Etzel R. A., Spierto F. W., Lilienfield D. E., Nussbaum M., Jacobson M. S. (1991) Passive smoking alters lipid profiles in adolescents. Pediatrics 88, 259–264 [PubMed] [Google Scholar]
- 19.Moffatt R. J., Chelland S. A., Pecott D. L., Stamford B. A. (2004) Acute exposure to environmental tobacco smoke reduces HDL-C and HDL2-C. Prev. Med. 38, 637–641 [DOI] [PubMed] [Google Scholar]
- 20.Cirillo D. J., Agrawal Y., Cassano P. A. (2002) Lipids and pulmonary function in the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 155, 842–848 [DOI] [PubMed] [Google Scholar]
- 21.Ortiz-Muñoz G., Houard X., Martín-Ventura J. L., Ishida B. Y., Loyau S., Rossignol P., Moreno J. A., Kane J. P., Chalkley R. J., Burlingame A. L., Michel J. B., Meilhac O. (2009) HDL antielastase activity prevents smooth muscle cell anoikis, a potential new antiatherogenic property. FASEB J. 23, 3129–3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreno J. A., Ortega-Gomez A., Rubio-Navarro A., Louedec L., Ho-Tin-Noé B., Caligiuri G., Nicoletti A., Levoye A., Plantier L., Meilhac O. (2014) High-density lipoproteins potentiate α1-antitrypsin therapy in elastase-induced pulmonary emphysema. Am. J. Respir. Cell Mol. Biol. 51, 536–549 [DOI] [PubMed] [Google Scholar]
- 23.Yvan-Charvet L., Wang N., Tall A. R. (2010) Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler. Thromb. Vasc. Biol. 30, 139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adorni M. P., Zimetti F., Billheimer J. T., Wang N., Rader D. J., Phillips M. C., Rothblat G. H. (2007) The roles of different pathways in the release of cholesterol from macrophages. J. Lipid Res. 48, 2453–2462 [DOI] [PubMed] [Google Scholar]
- 25.Out R., Jessup W., Le Goff W., Hoekstra M., Gelissen I. C., Zhao Y., Kritharides L., Chimini G., Kuiper J., Chapman M. J., Huby T., Van Berkel T. J., Van Eck M. (2008) Coexistence of foam cells and hypocholesterolemia in mice lacking the ABC transporters A1 and G1. Circ. Res. 102, 113–120 [DOI] [PubMed] [Google Scholar]
- 26.Wang N., Lan D., Chen W., Matsuura F., Tall A. R. (2004) ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. USA 101, 9774–9779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yvan-Charvet L., Ranalletta M., Wang N., Han S., Terasaka N., Li R., Welch C., Tall A. R. (2007) Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J. Clin. Invest. 117, 3900–3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldklang M., Golovatch P., Zelonina T., Trischler J., Rabinowitz D., Lemaître V., D’Armiento J. (2012) Activation of the TLR4 signaling pathway and abnormal cholesterol efflux lead to emphysema in ApoE-deficient mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 302, L1200–L1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Libby P. (2002) Inflammation in atherosclerosis. Nature 420, 868–874 [DOI] [PubMed] [Google Scholar]
- 30.Mercer B. A., Kolesnikova N., Sonett J., D’Armiento J. (2004) Extracellular regulated kinase/mitogen activated protein kinase is up-regulated in pulmonary emphysema and mediates matrix metalloproteinase-1 induction by cigarette smoke. J. Biol. Chem. 279, 17690–17696 [DOI] [PubMed] [Google Scholar]
- 31.Chavez-Santoscoy A. V., Huntimer L. M., Ramer-Tait A. E., Wannemuehler M., Narasimhan B. (2012) Harvesting murine alveolar macrophages and evaluating cellular activation induced by polyanhydride nanoparticles. J. Vis. Exp. 64, e3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foronjy R. F., Mercer B. A., Maxfield M. W., Powell C. A., D’Armiento J., Okada Y. (2005) Structural emphysema does not correlate with lung compliance: lessons from the mouse smoking model. Exp. Lung Res. 31, 547–562 [DOI] [PubMed] [Google Scholar]
- 33.Geraghty P., Dabo A. J., D’Armiento J. (2011) TLR4 protein contributes to cigarette smoke-induced matrix metalloproteinase-1 (MMP-1) expression in chronic obstructive pulmonary disease. J. Biol. Chem. 286, 30211–30218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schultz J. R., Tu H., Luk A., Repa J. J., Medina J. C., Li L., Schwendner S., Wang S., Thoolen M., Mangelsdorf D. J., Lustig K. D., Shan B. (2000) Role of LXRs in control of lipogenesis. Genes Dev. 14, 2831–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maes T., Bracke K. R., Vermaelen K. Y., Demedts I. K., Joos G. F., Pauwels R. A., Brusselle G. G. (2006) Murine TLR4 is implicated in cigarette smoke-induced pulmonary inflammation. Int. Arch. Allergy Immunol. 141, 354–368 [DOI] [PubMed] [Google Scholar]
- 36.Doz E., Noulin N., Boichot E., Guénon I., Fick L., Le Bert M., Lagente V., Ryffel B., Schnyder B., Quesniaux V. F., Couillin I. (2008) Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J. Immunol. 180, 1169–1178 [DOI] [PubMed] [Google Scholar]
- 37.Westerterp M., Murphy A. J., Wang M., Pagler T. A., Vengrenyuk Y., Kappus M. S., Gorman D. J., Nagareddy P. R., Zhu X., Abramowicz S., Parks J. S., Welch C., Fisher E. A., Wang N., Yvan-Charvet L., Tall A. R. (2013) Deficiency of ATP-binding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ. Res. 112, 1456–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu X., Lee J. Y., Timmins J. M., Brown J. M., Boudyguina E., Mulya A., Gebre A. K., Willingham M. C., Hiltbold E. M., Mishra N., Maeda N., Parks J. S. (2008) Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J. Biol. Chem. 283, 22930–22941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marcil M., Brooks-Wilson A., Clee S. M., Roomp K., Zhang L. H., Yu L., Collins J. A., van Dam M., Molhuizen H. O., Loubster O., Ouellette B. F., Sensen C. W., Fichter K., Mott S., Denis M., Boucher B., Pimstone S., Genest J., Jr., Kastelein J. J., Hayden M. R. (1999) Mutations in the ABC1 gene in familial HDL deficiency with defective cholesterol efflux. Lancet 354, 1341–1346 [DOI] [PubMed] [Google Scholar]
- 40.Koseki M., Hirano K., Masuda D., Ikegami C., Tanaka M., Ota A., Sandoval J. C., Nakagawa-Toyama Y., Sato S. B., Kobayashi T., Shimada Y., Ohno-Iwashita Y., Matsuura F., Shimomura I., Yamashita S. (2007) Increased lipid rafts and accelerated lipopolysaccharide-induced tumor necrosis factor-alpha secretion in Abca1-deficient macrophages. J. Lipid Res. 48, 299–306 [DOI] [PubMed] [Google Scholar]
- 41.Tang C., Liu Y., Kessler P. S., Vaughan A. M., Oram J. F. (2009) The macrophage cholesterol exporter ABCA1 functions as an anti-inflammatory receptor. J. Biol. Chem. 284, 32336–32343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baldán A., Gomes A. V., Ping P., Edwards P. A. (2008) Loss of ABCG1 results in chronic pulmonary inflammation. J. Immunol. 180, 3560–3568 [DOI] [PubMed] [Google Scholar]
- 43.Bortnick A. E., Favari E., Tao J. Q., Francone O. L., Reilly M., Zhang Y., Rothblat G. H., Bates S. R. (2003) Identification and characterization of rodent ABCA1 in isolated type II pneumocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 285, L869–L878 [DOI] [PubMed] [Google Scholar]
- 44.Speen A. M., Kim H. H., Bauer R. N., Meyer M., Gowdy K. M., Fessler M. B., Duncan K. E., Liu W., Porter N. A., Jaspers I. (2016) Ozone-derived oxysterols affect liver X receptor (LXR) signaling: a potential role for lipid-protein adducts. J. Biol. Chem. 291, 25192–25206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bates S. R., Tao J. Q., Collins H. L., Francone O. L., Rothblat G. H. (2005) Pulmonary abnormalities due to ABCA1 deficiency in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 289, L980–L989 [DOI] [PubMed] [Google Scholar]
- 46.Yvan-Charvet L., Welch C., Pagler T. A., Ranalletta M., Lamkanfi M., Han S., Ishibashi M., Li R., Wang N., Tall A. R. (2008) Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation 118, 1837–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai X., Ou X., Hao X., Cao D., Tang Y., Hu Y., Li X., Tang C. (2007) Effect of T0901317 on hepatic proinflammatory gene expression in apoE-/- mice fed a high-fat/high-cholesterol diet. Inflammation 30, 105–117 [DOI] [PubMed] [Google Scholar]
- 48.Laurencikiene J., Rydén M. (2012) Liver X receptors and fat cell metabolism. Int. J. Obes. 36, 1494–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng D., Hiipakka R. A., Xie J. T., Dai Q., Kokontis J. M., Reardon C. A., Getz G. S., Liao S. (2011) A novel potent synthetic steroidal liver X receptor agonist lowers plasma cholesterol and triglycerides and reduces atherosclerosis in LDLR(-/-) mice. Br. J. Pharmacol. 162, 1792–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu S., Li S., Henke A., Muse E. D., Cheng B., Welzel G., Chatterjee A. K., Wang D., Roland J., Glass C. K., Tremblay M. (2016) Dissociated sterol-based liver X receptor agonists as therapeutics for chronic inflammatory diseases. FASEB J. 30, 2570–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flaveny C. A., Griffett K., El-Gendy B.-D., Kazantzis M., Sengupta M., Amelio A. L., Chatterjee A., Walker J., Solt L. A., Kamenecka T. M., Burris T. P. (2015) Broad anti-tumor activity of a small molecule that selectively targets the Warburg effect and lipogenesis. Cancer Cell 28, 42–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bochem A. E., van Wijk D. F., Holleboom A. G., Duivenvoorden R., Motazacker M. M., Dallinga-Thie G. M., de Groot E., Kastelein J. J., Nederveen A. J., Hovingh G. K., Stroes E. S. (2013) ABCA1 mutation carriers with low high-density lipoprotein cholesterol are characterized by a larger atherosclerotic burden. Eur. Heart J. 34, 286–291 [DOI] [PubMed] [Google Scholar]
- 53.Brooks-Wilson A., Marcil M., Clee S. M., Zhang L. H., Roomp K., van Dam M., Yu L., Brewer C., Collins J. A., Molhuizen H. O., Loubser O., Ouelette B. F., Fichter K., Ashbourne-Excoffon K. J., Sensen C. W., Scherer S., Mott S., Denis M., Martindale D., Frohlich J., Morgan K., Koop B., Pimstone S., Kastelein J. J., Genest J., Jr., Hayden M. R. (1999) Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 22, 336–345 [DOI] [PubMed] [Google Scholar]
- 54.Willer C. J., Sanna S., Jackson A. U., Scuteri A., Bonnycastle L. L., Clarke R., Heath S. C., Timpson N. J., Najjar S. S., Stringham H. M., Strait J., Duren W. L., Maschio A., Busonero F., Mulas A., Albai G., Swift A. J., Morken M. A., Narisu N., Bennett D., Parish S., Shen H., Galan P., Meneton P., Hercberg S., Zelenika D., Chen W. M., Li Y., Scott L. J., Scheet P. A., Sundvall J., Watanabe R. M., Nagaraja R., Ebrahim S., Lawlor D. A., Ben-Shlomo Y., Davey-Smith G., Shuldiner A. R., Collins R., Bergman R. N., Uda M., Tuomilehto J., Cao A., Collins F. S., Lakatta E., Lathrop G. M., Boehnke M., Schlessinger D., Mohlke K. L., Abecasis G. R. (2008) Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat. Genet. 40, 161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edmondson A. C., Braund P. S., Stylianou I. M., Khera A. V., Nelson C. P., Wolfe M. L., Derohannessian S. L., Keating B. J., Qu L., He J., Tobin M. D., Tomaszewski M., Baumert J., Klopp N., Döring A., Thorand B., Li M., Reilly M. P., Koenig W., Samani N. J., Rader D. J. (2011) Dense genotyping of candidate gene loci identifies variants associated with high-density lipoprotein cholesterol. Circ Cardiovasc Genet 4, 145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jubinville É., Talbot M., Bérubé J. C., Hamel-Auger M., Maranda-Robitaille M., Beaulieu M. J., Aubin S., Paré M. E., Kallend D. G., Arsenault B., Bossé Y., Morissette M. C. (2017) Interplay between cigarette smoking and pulmonary reverse lipid transport. Eur. Respir. J. 50, 1700681 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.